Abstract
HNK-1 carbohydrate epitope is localized on the surface of avian neural crest cells (NCCs), and is necessary for their migration. However, it is still disputed whether the epitope works in similar ways in mammalian embryos. In this study, we found that HNK-1 carbohydrate epitope was specifically detected in some of the cranial ganglia, migrating trunk NCCs and some non-NCC derivatives in the rat embryo. Two genes encoding glucuronyltransferases that synthesize the HNK-1 epitope in vitro (GlcAT-P and GlcAT-D) were recently identified in the rat. Interestingly, the NCCs in the cranial ganglia expressed the GlcAT-D gene, whereas the migrating trunk NCCs expressed the GlcAT-P gene. To investigate in vivo functions of the GlcATs in the NCC migration further, we overexpressed GlcAT genes by electroporation in the cranial NCCs in cultured rat embryos. Transfection of both GlcAT genes resulted in efficient synthesis of the HNK-1 epitope in the NCCs. GlcAT-P overexpression increased distance of cranial NCC migration, whereas GlcAT-D overexpression did not show this effect. Our data suggest that the HNK-1 epitope synthesized by different GlcATs is involved in migration in the sublineages of the NCCs in the rat embryo, and that GlcAT-P and GlcAT-D mediate different effects on the NCC migration.
Keywords: electroporation, GlcAT genes, HNK-1, migration, neural crest cells, whole embryo culture
Introduction
Cell surface carbohydrates are considered to mediate fine-tuning of cell–cell interactions, and may have crucial roles in dynamic biological processes such as embryonic development (Schachner & Martini, 1995). The HNK-1 carbohydrate epitope is a residue on various glycoproteins and glycolipids, and is recognized by monoclonal antibody HNK-1 (Abo & Balch, 1981). The HNK-1 epitope is often found on cell adhesion molecules such as neural cell adhesion molecule (Kruse et al. 1984) and on extracellular matrices such as chondroitin sulphate proteoglycan (Domowicz et al. 1995; Pettway et al. 1996). Interestingly, only a subpopulation of these molecules expresses the HNK-1 epitope at a given time, and synthesis of the epitope is epigenetically modulated by the glycosylating enzymes. The structure of the HNK-1 epitope includes HSO3−3GlcAβ1–3Galβ1–4GlcNAc, which is common in both glycoprotein and glycolipid (Chou et al. 1986; Ariga et al. 1987; Voshol et al. 1996). The precursor of this residue, Galβ1–4GlcNAc, is non-specifically found in the various types of glycoproteins and glycolipids. Thus key enzymes in the HNK-1 epitope synthesis are glucuronyltransferase (GlcAT) and sulfotransferase. In the past few years, different types of GlcAT genes, GlcAT-P (Terayama et al. 1997) and GlcAT-D (GlcAT-S) (Seiki et al. 1999; Shimoda et al. 1999), have been identified. These GlcATs showed functional specificities in vitro; GlcAT-P has catalytic activity specifically on the HNK-1 epitope on Galβ1–4GlcNAc (Type II glycan), whereas GlcAT-D is active on both Galβ1–4GlcNAc (Type II) and Galβ1–3GlcNAc (Type I glycan) (Shimoda et al. 1999).
The HNK-1 epitope mediates cell–cell and/or cell–substrate interactions such as cell adhesion, migration or axonal pathfinding. In avian embryos, the HNK-1 epitope is found in odd-numbered rhombomeres (Kuratani, 1991). The HNK-1 epitope is also localized in the central and peripheral nervous systems (Zhao et al. 2000; Becker et al. 2001) and the cardiac conduction systems (Ikeda et al. 1990; Aoyama et al. 1995) in other vertebrate embryos. The HNK-1 epitope has attracted the interest of developmental biologists as a general marker for neural crest cells (NCCs) in the avian embryo (Tucker et al. 1984; Vincent & Thiery, 1984; Bronner-Fraser, 1986). Injection of HNK-1 antibody into chick embryos remarkably disturbed migration of cranial NCCs, indicating that the HNK-1 epitope is essential for proper NCC migration (Bronner-Fraser, 1987). HNK-1 reactivity of cultured quail trunk NCCs correlates with their adrenergic potential (Hennig & Maxwell, 1995), implying that it has a possible role also in NCC lineage specification in avians.
In the mouse embryo, HNK-1 staining is negative for NCCs (Tucker et al. 1988; our unpublished data). There is a controversy in the rat embryo: some reports have shown that HNK-1 immunoreactivity can detect NCCs in the ectomesenchyme (Nishida et al. 1997) and the trunk NCCs (Erickson et al. 1989), whereas others have indicated that an HNK-1 staining pattern is not specific to NCCs (Louryan et al. 1996; Bannerman et al. 1998). We previously confirmed that the rat ectomesenchyme was negative for HNK-1 immunoreactivity (Nagase et al. 2001), and that the HNK-1-positive cells at the limb bud level were the myoblasts, but not the NCCs (Nagase et al. 2000). Interestingly, genes of the two GlcATs synthesizing the HNK-1 epitope showed quite different expression patterns. For example, the GlcAT-P gene was selectively expressed in the hypaxial longitudinal fibres in the myotome, whereas the GlcAT-D gene was selectively positive in the epaxial migrating myoblasts within the limb bud (Nagase et al. 2000). This suggests that the differential expression of the GlcATs relates to different in vivo functions of the HNK-1 epitope.
In this study, we examined localization of the HNK-1 epitope in the rat embryo in detail, and compared it with other NCC markers such as CRABP-I (Ruberte et al. 1992) and AP-2 (Mitchell et al. 1991). HNK-1 staining was positive in the cranial ganglia and in migrating trunk NCCs. We also examined expression patterns of GlcAT genes to determine which of these genes is involved in synthesis of the HNK-1 epitope in situ. Expressions of GlcAT-P and GlcAT-D genes showed mutually exclusive patterns in the NCCs, generally in a lineage-specific manner. To know whether the HNK-1 epitope is also involved in NCC migration in the rat embryo, we further electroporated GlcAT genes into the cranial NCCs of the cultured rat embryos. The NCCs transfected with GlcAT genes showed marked up-regulation of HNK-1 epitope synthesis in vivo. Furthermore, overexpression of the two GlcATs resulted in differential effects on migration of the cranial NCCs. These results suggest that the HNK-1 epitope is involved in migration of the NCC subpopulation in the rat embryo, in a different manner to that for the avian embryo, via the differential in vivo functions of the two GlcATs.
Materials and methods
Animals
The experimental protocol was approved by the Ethics Review Committee for Animal Experimentation of the National Institute of Neuroscience. The day of detection of the vaginal plug was designated embryonic day (E) 0. Sprague–Dawley (SD) rat embryos were dissected out from anaesthetized mothers at E10.5 and 11.5, and fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) overnight at 4 °C. For whole-mount analysis, embryos were stored in 100% methanol at −30 °C until use. For cryosectioning, embryos were embedded in an optimal cutting temperature compound, cryosectioned into 14-µm-thick slices and thaw-mounted onto Vectabond (Vector Laboratories)-coated glass slides.
Immunostaining
Whole-mount immunostaining was performed using HNK-1 monoclonal antibody (CD57; Becton Dickinson) largely as described previously (Osumi et al. 1997; Inoue et al. 2001). In brief, embryos were incubated with HNK-1 antibody overnight at 1 : 10 dilution. After washing, samples were incubated overnight with horseradish peroxidase-conjugated antimouse IgM (Jackson ImmunoResearch Laboratories) at 1 : 200 dilution. Immunoreactivity was visualized by the diaminobenzidine (DAB) reaction.
Immunostaining on sections was performed generally as described previously (Nagase et al. 2000, 2001). Frozen sections were boiled in 0.01 m sodium citrate for antigen enhancement, and incubated overnight at 4 °C with HNK-1 antibody at 1 : 40 dilution. Biotinylated antimouse IgM (Zymed) was used as secondary antibody at 1 : 200 dilution, and the sections were treated with hydrogen peroxide. Immunoreactivity was detected using an ABC kit (Vector Laboratories) and a Metal Enhanced DAB Kit (Pierce).
Double-staining with HNK-1 antibody and anti Islet-1 antibody (40.2D6, Developmental Studies Hybridoma Bank) was also performed, at 1 : 100 dilution for Islet-1. Cy3-conjugated antimouse IgM and FITC-conjugated antimouse IgG (Jackson ImmunoResearch Laboratories) were used at 1 : 200 dilution.
In situ hybridization
RNA probes for rat GlcAT-P and GlcAT-D genes were generated according to the methods described by Shimoda et al. (1999) and Nagase et al. (2000, 2001). Probes for CRABP-I and AP-2 were kindly provided by Dr P. Chambon and Dr T. Williams, respectively (Mitchell et al. 1991; Ruberte et al. 1992).
Whole-mount in situ hybridization with a digoxygenin (DIG)-labelled RNA probe for the CRABP-I gene was performed as described previously (Osumi et al. 1997; Inoue et al. 2000). In brief, the whole-mount samples were treated with proteinase K (15 µg mL−1) at room temperature for 10 min, and hybridized with a DIG-labelled probe (20 ng mL−1) overnight at 60 °C. After washing, the samples were incubated overnight at 4 °C with anti-DIG Fab-alkaline phosphatase conjugate at 1 : 5000 dilution. The hybrids were visualized by the subsequent alkaline phosphatase reaction with nitro blue tetrazolium (NBT)/5-bromo-4-chloro-3-indolyl phosphate substrate (BCIP).
In situ hybridization on sections was performed as described previously (Nagase et al. 2000, 2001). After treatment with proteinase K (5 ng mL−1) at 37 °C for 2 min, the sections were hybridized with probes (1 µg mL−1) overnight at 60 °C. After washing, the slides were incubated overnight at 4 °C with anti-DIG Fab-alkaline phosphatase conjugate at 1: 2000 dilution. The hybrids were visualized by NBT/BCIP.
Rat whole embryo culture and transfection of GlcAT genes
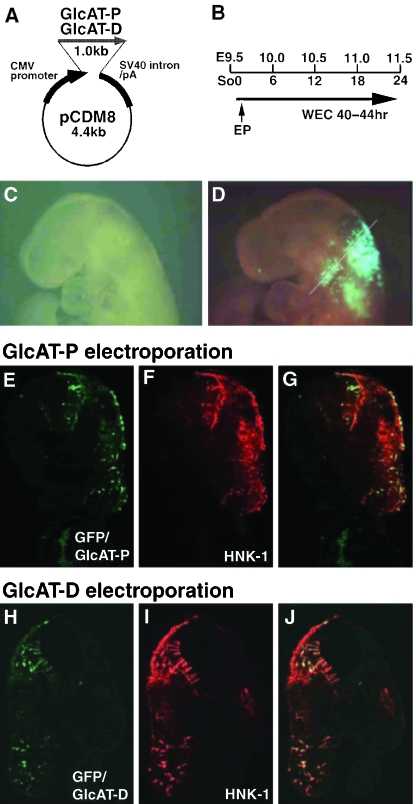
Two expression vectors, pCDM8-GlcAT-P and pCDM8-GlcAT-D, were generated as described previously (Shimoda et al. 1999). pCDM8 expression plasmid contains cytomegalovirus promotor (Shimoda et al. 1999). pCAX-GFP contains cytomegalovirus enhancer and chicken β-actin promotor (Inoue et al. 2001). Also, 5 µg µL−1 DNA–PBS solutions of pCDM8-GlcAT-P and pCAX-GFP (5 : 1), pCDM8-GlcAT-D and pCAX-GFP (5 : 1), and pure pCAX-GFP were prepared before transfection.
The method used for electroporation into cultured mammalian embryo was as described previously (Osumi & Inoue, 2001; Takahashi & Osumi, 2002). E9.5 SD rat embryos (0–3 somite stage) were dissected out and precultured for at least 1 h. Embryos were then transferred into a 35-mm dish, and the prepared DNA solutions were injected in the amniotic cavity using a micropipette. Square pulses (50 ms, 30 V, five times) were generated using an electroporator (BTX) and 3-mm-diameter platinum electrodes placed 6 mm apart, between which the embryo was placed so that negatively charged DNA flowed towards the cranial region. Using this set-up, we confirmed that the vectors were preferably expressed in the neuroepithelium and migrating NCCs in the cranial region (data not shown). The embryos were further cultured for 40–44 h, and then processed for further analyses.
Analyses of the transfected cranial NCCs of the cultured embryos
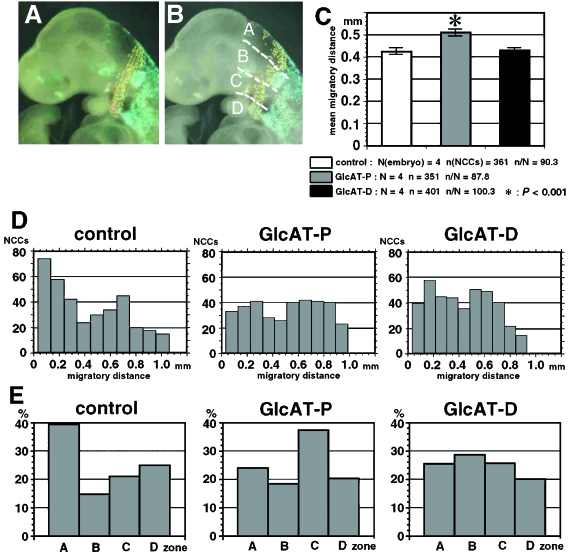
The transfected embryos without any morphological or physiological disorder were used for further analysis. We analysed migration of the NCCs within the second pharyngeal arch. The NCCs that were successfully transfected by the GlcAT-P or GlcAT-D gene can be detected under fluorescence illumination by the co-transfected green fluorescent protein (GFP). Lateral images of the embryos under the fluorescent dissection microscope were recorded, and all the NCCs in the second pharyngeal arch were marked on the images using the software MacSCOPE (Mitani Corp.).
Distance between a labelled NCC and the posterior edge of the neuroepithelium in the image was measured using the same software (see Fig. 5A). This distance may correspond to the projection of the migratory course of each NCC in three-dimensional space to the sagittal plane. Thus we regard this distance as an approximation of the true migratory distance. The data were summarized as the mean ± standard error (SEM) in the GlcAT-P group, the GlcAT-D group and the control (only GFP) group.
Fig. 5.
Altered migration of the second pharyngeal arch NCCs after electroporation with GlcAT genes. (A) Measurement of NCC migration. The distance of each NCC from the posterior edge of the neuroepithelium is measured in the lateral digital images (yellow lines). This distance is an approximate projection of the three-dimensional migratory course to the sagittal plane. (B) NCCs were counted in the four zones as follows: Zone A, from the neuroepithelium to the dorsal edge of the otic vesicle; Zone B, between the dorsal and ventral edges of the otic vesicle; Zone C, from the ventral edge of the otic vesicle to the base of the second pharyngeal arch; Zone D, within the second pharyngeal arch. (C) Average of the NCC migratory distance in the control group (GFP transfection only; white bars), the GlcAT-P transfection group (grey bars) and the GlcAT-D transfection group (black bars). NCCs in the GlcAT-P group migrate further than in the other groups. Asterisk: P < 0.001 by anova and Sheffe's test. (D) Distribution of NCC numbers along their migratory distance. NCCs show relatively shorter migration in the control group. Two peaks are observed at about 0.2 mm and 0.6 mm of migration in the GlcAT groups. The 0.2-mm peak is higher in the GlcAT-D group. (E) Percentages of NCCs in the four zones. Numbers are high in Zone A in the control group, in Zone C in the GlcAT-P group and in Zone B in the GlcAT-D group.
Localization of the NCCs on the lateral images was categorized into four zones (see Fig. 5B). Zone A is from the neuroepithelium to the dorsal edge of the otic vesicle. Zone B is between the dorsal and the ventral edges of the otic vesicle. Zone C is from the ventral edge of the otic vesicle to the base of the second phayngeal arch. Zone D is within the second pharyngeal arch. Numbers of NCCs within the four zones were determined using the MacSCOPE, summarized as mean ± SEM and compared among the three groups.
One-way anova was performed to determine whether there were significant differences in the migratory distance of the NCCs among the three groups.
Results
The HNK-1 epitope is positive in sublineages of both NCC and non-NCC derivatives in the rat embryo
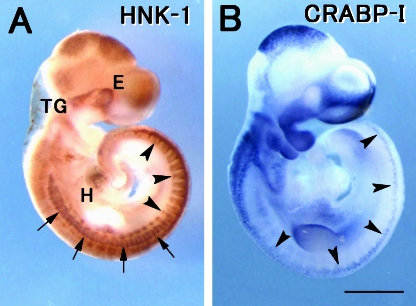
Localization of the HNK-1 epitope in the E11.5 rat embryo was first investigated by whole mount immunostaining (Fig. 1A). In the cranial region, clear staining was observed in the eye primordium, the forebrain/midbrain neuroepithelium, and some of the cranial ganglia. Expression of CRABP-I, a marker for NCCs, was clearly positive in the facial primordia (Fig. 1B), where HNK-1 staining was not detected. These staining patterns were consistent with our previous observations in sections of the rat embryo (Nagase et al. 2001). At the 0–15 somite level, HNK-1 staining was seen in the dermatome and the longitudinal fibres in the myotome (arrows in Fig. 1A), as reported previously (Nagase et al. 2000). HNK-1 was positive in the migrating NCCs in the trunk more caudally than the 15 somite level (arrowheads in Fig. 1A), as described by Erickson et al. (1989). The NCCs at the more rostral level were not stained by HNK-1 antibody, although they were positive for CRABP-I (arrowheads in Fig. 1B). HNK-1 staining was also positive in the heart, probably in the putative cardiac conduction system (Ikeda et al. 1990; Aoyama et al. 1995). These findings suggest that the HNK-1 epitope is localized in subpopulations of NCCs and non-NCC derivatives in the rat embryo.
Fig. 1.
Whole mount immunostaining using HNK-1 monoclonal antibody (A) and in situ hybridization with a probe of the CRABP-I gene (B) in the E11.5 rat embryo. (A) HNK-1 is localized in the trigeminal ganglia (TG) and the migrating trunk NCCs more caudally than somite 15 level (arrowheads). HNK-1 staining is also positive in the eye primordia (E), the midbrain neuroepithelium, the longitudinal fibres (arrows) in the myotome, the limb bud and the heart primordia (H). (B) CRABP-I transcripts are localized in the facial primodia and the NCCs in the trunk (arrowheads). Note that the CRABP-I-positive facial primordia are devoid of the HNK-1 epitope. Scale bar = 1 mm.
The HNK-1 epitope and GlcAT-D mRNA are positive in the cranial gangliogenic NCCs
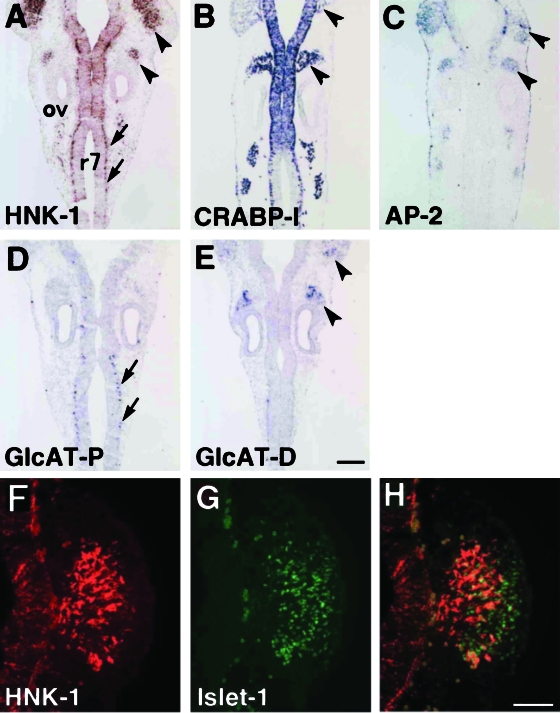
To observe localization of the HNK-1 epitope in the NCCs in more detail, we next performed HNK-1 immunostaining on sections, and compared them with the distribution of other NCC markers, CRABP-I and AP-2. In the E11.5 cranial region, a stage at which most cranial NCCs have already reached their destination, HNK-1 was positive in the trigeminal and vestibulocochlear ganglia, neuroepithelium of rhombomeres and cells in the basal layer of the rhombomere 7 (r7) (Fig. 2A). The cells in r7 were co-stained with antineurofilament antibody (data not shown), indicating that they are neurons. Expression of the CRABP-I gene was positive both in the all-cranial ganglia and the neuroepithelium (Fig. 2B), and transcripts of AP-2, another marker for NCCs, were also observed in the cranial ganglia (Fig. 2C). To investigate the extent to which the HNK-1 epitope is localized in the trigeminal ganglia, we carried out double-staining with antibodies for the HNK-1 epitope and Islet-1, a marker for neurogenic cells in the cranial and dorsal root ganglia (Cui & Goldstein, 2000). HNK-1 and Islet-1 double-positive cells were found in the proximal part of the trigeminal ganglion (Fig. 2F–H), suggesting that the HNK-1 epitope is localized only in the NCC-derived component of the ganglia, and not in the placodal portion (Noden, 1993). This observation is consistent with another finding that the ganglia derived from epibranchial placodes (such as geniculate, petrosal and nodose) also seem to be HNK-1 negative (Fig. 2A).
Fig. 2.
Localization of the HNK-1 epitope and expressions of other NCCs markers and GlcAT genes in the coronal sections of E11.5 rat embryos. (A) The HNK-1 epitope is localized in the trigeminal and vestibulocochlear ganglia (arrowheads), the rhombomeric neuroepithelium and the neurons in rhombomere 7 (r7) (arrows). (B,C) CRABP-I and AP-2 transcripts are positive in the cranial ganglia (arrowheads). CRABP-I expression is additionally detected in the neuroepithelium and r7 neurons. (D) The GlcAT-P gene is specifically expressed in r7 neurons (arrows). (E) The GlcAT-D gene is specifically expressed in the cranial ganglia (arrowheads). (F–H) Close-up views of the HNK-1-positive cells in the trigeminal ganglia. (F) HNK-1 immunostaining. (G) Immunostaining using anti Islet-1 antibody, a marker for sensory neurons in the ganglia. (H) An overlay image of HNK-1/anti Islet-1 immunostaining. Note that the HNK-1-positive cells are localized in the proximal portion of the Islet-1-positive early neurons, suggesting that placodal-derived cells in the ganglia are HNK-1 negative. ov, otic vesicle. Scale bars: 200 µm (A–E), 100 µm (F–H).
To determine which subtype of GlcATs is involved in synthesis of the HNK-1 epitope in situ, we then investigated the expression of the GlcAT-P and GlcAT-D genes. Interestingly, GlcAT-P mRNA was confined to r7 neurons (arrows in Fig. 2D), and negative for cranial ganglia. In contrast, cells in the trigeminal and vestibulocochlear ganglia were strongly positive for GlcAT-D (arrowheads in Fig. 2E). Transcripts of both GlcAT-P and GlcAT-D were negative in the neuroepithelium of the rhombomeres except for r7 neurons, suggesting a possible contribution of other glucuronyltransferases in this part.
In summary, the HNK-1 epitope was positive in the postmigratory NCCs in the cranial ganglia, and these cells specifically expressed GlcAT-D gene.
The HNK-1 epitope and GlcAT-P mRNA are positive in the migrating NCCs in the trunk
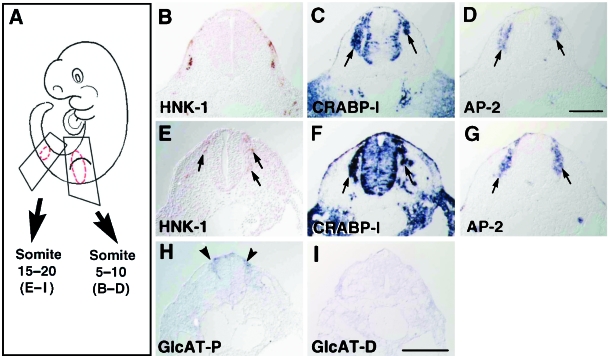
We then investigated the localization pattern of the HNK-1 epitope and expressions of GlcAT genes in serial sections of the trunk at E11.5. The rostral NCCs generally migrate earlier than the caudal NCCs. In E11.5 rat embryos, the NCCs at the somite 5–10 level have nearly finished their migration (except for melanogenic NCCs), condensing to form the dorsal root ganglia, whereas the NCCs at the somite 15–20 level are just starting to migrate.
In the rostral trunk (5–10 somite level), HNK-1 staining was found in the somite but not in the dorsal root ganglia (Fig. 3B), where transcripts of CRABP-I and AP-2 were found (arrows in Fig. 3C,D). In the caudal trunk (15–20 somite level), immunolocalization of the HNK-1 epitope was clearly observed in the cells near the neural tube (arrows in Fig. 3E). HNK-1 localization was included within the area of positive expression for NCC markers, CRABP-I and AP-2 (arrows in Fig. 3F,G), suggesting that HNK-1-positive cells are certainly migrating NCCs. It is known that the trunk NCCs migrate along the dorsolateral, ventrolateral and ventromedial pathways, and the melanogenic NCCs migrate in the dorsolateral pathway in later stages (Serbedzija et al. 1990). HNK-1 immunoreactivity was seen only in a subpopulation of the NCCs in both the ventromedial and the ventrolateral pathways. Expression of the GlcAT-P gene was detected in the NCCs (arrowheads in Fig. 3G), whereas GlcAT-D transcripts were not detected in this section (Fig. 3I). GlcAT-P gene expression was confined to the subpopulation of HNK-1-positive NCCs just after the delamination from the neural tube (compare Fig. 3E and H).
Fig. 3.
Localization of the HNK-1 epitope and expressions of other NCCs markers and GlcAT genes in the trunk. (A) Schematic drawing of E11.5 rat embryo showing sections of somite 5–10 level (B–D) and somite15–20 level (E–I) of the trunk. (B) HNK-1 staining is positive in the dermatome, myotome (corresponding to longitudinal fibres) and migrating myoblasts in the limb bud, but not in the NCCs in the rostral trunk. (C,D) CRABP-I and AP-2 transcripts are found in the dorsal root ganglia. (E) In the caudal trunk, the HNK-1 epitope is found in the migrating NCCs (arrows). (F,G) Expressions of CRABP-I and AP-2 genes are also found in the NCCs (arrows). Not all the CRABP-I/AP-2-positive NCCs are stained with HNK-1 antibody. (H) The GlcAT-P gene is expressed in the NCCs just starting their migration (arrowheads). (I) The GlcAT-D gene expression is not detected in the NCCs. Scale bar = 200 µm.
In summary, the HNK-1 epitope was positive in the migrating NCCs in the trunk, and these cells specifically expressed the GlcAT-P gene.
Synthesis of the HNK-1 epitope is up-regulated by electroporation of GlcAT genes
The data above show that the GlcAT-P-positive NCCs in the trunk have just started their migration, whereas the GlcAT-D-positive NCCs in the ganglia are not actively migrating. To investigate whether different GlcATs have any role in regulating such differential patterns of NCC migration, we transfected GlcAT genes in the cranial NCCs by in vivo electroporation using the whole embryo culture system (Inoue et al. 2001; Osumi & Inoue, 2001). We targeted the NCCs in the second pharyngeal arch because their migratory pattern is the simplest and the most typical, and thus suitable for morphological analysis.
We first examined whether overexpression of GlcAT genes effectively promotes synthesis of the HNK-1 epitope. pCDM8-GlcAT-P or pCDM8-GlcAT-D expression vector, together with pCAX-GFP, was injected into the amniotic cavity of the 1–3 somite stage embryos at about E9.5–9.6, and electroporated into the head fold region (Fig. 4A,B). After the culture (corresponding to the E11.5 stage), GFP was detected within the neuroepithelium and NCCs in the mesenchyme (Fig. 4C,D). To examine synthesis of the HNK-1 epitope, we compared GFP expression and HNK-1 staining on sections of the second pharyngeal arch. The overlay images of GFP and HNK-1 staining showed remarkable co-localization in the neuroepithelium and NCCs (Fig. 4E–J), indicating that the transfected GlcAT genes induced the HNK-1 epitope synthesis effectively in these tissues. There were no obvious differences between electroporation of GlcAT-P and GlcAT-D genes in HNK-1 synthesis.
Fig. 4.
Transfection of GlcAT genes in the cranial NCCs. (A) Structures of expression vectors (pCDM8-GlcAT-P/D). (B) Time course of the electroporation experiment. So: somite number; EP: electroporation; WEC: whole embryo culture. (C,D) A representative sample successfully transfected with the GlcAT-P gene in the NCCs, which are detected by GFP due to co-transfection of the pCAX-GFP gene. The samples are subsequently sectioned at the level of the second pharyngeal arch (white line). (E–G) Transfection of the GlcAT-P gene. (E) The migrating NCCs positive for GFP, showing successful electroporation. (F) HNK-1 immunostaining is positive in the migrating NCCs. (G) An overlay image showing co-localization of HNK-1 staining and GFP. (H–J) Transfection of the GlcAT-D gene. HNK-1 synthesis is qualitatively almost similar to GlcAT-P transfection.
Overexpression of GlcAT genes alters migratory distance of the cranial NCCs
We further examined whether up-regulation of GlcAT genes effects NCC migration in a differential mode. For quantitative analyses, we measured the migratory distances of each NCC (Fig. 5A) and the numbers of NCCs within Zones A–D (Fig. 5B; see details in Materials and methods). The data were obtained from four samples of the GlcAT-P transfected group, four samples of the GlcAT-D transfected group and four samples of the control (GFP only) group.
Averages of the migratory distances were statistically analysed among the three groups (Fig. 5C). The distance was significantly larger in the GlcAT-P group than in the other two groups (P < 0.001 in anova and Scheffe's test). The average distance for the control and the GlcAT-D groups was similar. However, the distribution profile of the NCCs (obtained by dividing the group into 10 subgroups according to their migratory distance) revealed the differential tendency of their migration among the three groups (Fig. 5D). In the control group, many NCCs migrated short distances, with a small distribution peak at 0.7 mm. By contrast, electroporation with GlcAT-P and GlcAT-D showed a biphasic distribution, with peaks at 0.2 mm and 0.6 mm. The peak at 0.2 mm was larger in the GlcAT-D group, whereas the peak at 0.7 mm was larger in the GlcAT-P group, reflecting the higher average migratory distance in this group. This different tendency was detected more clearly in the NCC number data in the four zones (Fig. 5E). Although the number of NCCs within Zone A was the largest in the control group, those in Zone C and Zone B were largest in the GlcAT-P and GlcAT-D groups, respectively.
Taken together, there was a tendency for the NCCs transfected with the GlcAT-P gene to show elongated migration. By contrast, the NCCs transfected with GlcAT-D seemed to gather around Zone B, although their average migratory distance was not altered. Considering that the GlcAT-P-positive NCCs in the trunk are at the stage of active migration and that the GlcAT-D-positive vestibulocochlear ganglia are located in the region corresponding to Zone B, it is suggested that the distinct results of GlcAT gene overexpression in the present study may reflect the differential in vivo roles of GlcATs on NCC migration in the normal development.
Discussion
Unique expression of the HNK-1 epitope in the NCCs of the rat embryo
Although the HNK-1 epitope is a general marker for avian NCCs (Bronner-Fraser, 1986), there is disagreement among researchers regarding localization of the HNK-1 epitope in NCCs of the rat embryos. Erickson et al. (1989) emphasized that HNK-1 immunostaining can detect broad populations of NCCs in the rat embryo, as seen in avians, although they mentioned that the rostral trunk NCCs are HNK-1 negative. Louryan et al. (1996) reported negative staining of HNK-1 in the E10.5 and E11.5 rat ectomesenchyme. We also reported previously that HNK-1 staining was not detected in the ectomesenchyme NCCs in the rat embryo (Nagase et al. 2001). In the present study, we performed detailed analyses on the localization of the HNK-1 epitope in the rat embryo, making comparisons with other frequently used NCC markers, CRABP-I and AP-2. Our results clearly demonstrate that HNK-1 is positive in some subpopulations, but not all, of the rat NCCs: namely, the cranial gangliogenic NCCs and the migrating trunk NCCs.
The cranial ganglia are derived from NCCs and/or epibranchial placodes (Noden, 1993). A proximal portion of the trigeminal and vestibulocochlear ganglia contains NCC derivatives, whereas a distal portion of these ganglia includes placodal derivatives. We found that HNK-1-positive cells in the trigeminal ganglia were confined to the NCC-derived portion by double immunostaining with HNK-1 and anti-Islet-1 antibody. The ganglia of placodal origin (putatively geniculate, petrosal and nodose) also appeared HNK-1 negative. In the trunk, the HNK-1 epitope was localized in the migrating NCCs in the somite 15–20 level. It is quite noteworthy that HNK-1 immunostaining was negative in the NCCs in the dorsal root ganglia, where CRABP-I and AP-2 transcripts were detected. We also noted that HNK-1-positive NCCs were only the subpopulation in the ventromedial and ventrolateral pathways, because expression patterns of CRABP-I/AP-2 in the migrating NCCs are broader and include HNK-1-positive NCCs at this level (see Fig. 3E–G).
We have also shown that the HNK-1 epitope is positive in some of the non-NCC derivatives. The dermatome, the longitudinal fibres of the myotome, migrating myoblasts within the limb bud and the eye primordium were HNK-1 positive, as reported previously (Nagase et al. 2000, 2001). In addition, the present study showed that the rhombomeric neuroepithelium and the r7 neurons were also positive for HNK-1. Taken together, there is a clear difference in localization of HNK-1 between avian and rodent embryos, which might reflect different roles of HNK-1-bearing molecules in the development of these animal groups.
GlcAT subtypes and synthesis of the HNK-1 epitope
The present study demonstrated the mutually exclusive expression patterns of two GlcAT genes. GlcAT-P transcripts were detected in the migrating trunk NCCs, whereas GlcAT-D transcripts were in the cranial ganglia where NCCs colonized and started to differentiate. Our previous reports also described differential expressions of these two GlcAT genes; GlcAT-P is specifically expressed in the myotomal longitudinal fibres, and GlcAT-D is expressed in the retina, dermatome and myoblasts migrating in the limb bud (Nagase et al. 2000, 2001). These results suggest a possible difference of in vivo functions between the two GlcATs, reflecting their differential expressions.
Although the rhombomeric neuroepithelium is HNK-1 positive, neither GlcAT-P nor GlcAT-D were expressed in this portion. This suggests that the HNK-1 epitope can be synthesized by other type(s) of GlcAT than GlcAT-P or GlcAT-D. A glycolipid-specific subtype of GlcAT, GlcAT-L, has already been reported (Oka et al. 1992), although its gene has not yet been cloned. Whether this GlcAT-L is involved in HNK-1 synthesis in the rhombomere awaits further investigation. It is also of note that HNK-1 epitope synthesis requires not only GlcATs, but also sulfotransferase. HNK-1 synthetic sulfotransferase has been already identified (Bakker et al. 1997), and its expression is clearly detected in the E13.5 rat brain by in situ hybridization (our unpublished data). This sulfotransferase gene may be expressed at a low level in the E11.5 rat limb bud, as we reported previously (Nagase et al. 2000). Because we could not ascertain this expression by RT-PCR in total RNA of E11.5 whole rat embryo (data not shown), it is unclear at present whether expression of this sulfotransferase gene is below a detection level, or another unknown subtype of sulfotransferase is involved in HNK-1 epitope synthesis in E11.5 rat embryo.
HNK-1 epitope, GlcATs and migration of NCCs
Bronner-Fraser (1987) reported that HNK-1 antibody injection disturbs the migration of cranial NCCs, suggesting an involvement of the HNK-1 epitope in NCC migration. A putative macromolecule bearing the HNK-1 epitope on the surface of NCCs may be α1β1 integrin, which may interact with extracellular matrices during migration (Lallier & Bronner-Fraser, 1992; Perris & Perissinotto, 2000). In the present study, overexpression of GlcAT genes up-regulated HNK-1 synthesis, and changed migratory patterns of the cranial NCCs in vivo. These results suggest that the HNK-1 epitope has an important role in NCC migration in mammalian embryos as well.
There was a differential tendency between the NCCs transfected with two GlcATs: the NCCs transfected with GlcAT-P migrated further than the NCCs transfected with GlcAT-D, which gathered around the otic placode. The differential effects of electroporation of GlcAT-P and GlcAT-D genes might be based on the substrate specificity of the two GlcATs. GlcAT-P acts on the HNK-1 epitope on Type II glycan, and GlcAT-D acts on both Type I and Type II glycan in vitro (Shimoda et al. 1999). Our results suggest that HNK-1-bearing macromolecules are different between the GlcAT-D-positive NCCs and the GlcAT-P-positive NCCs, and this question should be investigated in the future.
How can we interpret the results of this electroporation experiment in relation to the aforementioned differential patterns of GlcAT expressions in the NCCs in normal development? The GlcAT-D-positive NCCs in the cranial ganglia were considered to be post-migratory, and the GlcAT-P-positive NCCs in the trunk were considered to be migratory. These observations imply a possible hypothesis that GlcAT-D-dependent HNK-1 epitope in the cranial ganglia mediates cessation of their migration, whereas GlcAT-P-dependent HNK-1 epitope in the trunk NCCs mediates initiation and/or maintenance of their migration. We have demonstrated that overexpression of GlcAT-D caused an accumulation of the transfected NCCs in Zone B, near the acousticofacial ganglia, and this result is consistent with the hypothetical function of GlcAT-D. Also, the ectopic expression of GlcAT-P in the cranial NCCs resulted in induction of their elongated migration. To substantiate this hypothesis, several additional studies will be required, for example looking at the overexpression of GlcAT genes in the trunk NCCs or inhibition of GlcAT functions.
Acknowledgments
We thank Dr T. Inoue, Dr Y. Ishii and all the members of the Shindan Laboratory, NCNP, for discussions and helpful suggestions, and Miss J. Asami for technical assistance. The 40.2D6 antibody developed by J. Dodd was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences at Johns Hopkins University School of Medicine, Baltimore, MD, and the Department of Biology at the University of Iowa, Iowa City, IA, under contact number NO-HP-2-3144 from the NICHD. This work was supported by a Grant-in Aid for Scientific Research from the Japan Society for Promotion of Science (No.12-9223) given to T.N., by a Grant-in Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture (No.14370569) given to H.A., and by a Grant-in Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture (Nos. 1022029, 11470002 and 1158090), and CREST from Japanese Science and Technology Corporation given to N.O.
References
- Abo T, Balch CM. A differentiation antigen of human NK and K cells identified by a monoclonal antibody (HNK-1) J. Immunol. 1981;127:1024–1029. [PubMed] [Google Scholar]
- Aoyama N, Tamaki H, Kikawada R, Yamashina S. Development of the conduction system in the rat heart as determined by Leu-7 (HNK-1) immunohistochemistry and computer graphics reconstruction. Lab. Invest. 1995;72:355–366. [PubMed] [Google Scholar]
- Ariga T, Kohriyama T, Freddo L, Latov N, Saito M, Kon K, et al. Characterization of sulfated glucuronic acid containing glycolipids reacting with IgM M-proteins in patients with neuropathy. J. Biol. Chem. 1987;262:848–853. [PubMed] [Google Scholar]
- Bakker H, Friedmann I, Oka S, Kawasaki T, Nifant’ev N, Schachner M, et al. Expression cloning of a cDNA encoding a sulfotransferase involved in the biosynthesis of the HNK-1 carbohydrate epitope. J. Biol. Chem. 1997;272:29942–29946. doi: 10.1074/jbc.272.47.29942. [DOI] [PubMed] [Google Scholar]
- Bannerman PG, Oliver TM, Nichols WL, Jr, Xu Z. The spatial and temporal expression of HNK-1 by myogenic and skeletogenic cells in the embryonic rat. Cell Tissue Res. 1998;294:289–295. doi: 10.1007/s004410051179. [DOI] [PubMed] [Google Scholar]
- Becker T, Becker CG, Schachner M, Bernhardt RR. Antibody to the HNK-1 glycoepitope affects fasciculation and axonal pathfinding in the developing posterior lateral line nerve of embryonic zebrafish. Mech. Dev. 2001;109:37–49. doi: 10.1016/s0925-4773(01)00504-4. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Analysis of the early stages of trunk neural crest migration in avian embryos using monoclonal antibody HNK-1. Dev. Biol. 1986;115:44–55. doi: 10.1016/0012-1606(86)90226-5. [DOI] [PubMed] [Google Scholar]
- Bronner-Fraser M. Perturbation of cranial neural crest migration by the HNK-1 antibody. Dev. Biol. 1987;123:321–331. doi: 10.1016/0012-1606(87)90390-3. [DOI] [PubMed] [Google Scholar]
- Chou DK, Ilyas AA, Evans JE, Costello C, Quarles RH, Jungalwala FB. Structure of sulfated glucuronyl glycolipids in the nervous system reacting with HNK-1 antibody and some IgM paraproteins in neuropathy. J. Biol. Chem. 1986;261:11717–11725. [PubMed] [Google Scholar]
- Cui S, Goldstein RS. Early markers of neuronal differentiation in DRG: islet-1 expression precedes that of Hu. Brain Res. Dev. Brain Res. 2000;121:209–212. doi: 10.1016/s0165-3806(00)00034-1. [DOI] [PubMed] [Google Scholar]
- Domowicz M, Li H, Hennig A, Henry J, Vertel BM, Schwartz NB. The biochemically and immunologically distinct CSPG of notochord is a product of the aggrecan gene. Dev. Biol. 1995;171:655–664. doi: 10.1006/dbio.1995.1312. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Loring JF, Lester SM. Migratory pathways of HNK-1-immunoreactive neural crest cells in the rat embryo. Dev. Biol. 1989;134:112–118. doi: 10.1016/0012-1606(89)90082-1. [DOI] [PubMed] [Google Scholar]
- Hennig AK, Maxwell GD. Persistent correlation between expression of a sulfated carbohydrate antigen and adrenergic differentiation in cultures of quail trunk neural crest cells. Differentiation. 1995;59:299–306. doi: 10.1046/j.1432-0436.1996.5950299.x. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Iwasaki K, Shimokawa I, Sakai H, Ito H, Matsuo T. Leu-7 immunoreactivity in human and rat embryonic hearts, with special reference to the development of the conduction tissue. Anat. Embryol. (Berl.) 1990;182:553–562. doi: 10.1007/BF00186462. [DOI] [PubMed] [Google Scholar]
- Inoue T, Nakamura S, Osumi N. Fate mapping of the mouse prosencephalic neural plate. Dev. Biol. 2000;219:373–383. doi: 10.1006/dbio.2000.9616. [DOI] [PubMed] [Google Scholar]
- Inoue T, Tanaka T, Takeichi M, Chisaka O, Nakamura S, Osumi N. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development. 2001;128:561–569. doi: 10.1242/dev.128.4.561. [DOI] [PubMed] [Google Scholar]
- Kruse J, Mailhammer R, Wernecke H, Faissner A, Sommer I, Goridis C, et al. Neural cell adhesion molecules and myelin-associated glycoprotein share a common carbohydrate moiety recognized by monoclonal antibodies L2 and HNK-1. Nature. 1984;311:153–155. doi: 10.1038/311153a0. [DOI] [PubMed] [Google Scholar]
- Kuratani SC. Alternate expression of the HNK-1 epitope in rhombomeres of the chick embryo. Dev. Biol. 1991;144:215–219. doi: 10.1016/0012-1606(91)90493-m. [DOI] [PubMed] [Google Scholar]
- Lallier T, Bronner-Fraser M. α1β1 integrin on neural crest cells recognizes some laminin substrata in a Ca2+-independent manner. J. Cell Biol. 1992;119:1335–1345. doi: 10.1083/jcb.119.5.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louryan S, Werry-Huet A, Van Sint Jan S. Comparison between two HNK-1-related antibodies immunoreactivity (HNK-1-anti-leu 7 and anti-HNK-1/N-CAM) during rat cephalogenesis. Eur. J. Morph. 1996;34:295–300. doi: 10.1076/ejom.34.4.295.13045. [DOI] [PubMed] [Google Scholar]
- Mitchell PJ, Timmons PM, Hebert JM, Rigby PW, Tjian R. Transcription factor AP-2 is expressed in neural crest cell lineages during mouse embryogenesis. Genes Dev. 1991;5:105–119. doi: 10.1101/gad.5.1.105. [DOI] [PubMed] [Google Scholar]
- Nagase T, Shimoda Y, Sanai Y, Nakamura S, Harii K, Osumi N. Differential expression of two glucuronyltransferases synthesizing HNK-1 carbohydrate epitope in the sublineages of the rat myogenic progenitors. Mech. Dev. 2000;98:145–149. doi: 10.1016/s0925-4773(00)00449-4. [DOI] [PubMed] [Google Scholar]
- Nagase T, Nakamura S, Harii K, Osumi N. Ectopically localized HNK-1 epitope perturbs migration of the midbrain neural crest cells in Pax6 mutant rat. Dev. Growth Differ. 2001;43:683–692. doi: 10.1046/j.1440-169x.2001.00611.x. [DOI] [PubMed] [Google Scholar]
- Nishida A, Kobayashi T, Ariyuki F. In vitro developmental toxicity of concanavalin A in rat embryos: analysis of neural crest cell migration using monoclonal antibody HNK-1. Teratog. Carcinog. Mutagen. 1997;17:103–114. [PubMed] [Google Scholar]
- Noden DM. Spatial integration among cells forming the cranial peripheral nervous system. J. Neurobiol. 1993;24:248–261. doi: 10.1002/neu.480240210. [DOI] [PubMed] [Google Scholar]
- Oka S, Terayama K, Kawashima C, Kawasaki T. A novel glucuronyltransferase in nervous system presumably associated with the biosynthesis of HNK-1 carbohydrate epitope on glycoproteins. J. Biol. Chem. 1992;267:22711–22714. [PubMed] [Google Scholar]
- Osumi N, Hirota A, Ohuchi H, Nakafuku M, Iimura T, Kuratani S, et al. Pax-6 is involved in the specification of hindbrain motor neuron subtype. Development. 1997;124:2961–2972. doi: 10.1242/dev.124.15.2961. [DOI] [PubMed] [Google Scholar]
- Osumi N, Inoue T. Gene transfer into cultured mammalian embryos by electroporation. Methods. 2001;24:35–42. doi: 10.1006/meth.2001.1154. [DOI] [PubMed] [Google Scholar]
- Perris R, Perissinotto D. Role of the extracellular matrix during neural crest cell migration. Mech. Dev. 2000;95:3–21. doi: 10.1016/s0925-4773(00)00365-8. [DOI] [PubMed] [Google Scholar]
- Pettway Z, Domowicz M, Schwartz NB, Bronner-Fraser M. Age-dependent inhibition of neural crest migration by the notochord correlates with alterations in the S103L chondroitin sulfate proteoglycan. Exp. Cell Res. 1996;225:195–206. doi: 10.1006/excr.1996.0170. [DOI] [PubMed] [Google Scholar]
- Ruberte E, Friederich V, Morriss-Kay G, Chambon P. Differential distribution patterns of CRABP I and CRABP II transcripts during mouse embryogenesis. Development. 1992;115:973–987. doi: 10.1242/dev.115.4.973. [DOI] [PubMed] [Google Scholar]
- Schachner M, Martini R. Glycans and the modulation of neural-recognition molecule function. Trends Neurosci. 1995;18:183–191. doi: 10.1016/0166-2236(95)93899-9. [DOI] [PubMed] [Google Scholar]
- Seiki T, Oka S, Terayama K, Imiya K, Kawasaki T. Molecular cloning and expression of a second glucuronyltransferase involved in the biosynthesis of the HNK-1 carbohydrate epitope. Biochem. Biophys. Res. Commun. 1999;255:182–187. doi: 10.1006/bbrc.1999.0151. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Shimoda Y, Tajima Y, Nagase T, Harii K, Osumi N, Sanai Y. Cloning and expression of a novel galactoside β1, 3-glucuronyltransferase involved in the biosynthesis of HNK-1 epitope. J. Biol. Chem. 1999;274:17115–17122. doi: 10.1074/jbc.274.24.17115. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Osumi N. Pax6 regulates specification of ventral neurone subtypes in the hindbrain by establishing progenitor domains. Development. 2002;129:1327–1338. doi: 10.1242/dev.129.6.1327. [DOI] [PubMed] [Google Scholar]
- Terayama K, Oka S, Seiki T, Miki Y, Nakamura A, Kozutsumi Y, et al. Cloning and functional expression of a novel glucuronyltransferase involved in the biosynthesis of the carbohydrate epitope HNK-1. Proc. Natl. Acad. Sci. USA. 1997;94:6093–6098. doi: 10.1073/pnas.94.12.6093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker GC, Aoyama H, Lipinski M, Tursz T, Thiery JP. Identical reactivity of monoclonal antibodies HNK-1 and NC-1: conservation in vertebrates on cells derived from the neural primordium and on some leukocytes. Cell Differ. 1984;14:223–230. doi: 10.1016/0045-6039(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Delarue M, Zada S, Boucaut JC, Thiery JP. Expression of the HNK-1/NC-1 epitope in early vertebrate neurogenesis. Cell Tissue Res. 1988;251:457–465. doi: 10.1007/BF00215855. [DOI] [PubMed] [Google Scholar]
- Vincent M, Thiery JP. A cell surface marker for neural crest and placodal cells: further evolution in peripheral and central nervous system. Dev. Biol. 1984;103:468–481. doi: 10.1016/0012-1606(84)90334-8. [DOI] [PubMed] [Google Scholar]
- Voshol H, van Zuylen CW, Orberger G, Vliegenthart JF, Schachner M. Structure of the HNK-1 carbohydrate epitope on bovine peripheral myelin glycoprotein P0. J. Biol. Chem. 1996;271:22957–22960. doi: 10.1074/jbc.271.38.22957. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Nair SM, Chou DK, Tobet SA, Jungalwala FB. Expression and role of sulfoglucuronyl (HNK-1) carbohydrate and its binding protein SBP-1 in developing rat cerebral cortex. J. Neurosci. Res. 2000;62:186–205. doi: 10.1002/1097-4547(20001015)62:2<186::AID-JNR4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]