Abstract
The distribution of T- and B-cells in the developing lymphoid and immunohaematopoietic tissues of the tammar wallaby were investigated using antibodies to the mature cell surface markers, CD3, CD5 and CD79b. In the thymus, CD3- and CD5-positive T-cells were first observed at day 12 postpartum whilst rare B-cells were first detected at day 23. Both T- and B-lymphocytes were first stained on day 21 postpartum in the spleen and day 24 in lymph nodes. In one sample from a 7-day-old animal, rare CD79b-positive (CD79b+) lymphocytes were observed in the gut-associated lymphoid tissues. However, CD3+ cells were not apparent until day 12 and CD5+ cells were not detected until day 74 postpartum. No lymphocytes were detected in liver or bone marrow samples and no bronchus-associated lymphoid tissues were observed. The pattern of development and the distribution of T- and B-cells in the lymphoid and immunohaematopoietic tissues were similar to those observed in eutherian mammals and in limited studies of other metatherians. However, the detection of apparently mature T- and B-cells in the thymus and gut-associated lymphoid tissues (GALT) at the same postnatal age highlights the need for a more substantial study of the development of GALT. This is, at present, limited by availability of marsupial-specific antibodies.
Keywords: B-cells, development, immune system, marsupials, T-cells
Introduction
Marsupials are ideal models for studying the development of the immune system. They are born with no mature or functional lymphoid tissue (reviewed by Old & Deane, 2000) and subsequently develop in a maternal pouch (or marsupium), where they are readily accessible for study. Moreover, during these early stages of development, and in contrast to eutherian mammals, they are exposed to a range of potentially pathogenic micro-organisms (Old & Deane, 1998). Despite these unique characteristics, comprehensive studies of the development of the tissues of the immune system of these animals are few, primarily because of the lack of reagents that allow identification of specific cell populations.
To date, the development of the immunohaematopoietic and lymphoid tissues of the tammar wallaby (Macropus eugenii) have been described using histological techniques (Basden et al. 1996, 1997). Recently, we documented the capacity of antibodies to the surface markers, CD3, CD5 and CD79b, to recognize T- and B-cells in adult tammar wallaby tissues (Old & Deane, 2000). This study reports the use of these antibodies to document the appearance and distribution of T- and B-cells in the lymphoid and immunohaematopoietic tissues of the developing tammar wallaby and aims to clarify the time at which these tissues may be assumed to have achieved functional competence.
Methods
Animals and sample tissues
Tissues were collected opportunistically from 54 pouch young tammar wallabies from the Macquarie University Fauna Park, Macquarie University, NSW, Australia. These were primarily males removed for husbandry purposes and were classified as surplus to need. Ages were determined by measuring the head lengths and subsequent comparison with the values of Murphy & Smith (1970) relating head length to age. Depending on size, animals were killed by either decapitation or a lethal dose of pentobarbital (Lethabarb, Arnolds of Reading, Boronia, Victoria).
The methods used for the dissection and preservation of the tissues were dependent on the age and size of the animal and the target organ. In larger animals, where possible, individual sample tissues were dissected and preserved individually, but in many cases with small animals this was not possible and whole animals were preserved in fixative. Tissues collected included the liver, bone marrow, thymus (both cervical and thoracic), spleen, intestine and lung. All samples were immersed in 10% neutral buffered formalin and then treated as described previously (Old & Deane, 2000).
Antibodies
The primary antibodies used and their dilutions were the same as those described previously (Old & Deane, 2000). These included antibodies to CD3, CD5 and CD79b. Antibodies were donated by Dr Margaret Jones of the Immunodiagnostic Unit (Radcliffe Hospital, Oxford, UK) with the exception of polyclonal anti-CD3, which was obtained commercially from DAKO corporation (Carpenteria, USA).
Histology and immunohistochemistry
For immunohistochemistal studies, 4-µm sections were cut and treated as described previously (Old & Deane, 2002a). In addition, the lung sections were investigated for bronchus-associated lymphoid tissue (BALT) using standard histological techniques (Bancroft & Stevens, 1982). Tissue section integrity was assessed prior to immunohistochemistry using standard staining with haematoxylin and eosin. Positive and negative controls were undertaken to identify non-specific staining. In some cases the tests were limited due to the amount of tissue available from very small animals. All stained sections were viewed using an Olympus CX40RF200 microscope and representative photomicrographs taken using a Leica DMR DAS light microscope and Zeiss Axiovision software.
Results
Liver
Eutherian liver is known to contain biotin and therefore an avidin/biotin-blocking step was included to decrease any background due to endogenous biotin. Immunohistochemistry was conducted on six pouch young liver samples from animals aged 1, 5, 12, 14, 18 and 27 days postpartum. No positive cells were observed in any of the tissues (data not shown).
Bone marrow
Five bone marrow samples were collected from tammar wallabies over a range of ages including 5 (×2), 10, 12 and 75 days postpartum. Although lymphocyte-like cells were visible in serial sections stained with haematoxylin and eosin (data not shown), all test antibodies failed to react with bone marrow cells in these specimens.
Thoracic and cervical thymus
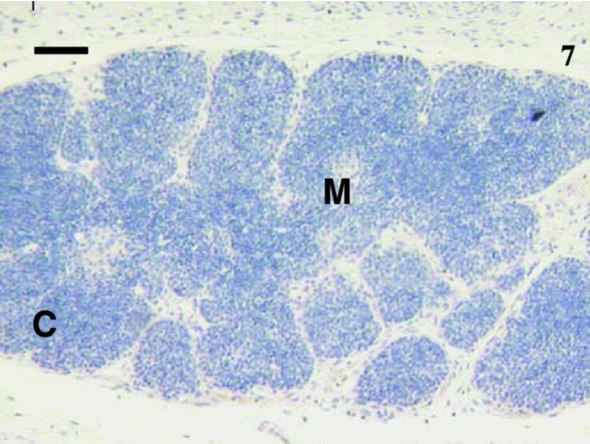
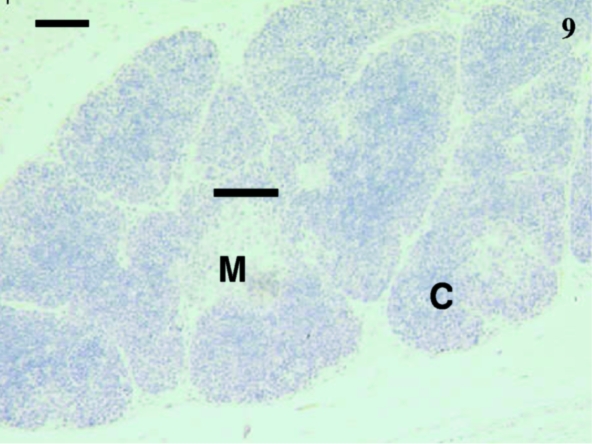
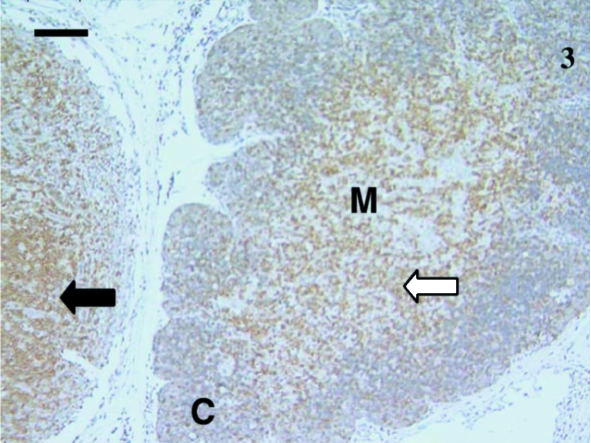
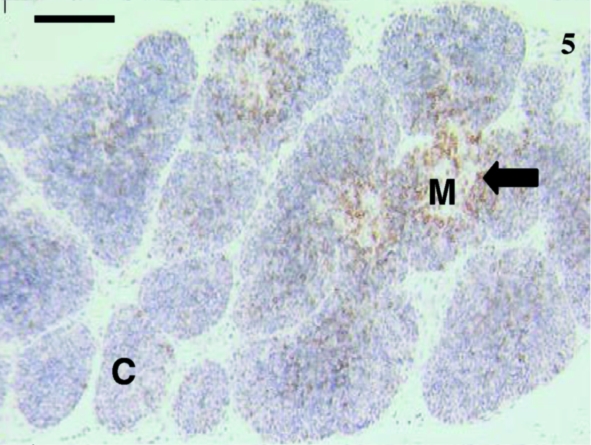
Sixteen samples were investigated from animals aged 1, 4, 5, 12, 14, 23, 24, 27 (×2), 30 (×2), 35, 39, 48, 73 and 75 days postpartum. This included both cervical and thoracic thymus samples. The histological appearance of the thoracic and cervical thymus in the pouch young tammar wallabies was similar to that described previously (Basden et al. 1997). Samples from animals aged 1, 4 and 5 days postpartum did not react with antibodies directed against CD3 (Fig. 1), CD5 (data not shown) or CD79b (data not shown). The youngest sample to demonstrate positive staining with anti-CD3 (Fig. 2) and anti-CD5 (data not shown) was aged 12 days postpartum. Histologically, the sample from an animal aged 12 days had a more defined cortico-medullary structure than observed in samples from younger animals. The majority of the CD3-positive (CD3+) lymphocytes appeared to be in the medulla rather than the heavily packed cortex. Anti-CD5 also stained more lymphocytes in the medulla, rather than the cortex. Similar numbers of CD3+ and CD5+ cells were stained in the thymus. T-cells were consistently observed from day 12 postpartum (Figs 4, 6, 7 and 9).
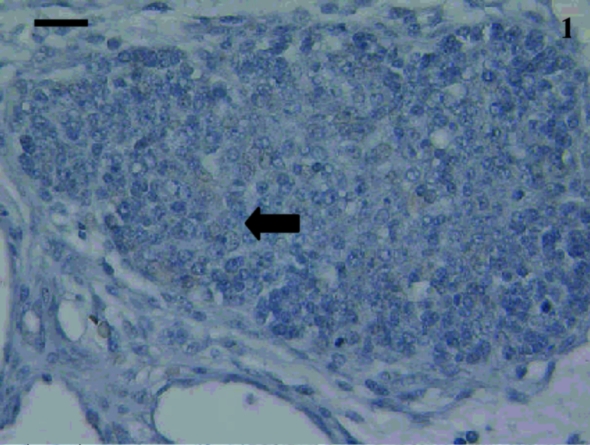
Fig. 1.
Anti-CD3 stained no cells in this thymus from a 5-day-old animal. Similar results were obtained when using anti-CD5 and anti-CD79b. A reticular epithelial cell was visible (arrow). Scale bar, 20 µm.
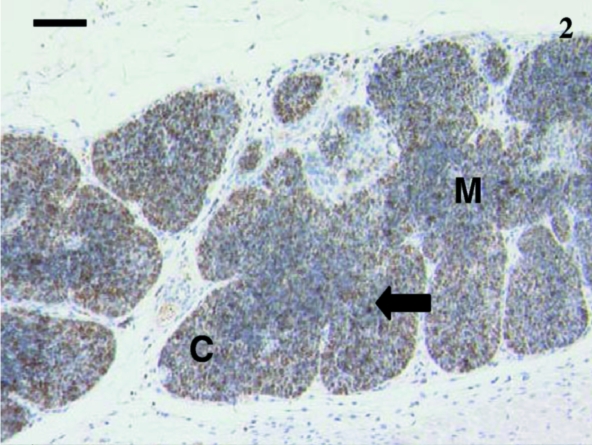
Fig. 2.
Thymus from a 12-day animal with lobules having defined cortical (C) and medullary (M) areas. Anti-CD3-stained cells (arrow) were scattered throughout the thymus. Scale bar, 80 µm.
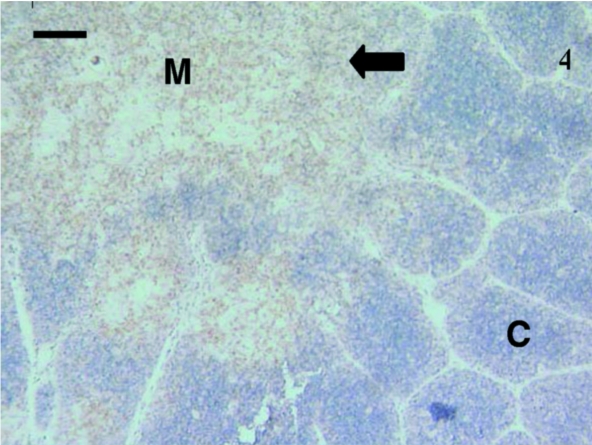
Fig. 4.
In this thymus from a 30-day-old animal, anti-CD3 antibodies stained the majority of lymphocytes (arrow) in the medulla (M) with a few stained lymphocytes in the cortex (C). The lobules were clearly visible. Scale bar, 90 µm.
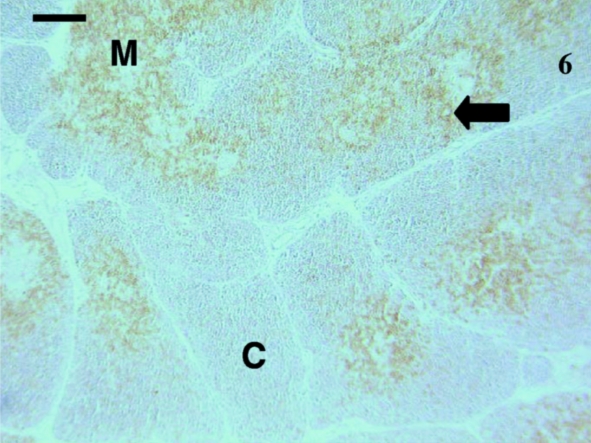
Fig. 6.
Thymus sample from 73-day-old animal with cells stained with anti-CD5 (arrow). The medulla (M) contained more cells stained than the cortex (C). A similar pattern was observed with anti-CD3 antibodies. Scale bar, 120 μm.
Fig. 7.
Sample of a thymus from an animal aged 12 days postpartum with no CD79b+ cells stained. The cortex (C) and medulla (M) were well defined and lobules were prominent. Scale bar, 15 μm
Fig. 9.
Thymus from a 35-day-old animal with no cells stained using anti-CD79b. The cortex (C) and medulla (M)were obvious. The lobules were well defined. Scale bar, 60 μm.
Anti-CD79b failed to recognize B-cells in the thymus from a 12-day-old animal (Fig. 3). Thymus samples from animals aged from 14 days and older displayed variable staining, with the majority containing extremely rare numbers of CD79b+ cells, whereas others had no staining with anti-CD79b (Figs 5 and 8).
Fig. 3.
Lymphocytes (white arrow) were heavily stained with anti-CD5 in this thymus sample from a 24-day-old animal. Most stained lymphocytes were in the medulla (M) with a few stained lymphocytes in the cortex (C). Anti-CD3 stained similarly. A black arrow indicates stained cells in the lymph node. Scale bar, 100 µm.
Fig. 5.
This thymus sample from an animal aged 35 days was extensively stained using anti-CD5. Scattered lymphocytes (arrow) were visible throughout, although the medullary (M) areas were more heavily infiltrated with lymphocytes than the cortical (C) areas. The majority of stained lymphocytes were at the periphery of the medulla. Similar staining was observed using anti-CD3. Scale bar, 120 µm.
Fig. 8.
No lymphocytes were stained using anti-CD79b in this 24-day-old thymus sample. However, the lymph node on the left has a few lymphocytes (arrow) stained. The cortex (C) and medulla (M) were evident. Scale bar, 100 µm.
Spleen
Owing to the friability of the spleen samples, immunohistochemistry was difficult. Furthermore, the small size of the spleen in very young animals limited the number of samples available for study. No analysis was conducted without negative controls, also reducing the numbers of sections available for immunohistochemical tests.
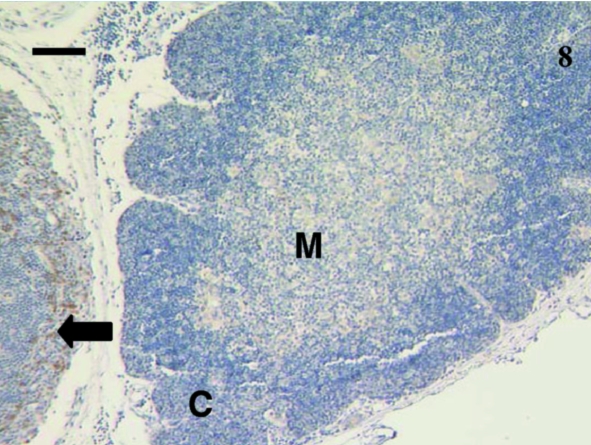
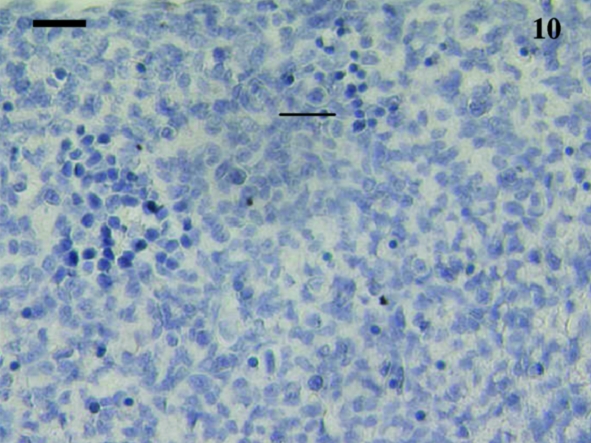
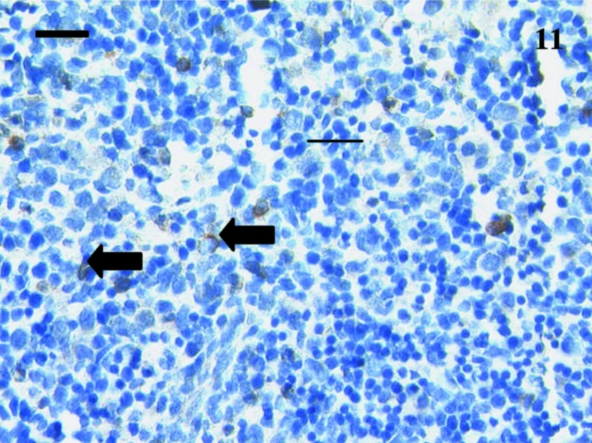
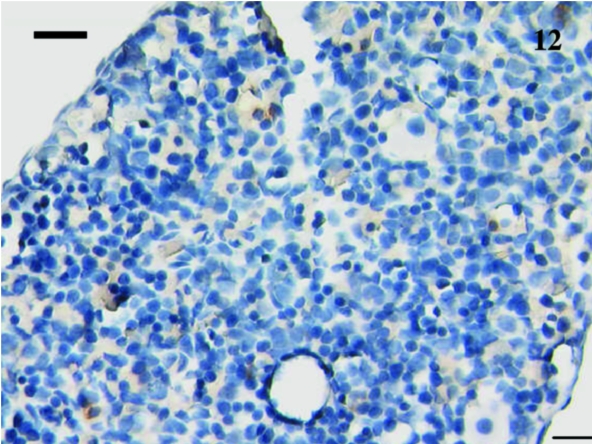
A total of 12 spleen samples were investigated from animals ranging in age from 10 to 110 days postpartum, as follows: 10, 11, 18, 21, 30, 37–38, 38, 48, 49–50, 50, 75 and 110 days. No lymphocytes were detected using anti-CD3, anti-CD5 or anti-CD79b in any samples taken from animals younger than 21 days postpartum. Figure 10 shows a representative section from a 10-day-old animal stained with anti-CD3. Although lymphocyte-like cells are apparent, the tissue bed lacks mature structure with no areas of red or white pulp apparent. Anti-CD3- and anti-CD79b-stained lymphocytes were first observed in a sample from a 21-day-old animal (Figs 11 and 12). Unfortunately, owing to the small size of the sample, no anti-CD5 could be tested. The next oldest age tested was 30 days, and CD5+ lymphocytes (data not shown) were observed in this sample, as were CD3+ (Fig. 13) and CD79b+ (Fig. 14) lymphocytes.
Fig. 10.
Spleen sample from a 10-day-old animal treated with polyclonal anti-CD3 did not stain any cells. No red and white pulp differentiation was apparent. Scale bar, 20 µm.
Fig. 11.
This 21-day-old spleen sample stained with anti-CD3 had a few scattered lymphocytes (arrows) positively stained. Scale bar, 25 µm.
Fig. 12.
12 No CD79b+ cells were observed in this spleen from a 21-day-old animal. Scale bar, 20 μm.
Fig. 13.
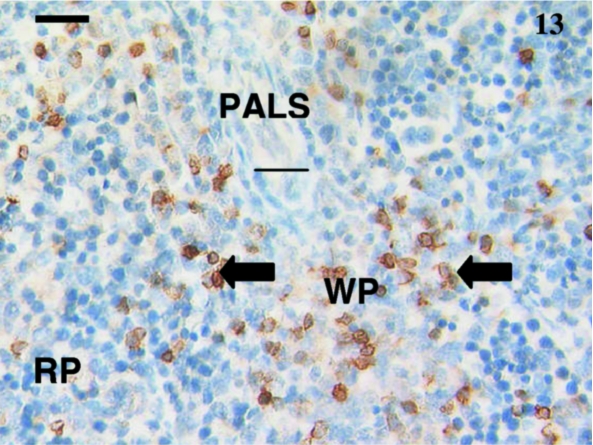
This spleen from a 30-day-old animal shows clearly defined white pulp (WP) and red pulp (RP) areas and an obvious periarterial lymphatic sheath (PALS). Anti-CD3-positively reacted with several scattered lymphocytes (arrows) in the white pulp and fewer in the red pulp areas. Scale bar, 20 µm.
Fig. 14.
A few lymphocytes were stained (arrows) with anti-CD79b throughout the red pulp areas of this spleen sample from an animal aged 30 days postpartum. Scale bar, 25 µm.
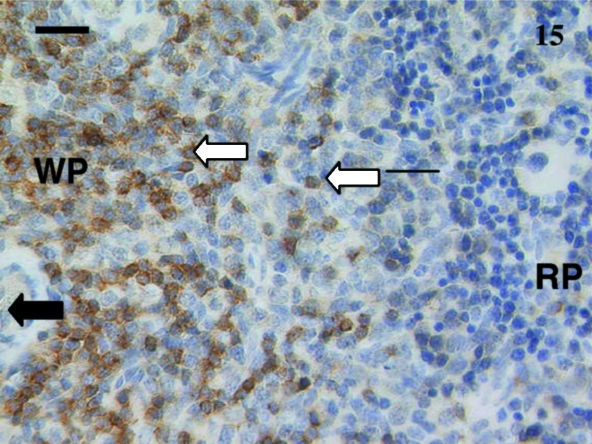
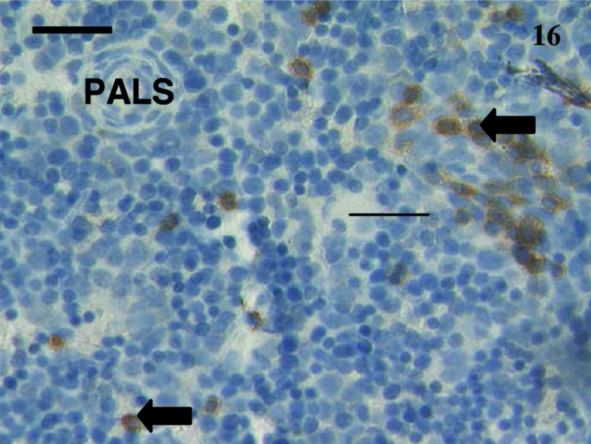
As animals aged, the numbers of positive cells increased and the tissue distribution of these cells assumed an adult pattern. Anti-CD3 and anti-CD5 stained most cells in the white pulp surrounding a periarterial lymphatic sheath (Fig. 15). Anti-CD79B-stained scattered cells mainly in the red pulp as well as throughout the primary follicles and mantle of secondary follicles (Fig. 16).
Fig. 15.
Spleen from a 38-day-old animal stained using anti-CD5. Obvious stained lymphocytes (white arrows) were seen in the white pulp (WP) areas compared with the red pulp (RP) area. Periarterial lymphatic sheaths (PALS) were evident in the white pulp as well as large cells (black arrow) in the red pulp. Scale bar, 25 µm.
Fig. 16.
A few scattered lymphocytes (arrows) were positively stained using anti-CD79b in this spleen from a 38-day-old animal. A periarterial lymphatic sheath (PALS) was evident. Scale bar, 20 µm.
Lymph nodes
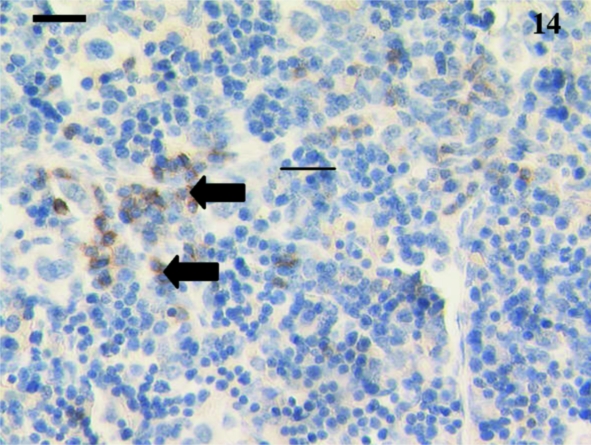
Owing to both the small size of the animal and the target tissue, lymph nodes were difficult to locate in pouch young. Five lymph nodes were studied from animals aged 24, 35, 39, and 48 days and 2½ months postpartum. Anti-CD3+ (Fig. 17) and anti-CD5+ stained cells were visible in the youngest node sample tested (24 days). At 35 days, anti-CD3 stained lymphocytes in the cortex, as well as a few in the medulla (Fig. 18), whilst anti-CD5 antibody stained lymphocytes in a similar pattern to that observed with anti-CD3. CD79b+ lymphocytes were visible in a 35-day-old sample (Fig. 19) at the periphery. CD79b+ cells were also observed in nodal tissue adjacent to a thymus in a 24-day-old animal (Fig. 8). No tissue from younger animals was available for study. Anti-CD79b stained cells in the medulla rather than the cortical areas with the exception of primary follicles and mantles of secondary follicles in older animals (Figs 19 and 20).
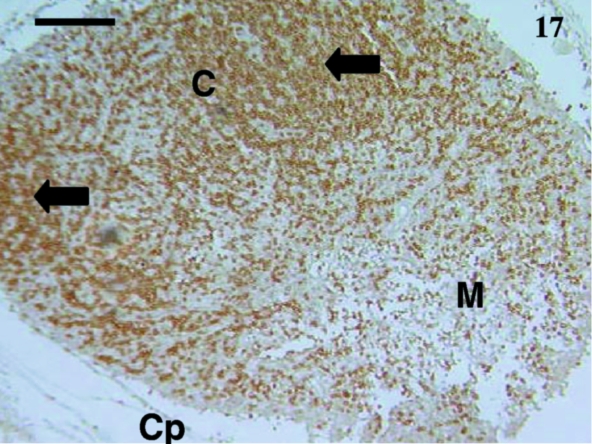
Fig. 17.
Lymph node from a 24-day-old animal stained with anti-CD3. Many cells were heavily stained (arrows) in the cortex (C) region. A few scattered lymphocytes were also stained in the medulla (M). The capsule (Cp) was evident. Scale bar, 120 µm.
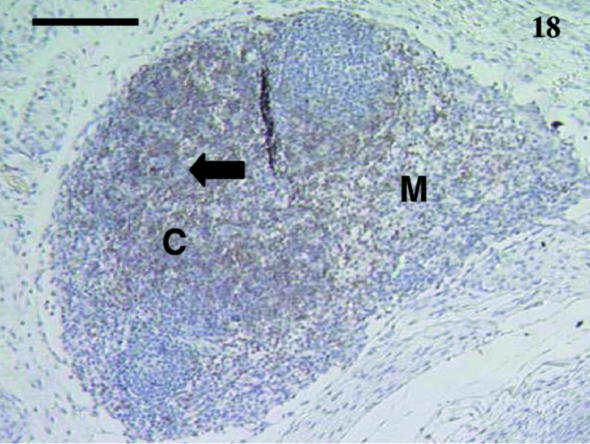
Fig. 18.
Lymph node from a 35-day-old animal stained with anti-CD5. The cortex (C) has more stained lymphocytes (arrow) than the medulla (M). Anti-CD3 stained cells similarly. Scale bar, 120 µm.
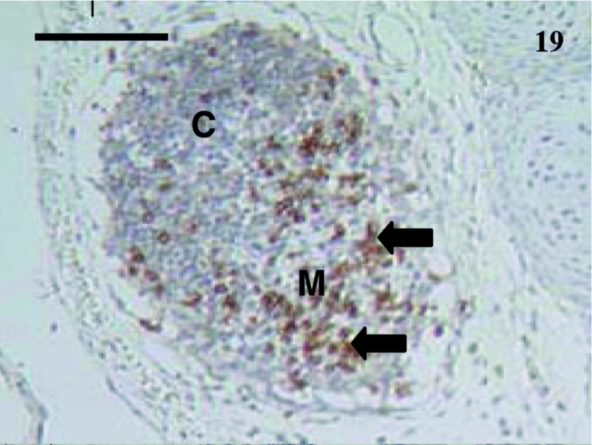
Fig. 19.
CD79b+ lymphocytes (arrows) in this lymph node from a 35-day-old animal. The cortex (C) and medulla (M) were obvious. Scale bar, 110 µm.
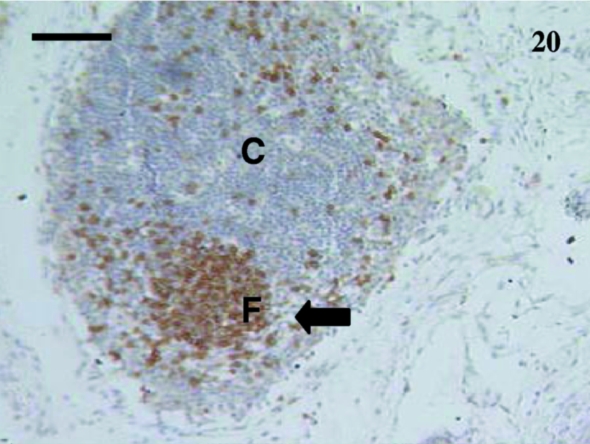
Fig. 20.
A primary follicle (F) was apparent in this lymph node from a 35-day-old sample. Lymphocytes in the follicle were stained with anti-CD79b (arrow). Thissection had mainly cortical (C) tissue. Scale bar, 100 μm.
Gut-associated lymphoid tissues
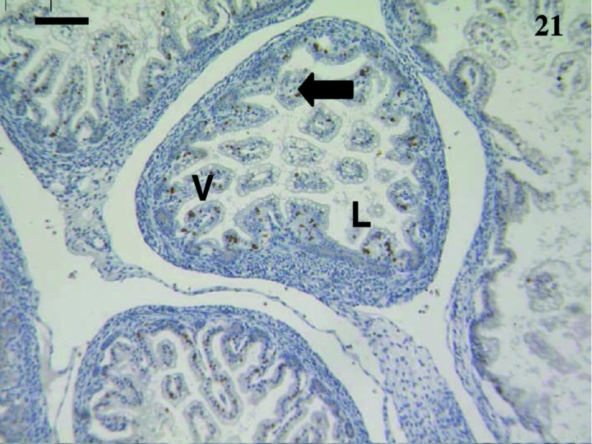
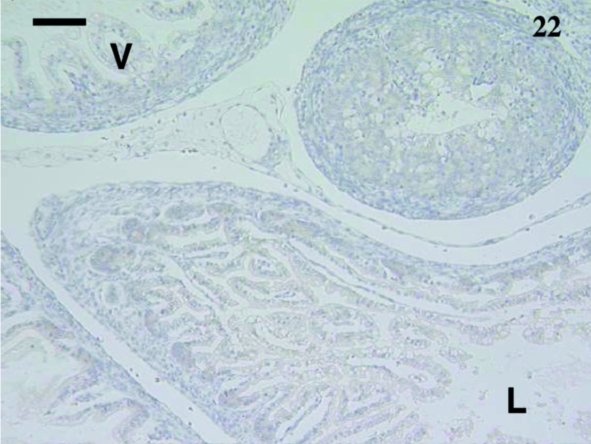
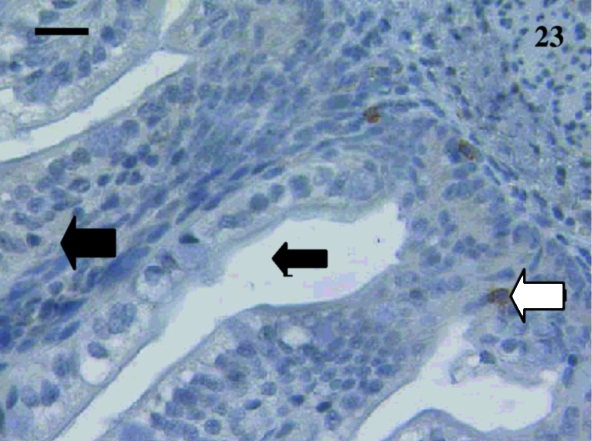
A total of 10 samples from animals aged from 5 days to 2½ months were examined including: 5, 7, 11, 12, 14, 18, 27, 74 and 75 days and 2½ months. Staining was extremely variable. Anti-CD3-stained lymphocytes were detected in the villi of an animal aged 12 days (Fig. 21) but no CD5+ cells were observed (Fig. 22). One sample aged 74 days stained with anti-CD5 (Fig. 23).
Fig. 21.
Gut sample from an animal aged 12 days with a few CD3 +-stained lymphocytes (arrow) throughout the villi (V). The villi were small and the lumen (L) large. Scale bar, 100 µm.
Fig. 22.
No lymphocytes were stained in this gut sample from an animal aged 12 days using anti-CD5. The villi (V) and lumen (L) were evident. Scale bar, 100 µm.
Fig. 23.
Rare lymphocytes (white arrow) were stained in the villi (large black arrow) using anti-CD5 in a gut sample from an animal aged 74 days. The lumen (small black arrow) was apparent. Scale bar, 250 µm.
Anti-CD79b+ cells were observed in only one sample from an animal aged 7 days (data not shown). These stained cells may have been plasma cells because they were large.
All other samples did not stain with anti-CD3, anti-CD5 or anti-CD79b.
Lungs and bronchus-associated lymphoid tissues
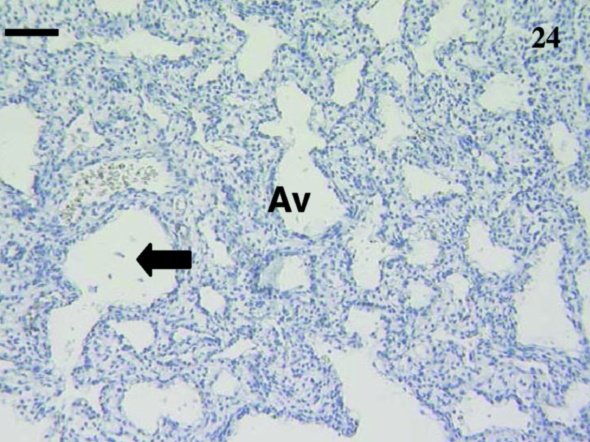
Nine lung samples were investigated from animals ranging in age including: 1, 5, 12 (×2), 14, 23, 24, 35 and 75 days postpartum. No BALT was observed in this lung tissue. Some CD3+, CD5+ and CD79b+ cells were present in the lung samples but were in blood vessels rather than in the lung tissue itself (data not shown). Most lung sections were unstained (Fig. 24).
Fig. 24.
Lung sample from an animal aged 12 days with no staining using polyclonal anti-CD3. Early alveoli (Avenue) are present. Atria (arrows) are also present. Scale bar, 80 µm.
Discussion
The antibodies used in this study have been shown to recognize mature T- and B-cells in the lymphoid tissues of the adult tammar wallaby (Old & Deane, 2000). This study has documented the appearance of these cell populations in the developing animal, and Table 1 presents a summary of when these surface markers, indicative of mature lymphocytes, were first detected.
Table 1.
Timing of first appearance of T- and B-lymphocytes in the tammar wallaby
| Lymphocytes initially detected in tammar wallaby | |||
|---|---|---|---|
| Tissue | Anti-CD3 | Anti-CD5 | Anti-CD79b |
| Liver | NS | NS | NS |
| Bone marrow | NS | NS | NS |
| Thymus | 12 days | 12 days | 23 days |
| Spleen | 21 days | 30 days | 21 days |
| Lymph nodes | 24 days | 24 days | 24 days |
| GALT | 12 days | 74 days | 7 days* |
| BALT | NS | NS | NS |
NS, no staining at any age.
One sample only.
Liver
Previous histological studies have indicated that at birth the liver is the major site of immunohaematopoiesis in the tammar wallaby and that this function is later transferred to the bone marrow (Basden et al. 1996). However, histological studies failed to detect lymphocytopoiesis in the liver of the Virginian opossum (Block, 1964), the quokka (Ashman & Papadimitriou, 1975) or the tammar wallaby (Basden et al. 1996). In contrast in young humans, lymphocytopoiesis is seen scattered throughout the liver sinusoids, most of which are B-cell precursors, with T-cell precursors considered rare (Timens & Kamps, 1997). In this study, antibodies directed to CD3, CD5 and CD79b failed to recognize any cells in the liver or bone marrow in any sample. Our results suggest that the cells may be too immature and not expressing the CD3, CD5 and CD79b antigens. However, this does not rule out the possibility of the presence of early cell precursors in these tissues.
Bone marrow
Bone marrow samples were observed to contain large areas of haematopoietic cells, but no CD79b+, CD3+ or CD5+ cells were identified. Using histological techniques, Ashman & Papadimitriou (1975) described lymphocytes in the quokka from 2 weeks to 2 months after birth in the bone marrow sinusoids, and Basden et al. (1996) described lymphocytes in the tammar wallaby bone marrow at day 50 postpartum but did not report the timing of the first appearance of these cells. Block (1964) described many granulocyte precursors and a variety of erythrocytic precursors but did not describe any lymphocytic precursors or lymphocytes in the bone marrow of the Virginian opossum. Again, the presence of early precursor cells cannot be ruled out, given the limited capacity of our test antibodies. In eutherians, bone marrow is a site of B-cell development and early B-cells in the tammar wallaby do not appear to express the B-cell marker, CD79b.
Currently, no antibodies are available that recognize early B-cells in marsupials. In humans, however, antibodies to terminal deoxytransferase (TdT), CD10, CD19, CD20 and CD21 as well as the MHC Class II molecule recognize B-cells in different stages of development from the pro-B-cell to the mature B-cell stage (Roitt, 1991). TdT has recently been cloned in the grey short tail opossum (Guth & Miller, 1998) and MHC Class II DRB cloned in the brushtail possum (Lam et al. 2001). Antibodies to these molecules may allow recognition of early B-cells in metatherian bone marrow samples and could also be used to examine B-cell ontogeny in other tissues. A recent study (Baker et al. 1999) also suggests that an anti-HLA-DR antibody may react with marsupial immune cells.
It has been shown that the decalcification process can affect antibody recognition by changing the tertiary structure of the epitope sequence and this could have accounted for the lack of recognition in bone marrow samples. In this case, smears of marrow may have shown some positive results. However, in this study CD3+, CD5+ and CD79b+ lymphocytes were stained in the thymus and lymph node samples in thoracic sections from young animals, despite decalcification. This suggests that the lack of recognition is not an artefact of preparation.
Thymus
The tammar wallaby, a diprotodont marsupial, possesses both the cervical and the thoracic thymus. In these animals, the cervical thymus is the larger and develops earlier (Old & Deane, 2000). In this study, CD3+ T-cells were first observed in the cervical thymus at day 12 and the thoracic thymus at day 24. CD3 and CD5 were expressed mainly on lymphocytes in the medulla and cortico-medullary junction and this may correlate with the arrival and maturation of thymocytes in the cortex and their subsequent migration to the medulla. In the adult white-bellied opossum, a greater number of CD3+ thymocytes were observed in the medulla compared with the cortex (Coutinho et al. 1995).
Our observation of mature T-cells at 24 days postpartum is consistent with those of Basden et al. (1997), who detected Hassall's corpuscles in the cervical thymus by day 21 and in the thoracic thymus by day 30 postpartum. These structures are characteristic of mature thymic tissue.
Rare B-cells were observed in thymus samples from 23 days of age. This was not unexpected as, in eutherians, the thymus is a T-cell development area rather than a B-cell development area.
B-cells and plasma cells can occur in diseased as well as normal human thymuses (Kendall, 1995). B-cells have a high rate of proliferation in the human thymus and are generally concentrated in the medulla and cortico-medullary junction whereas plasma cells in eutherian mammals are primarily found in the cortex (Kendall, 1995). Virtually no B-cells were detected in the thymus of the tammar wallaby, which may be due to low numbers of B-cells or the low level of expression of CD79b in cells in the thymus. Baker et al. (1999) detected only a few CD79a+ lymphocytes in the medulla of the thoracic thymus of the brushtail possum. In contrast in the northern brown bandicoot, Cisternas & Armati (2000) could not detect CD79a+ cells in the thymus and only a few CD79b+ cells were observed in the aged thymus, whereas Canfield et al. (1996) observed a few stained CD79b+ lymphocytes and plasma cells in the thymus of the adult koala. Monoclonal anti-CD79a does not cross-react with tammar wallaby tissues (Old & Deane, 2002a).
Polyclonal anti-CD79a may allow identification of B-cells in the thymus of pouch young marsupials rather than the monoclonal antibodies.
Spleen
CD3+ T-cells and CD 79b+ B-cells were present from day 21 in the tammar wallaby spleen even though the animal lacked defined red and white pulp areas. Unfortunately, not enough tissue was available to test with anti-CD5 antibodies but it is likely, given the usual coincidental staining of CD3 and CD5, that such cells were present at this age. In older pouch young, T- and B-cell distributions were similar to those observed in adult marsupials (Old & Deane, 2000).
The spleen was perhaps the most troublesome tissue to study, as it was extremely friable and this characteristic, as well as size of the tissue, decreased the number of samples able to be tested.
Lymph nodes
Lymph nodes were hard to locate in the tammar wallaby and so the number of samples was limited. The youngest lymph node available for study was from a 24-day-old animal. T-cells and B-cells were first detected by anti-CD3, anti-CD5 and CD79b antibodies on day 24 when the lymph nodes had a clearly defined cortex and medulla. By day 35 postpartum, the distribution of T- and B-cells in pouch young tammar wallaby lymph nodes was similar to that observed in adult tammar wallabies (Old & Deane, 2000).
Gut-associated lymphoid tissues (GALT)
In the samples examined in this study mature T- and B-cells were distributed throughout the gut of young animals and no follicle structures were observed. Basden et al. (1997) observed that the gut was the last of the lymphoid tissues to develop. From a structural perspective, this study supports that observation. In general, in gut samples from juvenile and adult tammar wallabies, T-cells were spread throughout the villi in large numbers whereas B-cells were virtually absent. As tammar wallabies lack follicles in the gut, this may explain the low numbers of B-cells in the gut. Alternatively, the B-cells present in the gut might not express CD79b.
However, the apparent expression of the CD3 surface marker on thymus cells at day 12, the same time as they appear in the thymus, raises issues at odds with a mammalian developmental model that positions the thymus as the first tissue to develop. Moreover, the detection of CD79b+ cells in the GALT from one animal, at 7 days postpartum, clearly signals a need to investigate further the development of this important tissue.
Balt
BALT was not observed in the pouch young tammar wallaby tissues examined in this study. This was similar to that observed in adult tammar wallabies (Old & Deane, 2002). Occasional positive cells were detected but these were located within the blood vessels transversing the pulmonary tissue. There has been very little investigation of BALT in marsupials and of the failure to detect mature lymphoid cells at this mucosal site (Old & Deane, 2002). The further investigation of the mucosal immune tissues, GALT and BALT awaits the development of more specific antibodies.
Summary
The young tammar wallaby lacks mature T- and B-cells in the liver, bone marrow, thymus, spleen, gut-associated and bronchial-associated lymphoid tissues during early postnatal life. Our observations suggest that mature T- and B-cell populations are present in all the lymphoid tissue beds by 35 days after birth and are consistent with previous histological studies (Basden et al. 1997). Such results imply that the young animal is competent to mount an active immunological response around this time.
T-cells, expressing CD3 and CD5, were first detected at day 12 in the thymus and thence in the spleen and lymph nodes whereas CD79b+ B-cells were first observed in the GALT in one animal at day 7 and subsequently appeared in the spleen, thymus and lymph nodes (Table 1). These limited observations suggest early possible appearance of mature T- and B-cells in the GALT of some animals before their appearance in other tissues, particularly the thymus and lymph nodes. Given the unique extrauterine development of this mammal, this may be related to early exposure to microbes via the oral portal. However, it does suggest that investigation of the development of GALT of young marsupials would be of value as a possible model for mammal systems, particularly the role of antigenic challenge and the development of the mucosal immune system.
References
- Ashman R, Papadimitriou J. Development of the lymphoid tissue in a marsupial, S. brachyurus. Acta Anat. 1975;91:594–611. [PubMed] [Google Scholar]
- Baker ML, Gemmell E, Gemmell RT. Ontogeny of the brushtail possum, Trichosurus vulpecula. Anat. Rec. 1999;256:354–365. doi: 10.1002/(SICI)1097-0185(19991201)256:4<354::AID-AR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bancroft JD, Stevens AS. Theory and Practice of Histological Techniques. 2. New York: Churchill Livingstone; 1982. [Google Scholar]
- Basden K, Cooper DW, Deane EM. Development of the blood-forming tissues of the tammar wallaby Macropus eugenii. Reprod. Fert. Dev. 1996;8:989–994. doi: 10.1071/rd9960989. [DOI] [PubMed] [Google Scholar]
- Basden K, Cooper DW, Deane EM. Development of the lymphoid tissues of the tammar wallaby Macropus eugenii. Reprod. Fert. Dev. 1997;9:243–254. doi: 10.1071/r96032. [DOI] [PubMed] [Google Scholar]
- Block M. The blood forming tissues of the newborn opossum (Didelphys virginiana) I. Normal development through about the one hundredth day of life. Ergeb. Anat. Entwick. 1964;37:1–237. [PubMed] [Google Scholar]
- Canfield P, Hemsley S, Connolly J. Histological and immunological study of the developing and involuting superficial cervical thymus in the koala (Phascolarctos cinereus) J. Anat. 1996;189:159–169. [PMC free article] [PubMed] [Google Scholar]
- Cisternas PA, Armati PJ. Immune system cell markers in the northern brown bandicoot, Isoodon macrourus. Dev. Comp. Immunol. 2000;24:771–782. doi: 10.1016/s0145-305x(00)00030-6. [DOI] [PubMed] [Google Scholar]
- Coutinho HB, Sewell HF, Tighe P, King G, Nogueira JC, Robalinho TI, et al. Immunocytochemical study of the ontogeny of the marsupial Didelphis albiventris immune system. J. Anat. 1995;187:37–46. [PMC free article] [PubMed] [Google Scholar]
- Guth AM, Miller RD. Opossum (Monodelphis domestica) terminal deoxynucleotidyl transferase gene. Immunogenetics. 1998;47:483–486. doi: 10.1007/s002510050386. [DOI] [PubMed] [Google Scholar]
- Kendall MD. Hemopoiesis in the thymus. Dev. Immunol. 1995;4:157–168. doi: 10.1155/1995/69454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam MKP, Belov K, Harrison GA, Cooper DW. Cloning of the MHC class II DRB cDNA from the brushtail possum (Trichosurus vulpecula) Immunol. Lett. 2001;76:31–36. doi: 10.1016/s0165-2478(00)00314-x. [DOI] [PubMed] [Google Scholar]
- Murphy CR, Smith JR. Age determination of pouch young and juvenile Kangaroo Island wallabies. Trans. Royal Soc. South Aust. 1970;94:15–20. [Google Scholar]
- Old JM, Deane EM. Effect of oestrus and the presence of pouch young on the aerobic bacterial flora of the pouch of the Tammar wallaby. Comparative Immunol. Infectious Dis. 1998;21:237–245. doi: 10.1016/s0147-9571(98)00022-8. [DOI] [PubMed] [Google Scholar]
- Old JM, Deane EM. Development of the immune system and immunological protection in marsupial pouch young. Dev. Comparative Immunol. 2000;24:445–472. doi: 10.1016/s0145-305x(00)00008-2. [DOI] [PubMed] [Google Scholar]
- Old JM, Deane EM. An immunohistochemistry study of the immunological tissues of the tammar wallaby, Macropus eugenii. J. Anat. 2002;201:257–266. doi: 10.1046/j.1469-7580.2002.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roitt I. Essential Immunology. 7. Oxford: Blackwell Scientific Publications; 1991. [Google Scholar]
- Timens W, Kamps WA. Hemopoiesis in human fetal and embryonic liver. Microsc. Res. Techn. 1997;39:387–397. doi: 10.1002/(SICI)1097-0029(19971201)39:5<387::AID-JEMT1>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]