Abstract
The peroneal (fibularis) tendons are held in place within the malleolar groove by the superior peroneal retinaculum. If this is torn, the tendons can subluxate or dislocate. Understanding the anatomy of the region is important for treating these injuries when it becomes necessary to reconstruct the malleolar groove surgically. Serial transverse sections of the groove were cut from 10 dissecting room cadavers after routine histology processing. The structure of the malleolar groove differed significantly in its proximal and distal parts. Distally, the bone is convex and the shape of the groove is determined by a thick periosteal cushion of fibrocartilage that covers the bone surface. Proximally, the groove shape is determined by the bone itself, and the periosteum is thin and fibrous. The restriction of a periosteal fibrocartilage to the distal end suggests that it serves to adapt the shape of the malleolar groove to that of the tendons within it and thus promotes stress dissipation. Paradoxically, however, it increases the risk of damage to subluxated tendons, because these can be sliced longitudinally by a sharp ridge created from periosteal fibrocartilage when the retinaculum is torn. Our results suggest that if bone-block surgical procedures are used to reconstruct the malleolar groove, they are best restricted to its proximal part.
Keywords: fibrocartilage, periosteum, peroneal retinaculum, sports injury
Introduction
The tendons of peroneus (fibularis) longus and brevis pass from the leg to the foot by wrapping around the lateral malleolus. As they turn around this bony pulley, they lie in a fibro-osseous tunnel, formed by the bone and the superior peroneal retinaculum (SPR). The SPR helps to maintain the tendons in their correct position within the malleolar groove (MG), behind the lateral malleolus of the fibula. Nevertheless, dislocations or subluxations of the tendons can occur, and over 90% of such injuries happen in sport (Clanton & Schon, 1993). Skiers are at particular risk, but the injuries can also occur in soccer, American football, running, basketball and ice-skating. The damage is generally the result of a single traumatic event that occurs while the foot is dorsiflexed and everted, and the peroneal muscles strongly contracted. Typically, a ski digs into the snow and the skier is thrown forwards by the sudden deceleration, so that the foot is forced into extreme dorsiflexion (Stover & Bryan, 1962; Eckert & Davis, 1976; Clanton & Schon, 1993).
Understanding the bone structure in the region is important for sports physicians faced with treating such injuries, as it may be necessary to reconstruct the fibro-osseous tunnel housing the peroneal tendons by appropriate surgical intervention. Although the osteological features of the MG have been well documented (Edwards, 1928), we know little of its lining soft tissues. Here we report regional variations in the histology of the MG that are clearly adapted to functional requirements, have a direct bearing on peroneal subluxations and the choice of surgical procedure used for repair.
Materials and methods
Samples of the entire length (approximately 3 cm) of the MG and its contained tendons (peroneus longus and brevis) were removed from 10 dissecting room cadavers of both sexes (59–97 years of age) that had been donated to the Anatomy Unit of Cardiff University. The medical histories of the subjects (other than the cause of death) were not available. The cadavers were fixed for 72 h with embalming fluid containing 4% formaldehyde and 25% alcohol. Samples of the MG were taken from one limb only (six right and four left) and prepared for routine histology. All the feet were in a position of slight plantarflexion, and occasionally slight inversion. The tissue was post-fixed in 10% neutral buffered formal saline for at least 1 week, decalcified in 5% nitric acid, dehydrated with graded alcohols, cleared in chloroform and embedded in 58 °C paraffin wax. The MG, with its tendons in situ, was serially sectioned at 8 µm in the transverse plane, and eight sections collected systematically at regular intervals throughout the block. For the first two specimens examined, sections were collected at 1-mm intervals, but on the basis of the results obtained from this material, all subsequent sections were collected at sample points 0, 3, 5, 10, 15, 20 and 25 mm from the tip of the lateral malleolus. Adjacent slides were stained with alcian blue, haematoxylin and eosin, Masson's trichrome, toluidine blue and Weigert's elastic stain with van Gieson's connective tissue stain. No attempt was made to sample the tendons proximal or distal to the MG, as the primary focus of our study was the MG rather than the tendons within it.
Results
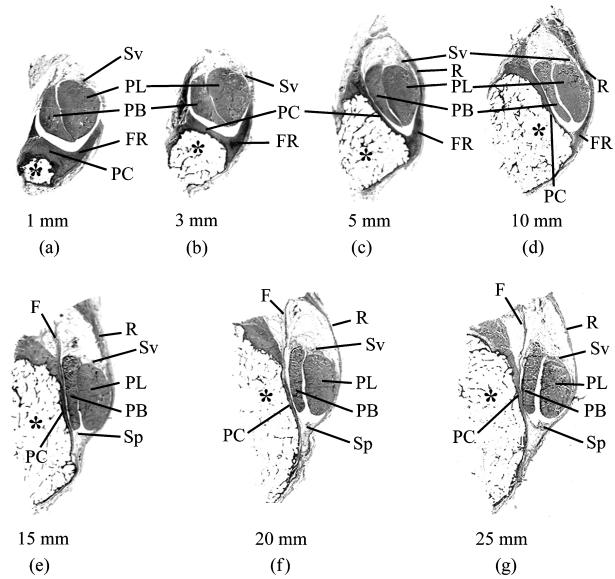
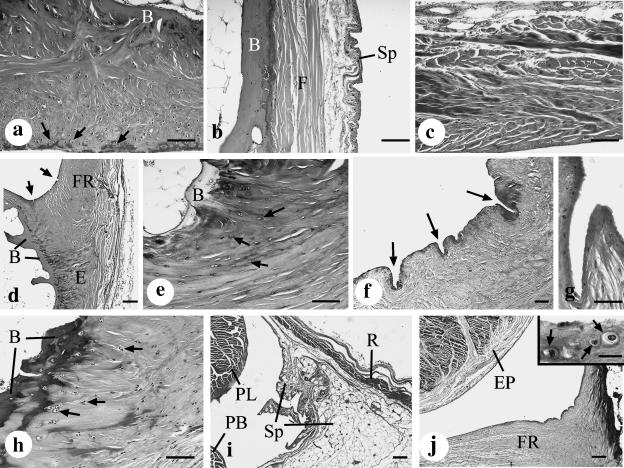
The MG is jointly formed by the lateral malleolus and its associated periosteum. The latter varied greatly in structure and thickness from the proximal to the distal end of the groove (Fig. 1). Towards the tip of the malleolus, it formed a thick, fibrocartilaginous cushion, which was primarily responsible for shaping the groove in a region where the bone itself was convex (Figs 1a,b and 2a). More proximally, however, the bone itself was grooved and the periosteum was thinner and purely fibrous (Figs 1c–g and 2b). The medial part of the periosteal cushion was formed by the posterior talofibular ligament distally and by the posterior inferior tibiofibular ligament proximally. Both of these also had fibrocartilaginous entheses (i.e. bony attachment sites; Fig. 2h), and thus the medial border of the MG was generally more fibrocartilaginous than the rest.
Fig. 1.
Macroscopic views of serial transverse sections through the malleolar groove and its contained tendons [peroneus brevis (PB) and peroneus longus (PL)]. The sections are taken at 1, 3, 5, 10, 15, 20 and 25 mm from the tip of the lateral malleolus (a–g, respectively). The top of each figure is posterior. At the distal end of the groove (1- and 3-mm sample points), the periosteal cushion (PC) is thick and together with the prominent fibrous ridge (FR) its shape compensates for the convexity of the lateral malleolus (*) in these regions. More proximally (i.e. at all other sample points), the groove is either flat or slightly concave, and the associated periosteal cushion is thus much less prominent. At all sample points 5 mm or more from the tip of the malleolus, the superior peroneal retinaculum (R) forms much of the lateral border of the groove. The synovial sheath associated with the peroneal tendons is present in all regions, but its parietal layer (Sp) is only visible at the 15- to 25-mm sample points. Although the visceral layer of the sheath (Sv) is present throughout, it is restricted to the sides of the tendons facing away from the bone. All sections are stained with Masson's trichrome. F is a band of fibrous tissue continuous with the purely fibrous periosteum at this proximal part of the malleolar groove.
Fig. 2.
(a) The distal part of the malleolar groove (3 mm from its tip) is lined by a thick, fibrocartilaginous periosteal cushion that largely accounts for the concavity or flat appearance of the groove in this region. Note the presence of fibrocartilage cells throughout the cushion and especially near the ‘articular’ surface of the malleolar groove (arrows). B, bone. Haematoxylin & alcian blue. Scale bar, 100 µm. (b) The proximal part of the malleolar groove (20 mm from its tip) is lined by a thinner and purely fibrous periosteum (F) that lacks cartilage cells. Fused with the periosteum is the parietal layer of the synovial sheath (Sp) that is associated with the peroneal tendons. B, bone. Haematoxylin & alcian blue. Scale bar, 100 µm. (c) The purely fibrous character of the superior peroneal retinaculum demonstrated in a section taken 10 mm from the tip of the malleolus. Masson's trichrome. Scale bar, 100 µm. (d) The base of the fibrous ridge (FR) on the lateral aspect of the malleolar groove, in a section taken 5 mm from the malleolar tip. The prominent fibrocartilage at its enthesis (E) is shown at higher magnification in (e) and the microscopic folds (arrows) in (f) and (g). B, bone. Haematoxylin & alcian blue. Scale bar, 200 µm. (e) High-magnification view of the fibrocartilaginous enthesis at the attachment of the fibrous ridge to the bone (B) 5 mm from the tip of the malleolus. Note the prominent fibrocartilage cells (arrows). Toluidine blue. Scale bar, 100 µm. (f) Numerous microscopic folds (arrows) are present at the angle between the periosteal cushion and the base of the fibrous ridge in this section taken 1 mm from the tip of the malleolus. Toluidine blue, Scale bar, 100 µm. (g) High-magnification view of a microscopic fold that is present at the angle between the periosteal cushion and the base of the fibrous ridge in this section taken 3 mm from the tip of the malleolus. Haematoxylin & alcian blue. Scale bar, 50 µm. (h) Numerous fibrocartilage cells (arrows) at the enthesis of the posterior talofibular ligament, which forms the medial border of the malleolar groove in this section taken 3 mm from the tip of the malleolus. B, bone. Masson's trichrome. Scale bar, 100 µm. (i) The parietal layer of the synovial sheath (Sp) associated with peroneus longus (PL) and peroneus brevis (PB) is prominent on the inner aspect of the superior peroneal retinaculum (R) in this section taken 25 mm from the tip of the malleolus. Masson's trichrome. Scale bar, 200 µm. (j) By contrast to the proximal end of the groove (see i above), there is no parietal layer of the synovial sheath in its distal half (this section is 10 mm from the malleolar tip). Instead the sheath is replaced by the fibrous ridge (FR). The visceral layer of the synovial sheath is represented by the epitenon (EP) on the surface of the peroneal tendons. Masson's trichrome. Scale bar, 200 µm. Inset: conspicuous fibrocartilage cells (arrows) are present near the articular surface of the fibrous ridge, but there is no synovial membrane. Masson's trichrome. Scale bar, 20 µm.
The lateral edge of the MG was prolonged into a ridge to which the SPR was attached (Figs 1c,d and 2c). The ridge was most prominent 3–10 mm from the tip of the malleolus, so that this part of the groove was deepest (Figs 1b–d and 2d). Fibrocartilage was conspicuous both at its enthesis and on the surface in contact with the peroneal tendons (Fig. 2d,e and j inset) – particularly in the distal part of the ridge. A conspicuous series of microscopic folds or wrinkles was present at the junction of the ridge and the rest of the periosteal cushion (Fig. 2f,g).
The remainder of the fibro-osseous tunnel was formed by the SPR (Fig. 1c–g). In its proximal part, it was lined by the parietal layer of the synovial sheath that surrounded the peroneal tendons (Fig. 2i). Distally, however, the parietal layer was generally lacking and the visceral layer of the sheath was restricted to the surface of the tendons that faced away from the bone (Fig. 2j).
Discussion
We suggest that regional variations in the structure of the MG reported in the present paper are of functional significance in reducing wear and tear and the associated risk of tendon displacement by subluxation or dislocation. The shape of the distal end of the groove is primarily determined by its thick, fibrocartilaginous periosteal cushion – in this region the bone itself is convex. Indeed, according to Edwards (1928) and Poll & Duijfjes (1984), the whole posterior surface of the fibula can be convex. The periosteum is probably thicker and fibrocartilaginous more distally, so that the MG and its tendons can mould their shape to each other and reduce the risk of tendon displacement. Because such injuries inevitably occur when the foot is on the ground, subluxation must start distally and extend proximally. The deformability of the distal part of the MG is further enhanced by a series of microscopic folds at the junction of the periosteal cushion with the vertical ridge. These could act as a hinge, allowing the ridge to accommodate its position to that of the foot. Although Eckert & Davis (1976) have described the ridge as a fibrous structure with a mixture of collagen and elastic fibres, it did not stain with Weigert's elastic stain in the specimens we examined, and was fibrocartilaginous, both at its enthesis and near the surface in contact with the peroneal tendons. Enthesis fibrocartilage is well documented as a promoter of insertional angle changes in tendons, ligaments and joint capsules (see Benjamin & Ralphs, 1998, for a review) and such changes are also likely to occur in the periosteal cushion, if this is moulded by peroneal tendons tensed by muscle contraction. Fibrocartilage near the ‘articular surface’ of the ridge (i.e. that which faces the tendons) enables the MG to withstand the compression and/or shear that comes from contact with the peroneal tendons. Fibrocartilage is known to cover regions of bones that are compressed by tendons and has been reported previously on the lateral malleolus, though no attempt was made to define regional variations in its prominence (Benjamin et al. 1995). The fibrocartilaginous ridge can damage subluxated peroneal tendons, as it can split away from the SPR to create a sharp blade of tissue capable of slicing through the tendons longitudinally (Larsen, 1987; Sobel et al. 1992; Bassett & Speer, 1993; Geppert et al. 1993). The presence of fibrocartilage in parts of the ridge would accentuate any tendon damage that occurs.
The risk of subluxation is likely to be highest at the distal end of the MG, because the three-dimensional movements of the foot at the subtalar and ankle joints first affect the peroneal tendons at their distal end. The relationship between the tendons and the MG is more stable proximally because this is further away from the ankle and subtalar joints. Damage is likely to be greatest when the foot is on the ground and the peroneal muscles work with a fixed insertion and a moveable origin. In this position, the forces acting on the SPR can be generated not only by active contraction of the muscle bellies, but also from external sources, for example when a ski is trapped in the snow.
A variety of surgical procedures are used to stabilize the peroneal tendons and repair the osteofibrous tunnel when the tendons are subluxated or dislocated. Some surgeons favour a soft tissue reconstruction of the tunnel, whereas others prefer to reconstruct the hard tissues (Clanton & Schon, 1993; Mason & Henderson, 1996). In the former technique, neighbouring soft tissues are transferred to the region of the MG. Thus a new retinaculum may be created from a strip of tissue re-routed from the Achilles tendon or the calcaneo-fibular ligament (Jones, 1932; Eckert & Davis, 1976; Martens et al. 1986). In one of the hard tissue reconstruction techniques commonly employed, a ‘sliding bone-block procedure’ may be used (Kelly, 1920; DuVries, 1965). In this technique, a piece of bone is excised from the lateral side of the fibula and repositioned more posteriorly, so as to deepen the MG. Because we have shown that the MG is largely formed from soft tissue distally and hard tissue proximally, we suggest that if bone-block procedures are favoured by a surgeon, they are best performed on the proximal part of the groove. A potential problem arising from bone-block procedures is excessive post-operative wear and tear on the peroneal tendons. This is likely to be worse if the procedure is performed too distally, because compression and shear are greater in the distal part of the MG, and a bone-block procedure performed here would make this region less compliant.
Most standard anatomy texts imply that the typical synovial sheath of a tendon has an inner visceral and an outer parietal layer along its entire length. The two are continuous with each other and form a flattened sac with a small quantity of lubricating, synovial fluid. However, the synovial membrane of the sheath associated with the peroneal tendons is absent where the tendon is compressed against the bone – though it is present both proximally and distally. Instead, this part of the sheath is lined directly by two fibrocartilages, one on the deep surface of the tendon (Benjamin et al. 1995) and a complimentary periosteal fibrocartilage on the bone. This is a common adaptation in such regions for resisting compression and/or shear (Benjamin et al. 1995) and is in line with the suggestion of Benjamin & McGonagle (2001) that there are striking parallels between regions of tendons that press against bone and typical synovial joints. In the latter, a synovial membrane is also absent over the articular surfaces themselves.
We have reported regional variations of clinical significance in the structure of the MG of the fibula. Whether there are comparable variations in the structure of the MG on the medial malleolus is unclear. However, fibrocartilage is known to be present at the former site (Benjamin et al. 1995). Both the location of periosteal fibrocartilage in relation to the mechanics of the peroneal tendons and the disappearance of fibrocartilage from bony grooves when the associated tendon is ruptured (Benjamin et al. 1993) suggest that periosteal fibrocartilage is a dynamic tissue that is developed and maintained in response to compression and/or shear.
References
- Bassett FH, Speer KP. Longitudinal rupture of the peroneal tendons. Am. J. Sports Med. 1993;21:354–357. doi: 10.1177/036354659302100305. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR, Newell RLM, Evans EJ. Loss of the fibrocartilaginous lining of the intertubercular sulcus associated with rupture of the tendon of the long head of biceps brachii. J. Anat. 1993;182:281–285. 10.1046/j.1469-7580.2003.00209.x. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Qin S, Ralphs JR. Fibrocartilage associated with human tendons and their pulleys. J. Anat. 1995;187:625–633. 10.1046/j.1469-7580.2003.00209.x. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments – an adaptation to compressive load. J. Anat. 1998;193:481–494. doi: 10.1046/j.1469-7580.1998.19340481.x. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J. Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clanton TO, Schon LC. Athletic injuries to the soft tissues of the foot and ankle. In: Mann RA, Coughlin MJ, editors. Surgery of the Foot and Ankle. 6. Vol. 2. St Louis: Mosby-Year Book; 1993. pp. 1167–1177. 10.1046/j.1469-7580.2003.00209.x. [Google Scholar]
- DuVries HL. Surgery of the Foot. St Louis: CV Mosby Co; 1965. pp. 256–257. 10.1046/j.1469-7580.2003.00209.x. [Google Scholar]
- Eckert WR, Davis EA. Acute rupture of the peroneal retinaculum. J. Bone Joint Surg. 1976;58-A:670–673. 10.1046/j.1469-7580.2003.00209.x. [PubMed] [Google Scholar]
- Edwards ME. The relation of the peroneal tendons to the fibula, calcaneus and cuboideum. Am. J. Anat. 1928;42:213–253. 10.1046/j.1469-7580.2003.00209.x. [Google Scholar]
- Geppert M, Sobel M, Bohne WHO. Lateral ankle instability as a cause of superior peroneal retinacular laxity: an anatomic and biomechanical study of cadaveric feet. Foot Ankle. 1993;14:330–334. doi: 10.1177/107110079301400604. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Jones E. Operative treatment of chronic dislocation of the peroneal tendons. J. Bone Joint Surg. 1932;14:574–576. 10.1046/j.1469-7580.2003.00209.x. [Google Scholar]
- Kelly RE. An operation for the chronic dislocation of the peroneal tendons. Br. J. Surg. 1920;7:502–504. 10.1046/j.1469-7580.2003.00209.x. [Google Scholar]
- Larsen E. Longitudinal rupture of the peroneus brevis tendon. J. Bone Joint Surg. 1987;69-B:340–341. doi: 10.1302/0301-620X.69B2.3818773. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Martens MA, Noyez JF, Mulier JC. Recurrent dislocation of the peroneal tendons: results of rerouting the tendons under the calcaneofibular ligament. Am. J. Sports Med. 1986;14:148–150. doi: 10.1177/036354658601400210. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Mason RB, Henderson IJP. Traumatic peroneal tendon instability. Am. J. Sports Med. 1996;24:652–658. doi: 10.1177/036354659602400515. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Poll RG, Duijfjes F. The treatment of recurrent dislocation of the peroneal tendons. J. Bone Joint Surg. 1984;66-B:98–100. doi: 10.1302/0301-620X.66B1.6693487. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Sobel M, Geppert MJ, Olson EJ, Bohne WH, Arnoczky SP. The dynamics of peroneus brevis tendon splits: a proposed mechanism, technique of diagnosis, and classification of injury. Foot Ankle. 1992;13:413–422. doi: 10.1177/107110079201300710. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]
- Stover CN, Bryan DR. Traumatic dislocation of the peroneal tendons. Am. J. Surg. 1962;103:180–186. doi: 10.1016/0002-9610(62)90483-x. 10.1046/j.1469-7580.2003.00209.x. [DOI] [PubMed] [Google Scholar]