Abstract
A domestic ferret was presented for episodic regurgitation. Cytologic examination and culture of an enlarged submandibular lymph node revealed Cryptococcus neoformans variety grubii (serotype A). The ferret was successfully treated with itraconazole. This is the first documented case of Cryptococcus neoformans variety grubii in a ferret in the United States.
Résumé
Diagnostic et traitement efficaces de Cryptococcus neoformans variété grubii chez un furet domestique. Un furet domestique a été présenté pour régurgitation épisodique. Un examen cytologique et une culture d’un ganglion submandibulaire tuméfié a révélé la présence de Cryptococcus neoformans variété grubii (sérotype A). Le furet a été traité avec succès à l’itraconazole. Il s’agit du premier cas authentifié de Cryptococcus neoformans variété grubii chez un furet aux Etats-Unis.
(Traduit par Docteur André Blouin)
An 18-month-old, castrated male, domestic ferret (Mustela putorius furo), adopted from a Wisconsin animal shelter 2 mo earlier, was presented to the Special Species Service of the University of Wisconsin Veterinary Medical Teaching Hospital with a history of regurgitation. The ferret had regurgitated clear, watery fluid 3 times in the 48 h prior to presentation, possibly in association with drinking. The animal was otherwise active and was eating and defecating normally.
Case description
On examination, the ferret weighed 1.07 kg and was in good body condition with a full hair coat. The only abnormal finding was a marked right submandibular lymphadenopathy. Oral examination failed to reveal fractured teeth, gingivitis, or inflammation, and only minimal tartar was present on the carnassial teeth.
The ferret was anesthetized with isoflurane (Isoflo; Abbot Laboratories, Chicago, Illinois, USA) administered via facemask. No abnormalities were identified on whole body radiographs. Complete blood cell count and serum biochemical profile parameters were within reference ranges, except for a monocytosis (0.64 × 109/L; reference interval, 0 to 0.432 × 109/L), hypocalcemia (2.0 mmol/L; reference interval, 2.15 to 2.62 mmol/L), hypophosphatemia (1.61 mmol/L; reference interval, 1.81 to 2.81 mmol/L), hypoproteinemia (52 g/L; reference interval, 53 to 72 g/L), and hypoalbuminemia (27 g/L; reference interval, 33 to 41 g/L) (1). The right submandibular lymph node was aspirated and on cytologic examination, a predominance of small lymphocytes and a moderate increase in immature lymphocytes was observed. A large number of large foamy macrophages containing phagocytosed debris, granules, and small yeast organisms were observed. Large encapsulated yeast organisms, some with narrow-based budding, were present in small groups (Figure 1). A diagnosis of granulomatous lymphadenitis with moderate reactive hyperplasia due to a Cryptococcus sp. infection was made. A Cryptococcus latex antigen agglutination test (Cryptococcal Antigen Latex Agglutination System [CALAS]; Meridian Bioscience, Cincinnati, Ohio, USA) was positive with a titer of > 1:4096. The aspirate was submitted for fungal culture and yielded Cryptococcus neoformans. The organism was then sent to an outside laboratory (Duke University Medical Center, Durham, North Carolina, USA) for further classification. Serotype and mating type of the isolate were determined by using a slide agglutination assay (Cryptocheck; Mitsubishi Kagaku Iatron, Tokyo, Japan) and a mating type assay with the Kn99a (MATa) and Kn99alpha (MATalpha) strains, respectively. Melanin production was assessed by growing cells on L-dopa agar and visualizing changes in the coloration of Cryptococcus sp. colonies. Analysis of the ferret isolate revealed that it was a melanin-positive C. neoformans variety (var.) grubii (serotype A) strain of the MATalpha mating type. After discussing potential zoonotic concerns and the possibility of an environmental source that the owners could be exposed to, the ferret was discharged on itraconazole suspension (Sporonox; Janssen Pharmaceutica, Titusville, New Jersey, USA), 15 mg/kg bodyweight (BW), PO, q24h). The owners were instructed to monitor the size of the lymph node, watch for regurgitation, anorexia, diarrhea, weight loss, or lethargy, and to return for reevaluation.
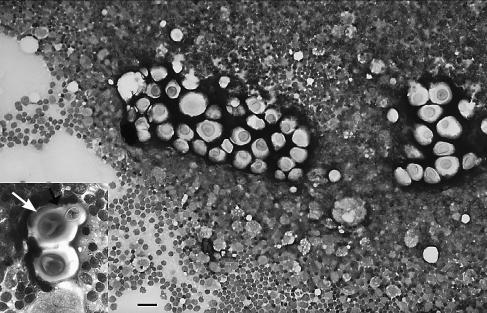
Figure 1.
Photomicrograph of a fine needle aspirate from the submandibular lymph node of a ferret with cryptococcosis. Cryptococcus neoformans vary in size, are surrounded by a negative staining capsule, and highlighted by a background population of lymphocytes and a few large macrophages. Wright-Giemsa stain; bar = 32 μm. Insert: Higher magnification of 2 Cryptococcus neoformans surrounded by lymphocytes. A bud with narrow base and surrounding capsule can be seen in the uppermost organism (arrow). Wright-Giemsa stain; bar = 14 μm.
Three weeks later, the owners reported no further regurgitation and no other symptoms had developed. The right submandibular lymph node, although still enlarged, was now only 1/3 of the size at initial presentation, and a physical examination, including a fundic examination, was otherwise unremarkable. The Cryptococcus antigen titer had dropped to 1:64. As the itraconazole suspension was difficult to administer, the ferret was switched to itraconazole capsules. Each 100 mg capsule contained approximately 700 beads (7 beads/mg). Thus, the owner would open a capsule, count 105 beads, and hide them in canned cat food or meat-based baby food. This method made daily compliance much easier.
At the 6-week reevaluation, the lymph node had returned to normal size and no signs had been noted by the owner since the original presentation. At each subsequent reevaluation, titers declined steadily until the Cryptococcus antigen titer was negative, nearly 10 mo after presentation. At that time, a repeat biochemical profile revealed resolution of all abnormalities. Although the ferret’s alanine aminotransferase was elevated from baseline levels (231 U/L up from 76 U/L), it was still within reference range (82–289 U/L) (1). Treatment was continued for 3 wk after the negative titer had been achieved.
Discussion
Cryptococcus neoformans is found worldwide and can infect a variety of hosts including humans, and domestic and wild mammals (2). Recent DNA sequencing has led to reclassification of the 2 previously identified varieties, neoformans (serotypes A, D, and AD) and gatti (serotypes B and C) (2). Serotype A is now considered C. neoformans var. grubii, and serotype D is C. neoformans var. neoformans. Cryptococcus neoformans var. gatti (sometimes referred to as C. bacillisporus) still included serotypes B and C, but it has recently been reclassified as a new species, C. gatti (3–5).
While C. neoformans var. grubii can be isolated from multiple environmental sources, including soil, fruits, and the oropharynx, gastrointestinal tract, and skin of healthy humans, the most common source is avian excreta, especially that of pigeons (2). Infection most likely occurs through inhalation of the organism, as suggested by the high prevalence of nasal cryptococcosis in cats (80%) (2). From there, the organism can disseminate hematogeneously, most often to the central nervous system.
While C. neoformans var. grubii infections usually occur in immunocompromised humans (6), the fungus appears to act as a primary pathogen in dogs and cats (2,7). There have been multiple reported cases of cryptococcosis in ferrets (3 were var. gatti, 3 were var. grubii, and the remainder were not identified beyond C. neoformans) (5,7–9) and presenting signs varied in each case, ranging from localized masses on the nose or digit to lymphadenopathy, swollen limbs, dyspnea, acute paralysis, and death. All animals were generally in good health and did not appear to be immunocompromised. Therefore, it appears that C. neoformans should also be considered a primary pathogen in ferrets.
While cryptococcosis in ferrets has been seen repeatedly in Australia (5) and Canada (5,7,9), there have been very limited reports regarding ferret cryptococcosis in the United States (8). Ferrets in Australia are usually infected with C. neoformans var. gatti, which is associated with the bark and leaf litter of eucalyptus trees (2), and ferrets in Canada have been infected with C. neoformans var. grubii. Prior cases in the United States have not been keyed to variety or serotype, and were diagnosed after death (8). This case is not only the first documented ante mortem diagnosis and successful treatment of cryptococcosis in a ferret in the United States, but the first documented case of C. neoformans var. grubii in a ferret in the United States.
Clinical signs of cryptococcosis vary, based on location and severity of the disease. Cats often have submandibular lymphadenopathy (2), the only abnormality on physical examination in this ferret. Diagnosis of cryptococcosis is often made, initially, on cytologic examination of aspirates or exudates and, subsequently, substantiated by using a latex cryptococcal antigen agglutination test (LCAT). The LCAT is widely utilized, has high specificity and sensitivity, and can be used to monitor response to treatment, as it measures antigen levels. Decreasing titers correlate with therapeutic efficacy and indicate a favorable prognosis (2). Treatment should be continued until the LCAT titer is undetectable (2). Isolation of Cryptococcus from clinical samples is relatively easy and allows for a definite diagnosis of cryptococcosis, but most laboratories do not offer identification of the variety or serotype.
Antifungal medication is the mainstay of therapy, although surgical debulking of large masses can be a useful adjunct. Itraconazole is usually the drug of choice to treat cryptococcosis. In cats, there are fewer side effects with itraconazole than with other imidazoles (2) and this seems to hold true for ferrets. Fluconazole is used for central nervous cryptococcosis in cats and ferrets have been treated with this drug as well (5). Itraconazole dosage in this ferret (15 mg/kg BW, PO, q24h) was based on that used in other ferrets (5) and is similar to the dose used for cats (5–10 mg/kg BW, PO, q12h or 20 mg/kg BW, PO, q24h for 6–10 mo) (2).
In this ferret, cryptococcosis appeared to be confined to the right submandibular lymph node, while other organ systems remained unaffected. There was no evidence to support the possibility of direct inoculation from a penetrating injury or bite wound, and the route of exposure remains unknown. While cryptococcosis can be focal, especially in cats and ferrets (2,5), it seems unlikely that the organism could have reached the lymph node without causing infection elsewhere. The progressive decline in serum cryptococcal antigen titers, combined with rapid resolution of the clinical signs, were useful indicators of a positive outcome. Early, aggressive, and lengthy therapy is necessary to treat cryptococcosis successfully (2,5), and there were no side effects from 10 mo of itraconazole therapy in this animal.
The source of this ferret’s infection remains unknown. There was nothing unusual in the history or activity of the ferret in the 2 mo it was with its owner. No members of the household or any of the owner’s other pets appear to have been infected. The owner’s local public health department did not report a case of human cryptococcosis during the ferret’s illness (Whitehouse J, Washington County Health Department, West Bend, Wisconsin, USA: personal communication, 2005). This animal may have been infected prior to entering the household, as a protracted latency period has been described in koalas (10) and humans (11), and postulated in ferrets (5). However, the animal shelter from which the ferret was acquired reported that it had been housed indoors, had had no prior illnesses, and had never been outside the state of Wisconsin.
This case serves as a reminder that, while sporadically reported, ferrets are susceptible to infection with C. neoformans and clinicians should include it on their list of differential diagnoses when presented with a ferret with lymphadenopathy, respiratory signs, or swollen limbs. Further investigation into the pathogenicity of Cryptococcus in ferrets is warranted.
Footnotes
Dr. Hanley’s current address is The Toledozoo, PO Box 140130, Toledo, Ohio, 43614, USA.
References
- 1.Quesenberry KE, Orcutt C. Basic approach to veterinary care. In: Quesenberry KE, Carpenter JW, eds. Ferrets, Rabbits, and Rodents. 2nd ed. Philadelphia: WB Saunders, 2004:13–24.
- 2.Jacobs GJ, Medleau L. Cryptococcosis. In: Greene CE, ed. Infectious Diseases of the Dog and Cat. 2nd ed. Philadelphia: WB Saunders, 1998:383–390.
- 3.Franzot SP, Salkin IF, Casadevall A. Cryptococcus neoformans var grubii: separate varietal status for Cryptococcus neoformans serotype A isolates. J Clin Microbiol. 1999;37:838–840. doi: 10.1128/jcm.37.3.838-840.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kwon-Chung KJ, Boekhout T, Fell JW, Diaz M. Proposal to conserve the name Cryptococcus gatii against C. hondurianus and C. bacillisporus (Basidiomycota, Hymenomycetes, Tremellomycetiadae) Taxon. 2002;51:804–806. [Google Scholar]
- 5.Malik R, Alderton B, Finlaison D, et al. Cryptococcosis in ferrets: A diverse spectrum of clinical disease. Aust Vet J. 2002;80:749–755. doi: 10.1111/j.1751-0813.2002.tb11343.x. [DOI] [PubMed] [Google Scholar]
- 6.Comaru-Pasqualotto A, Bittencourt-Severo C, de Mattos-Oliveira F, Severo LC. Cryptococcemia. An analysis of 28 cases with emphasis on the clinical outcome and its etiologic agent. Rev Iberoam Micol. 2004;21:143–146. [PubMed] [Google Scholar]
- 7.Lester SJ, Kowalewich NJ, Bartlett KH, Krockenberger MB, Fairfax TM, Malik R. Clinicopathologic features of an unusual outbreak of cryptococcosis in dogs, cats, ferrets, and a bird: 38 cases (January to July 2003) J Am Vet Med Assoc. 2004;225:1716–1722. doi: 10.2460/javma.2004.225.1716. [DOI] [PubMed] [Google Scholar]
- 8.Greenlee PG, Stephens E. Meningeal cryptococcosis and congestive cardiomyopathy in a ferret. J Am Vet Med Assoc. 1984;184:840–841. [PubMed] [Google Scholar]
- 9.Stephen C, Lester S, Black W, Fyfe M, Raverty S. Multispecies outbreak of cryptococcosis on southern Vancouver Island, British Columbia. Can Vet J. 2002;43:792–794. [PMC free article] [PubMed] [Google Scholar]
- 10.Krockenberger MB, Canfield PJ, Barnes J, et al. Cryptococcus neoformans variety gatti in the koala (Phascolarctos cinereus): Serological evidence for subclinical cryptococcosis. Med Mycol. 2002;40:273–282. doi: 10.1080/mmy.40.3.273.282. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Hermoso D, Janbon G, Dromer F. Epidemiological evidence for dormant Cryptococcus neoformans infection. J Clin Microbiol. 1999;37:3204–3209. doi: 10.1128/jcm.37.10.3204-3209.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]