Abstract
Three-dimensional reconstruction and BrdU incorporation have been used to quantify the development and growth of the mouse mandible and to analyse its relationship to Meckel's cartilage and the molar teeth. The mandible anlage is first histologically detectable at E13.5 as paired plates of osteoid tissue within condensed mesenchyme (∼0.9 mm long and ∼0.36 mm deep) that are lateral to the two arms of Meckel's cartilage. Over the next 3 days, each plate lengthens to ∼3.6 mm, and extends medially at its superior and inferior edges, folding over to enclose the alveolar nerve and Meckel's cartilage and producing additional processes that form the molar tooth sockets (E15.5). At around E15.5, the first molar tooth socket forms from two processes that extend from the medial and distal parts of the mandible to surround the tooth. By E16.5, this process is complete in the distal region where Meckel's cartilage is beginning to degenerate. Mandible ossification begins at E14 with proliferation restricted to the outer surface. BrdU incorporation rates are particularly high at the proximal and distal ends where lengthening occurs, and at the superior and inferior edges as they extend medially to surround Meckel's cartilage. Incorporation rates slow at the distal ends of each mandible at E16.5 as they approach each other at the symphysis. The results indicate that the mandible mainly grows at its periphery, and the pattern of mandibular growth and morphogenesis suggests that these processes are mainly directed and constrained by paracrine signalling from Meckel's cartilage and the tooth buds.
Keywords: BrdU, growth, mandible, Meckel's cartilage, morphogenesis, mouse, three-dimensional reconstruction
Introduction
The mandible or lower jaw is a paired bone that develops within the mandibular arch, embedding the teeth and forming an articulation of the jaw with the cranium, the tempero-mandibular joint. It is preceded by Meckel's cartilage, which mainly disappears as the mandible develops, but is thought to play an important but still unclear role in its morphogenesis, particularly because the mandible surrounds Meckel's cartilage as it grows (Bhaskar et al. 1953; Glasstone, 1971). The early mandible is formed by intramembranous ossification, but secondary cartilages at its proximal end contribute endochondral components at later stages.
The mouse mandible is first seen as a thin plate of condensed neural-crest-derived, mesenchymal cells in the lower jaw lateral to Meckel's cartilage (Chai et al. 1998, 2000); these cells proliferate and differentiate into osteogenic cells. Each plate lengthens and grows at its superior and inferior edges, which fold medially, eventually surrounding Meckel's cartilage (for a general review, see Meikle, 2002). Very little is known about how morphogenesis is controlled here: there are few reports about molecular receptors expressed by the mandible and virtually none on signal molecules expressed by Meckel's cartilage or nearby tissues, topics considered in more detail in the discussion. The growth of lower jaw tissues seems to have attracted little attention, although kinetic analysis has shown that cultured osteoprogenitor cells undergo 9–10 population doublings before the appearance of the first morphologically differentiated osteoblasts (Malaval et al. 1999).
Here, we use BrdU to investigate the pattern of cell proliferation in the developing mandible in the mouse embryo between E11.5 and E16.5 and describe how these proliferating cells contribute to the patterning and growth of this bone. By integrating the pattern of cell proliferation in the mandible with three-dimensional (3D) reconstruction of the tissues of the developing jaw, we are able to analyse the relationship between Meckel's cartilage, the mandible and the teeth that it surrounds, and show how the patterns of cell proliferation in the mandible generate its macroscopic growth. In the discussion, we consider the implications of these results for mandibular morphogenesis and suggest that the available molecular data are compatible with signalling from Meckel's cartilage and the tooth buds regulating mandibular growth.
Materials and methods
Tissue
The heads of E11.5–16.5 CBA/C57 mouse embryos were obtained from pregnant mice that had been injected intraperitoneally with BrdU (Sigma, 10 mg mL−1 in sterile saline; 0.2 mL per mouse). These heads were surplus to experiments carried out in other laboratories. One hour after BrdU injection, animals were killed and the embryos isolated. The heads of four embryos at each age were immediately removed and fixed in 4% paraformaldyhyde (PFA) overnight at 4 °C. The samples were rinsed in phosphate-buffered saline (PBS) and routinely processed through graded concentrations of ethanol and embedded in paraffin.
Immunostaining
Serial sections cut at 7 µm were mounted on poly-L-lysine-coated slides. Deparaffinized sections were treated with 100% ethanol and then incubated with 3% hydrogen peroxide for 20 min to remove endogenous peroxidase activity, then rehydrated with 70% ethanol and washed in PBS. The slides were heated in a microwave oven in citrate buffer (4 × 5 min) to denature the DNA. After washing in Tris-buffered saline (TBS), the sections were treated with normal rabbit serum for 30 min to block the non-specific binding of antibodies and then incubated overnight at 4 °C with primary anti-BrdU antibody (Becton Dickinson, stock diluted 1 : 200). The sections were next treated for 30 min with rabbit antimouse IgG, conjugated to biotin (Dako, stock diluted 1 : 200), washed in TBS and then incubated for 30 min with ABC complex (Dako) with the binding antibody visualized by 3,3′-diaminobenzidine (DAB, Sigma). All the sections were then lightly counterstained with haematoxylin. Control slides were incubated with normal rabbit serum but otherwise treated normally and found to lack nuclear staining.
The morphological changes and the distribution of BrdU-positive cells within the developing mandible were recorded chronologically from E11.5 to E16.5. The rate of cell proliferation in localized areas was determined by cell counts: using an eyepiece grid, the BrdU-positive and BrdU-negative nuclei were counted in a square area of 100 × 100 µm2 (100–150 cells) or a rectangular area of 200 × 100 µm2 in various regions of the developing mandible. For each set of counts, means and standard errors of percentages were routinely calculated and the results displayed using Deltagraph. Where required, pairwise comparisons were made using Mann–Whitney U-tests using StatView.
Computer-assisted 3D reconstruction
Three-dimensional reconstructions of the mandible were made from a head from an E13.5 (194 sections) embryo, and jaws from E14.5 (209 sections) and E16.5 (380 sections) embryos, choosing sectioned embryos that seemed normal on the basis of their histology (Kaufman, 1994). Serial histological sections of the BrdU-treated heads were digitized on a personal computer equipped with a PowerLook 3000 scanner (UMAX, UK) and UMAX software. The microscopic slides were directly scanned at 3040 dpi to give a pixel resolution of about 8 µm. The areas of interest were segmented from the background image using Adobe PhotoShop 6.0 and realigned. For this, each digitized section was superimposed on its underlying section and aligned to it, optimizing the fit to match up the ectoderm, the neural tube, the pharyngeal pouch and the oral cavity boundaries. Tissue alignment was done using the free transform and partial transparency facilities of PhotoShop-6. In this way, the complete stack of sections was aligned, although the final 3D block still included evidence of the sectioning because of the intrinsic and random distortions that occur in sectioning.
TDR-3dbase software (a 3D reconstruction program from the Hubrecht laboratory, Utrecht, courtesy of Dr Fons Verbeek) was used to delineate tissues and produce 3D reconstruction from the digital images of all the aligned serial sections. The contours of Meckel's cartilage, the developing mandible and the basement membrane of the mandibular dental epithelium were drawn directly on each digitized section using a tablet that displayed these images (Wacom, UK). The pattern of BrdU labelling around the developing mandible was then painted with the different levels of proliferation (see above) being displayed as different colours. TDR-3dbase was then used to generate 3D images from these stacks of delineated sections and to display them at arbitrary orientation.
Measurements of tissue sizes were made from the TDR images on the assumption that both Meckel's cartilage and the mandible were essentially straight. Each of the image blocks was enclosed in a box that could be freely rotated on the screen (a TDR facility) and measurements of the left and right tissues were made for each of the three dimensions. Full lengths were then calculated using Pythagoras’ theorem. As the true length of the z-axis of each box was given by the number of sections (assumed to be 7 µm, the cutting thickness), the corrected length could then be calculated. The lengths given in the text are the mean of the left and right (which differed by less than 5%), and should be considered as indicative.
Measurements of Meckel's cartilage were made from the histological sections using an eyepiece graticule. The thickness here was measured on each of these sections across the shortest diameter (to minimize effects due to the orientation of the section).
Results
The axis definitions and the terminology used to analyse Meckel's cartilage and the mandible are shown in Fig. 1. For descriptive purposes, the developing mandible is divided into three regions: a distal domain that includes the incisor region, a central domain in which the mandibular molar teeth form, and a proximal domain in which the condylar and angular cartilages form (Fig. 1).
Fig. 1.
The axis definitions and terminology used to describe (a) the mandibular process, (b) Meckel's cartilage and (c) the mandible. The shaded area shows gives the location of the first molar tooth and its socket.
The early development of the mandible and Meckel's cartilage
At E11.5, although the mandibular processes of the first arch have not yet fused, BrdU-positive cells were diffusely distributed in the mandibular processes, and in the condensations of presumptive Meckel's cartilage.
One day later (E12.5), after the mandibular processes have fused, the precartilage primordium of Meckel's cartilage is present as a rod within each mandibular process, together with a separate small rostral process at the distal end. BrdU-positive cells were mainly found in the rostral process and the distal and proximal ends of the rods, whereas the middle portions had few positive cells. Just proximal to the Meckel domain, BrdU-positive cells were also seen in the condensations that will later become the malleus bone. A nerve fibre bundle, the inferior alveolar nerve, is lateral to each side of the cartilaginous primordium, but there is no evidence of mandibular development. The first signs of the mandibular tooth buds are also seen at this stage: incisor rudiments are present at the distal end of the mandibular process, and the presumptive molar tooth buds can be identified as areas of thickened oral epithelium.
At E13.5, Meckel's cartilage is a single ‘V’-shaped structure (length ∼2.0 mm, diameter ∼150 µm decreasing to ∼80 µm at the distal end): the two rods are now joined to the short rostral process at the distal end, and the proximal end of each arm articulates with the malleus. The cells in both rods have started to display chondrocyte morphology, although the rostral process remained undifferentiated. As Meckel's cartilage differentiated, a few BrdU-positive cells were randomly distributed in the cartilaginous rods, whereas there was a focus of BrdU incorporation at the rostral process, where the two rods had joined to form the complete cartilage.
The developing mandible at E13.5 can be identified as ‘membranes’ or plates of condensed mesenchymal cells with small internal regions of osteoid tissue, lateral to Meckel's cartilage and with the inferior alveolar nerve between them. Three-dimensional reconstruction (Fig. 2a) showed that these plates were ∼0.9 mm long and ∼0.36 mm high and extended and curved medially from their superior and inferior borders in their distal domain. The mental branch of the inferior alveolar nerve passed through the distal region of each plate, initiating the future mental foramen. The mandibular first molar and incisor tooth buds can be identified in the oral epithelium and have, respectively, reached the bud and cap stages of development.
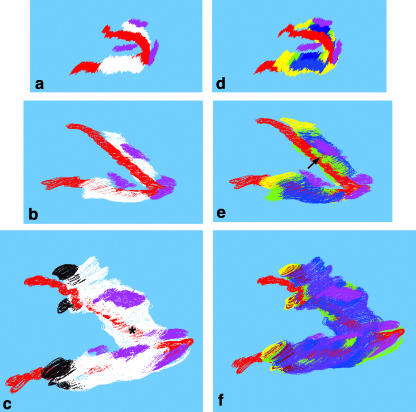
Fig. 2.
Three-dimensional reconstructions showing Meckel's cartilage, the developing mandible and the tooth germs (a, E13.5; b, E14.5; c, E16.5), and the associated levels of BrdU incorporation (d, E13.5; e, E14.5; f, E16.5). In these pictures, the lower ramus is nearer the viewer. The basic reconstructions (a–c) show the lengthening of Meckel's cartilage (red) and the mandible (white) together with their relationship to the incisor and first molar tooth rudiments (pink) and the secondary cartilages (black). At E16.5, the mandible surrounds the distal region of Meckel's cartilage (asterisk). One notable feature of the E14.5 reconstruction (e) is the high level of BrdU incorporation in the region where the mandible extends medially to form the molar socket (arrow). BrdU colour codes: yellow, very high (> 36% positive cells); green, high (26–35%); blue, medium (16–25%); purple, low (< 15% positive cells). Magnification ∼15×.
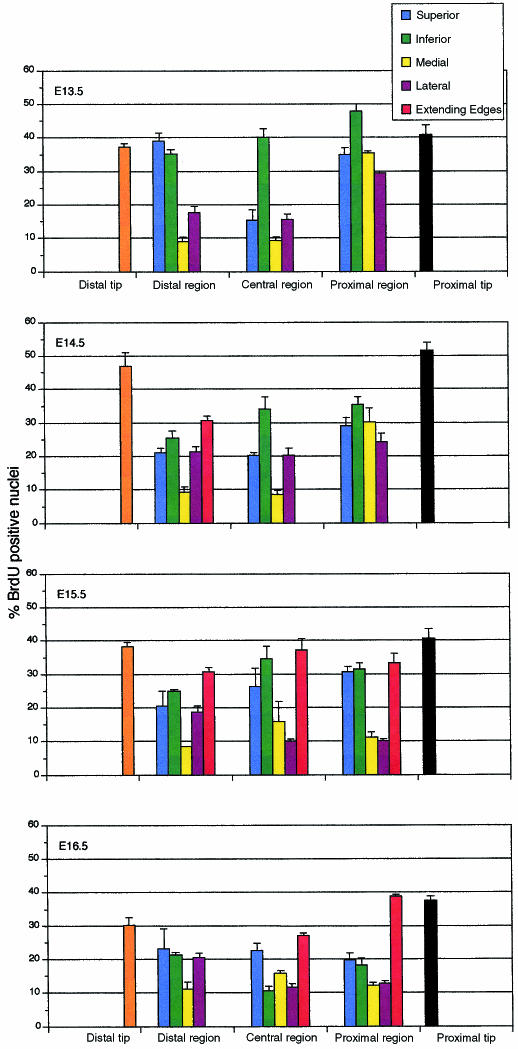
BrdU incorporation at this stage was almost entirely in the dense mesenchymal layer at the periphery of the bone rudiment (this is before a well-defined periosteum is apparent), particularly at the extending edges, with the very few BrdU-positive cells in the internal domain being randomly distributed. In this peripheral layer, the proportion of BrdU-positive cells was highly position-dependent (Figs 2d and 3), but was generally very high (> 30%) in all regions except the medial and lateral surfaces in the distal and central regions, and on the superior surface of the central region where the molars were forming (P = 0.04). Of particular note were the terminal regions (∼30 µm) at each end of the mandible (the proximal and distal tips): these were, and continued to be, characterized by dense mesenchyme and very high BrdU incorporation rates (Fig. 3). BrdU levels were low in the mesenchyme immediately surrounding the mandible, but higher in the developing jaw muscles.
Fig. 3.
Histograms (with standard errors) showing percentages of BrdU incorporation in domains of cells in the peripheral region of the developing mandible over the period E13.5–E16.5 (see Fig. 1). The extending-edge columns (red) refer specifically to the domains of the mandible that extend medially to surround Meckel's cartilage during E14.5–E16.5.
Growth of the mandible and formation of the tooth sockets
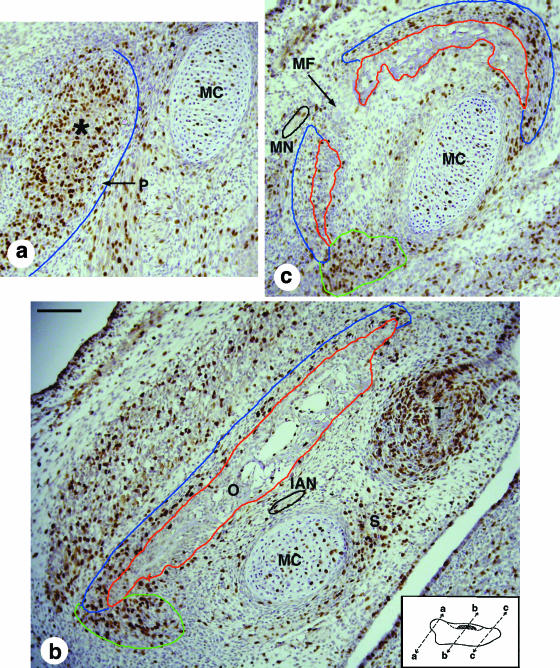
One day later at E14.5 (Fig. 2b), Meckels's cartilage is about 3.3 mm long and about 150 µm in diameter over most of its length, but decreases to about 110 µm at the distal end. The mandible now extends along ∼2 mm of Meckel's cartilage. A clear periosteum (Fig. 4a) can now be identified over much of the mandible as a highly condensed layer of flattened mesenchymal cells. The future secondary (condylar and angular) cartilages can be identified as mesenchyme condensations within the developing periosteum of the proximal region of the mandible (asterisk, Fig. 4a). In its central region, the mandible maintains its plate-like morphology (Figs 2b and 4b, depth ∼0.46 mm), but the distal region is now clearly C-shaped in transverse section as its ends surround about 50% of Meckel's cartilage (Fig. 4c).
Fig. 4.
Micrographs of BrdU-stained sections of the E14.5 mandible, whose differentiated bone and periosteum are outlined in red and blue, respectively. Inset: the location and orientation of the sections, where proximal is to the left and superior is at the top. (a) Proximal region. The mesenchymal condensation that will give rise to the condylar cartilage (asterisk) forms within the developing perichondrium; although most of this is well defined, its lateral boundary is unclear and not outlined in blue. This condensation shows a high level of BrdU incorporation, in contrast to the low level seen in Meckel's cartilage. (b) Central region. The mandible is lateral to Meckel's cartilage, the inferior alveolar nerve (outlined in black) and the first molar tooth germ. BrdU incorporation is particularly strong in the inferior region of the mandible where there is one projection (outlined in green) towards Meckel's cartilage and another towards the tooth germ in the region of mesenchyme where the socket will form. The large BrdU-positive mesenchymal mass lateral to the mandible is the developing masseter muscle. (c) Distal region. This micrograph shows the mental foramen that separates the C-shaped mandible into two parts in this section. The mental nerve (outlined in black) is on the lateral side of the mandible in this micrograph. Note the high levels of BrdU staining in the mesenchyme at the inferior (outlined in green) and superior ends of the mandible where it extends towards Meckel's cartilage. IAN, inferior alveolar nerve; MC, Meckel's cartilage; MF, mental foramen; MN, mental nerve; O, osteoid tissue; P, periosteum; S, tooth socket; T, molar tooth germ. Scale bars, 100 µm.
The generally high percentage of BrdU-positive cells seen at E13.5 (Fig. 2d) gradually diminished over the body of the mandible over the next 3 days (Fig. 2e,f). The noticeable exceptions at E14.5 were the proximal and distal ends where BrdU incorporation rates had increased significantly (P = 0.02). BrdU incorporation now marked the apparently undifferentiated mesenchyme beyond the inferior edge of the E14.5 mandible and indicated where the future bone would extend to surround Meckel's cartilage (outlined in green in Fig. 4). High levels of incorporation were also seen in the proximal region where the secondary cartilages were forming, and at the inferior edge of the mandible.
By E15.5, Meckel's cartilage has fully differentiated, having a diameter of ∼180 µm and a definitive perichondrium over most of its length, except at the distal region, where the perichondrium is beginning to disappear on the inferior-lateral side, and the rostral process, which remains as condensed mesenchyme. The body of the mandible has a well-defined periosteum on its lateral side and on the medial side of the proximal region adjacent to Meckel's cartilage (Fig. 5a). It is in this region that the condylar and angular cartilages are now beginning to chondrify.
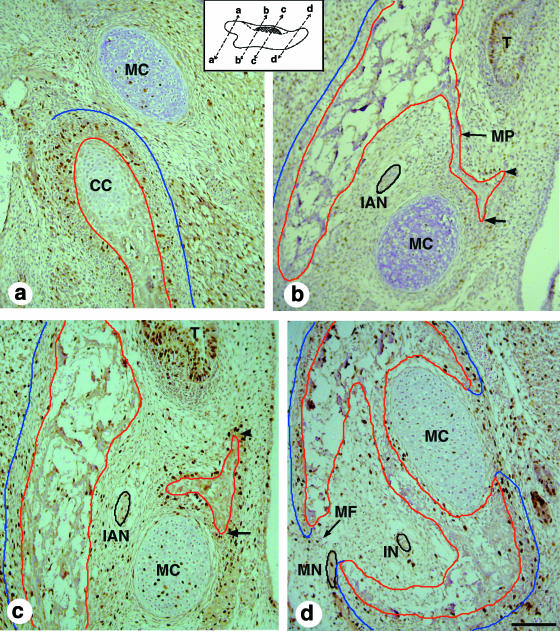
Fig. 5.
Micrographs of BrdU-stained sections of the E15.5 mandible (periosteum and bone tissue outlined in blue and red, respectively). Inset: the location and orientation of the sections, and, in each case, proximal is to the left and superior is at the top. (a) The proximal region. Condylar cartilage is differentiating lateral to Meckel's cartilage; the extent of BrdU staining in the condylar cartilage is now much less than it was at E14.5, whereas that in the periosteum has now increased. As at E14.5, most of the periosteum is well defined, but its lateral end is unclear and is not outlined in blue. (b) Proximal to the molar tooth bud. The mandible has extended to produce the medial process, which has bifurcated, with one arm extending towards the tooth germ (arrowhead) and the other towards Meckel's cartilage (arrow). Together, the mandible and the medial process start to enclose the inferior alveolar nerve (outlined in black). (c) The central region. This section is slightly distal to (b) and shows the second process that emerges from the distal part of the mandible as it extends over Meckel's cartilage. This process bifurcates, with one arm growing to surround Meckel's cartilage (arrow) and the other extending towards the tooth germ (arrowhead) where it will form the distal and most of the medial parts of the tooth socket. (d) The distal region. This section shows the foramen of the mental nerve (MN, outlined in black). Here, the developing mandible has almost completed its morphogenesis: the bone has created a cavity that encloses the incisive nerve (IN), before reaching Meckel's cartilage where it has split into arms that surround the cartilage on its medial and lateral sides. CC, condylar cartilage; IAN, inferior alveolar nerve; IN, incisive nerve; MC, Meckel's cartilage; MF, mental foramen; MN, mental nerve; MP, medial process; O, osteoid tissue; P, periosteum; S, tooth socket; T, molar tooth germ. Scale bars, 100 µm.
Two processes emerge from the superior part of the mandible at this stage: a medial process emerges from the side of the mandible immediately proximal to the molar tooth (Fig. 5b); a second process (Fig. 5c) emerges from the proximal edge of distal mandible that is surrounding Meckel's cartilage (Fig. 5d). Both of these processes contribute to the molar tooth socket that mainly forms once the tooth has reached the bell stage of development. The lateral side of the socket forms from the body of the mandible extending superiorly. The proximal side of the socket forms from the medial processes (Fig. 5b). The distal part of the molar tooth socket forms from the second process as it extends medially. The medial part of the socket mainly forms from an extension off this second process, but part comes from an extension of the medial process (Fig. 5b). Although much of the region at the base of the socket remains open, there is some bone that forms through additional growth of the lateral and medial sides of the socket.
Meckel's cartilage is now clearly beginning to be surrounded by mandible. In the distal region (∼0.7 mm), the mandible surrounds ∼75% of the cartilage (Fig. 5d); in the central region, the inferior surface is now extending around the cartilage, while, in the proximal region, the medial process extends towards the cartilage (Fig. 4b). BrdU incorporation in all three regions of extension (red histograms, Fig. 3) was > 30%, as they were at the proximal and distal ends. Elsewhere, however, BrdU incorporation rates had dropped as compared with E14.5, even in the proximal region where the secondary cartilages were now apparent.
One day later, at E16.5, the broader condylar and narrower angular secondary cartilages (each about 0.46 mm long), but not the coronoid cartilage, can clearly be seen in the proximal region of each side of the mandible (Fig. 2c), whereas most of the remaining mandible (now ∼3.7 mm in length, ∼0.85 mm in depth and ∼150 µm from the mandible) has ossified and is surrounded by periosteum. Its proximal region now partially encloses Meckel's cartilage and the inferior alveolar nerve. The central region of the mandible includes the socket for the first molar tooth and the domain where the socket will form for the second molar tooth bud, now just evident. The distal region (∼1 mm) completely encloses Meckel's cartilage (now ∼4.9 mm in length; Fig. 2c), which is now beginning to degenerate (its diameter had dropped to about 160 µm); this is particularly noticeable in the region near the mental foramen where the space previously occupied by the cartilage (there is a space of ∼0.4 mm between the rostral process and the cartilaginous rods) becomes occupied by the enlarging mandibular bone. By E16.5, BrdU incorporation rates had generally diminished except at the proximal tip and the extending edge of the medial process that now surrounds ∼50% of the proximal region of Meckel's cartilage; this latter region showed higher BrdU incorporation than the extending edge in the central region (P = 0.03).
Discussion
The traditional picture of mandibular development over the period E12.4–E16.5 is of two initially flat mesenchymal condensations that grow at their periphery and ossify internally, with this growth being linear along the proximo-distal axis, but extending medially to surround the adjacent Meckel's cartilage. Superimposed on this macroscopic development are some smaller-scale events: the bone surrounds the inferior alveolar nerve leaving a foramen for the mental nerve; it forms sockets around the tooth buds in its medial region and generates the secondary cartilages proximally. The BrdU (Fig. 3) and 3D reconstruction data presented in this paper expand and quantify our understanding of the growth of the mandible and Meckel's cartilage and indicate mechanisms that might control mandibular morphogenesis.
Meckel's cartilage
Our results confirm that Meckel's cartilage forms at about E12.5 as two short lateral rods and a rostral process that fuse together and grow (Chai et al. 1994; Miettinen et al. 1999), so lengthening the lower jaw (Kurihara et al. 1994). The external morphology confirms this growth, but the lengths of the cartilage and mandible determined from 3D reconstruction should be considered as only approximate. Although the heads chosen for 3D reconstruction were typical of their age, the amount of work required for reconstruction meant that it was not feasible to analyse more than a single lower jaw at a given age.
The pattern of BrdU uptake in Meckel's cartilage suggests that growth is due to lengthening (there is little diameter change) that mainly takes place at their ends, with the cartilage extending from 0.74 mm to ∼5 mm over E11.5–E16.5. Around E16, disintegration starts in the distal region near the mental foramen, just proximal to the rostral process. The mechanisms by which this occurs remain unclear: part may ossify (Bhaskar et al. 1953; Frommer & Margolies, 1971; Granstrom et al. 1988; Richman & Diewert, 1988; Yamazaki et al. 1997; Ishizeki et al. 1999) whereas other parts may apoptose (Trichilis & Wroblewski, 1997).
Growth of the mandible
Mandible development is complicated, even over the period examined here. Each side is first distinguishable as a thin plate of condensed mesenchyme cells lateral to Meckel's cartilage at E13.5. The peripheral cells of this tissue proliferate as the inner cells differentiate to become bone; the mandible thus grows in a manner typical of other membrane bones (e.g. Iseki et al. 1999). The cells at the mandibular periphery generally proliferate far faster than those in adjacent mesenchyme (Fig. 3). The initially small flat plate of neural-crest-derived mesoderm, < 1 mm in length and ∼0.36 mm deep, grows rapidly at its edges with its length increasing from about 2 to 4 mm over E13.5–E16.5, or at about the same rate as Meckel's cartilage. It is not therefore surprising that BrdU incorporation rates are particularly high (> 35%) at the distal and proximal ends where the jaw is extending. More interesting, however, is the behaviour of the mandible at its superior and inferior edges: here the two edges of the bone deepen and extend medially to enclose Meckel's cartilage and the inferior alveolar nerve and, in the central region, to surround the molar tooth primordia (Tomo et al. 1997). The BrdU incorporation observations show that this aspect of mandibular morphogenesis is driven by proliferation but give no clues as to the mechanism causing the bone to extend around Meckel's cartilage.
The relationship between the mandible, Meckel's cartilage and teeth
The mandible grows to surround the teeth, forming their sockets, the inferior alveolar nerve and Meckel's cartilage. The sockets start to form as the teeth of the lower jaw attain the bell stage of development at about the time when the inner enamel epithelium of the developing tooth undergoes progressive folding (Radlanski et al. 1998) (∼E15.5), well after their initiation at E12.5 (Thesleff & Aberg, 1999). The molar tooth socket forms as a result of localized vertical growth at the superior border of the mandible and from the growth of processes that extend from the mandible proximal and distal to the tooth bud. The various processes that extend and then fuse to give the complete socket are probably the result of signalling from the tooth rudiment, which is known to produce a wide range of ligands (see the tooth gene expression database http://bite-it.helsinki.fi/). Organ culture experiments using beads could be used to confirm this and to discover which of these ligands induce socket formation.
The formation of the mental foramen, the space through which the mental nerve leaves the mandible, seems due to unossified mesenchymal cells surrounding the nerve and there seems no need to look for a more sophisticated explanation. The mechanisms by which the mandible generates its final form are more complex and, because of its curvature, are different from the membrane bones of the skull vault, whose shape is mediated by brain enlargement and whose growth is mainly controlled by the edge-located sutures (Iseki et al. 1999).
Although it seems obvious that, because the mandible eventually surrounds most of Meckel's cartilage, this cartilage regulates mandibular morphogenesis, there seems to be no direct evidence to support this view. Indirect evidence comes from the endothelin-converting-enzyme-1 knockout mouse (Yanagisawa et al. 1998) that lacks Meckel's cartilage and has a severely hypoplastic mandible, but this may only indicate that the growth of Meckel's cartilage and the lower jaw domain provides space needed for the mandible to develop.
The pattern of BrdU incorporation (Fig. 3) provides some additional circumstantial evidence that Meckel's cartilage regulates mandibular growth and hence morphogenesis: BrdU incorporation around the developing mandible is generally highest at the superior and inferior edges, which act like sutures, lower on the lateral surface and least on the medial surface facing the cartilage. These BrdU observations could be explained on the basis of a generally disseminated growth factor and a location-specific density of growth-factor receptors on the periosteum that were derived from the initial patterning of the mandible mesenchyme. Such an explanation does not, however, explain why the edge of the mandible extends medially. The simplest possibility here is that Meckel's cartilage secretes a signal, perhaps the growth factor already mentioned, that directs this growth first medially and then toward itself.
There is, however, no evidence as yet that Meckel's cartilage secretes growth factors – current expression data mainly consist of transcription factors (see GXD, the mouse gene-expression database, http://www.informatics.jax.org/menus/expression_menu.shtml), with the roles of Msx1 and osterix being particularly important (Nakashima et al. 2002; Orestes-Cardoso et al. 2002). It is, however, worth noting that the addition of hepatocyte growth factor to cultured jaws can enhance mandible formation in vitro (Amano et al. 1999). The results presented here suggest that it would be worth investigating whether Meckel's cartilage does synthesize signalling molecules and, if so, what their effect is on the developing mandible.
Acknowledgments
We thank John West for material, and for help with the statistical analysis, Bill Sellers for advice on aligning images with Photoshop and Martin Collinson for advice on BrdU immunohistochemitstry.
References
- Amano O, Koshimizu U, Nakamura T, Iseki S. Enhancement by hepatocyte growth factor of bone and cartilage formation during embryonic mouse mandibular development in vitro. Arch. Oral Biol. 1999;44:935–946. doi: 10.1016/s0003-9969(99)00086-2. [DOI] [PubMed] [Google Scholar]
- Bhaskar S, Weinmann J, Schour I. Role of Meckel's cartilage in the development and growth of the rat mandible. J. Dental Res. 1953;32:398–409. doi: 10.1177/00220345530320031401. [DOI] [PubMed] [Google Scholar]
- Chai Y, Mah A, Crohin C, Groff S, Bringas P, Jr, Le T, et al. Specific transforming growth factor-beta subtypes regulate embryonic mouse Meckel's cartilage and tooth development. Dev. Biol. 1994;162:85–103. doi: 10.1006/dbio.1994.1069. [DOI] [PubMed] [Google Scholar]
- Chai Y, Bringas P, Jr, Shuler C, Devaney E, Grosschedl R, Slavkin HC. A mouse mandibular culture model permits the study of neural crest cell migration and tooth development. Int. J. Dev. Biol. 1998;42:87–94. [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Jr, Han J, Rowitch DH, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Frommer J, Margolies MR. Contribution of Meckel's cartilage to ossification of the mandible in mice. J. Dent. Res. 1971;50:1260–1267. doi: 10.1177/00220345710500052801. [DOI] [PubMed] [Google Scholar]
- Glasstone S. Differentiation of the mouse embryonic mandible and squamo-mandibular joint in organ culture. Arch. Oral Biol. 1971;16:723–729. doi: 10.1016/0003-9969(71)90117-8. [DOI] [PubMed] [Google Scholar]
- Granstrom G, Zellin G, Magnusson BC, Mangs H. Enzyme histochemical analysis of Meckel's cartilage. J. Anat. 1988;160:101–108. [PMC free article] [PubMed] [Google Scholar]
- Iseki S, Wilkie AO, Morriss-Kay GM. Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development. 1999;126:5611–5620. doi: 10.1242/dev.126.24.5611. [DOI] [PubMed] [Google Scholar]
- Ishizeki K, Saito H, Shinagawa T, Fujiwara N, Nawa T. Histochemical and immunohistochemical analysis of the mechanism of calcification of Meckel's cartilage during mandible development in rodents. J. Anat. 1999;194:265–277. doi: 10.1046/j.1469-7580.1999.19420265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman M. Atlas of Mouse Development. New York: Academic Press; 1994. [Google Scholar]
- Kurihara Y, Kurihara H, Suzuki H, Kodama T, Maemura K, Nagai R, et al. Elevated blood pressure and craniofacial abnormalities in mice deficient in endothelin-1. Nature. 1994;368:703–710. doi: 10.1038/368703a0. [DOI] [PubMed] [Google Scholar]
- Malaval L, Liu F, Roche P, Aubin JE. Kinetics of osteoprogenitor proliferation and osteoblast differentiation in vitro. J. Cell Biochem. 1999;74:616–627. [PubMed] [Google Scholar]
- Meikle MC. Craniofacial Development, Growth and Evolution. Bressingham: Bateson Publishing.; 2002. [Google Scholar]
- Miettinen PJ, Chin JR, Shum L, Slavkin HC, Shuler CF, Derynck R, et al. Epidermal growth factor receptor function is necessary for normal craniofacial development and palate closure. Nat. Genet. 1999;22:69–73. doi: 10.1038/8773. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Orestes-Cardoso S, Nefussi JR, Lezot F, Oboeuf M, Pereira M, Mesbah M, et al. Msx1 is a regulator of bone formation during development and postnatal growth: in vivo investigations in a transgenic mouse model. Connect. Tissue Res. 2002;43:153–160. doi: 10.1080/03008200290000547. [DOI] [PubMed] [Google Scholar]
- Radlanski RJ, Mocker E, Rahlfs D. Computer-aided graphical reconstructions of the development of murine dental primordia and surrounding structures from day 12 until birth. Eur. J. Oral Sci. 1998;106(Suppl. 1):71–79. doi: 10.1111/j.1600-0722.1998.tb02156.x. [DOI] [PubMed] [Google Scholar]
- Richman JM, Diewert VM. The fate of Meckel's cartilage chondrocytes in ocular culture. Dev. Biol. 1988;129:48–60. doi: 10.1016/0012-1606(88)90160-1. [DOI] [PubMed] [Google Scholar]
- Thesleff I, Aberg T. Molecular regulation of tooth development. Bone. 1999;25:123–125. doi: 10.1016/s8756-3282(99)00119-2. [DOI] [PubMed] [Google Scholar]
- Tomo S, Ogita M, Tomo I. Development of mandibular cartilages in the rat. Anat. Rec. 1997;249:233–239. doi: 10.1002/(SICI)1097-0185(199710)249:2<233::AID-AR10>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Trichilis A, Wroblewski J. Expression of p53 and hsp70 in relation to apoptosis during Meckel's cartilage development in the mouse. Anat. Embryol(Berlin) 1997;196:107–113. doi: 10.1007/s004290050083. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Suda N, Kuroda T. Immunohistochemical localization of parathyroid hormone-related protein in developing mouse Meckel's cartilage and mandible. Arch. Oral Biol. 1997;42:787–794. doi: 10.1016/s0003-9969(97)00096-4. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Yanagisawa M, Kapur RP, Richardson JA, Williams SC, Clouthier DE, et al. Dual genetic pathways of endothelin-mediated intercellular signaling revealed by targeted disruption of endothelin converting enzyme-1 gene. Development. 1998;125:825–836. doi: 10.1242/dev.125.5.825. [DOI] [PubMed] [Google Scholar]