Abstract
The present paper describes a novel structure, termed the peri-albumen layer, in the egg-envelopes of the quail Coturnix japonica. It reacts with Alcian blue and exists between the egg white and the shell membrane. Ultrastructurally, it is of fine granular structure and forms a fenestrate sheet, the width of which is 190 nm or less. Isolated materials of the peri-albumen layer include an Alcian-blue-positive polysaccharide of 260 kDa, and three glycoproteins of 160, 108 and 52 kDa. The layer is supplied to an egg when it passes through the magnum–isthmus junction, the normalized length of which is 0.62–0.63 of the oviduct. The mucosa of the junction consists exclusively of a luminal epithelium. It is apparently distinct from the mucosa of the magnum and the isthmus, which consist of a luminal epithelium and tubular glands. The luminal epithelium of the magnum–isthmus junction stains prominently with Alcian blue and consists of alternately distributed ciliated cells and granular cells. Immunohistochemistry with an antiserum raised against the materials of the peri-albumen layer revealed the staining of the peri-albumen layer of the egg, and secretory cells of the luminal epithelium at the magnum–isthmus junction. It was concluded that the materials of the peri-albumen layer are produced by secretory cells at the magnum–isthmus junction of the oviduct.
Keywords: immunohistochemistry, magnum–isthmus junction, oviduct, quail, shell membrane
Introduction
The avian ovum is supplied with various egg envelopes, such as the outer layer of the vitelline membrane (Bakst & Howarth 1977), the egg white, the shell membrane and the eggshell, during ovulation (Romanoff & Romanoff, 1949). They are all produced at the infundibulum, magnum, isthmus and uterus of the oviduct (Burley & Vadehra, 1989). Ultrastructural studies of these segments performed in chickens (Wyburn et al. 1970, 1973; Draper et al. 1972) and quails (Hoffer, 1971) show that there are several types of secretory cells in each. Despite such studies, the specific roles of these cells remain largely undefined. Recently, Gautron et al. (2001) succeeded in showing that ovocalyxin-32, the eggshell matrix protein, is secreted by surface epithelial cells at the isthmus and uterus. Fernandez et al. (2001) showed that type X collagen and several proteoglycans are produced by particular oviduct cell populations and at precise post-oviposition times, and Wang et al. (2002) independently reported the expression and production of collagen X in the tubular gland of the isthmus segment. Hincke et al. (2000) reported by Northern blotting and RT-PCR that there is a gradient to the expression of lysozyme message by different regions of the hen oviduct, with significant, albeit low, levels expressed in the isthmus and uterus. However, the avian oviduct may have other functions than previously thought, because production of proteolytic enzyme was also recently reported at the infundibulum of the quail oviduct where the 33-kDa glycoprotein of vitelline membrane is reduced in molecular size (Pan et al. 2000).
We found a structure separate from the shell membrane at the interface with the egg white in the quail egg, which to our knowledge has not been reported previously. Although Hoffer (1971) reported an Alcian-blue-positive, thin zone along the inner surface of the shell membrane, she was apparently referring to a different structure, i.e. an inner lining (Hoffer, 1971; Draper et al. 1972), a basement membrane (Dieckert et al. 1991) or a limiting membrane (Bellairs & Boyde, 1969; Tan et al. 1992; Hincke et al. 2000; Yoshizaki & Saito, 2002) of the shell membrane. By contrast, the magnum–isthmus (M-I) junction of the oviduct is a segment that is apparently different from those of the magnum and isthmus (Hoffer, 1971; Draper et al. 1972) but often is ignored (Romanoff & Romanoff, 1949; Burley & Vadehra, 1989; Fernandez et al. 2001) because it is small and because its function is unknown. Here we report a novel structure, the peri-albumen layer, in the envelopes of the quail egg, and we use immunohistochemistry to show that its site of production is the M-I junction of the oviduct.
Materials and methods
Japanese quails (Coturnix japonica) were housed individually in cages located in a poultry house, which was illuminated for 16 h each day. They were fed ad libitum on a layer's diet. The quails were killed by cervical dislocation; this protocol was approved by an Institutional Animal Care and Use Committee (No. 02017). The oviducts were removed from adult females 1 h before prospective spawning or several hours after prospective ovulation. The length of each segment of the oviduct from 12- to 20-week-old females was measured directly in 0.87% NaCl.
For light microscopy, the oviducts were fixed in Bouin's solution and cut into pieces 1 cm long. Paraffin sections (6 µm) were stained with Alcian blue (pH 0.4) and eosin. For electron microscopy, specimens were fixed with 2.5% glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4), cut into pieces 5 mm long and kept overnight at 4 °C in the same solution. Specimens were then rinsed with the buffer and post-fixed with 1% OsO4 in the same buffer for 3 h at 4 °C. After fixation, they were dehydrated in acetone and embedded in Epoxy resin for transmission electron microscopy. Ultrathin sections were stained with uranyl acetate and lead citrate. For scanning electron microscopy, specimens were dehydrated in acetone, dried in a critical point apparatus, HCP-1 (Hitachi, Japan), coated with a layer of gold in an IB-3 ion coater (Eiko, Japan) and examined under a model H-8000 electron microscope (Hitachi).
Integuments from spawned or ovulating eggs were also processed for light and electron microscopy after 1 week's fixation. Decalcification of eggshells was conducted according to the method of Gautron et al. (2001): the shells were treated with 4% formaldehyde in 0.1 m cacodylate buffer plus 150 mm EDTA (pH 7.7) over a period of 1 week at room temperature.
For the isolation of peri-albumen layer (PL) materials, the eggshell was cut open at the sharp-end, and the yolk and egg white were removed. Egg white adhering within the shell was washed out with running tap water. The shells were kept in distilled water for 30 min. The distilled water, 0.5 mL per shell, was added to the empty shell and the inner surface was scraped with a cosmetic brush. The crude solutions from 80 shells were collected into 50-mL test tubes and centrifuged at 7000 g for 10 min at 4 °C. The precipitate was suspended in 10 mL distilled water with an ultrasonic disruptor UR-200P (Tomy-Seiko, Japan). The suspension was centrifuged at 1000 g for 10 min and the supernatant was recentrifuged at 10 000 g for 10 min. The precipitate was suspended in 2 mL of distilled water again with the ultrasonic disruptor and the suspension was filtrated through a filter of 2 µm pore size (Toyo Roshi, Japan). The filtrate was centrifuged at 10 000 g for 10 min and the precipitate was stored at −30 °C until use.
For raising antiserum, isolated PL materials were suspended in phosphate-buffered saline with the ultrasonic disruptor, emulsified with Freund's complete adjuvant (Difco, MI, USA), and injected subscapularly into a rabbit. Paraffin sections of shell membranes or oviductal tissues were treated with the antiserum (1 : 50 dilution) for 1 h at 37 °C, after which they were washed thoroughly with saline and treated with fluorescein isothiocyanate (FITC)-conjugated goat antiserum against rabbit IgG (E-Y Laboratories, San Mateo, CA, USA) for 1 h at 37 °C. For immunoelectron microscopy, the glutaraldehyde-fixed specimens were embedded in Lowcryl K4M resin (Polysciences, Warrington, PA, USA). Thin sections were first treated with the antiserum for 2 h, and then with a gold-conjugated goat antiserum against rabbit IgG (E-Y Laboratories) for 1 h. Samples were next stained with uranyl acetate. Control sections were treated with pre-immune rabbit serum instead of the antiserum.
Isolated PL materials were separated through 7.5% sodium dodecylsulphate (SDS)–polyacrylamide gel electrophoresis (PAGE) (Laemmli, 1970). The gels were stained with Coomassie Brilliant Blue, periodic acid-Schiff (PAS) or Alcian blue. For immunoblotting analyses, the separated samples were electroblotted onto polyvinyliden difluoride membranes (Atto, Japan). The membranes were treated with the antiserum and then with horseradish peroxidase-conjugated sheep antirabbit IgG antiserum. The peroxidase was detected using a saturated concentration of 4-chloro-1-naphthol and 0.03% H2O2.
Results
Egg envelopes
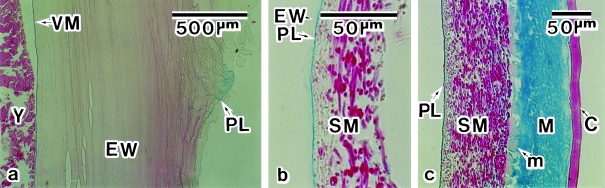
The avian ovum is surrounded by egg-envelopes such as, from inside outward, the vitelline membrane, the egg white, the shell membrane and the eggshell (Burley & Vadehra, 1989). We observed an Alcian-blue-positive layer in the outer surface of the egg white of quail egg obtained from the oviduct at the M-I junction (Fig. 1a). It was located between the egg white and the shell membrane (Fig. 1b) and adhered to the shell membrane so firmly that it was hardly dislodged during decalcification (Fig. 1c). We refer to this structure as the peri-albumen layer (PL).
Fig. 1.
Photomicrographs of envelopes of quail eggs. (a) An egg obtained at the magnum–isthmus junction. The vitelline membrane (VM) and peri-albumen layer (PL) are stained with Alcian blue. (b) Shell membrane (SM) from an ovulated egg. The PL at the interface between the egg white (EW) and SM is stained with Alcian blue. (c) Decalcified eggshell. The shell matrix (M) is stained with Alcian blue but the mammilla (m) is not. C, cuticle; Y, yolk. Stained with Alcian blue and eosin.
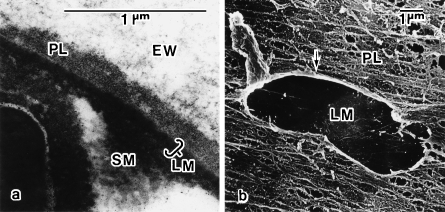
The width of the PL was variable, reaching 190 nm. Transmission electron microscopy showed that the PL was of fine granular structure (Fig. 2a). The image obtained by scanning electron microscopy was of a fenestrate sheet with ridges that run parallel to the long axis of the egg (Fig. 2b).
Fig. 2.
Electron micrographs of the peri-albumen layer (PL). (a) Transmission electron micrograph of shell membrane (SM) from an ovulated egg. The PL exists between the egg white (EW) and the limiting membrane (LM) of the SM. (b) Scanning electron micrograph of SM of an ovulated egg whose EW was removed. The PL is seen as a fenestrate sheet with ridges that run parallel to the long axis of the egg. Arrow indicates an artificially made hole in the PL.
Characterization of PL materials
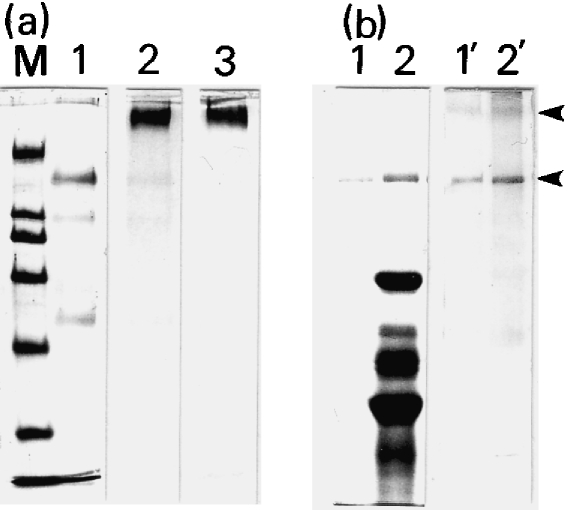
Isolated PL materials were electron-microscopically fine particles, not contaminated with components of the shell membrane, and were stained deeply with Alcian blue (not shown). An electrophoretic profile of the PL materials showed three bands, a major band at 160 kDa and minor bands at 108 and 52 kDa (Fig. 3a, lane 1), when stained with Coomassie Brilliant Blue. The 260-kDa band and these three bands all stained with PAS (lane 2), whereas only the 260-kDa band was stained with Alcian blue (lane 3). Thus the PL materials are a complex of three glycoproteins and one Alcian-blue-positive polysaccharide. Figure 3(b) shows that the antiserum raised against the PL materials specifically stained the 260- and 160-kDa bands.
Fig. 3.
SDS–PAGE and immunoblotting of peri-albumen layer (PL) materials. (a) Isolated PL materials were separated through 7.5% SDS–PAGE and stained with Coomassie Brilliant Blue (CBB; lane 1), PAS (lane 2) or Alcian blue (lane 3). M, marker proteins of 200, 116, 97.4, 66, 45 and 31 kDa from top to bottom. (b) SDS–PAGE profiles of isolated PL materials (lanes 1 and 1′) and their crude preparations (lanes 2 and 2′), stained with CBB (lanes 1 and 2) or the antiserum (lanes 1′ and 2′) against the PL materials. The 260- and 160-kDa bands were specifically stained with the antiserum (arrowheads).
Segmentation of the magnum–isthmus junction
The avian oviduct is traditionally segmented into an infundibulum, magnum, white isthmus, red isthmus, uterus and vagina (Burley & Vadehra, 1989). Subsequent descriptions here are concentrated on a distinct segment of the M-I junction in the quail oviduct.
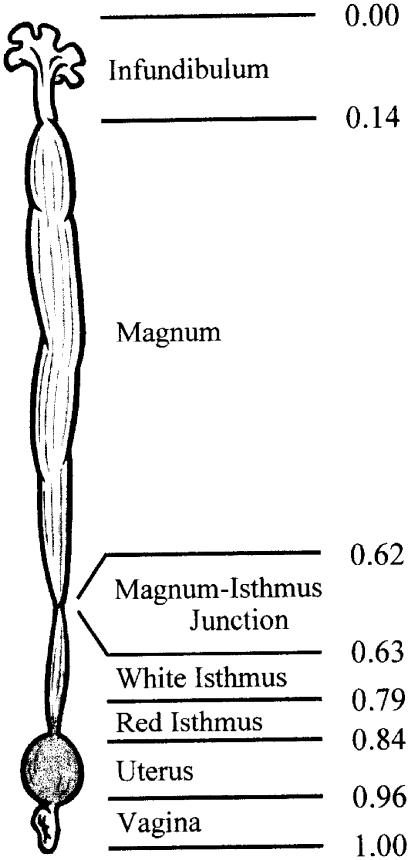
Three separate measurements were made in order to determine the relative length of each portion of the oviduct. Specific normalization lengths from anterior to posterior were as follows: infundibulum, 0.00–0.14; magnum, 0.14–0.62; M-I junction, 0.62–0.63; white isthmus, 0.63–0.79; red isthmus, 0.79–0.84; uterus, 0.84–0.96; vagina, 0.96–1.00 (Fig. 4).
Fig. 4.
Schematic representation of quail oviduct from 12- to 20-week-old females. Relative lengths from anterior to posterior are shown to the right.
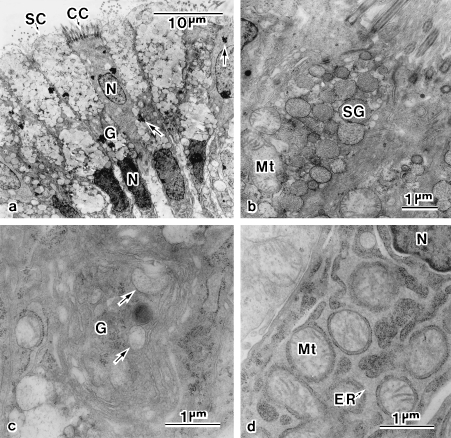
The M-I junction is a narrow ring-like constriction that is readily distinguished from the surrounding tissue by its translucency (Hoffer, 1971). It extends about 2 mm along the oviduct and its translucency is due to a deficiency of tubular glands. In cross-section, the luminal epithelium, which stains deeply with Alcian blue, exhibits a configuration of primary and secondary folds, supported by Alcian-blue-positive connective tissues and muscle tissues from beneath (Fig. 5a). It consists of alternately distributed ciliated cells and granular cells (Fig. 6a). The apical cytoplasm of the granular cells is occupied by secretory granules that are about 600 nm in size and contain fine particles interiorly (Fig. 6b). The middle portion of the cells is occupied by an electron-dense nucleus, a supranuclearly located Golgi complex and the forming secretory granules (Fig. 6c). The bottom cytoplasm of the granular cells is occupied by a huge, rough-surfaced endoplasmic reticulum, surrounding individual mitochondria (Fig. 6d). Aggregates of highly electron-dense materials are prominent in the cells, both ciliated and granular, at the M-I junction (Fig. 6a), but they are not exclusive to the junction; these were also observed in cells of the luminal epithelia at the infundibulum, anterior portion of the magnum and vagina (not shown). Thus their significance is still unknown.
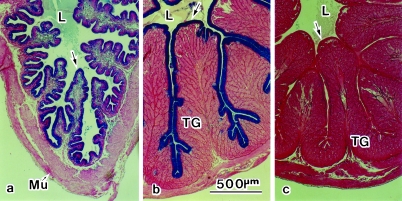
Fig. 5.
Cross-sections of oviduct, stained with Alcian blue and eosin. (a) Magnum–isthmus junction. The Alcian-blue-positive mucosa consists of a single layer of luminal epithelium (arrow). (b) Magnum. The mucosa consists of the luminal epithelium (arrow) and underlying tubular glands (TG). The luminal epithelium is reactive with Alcian blue. (c) Isthmus. Both the luminal epithelium (arrow) and TG are non-reactive with Alcian blue. L, lumen; Mu, muscle.
Fig. 6.
Electron micrographs of the magnum–isthmus junction. (a) The luminal epithelium consists of alternately distributed secretory cells (SC) and ciliated cells (CC). Arrows indicate unknown aggregates. (b) Apical cytoplasm of SC filled with secretory granules (SG). (c) Supranuclearly located Golgi complex (G) with immature SG (arrows). (d) Cytoplasm at the bottom of SC, filled with a huge, rough-surfaced endoplasmic reticulum (ER). Mt, mitochondrion; N, nucleus.
Morphological observations were extended to the segments of the magnum and isthmus to compare with those of the M-I junction described above. The mucosa at the posterior of the magnum are pushed up into longitudinally orientated folds. In cross-section, these folds are covered by a single layer of luminal epithelium with a thick layer of closely packed tubular glands beneath them. The luminal epithelium is stained with Alcian blue (Fig. 5b). It consists of alternately distributed ciliated cells and granular cells, both of which have a tall columnar shape of about 35 µm in height (Fig. 7a). The granular cells are packed from apex to base with secretory granules that have a size of about 1.5 µm and are filled with fibrillar material of low electron density (Fig. 7a). The tubular glands consist of cells packed with electron-dense, secretory granules of various sizes (Fig. 7b).
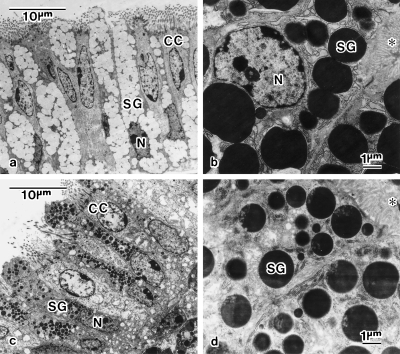
Fig. 7.
Ultrastructures of mucosa of the magnum (a,b) and isthmus (c,d). (a) Alternately distributed secretory cells and ciliated cells (CC) in luminal epithelium of the posterior portion of the magnum. The secretory granules (SG) are filled with fibrillar material of low electron density. (b) Secretory cells possessing electron-dense SG in tubular gland at the magnum. (c) Alternately distributed secretory cells and CC in luminal epithelium of the isthmus. SG is filled with materials of various electron density. (d) Secretory cells possessing electron-dense SG in tubular gland at the isthmus. Electron-lucent microstructures are seen in SG. The asterisk (*) indicates the glandular canal. N, nucleus.
The mucosa at the white isthmus are arranged as longitudinal folds composed of packed tubular glands covered by the luminal epithelium. The epithelium consists of alternately distributed ciliated cells and granular cells. All types of cells are stained deeply with eosin but not with Alcian blue (Fig. 5c). The granular cells are packed with secretory granules of various electron density and of various sizes smaller than 0.7 µm (Fig. 7c). The tubular glands consist of cells packed with electron-dense, secretory granules that possess electron-lucent microstructures interiorly (Fig. 7d).
These morphological studies apparently showed that the M-I junction is a distinct segment from those of the magnum and isthmus.
Immunohistochemistry of egg-envelopes and oviduct
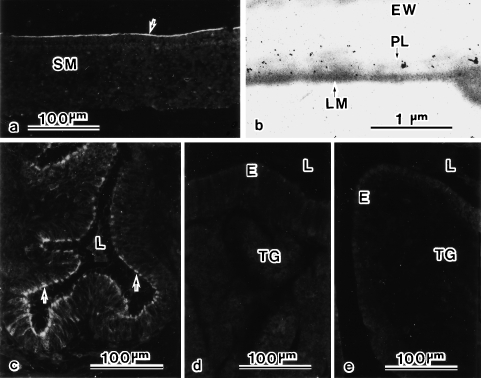
The antiserum against the PL materials provided a light microscopical stain for the inner surface of the shell membrane (Fig. 8a). Under the electron microscope, gold labels were specifically present on the PL (Fig. 8b). The antiserum also stained the secretory cells in the mucous epithelium of the M-I junction (Fig. 8c) but not of the magnum (Fig. 8d) or isthmus (Fig. 8e). Thus the PL materials are produced by secretory cells at the M-I junction of the oviduct.
Fig. 8.
Immunohistochemical labelling for the peri-albumen layer (PL) materials in shell membrane (a,b) and oviductal tissues (c,d,e). Under light microscopy, an antiserum against PL materials stains the inner surface of the shell membrane (SM) (arrow in a). Under electron microscopy, gold labels are present on the PL (b). Under light microscopy, the antiserum also stains secretory cells in epithelium of the magnum–isthmus junction (arrows in c) but not of the magnum (d) or isthmus (e). E, luminal epithelium; EW, egg white; L, lumen; LM, limiting membrane; PL, peri-albumen layer; TG, tubular gland.
Discussion
It has traditionally been viewed that the avian ovum is surrounded, from inside to outside, by the vitelline membrane, the egg white, the shell membrane and the eggshell (Burley & Vadehra, 1989). The present study revealed a separate structure, a peri-albumen layer (PL), between the egg white and the shell membrane. The PL was Alcian-blue-positive and was of fine granular structure. Although a thin zone exhibiting moderate reactivity with Alcian blue had been noticed along the inner surface of the shell membrane in quail (Hoffer, 1971), it has not been made clear whether this zone comprises a structure distinct from the shell membrane. Hoffer (1971) supposed it to correspond to the inner lining (Draper et al. 1972), the basement membrane (Dieckert et al. 1991) or the limiting membrane (Bellairs & Boyde, 1969; Tan et al. 1992; Hincke et al. 2000; Yoshizaki & Saito, 2002), an innermost component of the shell membrane. The PL materials consisted of three glycoproteins of 160, 108 and 52 kDa, and one Alcian-blue-positive polysaccharide of 260 kDa. It has yet to be determined how these components are arranged in the PL.
The avian oviduct may be divided structurally into five more or less distinct regions from the viewpoint of their fairly specific physiological functions in egg formation (Romanoff & Romanoff, 1949): (1) the infundibulum, where fertilization occurs (Olsen & Neher, 1948) and the outer layer of the vitelline membrane is produced (Bakst & Howarth, 1977); (2) the magnum producing the egg white; (3) the white isthmus forming the shell membrane; (4) the red isthmus and uterus, secreting the shell-matrix and the cuticle; and (5) the vagina. The present study has revealed a separate segment of the magnum–isthmus (M-I) junction, which has been noted structurally (Hoffer, 1971; Draper et al. 1972) but has been little studied because of a lack of evidence for its function. Anatomically, the mucous epithelium at the M-I junction is constructed of only a single-layered, luminal epithelium whereas that of both the magnum and the isthmus comprises a luminal epithelium and tubular glands. The mucous epithelium at the M-I junction is apparently distinct from the epithelia of the magnum and isthmus also in terms of histochemical stainability and ultrastructural characteristics. Immunohistochemical observations clearly indicated that the oviductal segment that produces the PL materials is the M-I junction, not the magnum or isthmus.
The PL materials are not soluble in physiological salt solutions. Their function may be in the intermediation between the water-soluble egg white and the water-insoluble shell membrane; for the formation of a well-defined shell membrane, it might be necessary to decorate the outermost surface of the sticky, amorphous egg white with these materials. As far as we can observe, the shape of the quail egg is determined the moment it passes through the narrow restriction of the M-I junction, being headed by its presumable sharp-end. Another possible function of the PL is the regulation of water permeation and/or air supply for fertile eggs. This function, mostly conducted by the shell membrane and shell, is indispensable for animals for which embryonic development occurs on land. In a previous study (Yoshizaki & Saito, 2002), we documented a small increase in water permeability through shell membranes on day 3 of incubation in the 17-day hatching period of quails, although we did not recognize its significance at that time. The PL disappeared from the inner surface of shell membranes in developing embryos on day 3 (our unpublished observations). In chickens, the oxygen permeability of shell membranes increases dramatically on days 2–5 of incubation in their 21-day hatching period, to a level sufficient for the oxygen-uptake rates required by the developing embryos (Kutchai & Steen, 1971). Physicochemical and biochemical characterization of the PL materials are required for an understanding of their functions.
Avian egg envelopes are the principal subjects of research in food science for the development of new food-stuffs and in medical science as a model of mineral deposition. In this respect, the PL materials found here might be the subject of further studies. However, there have been few cytological studies on the avian oviduct that have attempted to identify the cells participating in the production of specific materials (Lavelin et al. 2000; Fernandez et al. 2001; Gautron et al. 2001; Wang et al. 2002). Such studies might be needed, for the infundibulum in particular, where a novel function, the production of a protease that cleaves the ZPC glycoproteins of the vitelline membrane (Pan et al. 2000), is expected.
Acknowledgments
We wish to thank S. Ito for supplying the quail eggs.
References
- Bakst MR, Howarth B., Jr The fine structure of the hen's ovum at ovulation. Biol. Reprod. 1977;17:361–369. doi: 10.1095/biolreprod17.3.361. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Boyde A. Scanning electron microscopy of the shell membranes of the hen's egg. Z. Zellforsch. 1969;96:237–249. doi: 10.1007/BF00338771. [DOI] [PubMed] [Google Scholar]
- Burley RW, Vadehra DV. The Avian Egg: Chemistry and Biology. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- Dieckert JW, Dieckert MC, Creger CR. On the existence of a basement membrane at the inner surface of the avian eggshell membrane: implications for differentiation of the chorioallantoic membrane. SAAS Bull. Biochem. Biotechn. 1991;4:34–39. [Google Scholar]
- Draper MH, Davidson MF, Wyburn GM, Johnston HS. The fine structure of the fibrous membrane forming region of the isthmus of the oviduct of Gallus domesticus. Quart. J. Exper. Physiol. 1972;57:297–309. doi: 10.1113/expphysiol.1972.sp002163. [DOI] [PubMed] [Google Scholar]
- Fernandez MS, Moya A, Lopez L, Arias JL. Secretion pattern, ultrastructural localization and function of extracellular matrix molecules involved in eggshell formation. Matrix Biol. 2001;19:793–803. doi: 10.1016/s0945-053x(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Gautron J, Hincke MT, Mann K, Panheleux M, Bain M, McKee MD, et al. Ovocalyxin-32, a novel chicken eggshell matrix protein. J. Biol. Chem. 2001;276:39243–39252. doi: 10.1074/jbc.M104543200. [DOI] [PubMed] [Google Scholar]
- Hincke MT, Gautron J, Panheleux M, Garcia-Ruiz J, McKee MD, Nys Y. Identification and localization of lysozyme as a component of eggshell membranes and eggshell matrix. Matrix Biol. 2000;19:443–453. doi: 10.1016/s0945-053x(00)00095-0. [DOI] [PubMed] [Google Scholar]
- Hoffer AP. The ultrastructure and cytochemistry of the shell membrane-secreting region of the Japanese quail oviduct. Am. J. Anat. 1971;131:253–266. doi: 10.1002/aja.1001310302. [DOI] [PubMed] [Google Scholar]
- Kutchai H, Steen JB. Permeability of the shell and shell membranes of hen's eggs during development. Respir. Physiol. 1971;11:265–278. doi: 10.1016/0034-5687(71)90001-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lavelin I, Meiri N, Pines M. New insight in eggshell formation. Poult. Sci. 2000;79:1014–1017. doi: 10.1093/ps/79.7.1014. [DOI] [PubMed] [Google Scholar]
- Olsen MW, Neher BH. The site of fertilization in the domestic fowl. J. Exp. Zool. 1948;109:355–366. doi: 10.1002/jez.1401090303. [DOI] [PubMed] [Google Scholar]
- Pan J, Sasanami T, Nakajima S, Kido S, Doi Y, Mori M. Characterization of progressive changes in ZPC of the vitelline membrane of quail oocyte following oviductal transport. Molec. Reprod. Dev. 2000;55:175–181. doi: 10.1002/(SICI)1098-2795(200002)55:2<175::AID-MRD6>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Romanoff AL, Romanoff AJ. The Avian Egg. New York, NY: John Wiley and Sons; 1949. [Google Scholar]
- Tan CK, Chen TW, Chan HL, Ng LS. A scanning and transmission electron microscopic study of the membranes of chicken egg. Histol. Histopath. 1992;7:339–345. [PubMed] [Google Scholar]
- Wang X, Ford BC, Praul CA, Leach RM., Jr Collagen X expression in oviduct tissue during the different stages of the egg laying cycle. Poult. Sci. 2002;81:805–808. doi: 10.1093/ps/81.6.805. [DOI] [PubMed] [Google Scholar]
- Wyburn GM, Johnston HS, Draper MH, Davidson MF. The fine structure of the infundibulum and magnum of the oviduct of Gallus domesticus. Quart. J. Exp. Physiol. 1970;55:213–232. doi: 10.1113/expphysiol.1970.sp002071. [DOI] [PubMed] [Google Scholar]
- Wyburn GM, Johnston HS, Draper MH, Davidson MF. The ultrastructure of the shell forming region of the oviduct and the development of the shell of Gallus domesticus. Quart. J. Exp. Physiol. 1973;58:143–151. [PubMed] [Google Scholar]
- Yoshizaki N, Saito H. Changes in shell membranes during the development of quail embryos. Poult. Sci. 2002;81:246–251. doi: 10.1093/ps/81.2.246. [DOI] [PubMed] [Google Scholar]