Abstract
A province-wide, cross-sectional seroprevalence and agroecological risk factor study of Mycobacterium avium subspecies paratuberculosis (MAP), Neospora caninum (NC), Bovine leukemia virus (BLV), and Bovine viral diarrhea virus (BVDv) genotypes 1 and 2 (BVDv1 and BVDv2) infection in dairy cattle herds in Alberta was conducted. Among adults, the seroprevalence of MAP, NC, and BLV was 9.1%, 18.5%, and 26.9%, respectively. For MAP, based on a herd test cutpoint of 2 or more seropositive cows, 58.8% of herds were infected. Herd-level seroprevalence for NC and BLV was 98.7% and 86.7%, respectively, based on a herd-test cutpoint of 1 seropositive cow. Among unvaccinated dairy heifers, the seroprevalence for BVDv1 and BVDv2 infection was 28.4% and 8.9%, respectively, while herd-level infection was 53.4% and 19.7%. Seroprevalence for MAP varied moderately by agroecological region, whereas that for NC, BLV, and BVDv1 and BVDv2 did not. For MAP, aridity and soil pH (correlated features of the region) were also important.
Résumé
Séroprévalence des infections à Mycobacterium avium sous-espèce paratuberculosis, à Neospora caninum, au virus de la leucémie bovine et au virus de la diarrhée bovine virale parmi les bovins et les troupeaux laitiers de l’Alberta et facteurs de risques agroécologiques associés à la séropositivité. Une étude de séroprévalence transversale et de facteurs de risques agroécologiques a été menée à la grandeur de l’Alberta sur l’infection des troupeaux laitiers par Mycobacterium avium sous-espèce paratuberculosis (MAP), Neospora caninum (NC), le virus de la leucémie bovine (VLB) et le virus de la diarrhée bovine virale (VDBv) génotype 1 et 2 (VDBV1 et VDBV2). Parmi les adultes, la séroprévalence au MAP, NC et VLB était respectivement de 9,1, 18,5 et 26,9 %. Pour le MAP, avec un seuil de 2 vaches positives ou plus par troupeau, 58,8 % des troupeaux étaient infectés. Le niveau de séroprévalence de troupeaux pour NC et le VLB était respectivement à 98,7 et 86,7 %, avec un seuil d’une vache séropositive par troupeau. Parmi les génisses laitières non vaccinées, la séroprévalence pour les infections au VDBV1 et VDBV2 était respectivement de 28,4 et 8,9 % alors que le taux d’infection des troupeaux était de 53,4 et 19,7 %. La séroprévalence du MAP varie modérément selon les régions agroécologiques alors que pour NC, le VLB et le VDBV1 et VDBV2 la séroprévalence ne variait pas. Pour MAP, l’aridité et le pH du sol (caractéristiques combinées des régions) étaient également importants.
(Traduit par Docteur André Blouin)
Introduction
Losses due to infectious diseases of livestock generally fall into 3 categories: 1) mortality, morbidity, impaired productivity, or all three, 2) lack of livestock market access for regional, national, or international trade, and 3) public perceptions of food safety/public health risks associated with consumption of food products from infected animals or herds. In recent years, while focus has increasingly been on infectious diseases with implications for either national or international trade, or public health/food safety risks, concern has remained among various livestock industries about the so-called “production-limiting diseases.” In response to these ongoing concerns, national and provincial agencies and cattle livestock industry groups formed a Production Limiting Diseases Committee (PLDC), whose initial efforts to quantify and characterize the prevalence and risk factors for 4 major diseases in Canada have lead directly to the present study. Specifically, initial efforts to support inquiries into the magnitude of impacts from infections due to 1) Mycobacterium avium subspecies paratu-berculosis (MAP — the causative agent of Johne’s disease [JD]), 2) Neospora caninum (NC), 3) Bovine leukemia virus (BLV), and 4) Bovine viral diarrhea virus (BVDv) have been underway since 1998 nationally (1). Recent efforts to reinvigorate the Johne’s Disease Working Group and enact a voluntary Johne’s disease control program in Alberta have led to a multipronged approach to education and training, research, and extension (2).
Johne’s disease is a chronic, insidious disease of ruminants worldwide (3,4). Besides causing significant death, culling, and production and reproduction losses due to clinical and subclinical disease (3,5–7), MAP has also recently been implicated as a cause of Crohn’s disease in humans (8,9). While this association remains unproven and contentious (10), public perception of a causal link represents one of the most important economic risks to the milk and meat industries. In addition, paratuberculosis (another term for Johne’s disease) is on the list of “multiple species diseases” notifiable to the World Organization for Animal Health (4). A previous prevalence study in Alberta (11) suggested that levels of MAP infection in dairy herds are at least as high as in other jurisdictions in Canada and the United States (1,12–15). Some U.S. studies also have pointed to soil type and farm management as being associated with an increased prevalence of MAP (16–19).
Neosporosis is an infectious disease caused by NC, which can result in abortion or embryonic death in infected cattle. The disease is world-wide in distribution and is most commonly acquired via point-source exposure (3). No recent studies of the prevalence of NC in dairy cattle have been reported for Alberta. A recent study of beef cattle in northern Alberta found the seroprevalence to be 9.0%, compared with 13.5% of 260 samples collected earlier in the 1980s (20). Other historical data, this time from the Maritime Provinces (Maritimes), show that the seroprevalence in dairy cattle of those areas has also decreased in recent years (21). No published data were available for dairy herds in Alberta.
Bovine leukemia virus causes enzootic bovine leukosis (EBL), characterized by lymphosarcoma in cattle. Clinical signs of the disease include weight loss, pallor, decrease in milk production, and enlargement of all lymph nodes (3,4,22). Infection is often subclinical and can occur at any age, though tumors are typically found in animals over the age of 3 y (4). No recent prevalence studies of BLV have been reported for dairy herds in Alberta. In 1980, Samagh and Kellar (23) found that 12.5% of the dairy herds in Alberta harbored cows infected with BLV. In a 1997 study in Ontario, several farm management-related factors, including purchasing animals from outside sources, average weaning age, and housing practices for calves, were found to increase the likelihood of seropositivity for antibodies to BLV (24): 1330 cows from 102 dairy herds were tested: 23% of the cows tested were positive for BLV, and 69.6% of herds had at least 1 seropositive cow (24). Recently, in the Maritimes, the overall prevalence of BLV infection was found to be 20.8%, with a within-herd prevalence of 30.9% among infected herds; 70% of the herds tested had at least 1 seropositive cow (1).
Bovine viral diarrhea (BVD) is a multifaceted disease, variably characterized by fever, diarrhea, and oral erosions, caused by BVDv. The disease is world-wide in distribution and often subclinical in young cattle (3). Cattle are typically infected in 1 of 2 ways: acutely via infection shortly after birth or persistently via prenatal infection (25). Persistently infected cattle are the most insidious for causing transmission within and between farms, in that they are often subclinically infected (3,25). In the Maritimes, vaccination against BVDv was found to be associated with low herd level prevalence of infection (26). Other studies have shown that the most important farm-management practice that resulted in increased risk of disease was the presence of persistently infected cattle and calves not receiving adequate colostrum (3,27). There are no published estimates of BVDv prevalence among unvaccinated heifers in dairy herds in Alberta. However, in the Maritimes, 37.8% of unvaccinated calves > 6 mo of age were seropositive (titer ≥ 1:64) for antibodies to BVDv, and 46.1% of herds tested had at least 1 seropositive calf (1,26).
The objectives of the present study were as follows: 1) to estimate (a) the individual-level seroprevalence of antibodies to MAP, NC, and BLV among adult dairy cattle and to BVDv1 and BVDv2 among unvaccinated dairy heifers in Alberta, and (b) the herd-level seroprevalence of antibodies to MAP, NC, BLV, BVDv1, and BVDv2 among dairy herds in Alberta, and 2) to provide a preliminary examination of the major agroecological factors associated with seroprevalence of antibodies to MAP, NC, and BLV among individual dairy cattle and to MAP, NC, BLV, BVDv1, and BVDv2 among herds in Alberta. It is expected that this information will help to prioritize and direct future research and control programs in the province and, further, will be integrated into any national PLDC research and control campaigns.
Materials and methods
Study populations and sampling procedures
A stratified, 2-stage random sampling procedure was used to obtain valid estimates of individual- and herd-level seroprevalences for antibodies to the pathogens of interest in Alberta. Comprehensive individual cow as well as dairy herd sampling frames were unavailable. Instead, sampling was stratified, based on eligible and willing veterinarians (who subsequently randomly selected herds and collected random samples within herds), as described below.
The target population for this study comprised cattle in dairy herds in Alberta. The study population was limited to cattle in herds serviced by Johne’s disease accredited veterinarians; that is, veterinarians accredited by the Alberta Johne’s Control Program as of January 2002. The sampling frame was the listing of all dairy cattle owned by the client base of the accredited veterinarians.
The list included 102 veterinarians working throughout Alberta. A letter of introduction, a basic information packet, and enrollment forms for dairy and beef studies were mailed to each accredited veterinarian to gauge interest in the study. Sixty accredited veterinarians expressed interest in participating; of these, 27 indicated that their client base included dairy herds. All respondents indicated that they also had beef herds in their client base.
Twenty-four veterinarians formally agreed to participate in the study. Each veterinarian was asked to provide the number of dairy herds in his or her practice. The sampling frame comprised cattle in 288 dairy herds that were eligible to be selected into our study. To estimate the prevalence of herds expected to be infected with MAP, within ± 10% (of 40%, based on prior work [11]), 75 herds (adjusted for finite population) were required. The number of animals sampled per herd was consistent with prior work by PLDC investigators (1). The number of herds to be sampled per veterinarian was determined by researchers using probability proportional to size (weighted by the number of herds in the practice), with the exception being that each veterinarian would sample at least 1 herd. Where more than 1 accredited veterinarian from a practice volunteered, the number of herds in the practice was split evenly for the purposes of the weighted sampling. As a result, each of the veterinarians was asked to sample as few as 1 and as many as 14 dairy herds.
We provided each participating veterinarian with a further information package that included random selection procedures for dairy herds and cattle within herds. The participating veterinarians used a coding system to protect the herd identity and all research communication with the herd owners (reporting of results) was through the accredited veterinarian. The study ran from early February 2002 through January 2003.
The sampling protocol for randomly selecting the dairy herds was as follows: each veterinarian was advised to select a specified number of participating herds; herds were required to have more than 30 adult cattle (females, 2nd lactation and older; males, 3 y and older) in order to participate; herds were randomly selected from ordered client lists, which were assigned random number lists by the researchers; if a herd owner did not wish to participate, the veterinarian simply moved — in order — to the next random number until he or she reached his or her quota of eligible herds.
The sampling protocol for selecting the animals within each dairy herd was as follows. The clinic veterinarian(s) used systematic random sampling protocols (n/30 sampling interval between animals, with a random starting point) to select 30, 2nd lactation and older cows (or males over 36 mo), 7, 1st lactation cows, and 5 unvaccinated (for BVDv) heifers > 6 mo of age from the list of eligible animals in each of the selected herds.
Blood samples were collected from the randomly selected animals into serum-separator tubes. The veterinarian collected 5–8 mL of blood/vial from the median caudal (tail) vein of each selected animal (4 vials for adults, 2 vials for juveniles). The animal’s identification was recorded on each vial and on the submission form, along with the age, sex, and breed. Samples were packaged and submitted on ice (4°C) to the Alberta Agriculture, Food and Rural Development — Food Safety Division (AAFRD–FSD) Edmonton laboratories within 24 h after sampling.
Laboratory procedures and assays
Upon arrival at the AAFRD-FSD laboratories, blood samples were centrifuged and serum samples were harvested. Serum samples from individual test animals were assigned a unique bar code number for tracking purposes and stored frozen at −72°C until use. One vial of adult serum was stored for future confirmatory testing (if needed), 1 vial was sent for testing for antibodies to BLV at the laboratory of the Canadian Food Inspection Agency (CFIA) in St. Hyacinthe, Quebec, and 2 vials were forwarded to the AAFRD-FSD serology laboratory for testing for antibodies to MAP and NC. Sera from the dairy heifers only were shipped first to the CFIA laboratory in Lethbridge for testing for antibodies to BVDv1, and subsequently forwarded to the University of Guelph for testing for antibodies to BVDv2. Serological laboratory assays for MAP and NC were conducted in sequence as the samples arrived, and results reported to the herd veterinarians soon afterwards. Serological laboratory assays for BLV, BVDv1, and BVDv2 were conducted en masse, once all samples had been received.
An enzyme linked immunosorbent assay (ELISA) (BIOCOR® Parachek® ELISA; BIOCOR Animal Health, Omaha, Nebraska, USA) was used to analyze the samples for the presence of antibody to MAP. The procedure employed followed the directions in the product insert, except that the capacity, efficiency, and accuracy of the test was increased by using an automation workstation (Beckman Biomek; Beckman Coulter, Fullerton, California, USA). The test sample result for the ELISA was determined by using the optical density (O.D.) and the cut-off value. The cut-off value was the mean O.D. of the negative control plus 0.100. A positive result was an O.D. value > the cut-off value. A negative result was an O.D. value ≤ the cutoff value. The sensitivity and specificity of this test were estimated at 47.3% and 99%, respectively (28).
An ELISA kit (IDEXX® Herdchek® ELISA kit; IDEXX Laboratories, Westbrook, Maine, USA) was used to determine the presence of antibodies to NC. The procedure followed the manufacturer’s guidelines with the following exceptions: 1) the procedure was automated by using an automation workstation (Beckman Biomek 2000; Beckman Coulter); and 2) if the sample-to-positive (S/P) ratio was ≥ 0.4, the sample was classified as positive for antibodies to NC (0.5 is the S/P cutpoint recommended by the manufacturer). The 0.4 S/P test cutpoint has been validated in the laboratory with sensitivity estimated at 97.6% and specificity at 99.5% (29).
An ELISA kit for determining the presence of antibodies to BLV (IDEXX® Herdchek® Anti-BLV ELISA kit; IDEXX Laboratories) was used according to the manufacturer’s instructions, with sensitivity and specificity reported as 98.5% and 99.9%, respectively (30). The testing for antibodies to BLV was performed by the same CFIA Laboratory of Excellence utilized previously (1).
To determine the presence of BVDv1, a standard virus neutralization (VN) test (using the cytopathic Singer strain) involving initial screenings of sera at dilutions of 1/2 and 1/4 was used (31). Sera found positive at this stage were subsequently assayed by using dilutions of 1/2 to 1/256. The sensitivity (99.6%) and specificity (100%) were as described previously (31). To determine the presence of BVDv2, a standard VN assay (using the NVSL125 strain) was used in the Animal Health Laboratory, University of Guelph (32,33). Titers > 1:2 were considered positive.
Agroecological risk factors
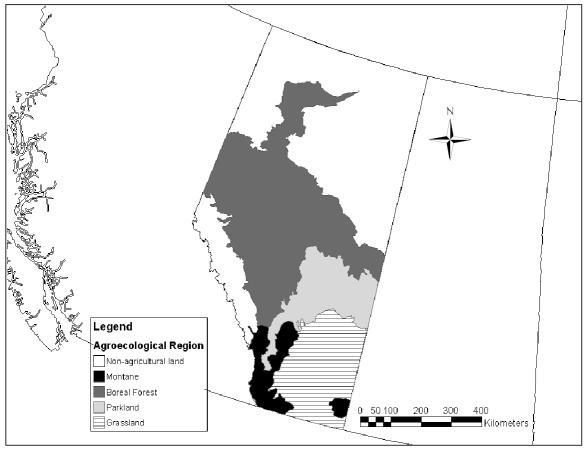
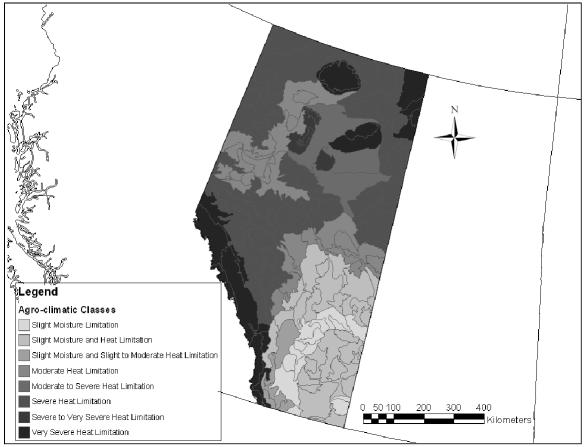
Farm and ranch locations were for the “quarter-section” of land representing the location of the dairy barn. The land locations — provided by the dairy producers — were converted to latitude and longitude with projection based on the North American datum of 1983 (NAD83) (an earth-centered datum based on the Geodetic Reference System of 1980). These locations were used to relate the herd seroprevalence data to: 1) agroecological regions (boreal forest, parkland, grassland, montane [Figure 1]); 2) agro-climatic features (heat and moisture limitations [Figure 2]); 3) landscape features (plains, valleys, uplands); and 4) soil features (soil orders, texture, pH) (34). By using the algorithms described in an Alberta Soil Database (34), these features were joined and related for agricultural regions of Alberta and then appended to each of the dairy herds in a software program (ArcMap 8.1; Environmental Systems Research Institute, Redlands, California, USA) as follows. Each farm was nested in the most refined polygon for each of the climate and soil landscape feature data sets. Where multiple features were applicable (multiple soil types per polygon), the following decision tree was followed: 1) the value for the uppermost soil horizon was always chosen over that for lower horizons; 2) the value for disturbed soils was chosen over that for undisturbed or natural soils; and 3) the dominant soil type was chosen over lesser soil types. In the end, a single soil feature representing the dominant soil class for the upper horizon in disturbed (cultivated or pastured) agricultural soils was applied to the herd database, along with the appropriate agroecological region and climatic features.
Figure 1.
Major agroecological regions in Alberta.
Figure 2.
Agro-climatic classes representing heat and moisture limitations in Alberta.
Statistical analyses
All analyses were performed by using standard statistical software packages (SPSS version 12.0; SPSS, Chicago, Illinois, USA); (Stata release 8; Stata Corporation, College Station, Texas, USA), or (SAS version 9.2; SAS Institute, Cary, North Carolina, USA).
Descriptive statistics were compiled as follows. The stratified 2-stage sampling scheme required that prevalence estimates and standard errors be adjusted for strata (veterinarian), primary sampling unit (herd), and sampling weights (inverse of the product of the probability of herd and cows being sampled) (35). The ‘svyset’ command (Stata release 8; Stata Corporation) permitted the combined adjustment of these and for correlated observations within herd. Individual- and herd-level seroprevalence (+/− 95% confidence interval [CI]) for infection with each of MAP, NC, BLV, BVDv1, and BVDv2 were estimated using ‘svyprop’ and ‘svytab’ (Stata release 8; Stata Corporation) commands (36,37). Because of the low sensitivity of the serological test for MAP, an estimate of the true individual-level prevalence (adjusted for imperfect sensitivity and specificity) was calculated (38).
In exploratory analyses, the bivariable association of the individual- and herd-level serological status for each of MAP, NC, BLV, BVDv1, and BVDv2 with each of the agroecological risk factors was modelled in a generalized linear model (GLM) (39) framework (SAS PROC GENMOD — SAS Institute) by using a binomial error distribution and a logit link function. Ratio statistics assessing the odds of seropositivity (individual or herd) for each level of the agroecological risk factor versus a baseline level were calculated. Agroecological risk factors significantly associated (based on likelihood ratio [LR] statistics at P < 0.05) with outcomes in bivariable analyses were then further assessed in multivariable models. For agroclimatic risk-factor analysis purposes, heat limitations for crop growth and climate aridity were broken-out and treated as separate risk factors.
Multivariable model-building approaches were as follows. Herd size (dichotomized at the median — 90 cows) was assessed in each model. Individual animal age, or demographic age structure of the herd, was not included in the models as a potential confounder, since an equal number of 1st (n = 7), and 2nd and greater (n = 30), lactation animals were drawn from each herd. Subsequently, saturated models were created by using all variables significant in the bivariable analyses. Variables (or groups of indicator variables) then were removed in a backwards-elimination strategy (model-based likelihood ratio statistics at P < 0.05). Contingency tables and basic chi-square tests illustrated the joint frequency distribution of agroecological risk factors by region at the farm level. Since the individual-level binary outcome responses were grouped (clustered) within herds, a generalized estimating equation (GEE) (40) within the GLM framework was used to assess the significance of each of the variables, based on robust estimates of the standard errors. Indicator variables with more than 2 levels (‘r’ levels) were retained in the final models when the P-values were less than 0.05 for the generalized ‘score’ statistic ‘T’, based on a chi-square distribution with ‘r-1’ df.
Results
Descriptive analyses
Of the 81 herds, there were 77 herds with complete samples and data for 2nd and greater lactation adult cattle and land location. Of these 77 herds, there were 4 herds from which no blood samples from the 7 randomly selected 1st lactation cows were received. There was a minimum of 30 and a maximum of 405 adult cows (mean = 110.9) on the sampled farms. In addition, there was a minimum of 0 and a maximum of 180 heifers (mean = 44.6) on these same farms. There was a total of 2819 adult dairy cows (2311 — 2nd and greater lactation; 508 — 1st lactation) sampled (no bulls were selected). For dairy heifers, 36 herds meeting the inclusion criteria were sampled for a total of 179 pre-BVD-vaccinated heifers.
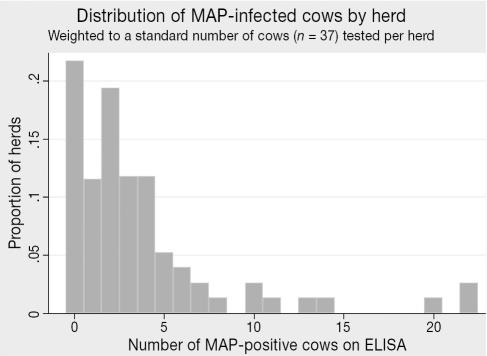
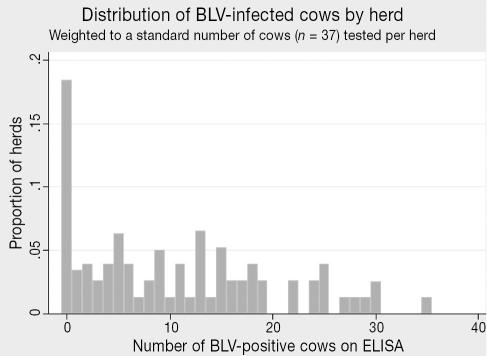
The seroprevalence of antibodies to MAP was 9.1% (95% CI: 6.3–13.0) for the adult dairy cows (Table 1). For 2nd and greater lactation cows the seroprevalence was 10.9%, while 1st lactation cows exhibited only 0.4% (Table 1). Based upon the imperfect performance of the serological test (47.3% sensitivity, 99% specificity), the true overall adult individual-level prevalence of MAP infection (38) was estimated to be 17.5%. Herd-prevalence estimates (dichotomized as infected herds and noninfected herds) based on cutpoints of both 1 or more (1+), and 2 or more (2+) ELISA positive cows are presented in Table 1. To minimize the possibility of false-positives at the herd-level, the 2+ cutpoint has been more widely applied in MAP seroprevalence studies (1,11,12), and on this basis, 58.8% of dairy herds in Alberta were estimated to harbor infected animals. The within-herd frequency distribution of MAP-positive cows (weighted to a standard of 37 cows tested per herd) is presented in Figure 3.
Table 1.
Individual animal and herd seroprevalence for Mycobacterium avium subsp. paratuberculosis (MAP), Neospora caninum (NC), Bovine leukemia virus (BLV), Bovine viral diarrhea virus genotype 1 (BVDv1), and Bovine viral diarrhea virus genotype 2 (BVDv2) among dairy cattle and herds in Alberta
| Pathogen | Animal type | Individual (seropositive) % (95% CI) | Herda,b (≥ 1 seropositive) % (95% CI) | Herda,b (≥ 2 seropositive) % (95% CI) |
|---|---|---|---|---|
| MAP | All adult cows (n = 2819 animals in 77 herds) | 9.1 (6.3–13.0) | 70.2 (53.7–86.6) | 58.8 (42.2–75.4) |
| — 2nd and greater lactation (n = 2311 animals in 77 herds) | 10.9 (7.4–15.7) | — | — | |
| — 1st lactation (n = 508 animals in 73 herds) | 0.4 (0.1–1.5) | — | — | |
| NC | All adult cows (n = 2816 animals in 77 herds) | 18.5 (12.0–27.5) | 98.7 (97.5–99.9) | 88.5 (77.7–99.2) |
| — 2nd and greater lactation (n = 2308 animals in 77 herds) | 19.0 (11.9–29.2) | — | — | |
| — 1st lactation (n = 508 animals in 73 herds) | 15.9 (11.3–21.9) | — | — | |
| BLV | All adult cows (n = 2814 animals in 77 herds) | 26.9 (22.1–32.2) | 86.7 (78.5–92.1) | 77.7 (67.2–88.2) |
| — 2nd and greater lactation (n = 2306 animals in 77 herds) | 28.3 (23.1–34.1) | — | — | |
| — 1st lactation (n = 508 animals in 73 herds) | 19.8 (12.6–29.6) | — | — | |
| BVDv1c | Unvaccinated heifers (n = 179 animals in 36 herds) | 28.4 (14.6–48.0) | 53.4 (30.4–75.0) | 32.2 (9.9–54.4) |
| BVDv2c | Unvaccinated heifers (n = 179 animals in 36 herds) | 8.9 (3.7–20.1) | 19.7 (7.4–43.0) | 9.2 (1.3–17.2) |
aHerd positivity for MAP, NC, and BLV is based on the entire sample including cows of all lactations
bHerd positivity for BVDv1 and BVDv2 is based on the 5 younger animals tested in each herd
cBased on a titer of ≥ 1:2
Figure 3.
Distribution of MAP-infected cows by herd (n = 77 herds tested).
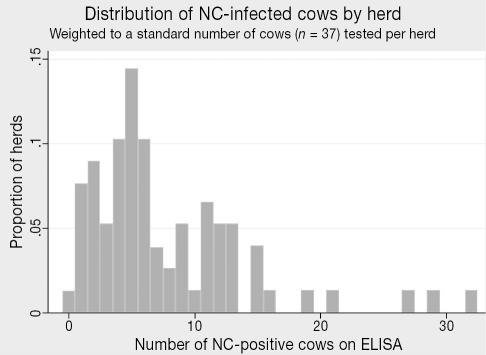
The province-wide seroprevalence of antibodies to NC was 18.5% (95% CI: 12.0–27.5) among all adult dairy cows (Table 1). There was no significant difference between 2nd and greater lactation and 1st lactation cows (19.0% and 15.9%, respectively), though older cows had a slightly higher seroprevalence level (Table 1). All but one of the herds (98.7%) in the province had at least 1 cow with antibodies to NC; 88.5% of herds had 2+ ELISA positive animals (Table 1). The within-herd frequency distribution of NC-positive cows is presented in Figure 4.
Figure 4.
Distribution of NC-infected cows by herd (n = 77 herds tested).
Seroprevalence of infection with BLV was 26.9% (95% CI: 22.1–32.2 [Table 1]). Cattle of 2nd and greater lactation were at a nonsignificantly (P > 0.05) higher risk of infection (28.3%) than 1st lactation cattle (19.8%) (Table 1). Most herds (86.7%, 95% CI: 78.5–92.1) had at least 1 infected animal in the herd; 77.7% had 2+ infected animals (Table 1). Beyond the 13.3% of herds with no BLV-infected animals, the within-herd frequency distribution of BLV-positive cows is uniform between 1 and 30 animals (Figure 5).
Figure 5.
Distribution of BLV-infected cows by herd (n = 77 herds tested).
The joint frequency distribution of apparent prevalence of infection with each of MAP, NC, and BLV among adult cattle is tabulated in Table 2. Of 2813 cattle with valid test results for all 3 assays, 50.9% of the animals were serologically negative for all 3 infectious agents, only 16 were positive for all 3.
Table 2.
Joint prevalence and standard errors of the mean (
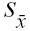 for infection/coinfection with Mycobacterium vium subsp. paratuberculosis (MAP), Neospora caninum (NC), and Bovine leukemia virus (BLV) among adult dairy cattle in Alberta (n = 2813)
for infection/coinfection with Mycobacterium vium subsp. paratuberculosis (MAP), Neospora caninum (NC), and Bovine leukemia virus (BLV) among adult dairy cattle in Alberta (n = 2813)
| MAP | NC | BLV | Observations | Joint Probability | |
|---|---|---|---|---|---|
| 0 | 0 | 0 | 1431 | 0.509 | 0.0094 |
| 0 | 0 | 1 | 580 | 0.206 | 0.0076 |
| 0 | 1 | 0 | 345 | 0.123 | 0.0062 |
| 0 | 1 | 1 | 173 | 0.062 | 0.0045 |
| 1 | 0 | 0 | 152 | 0.054 | 0.0043 |
| 1 | 0 | 1 | 58 | 0.021 | 0.0027 |
| 1 | 1 | 0 | 58 | 0.021 | 0.0027 |
| 1 | 1 | 1 | 16 | 0.006 | 0.0014 |
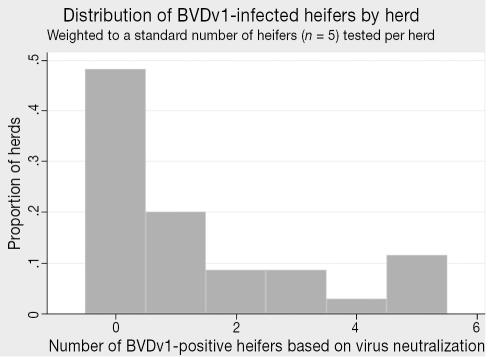
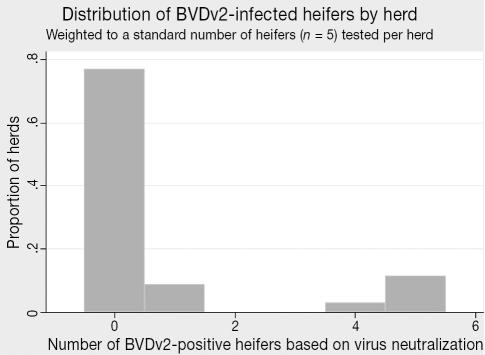
Among the smaller number of herds (n = 36 out of 77 total) that had not vaccinated their heifers before 6 mo of age, the seroprevalence for BVDv1 infection was 28.4% (95% CI: 14.5–48.0 [Table 1]). The herd-level seropositivity (at least a single heifer out of 5 sampled was positive) was 53.4% (32.2% based on a cutpoint of 2+) (Table 1). Considering only BVDv genotype 2 infection, the seroprevalence was markedly lower at 8.9% overall (95% CI: 3.7–20.1), with 19.7% of the herds containing infected calves (9.2% using a 2+ cutpoint) (Table 1). Within-herd frequency distributions of infected heifers (weighted to a standard sample of 5 animals per herd) for BVDv genotypes 1 and 2 are presented in Figures 6 and 7, respectively.
Figure 6.
Distribution of BVDv1-infected heifers by herd (n = 36 herds tested).
Figure 7.
Distribution of BVDv2-infected heifers by herd (n = 36 herds tested).
Agroecological risk-factors
Agroecological region (Figure 1) is a composite summary index of a variety of climatic, soil, and vegetative features of the landscape in Alberta. Several of the ecological risk factors, such as climatic aridity, heat limitations for plant growth, and soil pH, were highly associated (P < 0.001) with region (Table 3). Serological evidence of MAP infection at the herd level (2 or greater seropositive cows) was significantly associated with agroecological region in bivariable analysis (P = 0.026) and multivariable analysis adjusted for herd size (P = 0.034). For the agroecological region, with grassland as the referent level (Figure 1), the unadjusted odds ratios (OR) for the other regions were montane (OR: 4.78, 95% confidence interval [95% CI]: 0.74–30.93), parkland (OR: 5.85, 95% CI: 1.62–21.10), and boreal forest (OR: 1.44, 95% CI: 0.37–5.57). None of the other agroecological risk factors was associated (P < 0.05) with the herd-level risk of infection with MAP in bivariable analyses. Owing to NC’s wide distribution (seroprevalence of 98.7% of herds based on 1 or greater infected animals out of 37 tested), there were no agroecological factors associated with its herd-level risk of infection. Likewise, for BLV, BVDv1, and BVDv2, none of the agroecological risk factors was found to be significantly associated (P < 0.05) with the herd-level risk of infection.
Table 3.
Contingency table illustrating association of ecological risk factors with the aggregated index of agroecological region at the herd-level (n = 77 herds)
| Agroecological region
|
||||||
|---|---|---|---|---|---|---|
| Ecological risk factor | Level of risk factor | Grassland | Montane | Parkland | Boreal forest | Significance (P-value)a |
| pH | < 7.0 | 6 | 4 | 30 | 13 | < 0.001 |
| ≥ 7.0 | 14 | 5 | 3 | 2 | ||
| Arid climate | No | 0 | 6 | 28 | 15 | < 0.001 |
| Yes | 20 | 3 | 5 | 0 | ||
| Heat limitation | Area 2 | 14 | 3 | 30 | 2 | < 0.001 |
| Areas 3 & 4 | 6 | 6 | 3 | 13 | ||
aP-values were derived from asymptotic Pearson chi-square 2-sided tests of association comparing agroecological region to each of the other ecological risk factors
At the individual level, there were several agroecological risk factors associated (P < 0.05) with the risk of MAP seroprevalence in bivariable analyses. These included agroecological region, terrain morphology, soil pH, climate aridity, and heat limitations. When adjusted for herd size and within-herd dependence of the outcome variable, only agroecological region, soil pH, and climate aridity remained significant (P < 0.05 [Table 4]). Because several of the features (soil pH, aridity) within the indexed regions were associated (nesting), the 3 ecological risk factors could not be included in the same model. The 3 competing models (I, II, and III), representing agroecological region, climatic aridity, and soil pH, respectively, are presented in Table 4.
Table 4.
Three alternative models of associations of ecological risk factors with individual MAP seroprevalence among dairy cattle in Alberta. Model I: agroecological region, Model II: climate aridity, and Model III: soil pH. All models are generalized estimating equation-adjusted for within-herd dependence of outcomes. Intercept not shown
| Risk factor | Level of risk factor | Odds ratio (OR) | 95% confidence interval (OR) | P-valuea |
|---|---|---|---|---|
| Model I: | ||||
| Herd size | Small (< 90 cows) | 0.51 | 0.27–0.96 | 0.05 |
| Large (≥ 90 cows) | — | — | ||
| Agroecological region | Grassland | 0.33 | 0.14–0.76 | 0.05 |
| Montane | 0.55 | 0.32–0.95 | ||
| Boreal forest | 0.87 | 0.37–2.06 | ||
| Parkland | — | — | ||
| Model II: | ||||
| Herd size | Small (< 90 cows) | 0.55 | 0.30–1.01 | 0.07 |
| Large (≥ 90 cows) | — | — | ||
| Arid climate | No | 1.94 | 1.05–3.57 | 0.04 |
| Yes | — | — | ||
| Model III: | ||||
| Herd size | Small (< 90 cows) | 0.54 | 0.29–0.98 | 0.06 |
| Large (≥ 90 cows) | — | — | ||
| pH | < 7.0 | 1.92 | 1.14–3.22 | 0.03 |
| ≥ 7.0 | — | — | ||
aP-value was derived from a Type III generalized estimating equations (GEE) analysis in SAS (40) assessing the overall significance of each risk factor over all levels of the variable
MAP = Mycobacterium avium subspecies paratuberculosis
In bivariable analysis, individual-level seroprevalence to NC infection was significantly associated (P < 0.001) with agroecological region in a similar pattern to that seen for MAP. However, when adjusted for within-herd dependence of outcomes, agroecological region was no longer important (P = 0.21).
There were no significant agroecological factors associated with BLV, BVDv genotype 1, or BVDv genotype 2 seroprevalence at the individual level.
Discussion
The sampling frame and study design employed in this investigation were generally similar to those used in other published PLDC seroprevalence studies (1,41), with several exceptions. Whereas the herds eligible for inclusion in the earlier studies had to be Dairy Herd Improvement (DHI) subscribers, the herds in the present study were restricted to dairy clients of Johne’s control program veterinarians in Alberta, and not limited by DHI subscriber status. Our sampling frame represented approximately 288 out of the 817 herds licensed to operate as of January 1, 2002, with an average herd size of 111 adult cows among our random sample of 77 herds.
Another difference between the present and past PLDC studies was in the numbers and age of animals selected for inclusion. In the previous PLDC studies, any adult cow (1st or greater lactation) was eligible to be randomly selected to be 1 of the 30 animals bled. In the present study, 30, 2nd or greater lactation animals were randomly selected. To aid in future national prevalence comparisons, 7 additional 1st lactation cows were also randomly selected and will be used to randomly replace 7 of the 30 older cattle in a regional comparison of seroprevalence for MAP, NC, and BLV in Canada. Finally, the ELISAs used in the present study for MAP and NC differed from those used in previous studies (1,41). However, estimates of true prevalence can be used to compare results between studies.
The MAP seroprevalence estimated in this study is very similar to that estimated in a recent seroprevalence study of dairy herds in Alberta (11). This previous study utilized 1500 serum samples from adult dairy cattle in 50 herds tested with an ELISA test kit (IDEXX® Herdchek® ELISA kit; IDEXX Laboratories); 105 samples from 20 herds were positive for MAP for an apparent herd level prevalence of 40.0%, when a cutpoint of 2 or more positive test results was used for a herd to be classified as positive. The same study also included evaluation of pooled fecal cultures, giving a herd-level prevalence estimate ranging from 27.6% to 57.1%, depending on how many samples in each pool were positive — this was unknown (11).
Compared with historical data from other parts of Canada (1,41), the United States (17,42), Belgium, and The Netherlands (43,44), herd-level seroprevalence generally appears to be somewhat higher among dairy herds in Alberta. There are obvious differences in study design and diagnostic tests that should be considered when comparing these studies, such as the selection of older cows (2nd and older lactation) in the present study, which might shift the probability of obtaining positive test results higher, depending on the underlying demographic structure of herds in other jurisdictions.
Studies conducted elsewhere in Canada have found somewhat lower prevalence estimates than in this study (1,13,41). In 3 Maritime provinces (excluding Newfoundland), 30 dairy herds were randomly selected from each province, and 30 cows were randomly selected from each herd. These cows were surveyed by ELISA for the presence of antibodies to MAP; 2.6% of cows tested positive, for an apparent herd seroprevalence of 16.7%, assuming that a minimum of 2 positive cows constituted a positive herd (1). In a similar study in Saskatchewan, where 1530 cattle were tested, the province-wide seroprevalence for MAP was 2.7% and the herd prevalence (using the 2 or more positive cows cutpoint) was 24.3% (41). These results suggest that dairy cattle and herds in Alberta may exhibit a greater seroprevalence of MAP infection than that of other provinces in Canada.
In the United States, results have been almost uniformly of similar or lower seroprevalence than in the present study, with 1 major exception. In Colorado, dairy cows were surveyed using an ELISA, and 424 out of 10 280 (4.12%) cows were classified as seropositive; the within-herd prevalence was estimated to be up to 7.82% (42). In that study, of 15 large herds tested, only a single herd was classified as negative, based on seropositivity. However, the size and demographic dynamics of western large-scale dairy herds in the United States make comparisons with Canadian dairy herds difficult at the herd level. In Michigan, where the dairy industry is more similar to that in Alberta, many dairy cattle and herds also tested positive, with 55% of 121 herds tested having at least 2 positive cows (17). Nationally, however, the 1996 National Animal Health Monitoring System (NAHMS) study estimated that only 22% of U.S. herds were infected with MAP (12).
Northern European dairy production practices are somewhat similar to those in Canada. In The Netherlands, 2.5% of tested cows were seropositive, while 28% of herds had at least 2 seropositive cows (44). In Belgium, an absorbed ELISA kit was used on a stratified random sample of adult cattle 24 mo or older. The median seroprevalence of MAP infection among all herds was found to be 2.9%. The individual-animal seroprevalence was 0.87% — the herd seroprevalence was 18% (43).
The performance of the diagnostic and screening tests for MAP infection remains one of the most critical roadblocks to successful control of Johne’s disease. Commercial ELISAs are commonly used to identify antibodies to MAP in blood samples from cattle. One issue with this type of test is that its sensitivity greatly depends on the stage of the disease in infected animals (14,45–47). Most seroprevalence studies that attempt to classify both cattle and herds rely on previous estimates of sensitivity (47.3%) and specificity (99%) that may or may not be appropriate. It is important to understand that all seroprevalence studies rely on screening tests, such as ELISA, which it is the apparent prevalence presented; that is, even if a “true” prevalence is reported, it is an estimate calculated from assay performance criteria reported in the literature.
Some of the agroecological risk factors for seroprevalence identified in this study have been examined elsewhere (16,18,19). Two major inhibitors to MAP organism survival outside the host (increased soil pH and aridity) were noted in this study as moderately associated with a decreased risk of seropositivity. However, pH was not associated with risk in a strictly linear relationship, as noted elsewhere (16). Dichotomized pH (at 7.0) revealed a sparing effect on the risk of MAP seropositivity associated with more alkaline soils. This suggests that there may be a critical cutpoint of effect on organism viability, rather than a dose-response on a continuous linear scale. While Johnson-Ifearulundu and Kaneene (16) in Michigan and Ward and Perez (19) in Indiana found soil features — including pH — to be associated with risk of MAP seroprevalence, it is important to note that different approaches to assessing exposure were used in each of these studies as compared with those used herein. In the present study, more northern agroecological regions appeared to favor increased seroprevalence, possibly due to prolonged indoor confinement during winter months and calving periods, and variable freeze-thaw episodes during spring months. However, both soil pH and arid climatic conditions are correlated with agroecological region and latitude, and these effects could not be separated out in the present analysis.
In this study, seroprevalence for infection with NC was estimated at levels that are comparable with those for the seroprevalence of NC in other regions of Canada (20,21,48,49). Overall, the seroprevalence in Canada is similar to the prevalence in the United States and Great Britain (50,51). Almost all herds tested in Alberta have some evidence of infection. Agroecological regional associations with the risk of seropositivity to NC (though nonsignificant when adjusted for within-herd dependence) were similar in direction between MAP and NC, in that northern ecological regions had somewhat higher seroprevalences than southern regions for both pathogens. However, neither soil type nor pH had an effect on NC seroprevalence. This is compatible with known biological features of the disease that include both domestic and wild canid hosts and a point source infection. Certainly, the northern regions of Alberta exhibit greater forested lands, which could support a larger wild canid population, as well as bringing cattle and wildlife into direct contact more often in wooded grazing lands.
The seroprevalence of BLV in Alberta is virtually the same as that estimated for other regions of Canada (1,23,41). However, the seroprevalence in Canada is generally lower than that in the United States (52). It was not surprising to find no significant ecological risk factors for BLV seroprevalence, given that infection is generally associated with management practices pertaining to cattle purchases and use of dirty needles, gloves, and other objects that are contaminated with blood (53), rather than macroecological risk factors (climate, soil type).
In our study, the seroprevalence of BVDv1 infection of unvaccinated dairy heifers in Alberta was very similar to that in the Canadian Maritime Provinces (1,26) and Saskatchewan (41).
The unique aspect of the present round of PLDC studies is that comparisons and contrasts may be more readily made among provinces. However, even with the relative consistency in study design and application, there remain some differences in methodology, laboratory assays, and the time period during which the studies were performed. Therefore, comparisons between provinces should be made with some caution. That said, these data will provide a rich addition to further efforts to quantify the risks associated with these 4 diseases and to prioritize and customize control programs in Canada.
Acknowledgments
The authors thank the participating dairy farmers and their Alberta Johne’s Control Program-accredited veterinarians for their cooperation. In addition, we thank the scientists and technical staff of the Agri-Food Laboratories Branch of the Food Safety Division of Alberta Agriculture, Food and Rural Development (AAFRD) for their efforts (sample processing, MAP, and NC testing), as well as those of the Canadian Food Inspection Agency [CFIA] laboratories in St. Hyacinthe, Quebec, (BLV testing) and Lethbridge, Alberta (BVDv1 testing). The Animal Health Laboratory at the University of Guelph is acknowledged for the BVDv2 testing. Geographical information systems (GIS) and AGRASID 3.0 Alberta soil data analysis and support were graciously provided by resource specialists from the Conservation and Development Division of AAFRD and from Agriculture and Agri-food Canada. CVJ
Footnotes
This study was supported by Alberta Agriculture, Food and Rural Development, the Western Economic Partnership Agreement, the Canadian Food Inspection Agency, and the Production Limiting Diseases Committee.
References
- 1.VanLeeuwen JA, Keefe GP, Tremblay R, Power C, Wichtel JJ. Seroprevalence of infection with Mycobacterium avium subspecies paratuberculosis, bovine leukemia virus, and bovine viral diarrhea virus in Maritime Canada dairy cattle. Can Vet J. 2001;42:193–198. [PMC free article] [PubMed] [Google Scholar]
- 2.Alberta Johne’s Control Program [homepage on the Internet]. Edmonton, AB: Government of Alberta c2004–2005 [updated 2004 June 28]. Available from http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/afs5602?opendocument Last accessed 03/06/2006.
- 3.Radostits OM, Gay CC, Blood DC, Hinchcliff KW. Veterinary Medicine: a Textbook of the Diseases of Cattle, Sheep, Pigs, Goats and Horses, 9th ed. London, UK: WB Saunders, 2000:922–934, 1046–1058, 1085–1105, 1308–1310.
- 4.OIE. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals (Mammals, Birds and Bees). Paris, France: Office International des Epizooties, 2004:347–359, 464–473.
- 5.Stabel JR. Johne’s disease: a hidden threat. J Dairy Sci. 1998;81:283–288. doi: 10.3168/jds.S0022-0302(98)75577-8. [DOI] [PubMed] [Google Scholar]
- 6.Coussens PM. Mycobacterium paratuberculosis and the bovine immune system. Anim Health Res Rev. 2001;2:141–161. [PubMed] [Google Scholar]
- 7.Manning EJ, Collins MT. Mycobacterium avium subsp. paratuberculosis: Pathogen, pathogenesis and diagnosis. Rev Sci Tech. 2001;20:133–150. doi: 10.20506/rst.20.1.1275. [DOI] [PubMed] [Google Scholar]
- 8.Hermon-Taylor J. Causation of Crohn’s disease: The impact of clusters. Gastroenterology. 1993;104:643–646. doi: 10.1016/0016-5085(93)90438-I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiodini RJ, Rossiter CA. Paratuberculosis: A potential zoonosis? Vet Clin North Am Food Anim Pract. 1996;12:457–467. doi: 10.1016/s0749-0720(15)30417-5. [DOI] [PubMed] [Google Scholar]
- 10.Van Kruiningen HJ. Lack of support for a common etiology in Johne’s disease of animals and Crohn’s disease in humans. Inflamm Bowel Dis. 1999;5:183–191. doi: 10.1097/00054725-199908000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Sorensen O, Rawluk S, Wu J, Manninen K, Ollis G. Mycobacterium paratuberculosis in dairy herds in Alberta. Can Vet J. 2003;44:221–226. [PMC free article] [PubMed] [Google Scholar]
- 12.National Animal Health Monitoring System. Johne’s Disease on U.S. Dairy Operations. #N245.1097. Fort Collins, CO: United States Department of Agriculture, Animal and Plant Health Inspection Service; Veterinary Services; Centers for Epidemiology and Animal Health, 1997.
- 13.McNab WB, Meek AH, Duncan JR, Martin SW, Van Dreumel AA. An epidemiological study of paratuberculosis in dairy cattle in Ontario: Study design and prevalence estimates. Can J Vet Res. 1991;55:246–251. [PMC free article] [PubMed] [Google Scholar]
- 14.Adaska JM, Anderson RJ. Seroprevalence of Johne’s-disease infection in dairy cattle in California, USA. Prev Vet Med. 2003;60:255–261. doi: 10.1016/s0167-5877(03)00120-x. [DOI] [PubMed] [Google Scholar]
- 15.Pence M, Baldwin C, Black CC., III The seroprevalence of Johne’s disease in Georgia beef and dairy cull cattle. J Vet Diagn Invest. 2003;15:475–477. doi: 10.1177/104063870301500514. [DOI] [PubMed] [Google Scholar]
- 16.Johnson-Ifearulundu YJ, Kaneene JB. Relationship between soil type and Mycobacterium paratuberculosis. J Am Vet Med Assoc. 1997;210:1735–1740. [PubMed] [Google Scholar]
- 17.Johnson-Ifearulundu Y, Kaneene JB. Distribution and environmental risk factors for paratuberculosis in dairy cattle herds in Michigan. Am J Vet Res. 1999;60:589–596. [PubMed] [Google Scholar]
- 18.Wells SJ, Wagner BA. Herd-level risk factors for infection with Mycobacterium paratuberculosis in US dairies and association between familiarity of the herd manager with the disease or prior diagnosis of the disease in that herd and use of preventive measures. J Am Vet Med Assoc. 2000;216:1450–1457. doi: 10.2460/javma.2000.216.1450. [DOI] [PubMed] [Google Scholar]
- 19.Ward MP, Perez AM. Association between soil type and paratuberculosis in cattle herds. Am J Vet Res. 2004;65:10–14. doi: 10.2460/ajvr.2004.65.10. [DOI] [PubMed] [Google Scholar]
- 20.Waldner CL, Henderson J, Wu JT, Coupland R, Chow EY. Seroprevalence of Neospora caninum in beef cattle in northern Alberta. Can Vet J. 2001;42:130–132. [PMC free article] [PubMed] [Google Scholar]
- 21.Keefe GP, VanLeeuwen JA. Neospora then and now: Prevalence of Neospora caninum in Maritime Canada in 1979, 1989, and 1998. Can Vet J. 2000;41:864–866. [PMC free article] [PubMed] [Google Scholar]
- 22.Reed VI. Enzootic bovine leukosis. Can Vet J. 1981;22:95–102. [PMC free article] [PubMed] [Google Scholar]
- 23.Samagh BS, Kellar JA. Seroepidemiological survey of bovine leukaemia virus infection in Canadian cattle. Curr Top Vet Med Anim Sci. 1982;15:397–412. [Google Scholar]
- 24.Sargeant JM, Kelton DF, Martin SW, Mann ED. Associations between farm management practices, productivity, and bovine leukemia virus infection in Ontario dairy herds. Prev Vet Med. 1997;31:211–221. doi: 10.1016/s0167-5877(96)01140-3. [DOI] [PubMed] [Google Scholar]
- 25.Houe H. Epidemiology of bovine viral diarrhea virus. Vet Clin North Am Food Anim Pract. 1995;11:521–547. doi: 10.1016/s0749-0720(15)30465-5. [DOI] [PubMed] [Google Scholar]
- 26.Chi J, VanLeeuwen JA, Weersink A, Keefe GP. Management factors related to seroprevalences to Bovine viral-diarrhoea virus, Bovine-leukosis virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy herds in the Canadian Maritimes. Prev Vet Med. 2002;55:57–68. doi: 10.1016/s0167-5877(02)00067-3. [DOI] [PubMed] [Google Scholar]
- 27.Jones GF. BVD in dairy cattle. Mod Vet Pract. 1980;61:626–627. [PubMed] [Google Scholar]
- 28.Collins MT, Sockett DC, Ridge S, Cox JC. Evaluation of a commercial enzyme-linked immunosorbent assay for Johne’s disease. J Clin Micro. 1991;29:272–276. doi: 10.1128/jcm.29.2.272-276.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu JTY, Dreger S, Chow EYW, Bowlby EE. Validation of 2 commercial Neospora caninum antibody enzyme linked immunosorbent assays. Can J Vet Res. 2002;66:264–271. [PMC free article] [PubMed] [Google Scholar]
- 30.Johnson R, Kaneene JB. Bovine leukemia virus. Part 1. Descriptive epidemiology, clinical manifestations, and diagnostic tests. Compend Contin Educ Pract Vet. 1991;13:315–325. [Google Scholar]
- 31.Deregt D, Smithson S, Kozub GC. A short incubation serum neutralization test for bovine viral diarrhea virus. Can J Vet Res. 1992;56:161–164. [PMC free article] [PubMed] [Google Scholar]
- 32.Carman S, van Dreumel T, Ridpath J, et al. Severe acute bovine diarrhea in Ontario, 1993–1995. J Vet Diagn Invest. 1998;10:27–35. doi: 10.1177/104063879801000106. [DOI] [PubMed] [Google Scholar]
- 33.Wood RD, Goens SD, Carman PS, Deregt D, Jefferson B, Jacobs RM. Effect on hematopoietic tissue of experimental infection of calves with noncytopathic type 2 bovine viral diarrhea virus. Can J Vet Res. 2004;68:42–48. [PMC free article] [PubMed] [Google Scholar]
- 34.Agricultural Region of Alberta Soil Inventory Database (AGRASID) 3.0 [database on the Internet] Edmonton, AB: Government of Alberta c2005 [update 2005 March 1]. Available from http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/sag3249?opendocument Last accessed 03/06/2006.
- 35.Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. Charlottetown, Prince Edward Island, Canada: AVC Inc., 2003: 34–39.
- 36.Stata Corporation. Stata Survey Data Reference Manual: Release 8. College Station, Texas: Stata Corporation, 2003:42–89.
- 37.Stata Corporation. Stata User’s Guide: Release 8. College Station, Texas: Stata Corporation. 2003;13:338. [Google Scholar]
- 38.Martin SW, Meek AH, Willeberg P. Veterinary Epidemiology: Principles and Methods. Ames, Iowa: Iowa State Univ Pr, 1987:69.
- 39.McCullagh P, Nelder JA. Generalized Linear Models. Boca Raton, Florida: Chapman & Hall/CRC, 1989:1–32.
- 40.Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton, Florida: Chapman & Hall/CRC, 2003:55–95.
- 41.VanLeeuwen JA, Forsythe L, Tiwari A, Chartier R. Seroprevalence of antibodies against bovine leukemia virus, bovine viral diarrhea virus, Mycobacterium avium subspecies paratuberculosis, and Neospora caninum in dairy cattle in Saskatchewan. Can Vet J. 2005;46:56–58. [PMC free article] [PubMed] [Google Scholar]
- 42.Hirst HL, Garry FB, Morley PS, et al. Seroprevalence of Mycobacterium avium subsp paratuberculosis infection among dairy cows in Colorado and herd-level risk factors for seropositivity. J Am Vet Med Assoc. 2004;225:97–101. doi: 10.2460/javma.2004.225.97. [DOI] [PubMed] [Google Scholar]
- 43.Boelaert F, Walravens K, Biront P, Vermeersch JP, Berkvens D, Godfroid J. Prevalence of paratuberculosis (Johne’s disease) in the Belgian cattle population. Vet Microbiol. 2000;77:269–281. doi: 10.1016/s0378-1135(00)00312-6. [DOI] [PubMed] [Google Scholar]
- 44.Muskens J, Barkema HW, Russchen E, Van Maanen K, Schukken YH, Bakker D. Prevalence and regional distribution of paratuberculosis in dairy herds in The Netherlands. Vet Microbiol. 2000;77:253–261. doi: 10.1016/s0378-1135(00)00310-2. [DOI] [PubMed] [Google Scholar]
- 45.Dargatz DA, Byrum BA, Hennager SG, et al. Prevalence of antibodies against Mycobacterium avium subsp paratuberculosis among beef cow-calf herds. J Am Vet Med Assoc. 2001;219:497–501. doi: 10.2460/javma.2001.219.497. [DOI] [PubMed] [Google Scholar]
- 46.Kalis CH, Hesselink JW, Barkema HW, Collins MT. Culture of strategically pooled bovine fecal samples as a method to screen herds for paratuberculosis. J Vet Diagn Invest. 2000;12:547–551. doi: 10.1177/104063870001200609. [DOI] [PubMed] [Google Scholar]
- 47.Carpenter TE, Gardner IA, Collins MT, Whitlock RH. Effects of prevalence and testing by enzyme-linked immunosorbent assay and fecal culture on the risk of introduction of Mycobacterium avium subsp. paratuberculosis-infected cows into dairy herds. J Vet Diagn Invest. 2004;16:31–38. doi: 10.1177/104063870401600106. [DOI] [PubMed] [Google Scholar]
- 48.Paré J, Fecteau G, Fortin M, Marsolais G. Seroepidemiologic study of Neospora caninum in dairy herds. J Am Vet Med Assoc. 1998;213:1595–1598. [PubMed] [Google Scholar]
- 49.Cramer G, Kelton D, Duffield TF, et al. Neospora caninum serostatus and culling of Holstein cattle. J Am Vet Med Assoc. 2002;221:1165–1168. doi: 10.2460/javma.2002.221.1165. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez I, Choromanski L, Rodgers SJ, Weinstock D. Survey of Neospora caninum antibodies in dairy and beef cattle from five regions of the United States. Vet Therapeutics. 2002;3:396–401. [PubMed] [Google Scholar]
- 51.Davison HC, Otter A, Trees AJ. Significance of Neospora caninum in British dairy cattle determined by estimation of seroprevalence in normally calving cattle and aborting cattle. Int J Parasitol. 1996;29:1189–1194. doi: 10.1016/s0020-7519(99)00094-6. [DOI] [PubMed] [Google Scholar]
- 52.Ott SL, Johnson R, Wells SJ. Association between bovine-leukosis virus seroprevalence and herd-level productivity on US dairy farms. Prev Vet Med. 2003;61:249–262. doi: 10.1016/j.prevetmed.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 53.Hopkins SG, DiGiacomo RF. Natural transmission of bovine leukemia virus in dairy and beef cattle. Vet Clin North Am Food Anim Pract. 1997;13:107–128. doi: 10.1016/s0749-0720(15)30367-4. [DOI] [PubMed] [Google Scholar]