Abstract
The mammalian cerebellum is histologically uniform. However, underlying the simple laminar architecture is a complex arrangement of parasagittal stripes and transverse zones that can be revealed by the expression of zebrin II/aldolase C. The cerebellar cortex of rodents, for example, is organized into four transverse zones: anterior, central, posterior and nodular. Within the anterior and posterior zones, parasagittal stripes of Purkinje cells expressing zebrin II alternate with those that do not. Zonal boundaries appear to be independent of cerebellar lobulation. To explore this model further, and to broaden our understanding of the evolution of cerebellar patterning, zebrin II expression has been studied in the cerebellum of the Madagascan hedgehog tenrec (Echinops telfairi), a basal insectivore with a lissiform cerebellum with only five lobules. Zebrin II expression in the tenrec reveals an array of four transverse zones as in rodents, two with homogeneous zebrin II expression, two further subdivided into stripes, that closely resembles the expression pattern described in other mammals. We conclude that a zone-and-stripe organization may be a common feature of the mammalian cerebellar vermis and hemispheres, and that zonal boundaries and cerebellar lobules and fissures form independently.
Keywords: Afrotheria, lissiform cerebellum, Purkinje cell, whole mount immunohistochemistry, zonal organization
Introduction
The mammalian cerebellum is histologically uniform and the cytoarchitectural organization is similar through all lobules of the vermis and hemispheres. A complex arrangement of parasagittal stripes and transverse zones underlies this relatively simple cellular organization. This can be revealed using physiological mapping, anatomical tracing, mutational analysis and molecular markers (e.g. Voogd et al. 1996; Voogd & Glickstein, 1998). The antigen zebrin II (Brochu et al. 1990; Leclerc et al. 1992), which cloning studies have shown to be the respiratory isoenzyme aldolase C (Ahn et al. 1994), is the most extensively studied marker of cerebellar compartmentation (reviewed in Armstrong & Hawkes, 2000). Zebrin II expression is restricted to a subset of Purkinje cells that are organized into zones and stripes. In rodents, zebrin II expression domains divide the vermis into four transverse zones – the anterior zone (AZ: ∼ lobule I–V), the central zone (CZ: ∼ lobule VI–VII), the posterior zone (PZ: ∼ lobule VIII–IX) and the nodular zone (NZ: ∼ lobule X). The AZ and PZ are further subdivided from medial to lateral, into an array of parasagittal stripes (rat, Brochu et al. 1990; mouse, Eisenman & Hawkes, 1993; Ozol et al. 1999; Sillitoe & Hawkes, 2002; guinea-pig, Larouche et al. 2003; hamster, Marzban et al. 2003). A similar compartmentation is seen in opossum (Doré et al. 1990), rabbit (Sanchez et al. 2002) and cat (Sillitoe et al. 2003). The expression of zebrin II in the hemispheres of rodents suggests that similar transverse boundaries are present to those in the vermis. The parasagittal organization in the hemispheres is also homologous with that in the vermis – an anterior striped zone comprising the anterior lobe and crus I, a homogeneous central crus II, a striped posterior zone (the paramedian lobule) and a homogeneous paraflocculus and flocculus.
In the present investigation a whole mount immunohistochemical technique originally developed for the analysis of mouse cerebella (Sillitoe & Hawkes, 2002) has been applied to study compartmentation in the cerebellum of the Madagascan lesser hedgehog tenrec Echinops telfairi. Tenrecs are nocturnal animals, weighing slightly less than a rat, found exclusively in the western and south-western regions of Madagascar (Eisenberg & Gould, 1970). Echinops telfairi belongs to the subfamily Tenrecinae, themselves members of a large family of insectivores, the Tenrecidae, which has radiated such that they resemble widely diverse mammalian species, both morphologically and ecologically, including hedgehogs, shrews, opossums and mice. Classically, the tenrecs are considered insectivores, but more recent studies have placed them within the superorder Afrotheria (Mouchaty et al. 2000; Murphy et al. 2001; Douady et al. 2002; Malia et al. 2002). A growing body of molecular evidence supports the idea of polyphyletic insectivora, with tenrecs grouped within an ‘African clade’ of mammals that excludes several other insectivore families (Stanhope et al. 1998; van Dijk et al. 2001).
The tenrec cerebellum is of interest for two reasons. First, there are no previous reports of molecular compartmentation of the insectivore cerebellum. It thus affords the opportunity to extend our phylogenetic comparisons of cerebellar compartmentation. Secondly, unlike cerebella studied to date, the tenrec cerebellum lacks most lobulation in the vermis and hemispheres of the posterior lobe (Bauchot & Stephan, 1970; Stephan et al. 1991). Historically, the fissures and lobules in the cerebellum have been used to compartmentalize the cerebellum (e.g. Larsell, 1970; Altman & Bayer, 1997). Therefore, the tenrec allows us to consider cerebellar compartmentation in the absence of lobules, and to explore the potential reciprocal relationships between transverse expression boundaries and fissuration.
Materials and methods
Perfusion and sectioning
Eight Madagascan lesser hedgehog tenrecs (Echinops telfairi, weighing between 80 g and 160 g) obtained from our breeding colony in Munich, Germany (Künzle, 1998), were deeply anaesthetized with an intraperitoneal injection of tribromoethanol (1.0 mL/100 g) and killed by transcardiac perfusion with an initial rinse using 0.9% saline followed by 4% paraformaldehyde (pH 7.4: seven cases) or 10% formalin (one case). The brains were removed from the skull and immersion fixed and stored in 4% paraformaldehyde at 4 °C. Six cerebella were processed for whole mount immunohistochemistry and two were processed for free-floating immunohistochemistry and histological staining. All animal procedures conformed to German laws on the protection of animals.
Before sectioning, the cerebella were cryoprotected through graded sucrose solutions: 10% (2 h), 20% (2 h or until the cerebellum sank) and 30% (until the cerebellum sank) at 4 °C. The tissue was then frozen in OCT embedding compound (VWR, Mississauga, Ontario, Canada). Serial sections were cut in the sagittal (30 µm) and horizontal (40 µm) planes and collected on gelatin-coated slides for histological staining and in phosphate-buffered saline (PBS; Sigma, St Louis, MO, USA) for free-floating immunohistochemistry.
Histology
Frozen tissue sections were stained for myelin and counterstained with cresyl violet following the methods of Schmued (1990).
Immunohistochemistry
Sections were rinsed three times in PBS for 5 min each, incubated in 30% H2O2 for 10 min at room temperature (RT) and again rinsed three times in PBS (5 min each). Sections were then blocked in 10% normal goat serum in PBS for 3 h at RT. Following blocking, sections were incubated at RT overnight in anti-zebrin II antibody. Anti-zebrin II is a mouse monoclonal antibody produced by immunization with a crude cerebellar homogenate from the weakly electric fish Apteronotus (Brochu et al. 1990): it was used directly from spent hybridoma culture medium at a concentration of 1 : 200. Following three 5-min rinses in PBS, sections were incubated in horseradish peroxidase (HRP)-conjugated rabbit anti-mouse immunoglobulin (diluted 1 : 200 in 10% normal goat serum, Jackson ImmunoResearch Laboratory, West Grove PA, USA) for 3 h at RT. Staining was visualized by incubation in 0.5 mg mL−1 diaminobenzidine (DAB; 10 mg tablets, Sigma), 0.5 µL mL−1 30% H2O2 in PBS until the desired colour intensity was achieved. Sections were then mounted in Entellan mounting media (E.M. Science, Gibbstown, NJ, USA) and allowed to dry.
Photomicrographs were captured with a SPOT Cooled Color digital camera (Diagnostic Instruments Inc., Sterling Heights, MI, USA) mounted on a Zeiss Axioplan II microscope. Images were assembled in Adobe Photoshop 4.0, and then cropped and corrected for brightness and contrast but not otherwise manipulated.
Whole mount immunohistochemistry
Whole mount immunostained tissue was processed using a slightly modified protocol originally designed for screening mutations in the mouse cerebellum (Sillitoe & Hawkes, 2002). Cerebella were post-fixed overnight at 4 °C in Dent's fixative (four parts absolute methanol (MeOH): one part dimethysulphoxide (DMSO); Dent et al. 1989). Next cerebella were incubated in Dent's bleach (four parts MeOH: one part DMSO: one part 30% H2O2; Dent et al. 1989) for ∼6 h at RT then dehydrated twice for 30 min each in 100% MeOH at RT. The tissue was passed through 4–5 cycles of chilling to −80 °C and thawing to RT in 100% MeOH followed by overnight incubation in MeOH at −80 °C. Next, the tissue was rehydrated for 90 min each in 50% MeOH, 15% MeOH, and PBS then enzymatically digested in 10 µg mL−1 proteinase K (> 600 units mL−1; Boehringer Mannheim) in PBS for 5 min at RT. After rinsing three times for 30 min each in PBS, the tissue was incubated in blocking buffer (Davis, 1993) overnight at 4 °C. The tissue was then incubated for 48–96 h in anti-zebrin II antibody (Brochu et al. 1990; 1 : 200), rinsed three times for 2 h each at 4 °C, and incubated for 48–72 h at 4 °C in secondary antibody (1 : 200, Jackson ImmunoResearch). Finally, the tissue was rinsed four times for 3 h each at 4 °C followed by a final overnight rinse, incubated in PBT (0.2% bovine serum albumin, 0.1% Triton X-100 in PBS; Davis, 1993) for 2 h at RT, and antibody binding sites revealed with DAB and 0.5 µL mL−1 30% H2O2.
Whole mount photomicrographs were captured with a SPOT digital camera (Diagnostics Instruments Inc.) mounted on a Zeiss Stemi SV6 microscope. Cerebella were photographed immersed in PBT, with incident illumination. Montages were assembled in Adobe Photoshop 4.0. Schematics were drawn using Adobe Illustrator 8.0 and are based upon the morphology of whole mount immunoperoxidase-stained cerebella.
Results
The mammalian cerebellum, as described by Larsell (1970), is classified into 10 lobules (indicated by the Roman numerals I–X). Three principal fissures, the primary fissure (located between lobules V and VI), secondary fissure (located between lobules VIII and IX) and the posterolateral fissure (located between lobules IX and X), show strong homology across the mammalia and are easily recognized by their positions and prominence. Hemispheric lobules are named and linked to putative vermal equivalents: the anterior lobules are usually unpaired, lobule VI is paired with the lobulus simplex, lobule VII with the lobulus ansiformis (crus I and crus II), lobule VIII with the paramedian lobule, lobule IX with the paraflocculus, and lobule X with the flocculus.
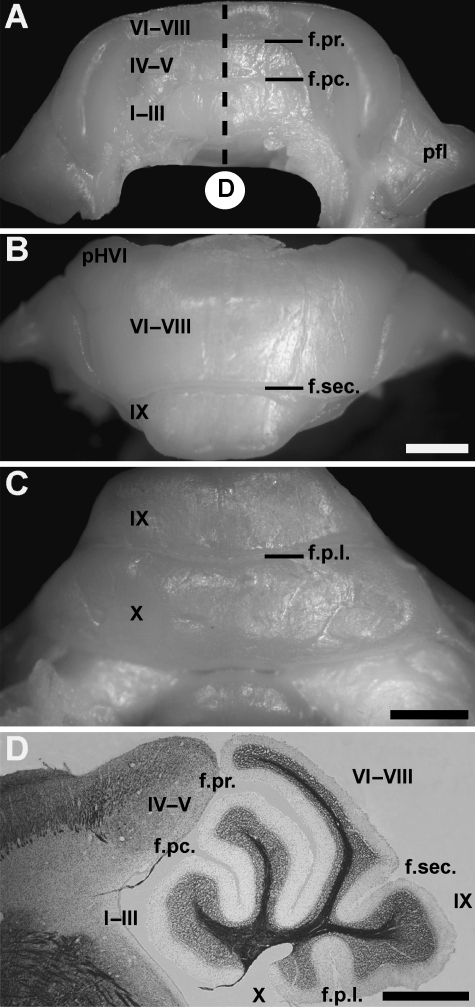
The tenrec cerebellum is only slightly larger than the cerebellum of mouse (∼8 mm in width). In general, the structure of the cerebellum resembles that of other small mammals. The vermis extends along the entire antero-posterior extent of the cerebellum. Laterally, the hemispheres are large and extend outwards from the anterior and posterior lobe vermis. Even more laterally, a prominent paraflocculus sits adjacent and partially ventral to the hemispheres. As in other mammals, lobules and fissures extend transversely across the vermis of the anterior and posterior lobe. However, in the central vermis and the hemispheres, fissures are absent and most of the posterior lobe vermis and all the hemispheres are lissiform (Fig. 1). Similarly, no longitudinal furrows separate the vermis and hemispheres of the posterior lobe. Because of the morphological similarities of the cerebellum, we have adapted the lobule nomenclature previously employed for another insectivore, the shrew Sorex cinereus (Larsell, 1970), despite the more modest lobulation in Echinops telfairi (see Bauchot & Stephan, 1970; Stephan et al. 1991). In the anterior lobe, lobule I–III are fused and separated from lobule IV–V (also undifferentiated) by the preculminate fissure (Fig. 1A,D). Lobules IV–V and VI–VIII are separated by the deep primary fissure (Fig. 1A,D). Lobules VI, VII and VIII cannot be distinguished (Fig. 1B; at the microscopic level there is occasionally an additional tiny fissure, possibly separating lobule VI–VII from lobule VIII: see also Bauchot & Stephan, 1970). The rostral portion of lobule VI–VIII (referred to below as the presumptive lobule VI (pVI)) overhangs the superior surface of lobule IV–V (Fig. 1D). Caudally, lobule VI–VIII are divided from the prominent lobule IX by the secondary fissure (Fig. 1B,D). The most posterior fissure is the posterolateral fissure (Fig. 1C,D) between lobule IX and X.
Fig. 1.
The morphology of the adult tenrec cerebellum views seen from the anterior (A), dorsal (B) and posteroventral (C) and on a midsagittal section stained for myelin and counterstained for cresyl violet (D, from the region indicated in A). (A) Lobules I–III, IV–V and VI–VIII are undifferentiated and are not separated by fissures. (B) In the hemispheres, the presumed hemisphere lobule VI (pHVI) is not separated from the posterior hemispheric lobules that are themselves fused. (C) The paraflocculus is prominent and extends laterally away from the hemispheres while the flocculus is hidden from view. (D) Five lobules are present in the tenrec cerebellum (I–III, IV–V, VI–VIII, IX and X). At the midsagittal level four fissures are prominent: preculminate, primary, secondary and the posterolateral fissure. The lobules are indicated by Roman numerals. Abbreviations: f.pc., preculminate fissure; f.pr., primary fissure; f.s., secondary fissure; f.pl., posterolateral fissure; pfl, paraflocculus. Scale bars: B = 1 mm (A, B, D); C = 1 mm, D = 1 mm.
The morphology of the anterior hemispheres in Echinops telfairi is similar to rodents (each vermian lobule is accompanied by a corresponding hemispheric lobule). The hemispheric extension of lobule VI is prominent in Echinops telfairi and is not separated by a posterior superior fissure from the more posterior crus I and crus II (Fig. 1B). As in the vermis, the posterior lobe hemispheres are poorly lobulated. No overt fissure separates the posterior lobe vermis from the hemispheres. Unlike other mammals (see Voogd et al. 1996), no discontinuity was observed at the transition from the vermis of presumed lobule VII (pVII) to the hemispheres of presumed lobule VII (pHVII). The posterolateral fissure separating lobules IX and X of the vermis extends laterally to separate the prominent paraflocculus (Fig. 1A) from the flocculus. The paraflocculus and flocculus are anatomically continuous with lobules IX and X, respectively. The flocculus is located ventrocaudal to the paraflocculus at its connection with the vermis and extends rostrally to lie medial to the paraflocculus.
Zebrin II expression in the tenrec cerebellum
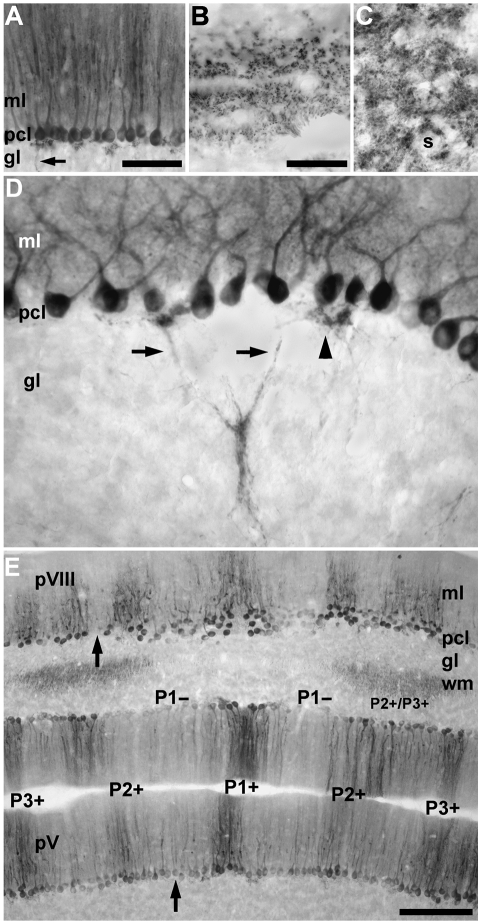
As in all mammals studied to date, zebrin II immunoreactivity is expressed in Purkinje cells of the tenrec cerebellum (Fig. 2A). Immunoperoxidase reaction product is deposited in all three laminae of the cerebellar cortex. In the molecular layer, reaction product is heavily deposited in the dendrites of Purkinje cells. In the Purkinje cell layer, the somata of Purkinje cells are strongly reactive for zebrin II (their nuclei are all non-reactive), and in the granular layer Purkinje cell axons also express zebrin II (arrow, Fig. 2A), which extend into the white matter tracts (Fig. 2B) and into the deep cerebellar nuclei (Fig. 2C). Zebrin II reactive axons are prominent from the point at which they exit the basal portion of the Purkinje cell somata. They then leave the dense plexus of stained processes located below the Purkinje cell layer (arrowhead, Fig. 2D), converge in the granular layer and extend as fascicles into the white matter. Reaction product is heavily deposited in Purkinje cell axon terminals within the cerebellar and vestibular nuclei (Fig. 2C). There is no apparent staining of astrocytes in the tenrec cerebellum: some glial immunoreactivity has been described in other species (e.g. mouse, Walther et al. 1998; hamster, Marzban et al. 2003).
Fig. 2.
Zebrin II expression by Purkinje cells in the tenrec cerebellum as viewed in immunoperoxidase-stained horizontal sections. (A) The expression of zebrin II in the vermis of lobule IV–V. All Purkinje cells are immunoreactive in this field. No other cells in the cerebellar cortex are stained. Reaction product is deposited heavily in the dendrites in the molecular layer (ml) and the somata in the Purkinje cell layer (pcl). Purkinje cell axons in the granular layer (gl) are also immunoreactive (arrow). (B) Zebrin II immunoreactivity in the cut profiles of Purkinje cell axons located in the white matter is seen as tracts. (C) Staining in the cerebellar and vestibular nuclei is as punctate deposits around the somata of neurons (s), consistent with the distribution of Purkinje cell axon terminals. (D) Zebrin II immunoreactivity is detected in the cytoplasm of the Purkinje cell somata but not the nuclei. Immunoreactive Purkinje cell axons (arrows) exit the somata and converge in the granular layer forming small fascicles. Occasionally, axons and recurrent axon collaterals form a dense plexus directly beneath the Purkinje cell layer (arrowhead). (E) Low-power view of the vermis of presumptive lobules V (pV) and VIII (pVIII). Zebrin II expression is restricted to a Purkinje cell subset that forms a symmetrical array of stripes. Stripes of non-reactive Purkinje cells (e.g. arrow in lobule pVIII) interrupt immunoreactive Purkinje cell stripes. The P1+ (midline) and two laterally located P2+ and P3+ stripes are labelled. Note that in the ‘negative’ stripes (e.g. P1−) the Purkinje cell somata may be weakly reactive for zebrin II (e.g. arrow in lobule pV) and the distinction between stripes depends largely on the dendritic immunoreactivity in the molecular layer. In the white matter (wm), the axons of zebrin II-immunoreactive Purkinje cells form symmetrically organized fascicles on either side of the midline (for the same in rat, see De Camilli et al. 1984; see also Voogd, 1969). Scale bar = 100 µm in A; 50 µm in B (also applies to C and D); 250 µm in E.
Not all Purkinje cells in the tenrec cerebellum express the same levels of zebrin II immunoreactivity. In the molecular and Purkinje cell layers, immunostaining in the dendrites and somata reveals a symmetrical array of bands interrupted by Purkinje cell stripes that are either weakly immunoreactive (e.g. the arrow in lobule IV–V in Fig. 2E) or completely non-reactive (e.g. the arrow in the caudal part of lobule VI–VIII (pVIII) in Fig. 2E). In other regions, all Purkinje cells are strongly reactive for zebrin II (in some regions of the cerebellar cortex, immunoreactivity was observed in Purkinje cell somata but was absent from the distal dendrites, e.g. stripes in the vermis of pVIII (Fig. 2E): from the point of view of defining zones and stripes these specific areas have all been deemed zebrin II-immunoreactive). Exclusion of the zebrin II antigen from dendrites has been noted in both guinea-pig (Larouche et al. 2003) and cat (Sillitoe et al. 2003), and reflects subcellular compartmentation of zebrin II rather than a staining artefact. The general proportions and distributions of the anti-zebrin II immunoreactive stripes are consistent with those previously described in other mammals (rat, Brochu et al. 1990; mouse, Eisenman & Hawkes, 1993; rabbit, Sanchez et al. 2002; hamster, Marzban et al. 2003; cat, Sillitoe et al. 2003). Therefore, the same nomenclature has been adopted for the tenrec, labelling the zebrin II-immunopositive regions of the vermis P1+ to P3+, and the positive regions of the hemispheres P4+ to P7+. Zebrin II-immunonegative regions are numbered P1− to P6− according to the neighbouring, medially located, immunopositive stripe.
Organization of the vermis
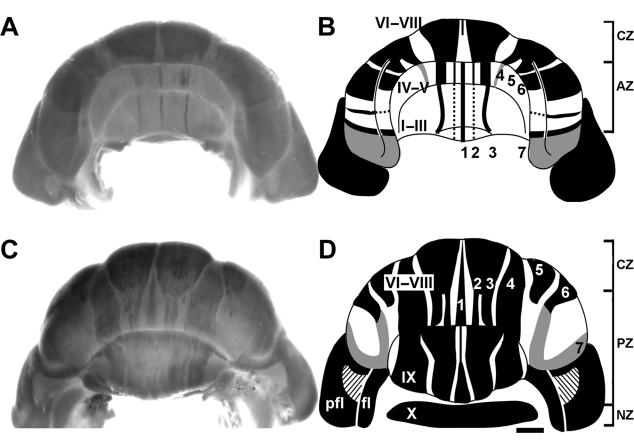
Four distinct zebrin II-expressing transverse domains can be identified in the tenrec vermis – the anterior zone (AZ: ∼ lobule I–V; Fig. 3A,B), the central zone (CZ: ∼ pVI–pVII; Fig. 3A–D), the posterior zone (PZ: ∼ pVIII, lobule IX; Fig. 3C,D) and the nodular zone (NZ: ∼ lobule X; Fig. 3C,D), similar to those in mice (Ozol et al. 1999). Transitions between zones, as in rodents (e.g. Ozol et al. 1999), are comprised of interdigitating zebrin II-positive and -negative stripes, and as a result the boundaries are not sharply delineated.
Fig. 3.
Whole mount zebrin II peroxidase immunohistochemistry of the adult tenrec cerebellum; (A,C) whole mounts, and (B,D) schematics. Peroxidase reaction product is deposited in a subset of Purkinje cells that are arranged into an elaborate array of zones and stripes. Heavily reactive stripes are shown in black and weakly reactive stripes are shown in grey. Dotted lines indicate inconsistent labelling. Whole mount views show staining of the Purkinje cell dendrites arranged parasagittally in the molecular layer. Although reaction product is also deposited in the somata and axons of Purkinje cells, this staining cannot be seen from the surface. Anterior (A,B) and posterior (C,D) views of the cerebellum. Each whole mount on the left corresponds to the schematic on the right. The schematics are a composite of data derived from high-power whole mount and tissue section immunostaining. Cerebellar vermian lobules I–IX are indicated by Roman numerals (lobule X is hidden from view in C but reflected outwards in D): pfl = paraflocculus, fl = flocculus. The zebrin II-immunoreactive stripes P1+ to P7+ are labelled (as 1–7 for clarity). Note that although all Purkinje cells in the paraflocculus express zebrin II, the level of expression is lower anteriorly (see Fig. 9). The slanted lines in the paraflocculus (D) indicate acellular regions, as found in many other mammals (see Voogd et al. 1996). The approximate extents of the transverse zones in the vermis are indicated in the schematics: anterior zone (AZ), central zone (CZ), posterior zone (PZ) and nodular zone (NZ). The scale bar in D = 1 mm (applies to whole mounts and schematics).
Anterior zone
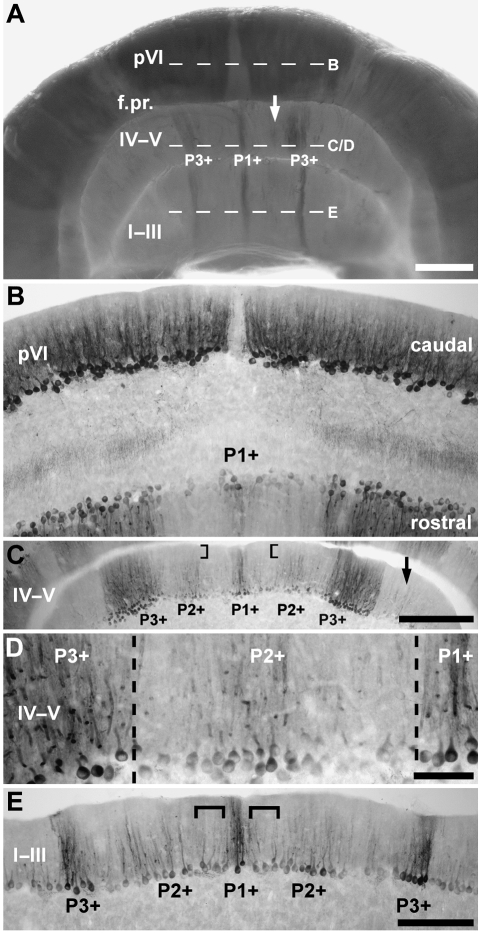
Despite the presence of undifferentiated lobules (lobule I–III and IV–V) in the anterior lobe, the AZ of tenrec (Fig. 4A,C,E) resembles that of mouse, with a strongly zebrin II-immunoreactive midline band (P1+) and a strong flanking band laterally to either side (called P3+: see Ozol et al. 1999). P1+ straddles the midline, and P3+ lies laterally (distance from the midline is ∼625 µm – lobule I–III; ∼750 µm – lobule IV–V). As in mouse, a short P2+ stripe is seen in lobule IV–V anterior to the primary fissure. In rodents, this cannot be traced into the more anterior lobules. The same is true in whole mount preparations of the tenrec cerebellum (e.g. Fig. 3A). However, in horizontal sections through the AZ a weak stripe of zebrin II expression is seen between P1+ and P3+ (e.g. Fig. 4C–E). We have interpreted this as the rostral extension of P2+. Thus, the stripes in lobule IV–V appear to be continuous with those in lobule I–III, despite minor differences in width and staining intensity (e.g. P3+ is much broader in lobule IV–V: Figs 3A,B and 4A). Finally, an inconsistent, very weak, P4+ stripe is seen laterally in sections (arrow, Fig. 4C: P4+ is described further with the hemispheres below).
Fig. 4.
The topography of the anterior zone (AZ) of the vermis. (A) Zebrin II-immunoreactive Purkinje cells in the AZ, shown in whole mount. An array of zebrin II parasagittal stripes extends through all lobules anterior to the primary fissure (f.pr.). The arrow indicates the weak P2+ stripe and dashed lines indicate the approximate levels of the sections shown in B–E. (B) Horizontal section through the boundary between the AZ and CZ in lobule pVI, immunoperoxidase-stained for zebrin II. A narrow stripe of zebrin II-reactive Purkinje cells (P1+) straddles the midline on the rostral aspect. (C,D) Horizontal sections through lobule IV–V immunoperoxidase-stained for zebrin II. (C) Seen at lower magnification, the most striking stripe in the AZ is the thick (∼375 µm) heavily reactive P3+. Note that almost no zebrin II immunoreactivity is detected in the distal dendrites of Purkinje cells located within P2+ (square brackets), perhaps explaining why P2+ is not seen in the whole mount view (A). Patches of zebrin II-immunoreactive Purkinje cells lateral to P3+ are inconsistently stained and may represent P4+ (arrow). (D) Seen at high magnification, P1+ at the midline is flanked by P2+ and P3+ laterally. (E) Horizontal section through lobule I–III immunoperoxidase-stained for zebrin II. P1+ and P3+ are both strongly zebrin II immunoreactive and equal in width. P2+ is more weakly immunoreactive. At this rostrocaudal level, P1− is difficult to discern as the lateral border of P1+ and the medial border of P2+ merge (square brackets). Scale bars: A = 1 mm; C = 500 µm; D = 100 µm; E = 250 µm (also applies to B).
Central zone
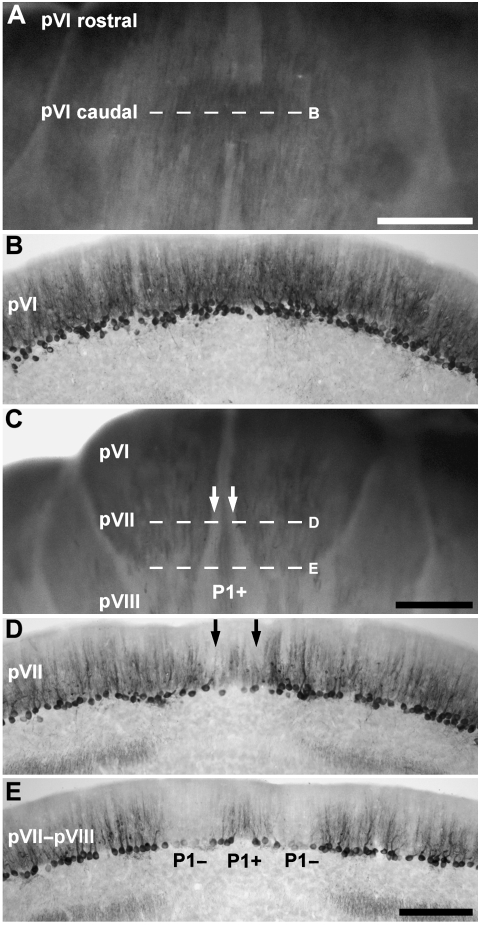
The alternating zebrin II +/− stripes seen in the AZ disappear in the CZ, which begins caudal to the primary fissure (Fig. 4A,B) in lobule VI–VIII (Fig. 5A), and occupies the rostral portion of lobule VI–VIII (accounts for ∼1/4 of the posterior lobe vermis). All Purkinje cells in the vermis of the CZ express zebrin II (Fig. 5B): at the caudal end of VI–VIII (pVIII) the P1+ stripe from the PZ interdigitates into the CZ as a thin immunoreactive stripe flanked by P1− stripes on either side (e.g. Fig. 5C–E). However, in some individuals a few Purkinje cells at the midline of the caudal aspects of pVI are only weakly immunoreactive (e.g. Fig. 5C) whereas the midline is non-reactive in the most rostral aspects of pVI (see Figs 3B and 4A,B). The P1− stripes (dashed line D in Fig. 5C; arrows in Fig. 5D) broaden caudally (Fig. 5C,E). The uniform CZ expression domain interdigitates with the AZ rostrally and with the PZ caudally, and the span where no zebrin II− Purkinje cells is seen is short.
Fig. 5.
The topography of the central zone (CZ) of the vermis. (A) Dorsal view of the rostral half of lobule VI–VIII, presumptive lobules VI and VII (pVI–pVII), shown in an anti-zebrin II-stained whole mount preparation. Zebrin II is expressed throughout the CZ. (B) Horizontal section through pVI–pVII, immunoperoxidase-stained for zebrin II (the corresponding region is indicated by dashed line B in panel A). All Purkinje cells express zebrin II. (C) Dorsoposterior view of lobule VI–VIII, shown in whole mount. Between lobules pVI and pVII, P1+/P1− stripes, which appears to derive from the caudal PZ, interrupts the homogeneous zebrin II expression domain. (D) Horizontal section through lobule pVII, immunoperoxidase-stained for zebrin II (indicated by dashed line D in panel C). Peroxidase reaction product is heavily deposited in most Purkinje cell dendrites, somata and axons but dendritic immunoreactivity is weak in a pair of stripes adjacent to the midline (arrows). (E) Horizontal section through lobules pVII–pVIII immunoperoxidase-stained for zebrin II (indicated by the dashed line E in panel C). P1+ becomes clearly differentiated from the immunopositive Purkinje cells located laterally. Scale bar in A = 1 mm; C = 1 mm; E = 250 µm (also applies to B and D).
Posterior zone
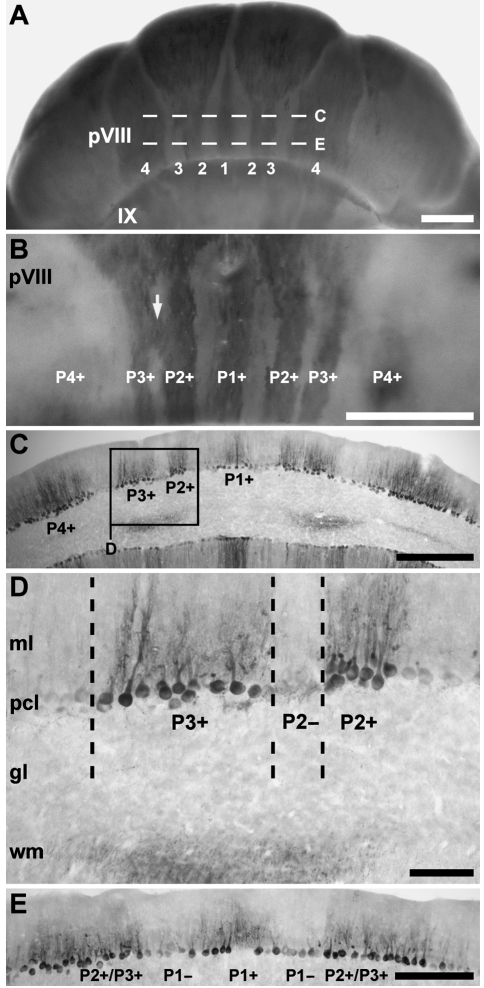
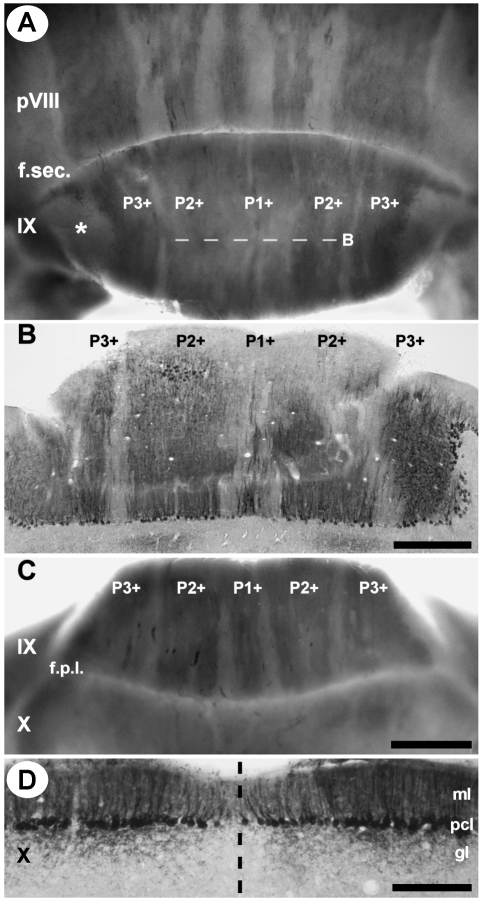
Zebrin II expression in the most caudal portion of lobule VI–VIII reveals a characteristic pattern of broad parasagittal bands (Figs 3C,D and 6) typical of the PZ in other species (e.g. rat, Brochu et al. 1990; mouse, Eisenman & Hawkes, 1993; Ozol et al. 1999; Sillitoe & Hawkes, 2002; rabbit, Sanchez et al. 2002; guinea-pig, Larouche et al. 2003; hamster, Marzban et al. 2003; cat, Sillitoe et al. 2003). One zebrin II immunoreactive stripe is located at the midline (P1+) and at least three lie laterally on either side (P2+ to P4+ Fig. 6A–C). P2+ and P3+ appear to diverge from a single thick stripe originating in the lateral CZ (Fig. 3D). Caudally in the PZ, P2+ and P3+ fuse into one broad stripe as the P2− tapers away (Fig. 6E). Finally, P4+ sits at the boundary between the vermis and the hemispheres (see below): the medial half of P4+ appears to form the lateral edge of the vermis and the lateral half of the stripe is paravermian. Caudal to the secondary fissure, P1+ tapers and P2+ increases in width towards the apex of lobule IX (Fig. 7A,B). Ventrally in lobule IX, P1− and P2− widen and again P1+ to P3+ can be clearly distinguished (Fig. 7C). In mouse, ventral IX is a zone of overlap between the PZ and the NZ (e.g. Armstrong et al. 2000), and the same may be true in tenrec as well.
Fig. 6.
The topography of the posterior zone (PZ) of the vermis. (A) The arrangement of zebrin II-immunoreactive Purkinje cells in the PZ, shown in whole mount. An array of zebrin II parasagittal stripes extends through the PZ. P1+ to P4+ are labelled (as 1–4 for clarity). (B) A higher power whole mount view of the caudal aspect of lobule VI–VIII (i.e. pVIII) immunoperoxidase-stained for zebrin II. Reaction product is heavily deposited in P1+ to P3+ of the vermis. All five stripes in the vermis of the PZ are approximately equal in width. P2+ and P3+ occasionally appear to fuse for a short distance (arrow). (C) Horizontal section through rostral pVIII, immunoperoxidase-stained for zebrin II (from the region indicated by dashed line C in panel A). P2+ and P3+ are separated by a thin zebrin II-immunonegative P2− stripe. (D) A high magnification view of the boxed area in C. The dendrites and somata of Purkinje cells in P2− are non-reactive for zebrin II. (E) Horizontal section through the caudal aspect of pVIII, immunoperoxidase-stained for zebrin II (indicated by dashed line E in panel A). P1+ at the midline and P1− on either side extend through the entire PZ. The P2+ and P3+ stripes are fused. Scale bars: A = 1 mm; B = 1 mm; C = 500 µm; D = 100 µm; E = 250 µm.
Fig. 7.
The topography of the caudal posterior zone (PZ) and the nodular zone (NZ) of the vermis. (A) Posterior view of lobules pVIII and IX zebrin II immunoperoxidase stained in whole mount. The pattern of zebrin II expression changes across the secondary fissure (f. sec.). In lobule IX P1+ is thin whereas P2+ is very broad and occupies ∼40% of the vermis. P3+ is the most lateral stripe in lobule IX and is located medial to a thick acellular region at the extreme edge of the cerebellum (asterisk). The P− stripes taper toward the apex of lobule IX. (B) Horizontal section through the apex of lobule IX, immunoperoxidase-stained for zebrin II (from the region indicated by dashed line B in panel A). (C) A ventral view of zebrin II expression in lobules IX and X shown in whole mount. The P− stripes thicken and P1+ to P3+ become distinctly separated from one another. Beyond the posterolateral fissure (f.p.l.), zebrin II is expressed uniformly and the P− stripes disappear. (D) Horizontal section through lobule X, immunoperoxidase-stained for zebrin II. All Purkinje cells in lobule X express zebrin II. Reaction product is heavily deposited in the dendrites, somata and axons. The dashed line indicates the midline. Abbreviations: ml = molecular layer; pcl = Purkinje cell layer; gl = granular layer. Scale bar = 500 µm in B; 1 mm in C (also applies to A); 250 µm in D.
Nodular zone
Posterior to the posterolateral fissure, in lobule X, all stripes disappear within the depths of the fissure as the parasagittal stripe pattern of zebrin II expression once again gives way to a uniform zone of Purkinje cells that all express zebrin II (the NZ: Fig. 7C,D). Although zebrin II staining intensity occasionally varied between Purkinje cells, no consistently zebrin II negative stripes were seen in lobule X.
Organization of the hemispheres
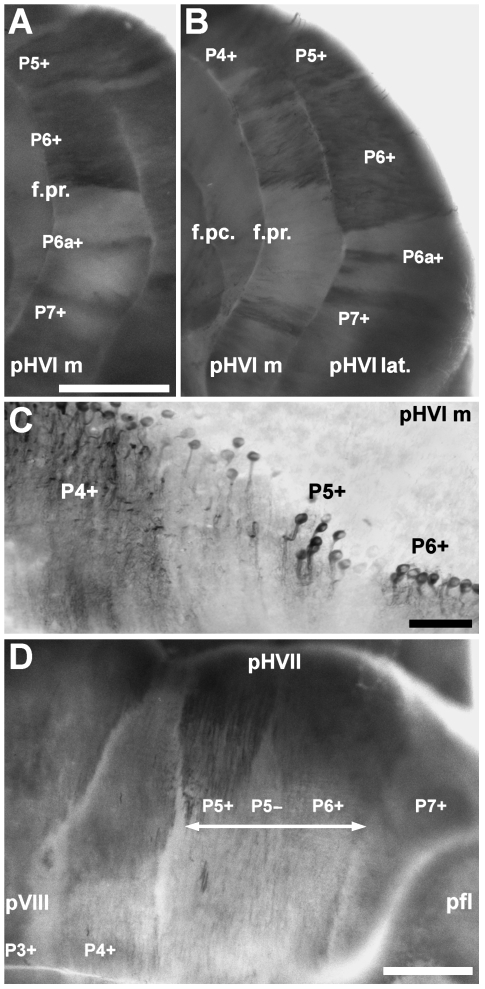
In rodents, the rostrocaudal pattern of zebrin II in the vermis is recapitulated in the hemispheres where zones with parasagittal stripes alternate with uniform expression domains. In the hemispheres of Echinops telfairi, the pattern of zebrin II expression reveals an array of parasagittal stripes that extends uninterrupted through all lobules. However, similar to rodents, the hemispheres have at least four zebrin II-immunoreactive stripes: P4+, P5+, P6+ and P7+ (Figs 3B and 8A,B). Medially, P4+ is located adjacent to the vermis and is often difficult to differentiate from staining in the vermis of the anterior lobules. P4+ gradually broadens caudally, becoming the thickest stripe in the posterior cerebellum (Fig. 8D). Both the lateral and the medial edges of P4+ are sharp. No thin P4a+ and P5b+ bands were found in tenrec, equivalent to those in crus II of other species (e.g. rat, Brochu et al. 1990; Hallem et al. 1999; mouse, Eisenman & Hawkes, 1993; Sillitoe & Hawkes, 2002). Laterally, P5+ is prominent and sometimes appears to be fused with P4+ (e.g. Fig. 8C). Immunoreactivity of P5+ gradually decreases and is difficult to distinguish from neighbouring stripes in the more posterior regions (Fig. 8D). Distinct P5+ and P6+ stripes cannot be distinguished in the most caudal part of the posterior hemisphere (double arrow, Fig. 8D). Lateral to P5+, a very broad P6+ occupies most of the lobule and is the most prominent stripe in the anterior hemispheres. The lateral edge of P6+ forms a sharp boundary with P6−. Within P6−, a thin, strongly reactive zebrin II-positive stripe (P6a+) appears inconsistently in the medial aspect of presumptive HVI (pHVI m: Fig. 8A) and consistently in the middle portion of pHVI (pHVI lat: Fig. 8B). P6a+ is not seen in the posterior hemispheres and appears to fuse with P7+ in the lateral aspects of pHVI. Even more laterally, P7+ skirts the extreme edge of the hemisphere. The medial edge of P7+ forms a sharp boundary with P6− and its lateral extent borders the paraflocculus (Fig. 8D).
Fig. 8.
Zebrin II expression domains in the cerebellar hemispheres. (A) Anterior view of the medial aspect of presumptive hemisphere lobule VI (pHVI m) shown in a whole mount immunoperoxidase-stained preparation. P4+, P5+, P6+ and P7+ are highly reproducible between individuals. P6a+ is thin, weaker in reactivity and inconsistently labelled. (B) The zebrin II immunoreactive stripes in the lateral aspects of pHVI (pHVI lat.) seem to be continuous with those in pHVI m. P6+ and P7+ are thicker and extend into the P6− stripe. P6a+ is thin and heavily reactive for zebrin II. In contrast to pHVI m, the P6a+ stripe in pHVI lat. is consistently labelled. (C) Immunoperoxidase-stained transverse section through pHVI m showing P4+ to P6+. (D) P4+ broadens in pHVII, eventually becoming the thickest stripe in the posterior lobe hemispheres (pHVIII). P5+ and P6+ are heavily reactive in pHVII and P7+ persists as a weakly reactive stripe. In the middleregion of the hemispheres (∼ pHVII–pHVIII), zebrin II expression levels in P5+ and P6+ decreases suddenly forming a broad, weakly reactive domain (double arrow) and leaving only the lateral edge of P6+ sharply defined. P5− broadens and is distinct. P7+ remains weakly immunoreactive. P3+ in the vermis is labelled for orientation. Abbreviations: f.pc., preculminate fissure; f.pr., primary fissure. Scale bars = 1 mm in A and D (scale bar in D also applies to B); C = 100 µm.
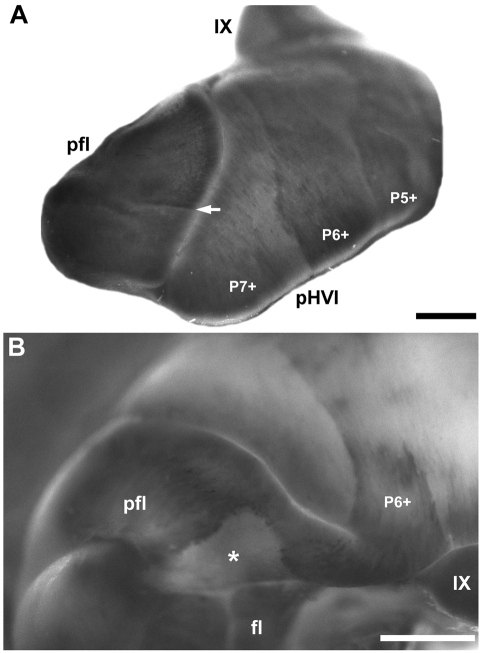
The anterior paraflocculus is weakly immunoreactive for zebrin II (Fig. 9A). Dorsally, an abrupt transition separates the anterior and posterior paraflocculus where reactivity of zebrin II in the posterior paraflocculus becomes stronger (Fig. 9A). The boundary between the two expression domains is sharp and divides the entire mediolateral extent of the paraflocculus (arrow, Fig. 9A). As seen from a ventroposterior view, the zebrin II expression domain in the posterior paraflocculus is apparently continuous with the P6+ stripe of the posterior hemispheres (Fig. 9B). A large acellular gap interrupts the expression of zebrin II in the paraflocculus (slanted lines, Fig. 3D; asterisk, Fig. 9B). This is a region with a complete absence of cerebellar cortex, which is found in many other mammals (Larsell, 1970; Voogd et al. 1996). All Purkinje cells in the flocculus are heavily reactive for zebrin II and no stripes are apparent (Fig. 9B).
Fig. 9.
Zebrin II expression in the paraflocculus and flocculus shown in whole mount immunoperoxidase-stained cerebella. (A) Lateral view of the paraflocculus (pfl). The heavily reactive Purkinje cells in the posterior paraflocculus form a sharp boundary (arrow) with the weakly reactive anterior paraflocculus. pHVI and vermian lobule IX are labelled. (B) Ventroposterior view of the paraflocculus and flocculus (fl). All Purkinje cells express zebrin II. The P6+ stripe extending over the hemispheres of the posterior hemispheric lobules appears to be continuous with the paraflocculus. A large cell-poor area in the posterior paraflocculus (asterisk) presents as an immunonegative gap in the zebrin II expression. Ventrally, lobules IX and X (hidden from view) of the vermis are morphologically continuous with the paraflocculus and the flocculus, respectively. Scale bars = 1 mm.
Discussion
The antigenic compartmentation of the cerebellar cortex has been correlated with many features of cerebellar biology. First, afferent terminal fields in the cerebellum are aligned with Purkinje cell stripes (mossy fibres, e.g. Ji & Hawkes, 1994; climbing fibres, Gravel et al. 1987; reviewed in Voogd et al. 1996). Second, tactile receptive field mapping has revealed correlations in rat between sensory modalities and zebrin II stripe boundaries (Chockkan & Hawkes, 1994; Ji & Hawkes, 1995; Hallem et al. 1999). Third, there is much evidence that these topographical relationships are established during embryogenesis through afferent growth cones using Purkinje cell compartmentation as a guide (e.g. Sotelo & Wassef, 1991; Armstrong & Hawkes, 2000). Thus a thorough understanding of cerebellar compartmentation is a necessary underpinning of studies of cerebellar function (e.g. Sanchez et al. 2002).
Traditionally, the transverse division of the cerebellum into lobes and lobules has been the standard compartmentation in the mammalian cerebellum (e.g. Larsell, 1970). More recently, the expression of several protein markers (e.g. zebrin II, calbindin, L7/pcp-2-lacZ transgene, Ozol et al. 1999; HSP25, Armstrong et al. 2000) during development and in the adult have indicated that the cerebellar vermis is divided along the rostrocaudal axis by three transverse boundaries: through the rostral face of lobule VI, in the caudal half of lobule VII, and across the posterolateral fissure between lobules IX and X. These three boundaries subdivide the newborn and adult vermis into four transverse zones: AZ, CZ, PZ and NZ. Within the transverse zones, unique combinations of Purkinje cell phenotypes, together with the topography of afferent terminal fields, identify further subdivisions into parasagittal stripes. As in rodent cerebella, four distinct transverse zones (AZ, CZ, PZ and NZ) may be identified in the cerebellum of Echinops telfairi. Compared with other mammals previously investigated, the location of boundaries between the transverse zones is comparable between tenrec and mammals with more differentiated cerebella. Thus, the presence of structurally distinct lobules does not appear to dictate the number and relative size of transverse compartments in the mammalian cerebellum.
The number and general organization of zebrin II transverse zones and parasagittal stripes is similar in tenrecs and rodents, although some features of tenrec cerebellar compartmentation are different. First, three thin zebrin II-immunoreactive stripes are a characteristic feature of the mammalian AZ (e.g. rat, Brochu et al. 1990; opossum, Doré et al. 1990; mouse, Eisenman & Hawkes, 1993; rabbit, Sanchez et al. 2002; guinea-pig, Larouche et al. 2003; cat, Sillitoe et al. 2003). In rodent, P1+ and two P3+ (previously referred to as P2+, see Ozol et al. 1999) stripes located laterally are the easiest to detect. In tenrec, P1+ and two P3+ stripes are prominent in lobules I–III and are very similar to those in the mouse AZ. In rodents, a P2+ stripe is present in lobule V but cannot be traced far beyond the primary fissure (e.g. Eisenman & Hawkes, 1993; see also Larouche et al. 2003). By contrast, in the tenrec, a weakly immunoreactive P2+ was detected throughout the AZ (e.g. Fig. 4). Although the extension of P2+ beyond the preculminate fissure is not seen in rats and mice, a tripartite organization within the P1− stripe of the rat was previously inferred from the mossy fibre terminal field distributions (e.g. Ji & Hawkes, 1994).
Secondly, the CZ, as defined by the uniform expression of zebrin II (Ozol et al. 1999), appears small in Echinops telfairi compared with other species studied to date, perhaps because the interdigitation with neighbouring zones is more extensive. The CZ receives substantial afferent projections originating from the cerebral cortex associated with the transmission of visual information (e.g. Glickstein et al. 1994; Serapide et al. 1994, 2001, 2002). It is interesting then that Echinops telfairi may have only rudimentary vision (Larsell, 1970).
Thirdly, a striking feature of the tenrec cerebellum is the lissiform posterior vermis and hemispheres. Lobulation is poor in the posterior lobe – a secondary fissure is visible macroscopically but there are no discrete lobules VI, VII and VIII apparent at the cerebellar surface. This presents an opportunity to investigate whether zebrin expression boundaries between transverse zones are determined by the pattern of lobulation. The mechanisms responsible for the placement of fissures remain obscure. It is generally assumed that the position of individual lobules is dependent on the underlying organization of Purkinje cells (e.g. Altman & Bayer, 1997). However, the relationship in the adult is not simple, and transverse expression boundaries and fissures do not coincide (e.g. Ozol et al. 1999). Similarly, in murine mutants in which lobule formation is interrupted, the normal underlying pattern of expression domains can still be identified (e.g. reeler, Edwards et al. 1994; disabled, Gallagher et al. 1998; cerebellar deficient folia, Beierbach et al. 2001). Similarly in the lissiform tenrec posterior lobe vermis, the transverse zone and parasagittal stripe expression pattern of zebrin II is clearly reminiscent of that in other mammals. Neither the transition from one transverse zone to another nor the pattern of stripes (seen also in the AZ as the parasagittal stripes are unaffected by the undifferentiated lobules) bears any constant relationship to a feature of the surface topography. Thus transverse boundaries between expression domains do not induce the formation of lobules and fissures, and lobulation is not required for transverse boundary formation.
Finally, in several mammals, a similarity is apparent between the transverse zones in the vermis and the rostrocaudal compartmentation of the hemispheres. For example, zebrin II expression in mouse reveals a similar alternation in vermis and hemispheres of striped (AZ ∼ lobulus simplex), uniform (CZ ∼ crus I), striped (PZ ∼ crus II and paramedian lobule) and uniform (NZ ∼ paraflocculus and flocculus). In tenrec, the expression of zebrin II reveals an array of parasagittal stripes that extend uninterrupted from the AZ hemispheres to the caudal edges of the PZ hemispheres. However, although no hemispheric equivalent to the CZ is obvious (as is often the case in the vermis of the AZ), changes in the width (AZ to CZ) and intensity (CZ to PZ) of individual stripes are consistent with the zonal transitions in the vermis. In the caudal PZ, the broad domains of zebrin II expression in the paraflocculus are consistent with the broad stripes evident in lobule IX (the paraflocculus may serve as hemisphere lobule IX (HIX), whereas in the NZ uniform expression of zebrin II in the flocculus matches the expression in lobule X (the flocculus may serve as hemisphere lobule X (HX).
Acknowledgments
This investigation received financial support from the Canadian Institutes for Health Research (R.H.) and the DFG (H.K.). R.V.S. was supported by a studentship from the CIHR strategic Training Program in Genetics, Child Development and Health.
References
- Ahn AH, Dziennis S, Hawkes R, Herrup K. The cloning of zebrin II reveals its identity with aldolase C. Development. 1994;120:2081–2090. doi: 10.1242/dev.120.8.2081. [DOI] [PubMed] [Google Scholar]
- Altman J, Bayer S. In: Development of the Cerebellar System in Relation to its Evolution, Structure, and Functions. Petralia P, editor. Boca Raton: CRC Press; 1997. pp. 2–25.pp. 230–251. [Google Scholar]
- Armstrong CL, Hawkes R. Pattern formation in the cerebellar cortex. Biochem. Cell Biol. 2000;78:551–562. [PubMed] [Google Scholar]
- Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of Purkinje cells in the adult mouse cerebellar cortex. J. Comp. Neurol. 2000;416:383–397. doi: 10.1002/(sici)1096-9861(20000117)416:3<383::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bauchot R, Stephan H. Morphologie comparée de l'encephale des insectivores tenrecidae. Mammalia. 1970;34:514–541. [Google Scholar]
- Beierbach E, Park C, Ackerman SL, Goldowitz D, Hawkes R. Abnormal dispersion of a Purkinje cell subset in the mouse mutant cerebellar deficient folia (cdf) J. Comp. Neurol. 2001;436:42–51. [PubMed] [Google Scholar]
- Brochu G, Maler L, Hawkes R. Zebrin II: a polypeptide antigen expressed selectively by Purkinje cells reveals compartments in rat and fish cerebellum. J. Comp. Neurol. 1990;291:538–552. doi: 10.1002/cne.902910405. [DOI] [PubMed] [Google Scholar]
- Chockkan V, Hawkes R. Functional and antigenic maps in the rat cerebellum: zebrin II and vibrissal receptive fields in lobule IXa. J. Comp. Neurol. 1994;345:33–45. doi: 10.1002/cne.903450103. [DOI] [PubMed] [Google Scholar]
- Davis CA. Whole-mount immunohistochemistry. Meth. Enzymol. 1993;225:502–516. doi: 10.1016/0076-6879(93)25034-y. [DOI] [PubMed] [Google Scholar]
- De Camilli P, Miller P, Levitt P, Walter U, Greengard P. Anatomy of cerebellar Purkinje cells in the rat determined by a specific immunohistochemical marker. Neuroscience. 1984;11:761–817. doi: 10.1016/0306-4522(84)90193-3. [DOI] [PubMed] [Google Scholar]
- Dent JA, Polson AG, Klymkowsky MW. A whole-mount immunocytochemical analysis of the expression of the intermediate filament protein vimentin in Xenopus. Development. 1989;105:61–74. doi: 10.1242/dev.105.1.61. [DOI] [PubMed] [Google Scholar]
- van Dijk MA, Madsen O, Catzeflis F, Stanhope MJ, de Jong WW, Pagel M. Protein sequence signatures support the African clade of mammals. Proc. Natl. Acad. Sci. USA. 2001;98:188–193. doi: 10.1073/pnas.250216797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré L, Jacobson CD, Hawkes R. Organization and postnatal development of zebrin II antigenic compartmentation in the cerebellar vermis of the grey opossum, Monodelphis domestica. J. Comp. Neurol. 1990;291:431–449. doi: 10.1002/cne.902910309. [DOI] [PubMed] [Google Scholar]
- Douady CJ, Catzeflies F, Kao DJ, Springer MS, Stanhope MJ. Molecular evidence for the monophyly of tenrecidae (Mammalia) and the timing of the colonization of Madagascar by Malagasy tenrecs. Mol. Phylogen. Evol. 2002;22:357–363. doi: 10.1006/mpev.2001.1055. [DOI] [PubMed] [Google Scholar]
- Edwards MA, Leclerc N, Crandall JE, Yamamoto M. Purkinje cell compartments in the reeler mutant mouse as revealed by zebrin II and 9–0-acetylated glycolipid antigen expression. Anat. Embryol. (Berlin) 1994;190:417–428. doi: 10.1007/BF00235488. [DOI] [PubMed] [Google Scholar]
- Eisenberg JF, Gould E. The tenrecs: a study in mammalian behavior and evolution. Smithsonian Contrib. Zool. 1970;27:1–137. [Google Scholar]
- Eisenman LM, Hawkes R. Antigenic compartmentation in the mouse cerebellar cortex: zebrin and HNK-1 reveal a complex, overlapping molecular topography. J. Comp. Neurol. 1993;335:586–605. doi: 10.1002/cne.903350410. [DOI] [PubMed] [Google Scholar]
- Gallagher E, Howell BW, Soriano P, Cooper JA, Hawkes R. Cerebellar abnormalities in the disabled (mdab1–1) mouse. J. Comp. Neurol. 1998;402:238–251. [PubMed] [Google Scholar]
- Glickstein M, Gerrits N, Kralj-Hans I, Mercier B, Stein J, Voogd J. Visual pontocerebellar projections in the macaque. J. Comp. Neurol. 1994;349:51–72. doi: 10.1002/cne.903490105. [DOI] [PubMed] [Google Scholar]
- Gravel C, Eisenman LE, Sasseville R, Hawkes R. Parasagittal organization of the rat cerebellar cortex: a direct correlation between antigenic Purkinje cell bands revealed by mabQ113 and the organization of the olivocerebellar projection. J. Comp. Neurol. 1987;263:294–310. doi: 10.1002/cne.902650211. [DOI] [PubMed] [Google Scholar]
- Hallem JS, Thompson J, Gundappa-Sulur S, Hawkes R, Bjaalie JG, Bower JM. Spatial correspondence between tactile projection patterns and the distribution of the antigenic Purkinje cell markers anti-zebrin I and anti-zebrin II in the cerebellar folium crus IIa of the rat. Neuroscience. 1999;93:1083–1094. doi: 10.1016/s0306-4522(99)00144-x. [DOI] [PubMed] [Google Scholar]
- Ji Z, Hawkes R. Topography of Purkinje cell compartments and mossy fiber terminal fields in lobules II and III of the rat cerebellar cortex: spinocerebellar and cuneocerebellar projections. Neuroscience. 1994;61:935–954. doi: 10.1016/0306-4522(94)90414-6. [DOI] [PubMed] [Google Scholar]
- Ji Z, Hawkes R. Developing mossy fiber terminal fields in the rat cerebellar cortex may segregate because of Purkinje cell compartmentation and not competition. J. Comp. Neurol. 1995;359:197–212. doi: 10.1002/cne.903590202. [DOI] [PubMed] [Google Scholar]
- Künzle H. Care and breeding of the Madagascan hedgehog tenrec, Echinops telfairi, under laboratory conditions. Der Tierschutzbeauftragte. 1998;7:5–12. 113–115. [Google Scholar]
- Larouche M, Diep C, Sillitoe RV, Hawkes R. The topographical anatomy of the cerebellum in the guinea pig, Cavia porcellus. Brain Res. 2003;965:159–169. doi: 10.1016/s0006-8993(02)04160-4. [DOI] [PubMed] [Google Scholar]
- Larsell O. In: The Comparative Anatomy and Histology of the Cerebellum from Monotremes Through Apes. Jansen J, editor. Minneapolis: University of Minnesota Press; 1970. pp. 3–269. [Google Scholar]
- Leclerc N, Schwarting GA, Herrup K, Hawkes R, Yamamoto M. Compartmentation in mammalian cerebellum: Zebrin II and P-path antibodies define three classes of sagittally organized bands of Purkinje cells. Proc. Natl Acad. Sci. USA. 1992;89:5006–5010. doi: 10.1073/pnas.89.11.5006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malia MJ, Adkins RM, Allard MW. Molecular support for Afrotheria and the polyphyly of Lipotyphla based on analyses of the growth hormone receptor gene. Mol. Phylogenet. Evol. 2002;24:91–101. doi: 10.1016/s1055-7903(02)00219-1. [DOI] [PubMed] [Google Scholar]
- Marzban H, Zahedi SM, Sanchez M, Hawkes R. Antigenic compartmentation of the cerebellar cortex of the Syrian hamster, Mesocricetus auratus. Brain Res. 2003;974:176–183. doi: 10.1016/s0006-8993(03)02576-9. [DOI] [PubMed] [Google Scholar]
- Mouchaty SK, Gullberg A, Janke A, Arnason U. Phylogenetic position of the tenrecs (Mammalia: Tenrecidae) of Madagascar based on analysis of the complete mitochondrial genome sequence of Echinops telfairi. Zool. Scripta. 2000;29:307–317. [Google Scholar]
- Murphy WJ, Eizirik E, Johnson WE, Zhang YP, Ryderk OA, O'Brien SJ. Molecular phylogenetics and the origins of placental mammals. Nature. 2001;409:614–618. doi: 10.1038/35054550. [DOI] [PubMed] [Google Scholar]
- Ozol K, Hayden JM, Oberdick J, Hawkes R. Transverse zones in the vermis of the mouse cerebellum. J. Comp. Neurol. 1999;412:95–111. [PubMed] [Google Scholar]
- Sanchez M, Sillitoe RV, Attwell PJ, Ivarsson M, Rahman S, Yeo CH, Hawkes R. Compartmentation of the rabbit cerebellar cortex. J. Comp. Neurol. 2002;444:159–173. doi: 10.1002/cne.10144. [DOI] [PubMed] [Google Scholar]
- Schmued LC. A rapid, sensitive histochemical stain for myelin in frozen brain sections. J. Histochem. Cytochem. 1990;38:717–720. doi: 10.1177/38.5.1692056. [DOI] [PubMed] [Google Scholar]
- Serapide MF, Cicirata F, Sotelo C, Panto MR, Parenti R. The pontocerebellar projection: longitudinal zonal distribution of fibers from discrete regions of the pontine nuclei to vermal and parafloccular cortices in the rat. Brain Res. 1994;644:175–180. doi: 10.1016/0006-8993(94)90362-x. [DOI] [PubMed] [Google Scholar]
- Serapide MF, Panto MR, Parenti R, Zappala A, Cicirata F. Multiple zonal projections of the basilar pontine nuclei to the cerebellar cortex of the rat. J. Comp. Neurol. 2001;430:471–484. doi: 10.1002/1096-9861(20010219)430:4<471::aid-cne1044>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Serapide MF, Parenti R, Panto MR, Zappala A, Cicirata F. Multiple zonal projections of the nucleus reticularis tegmenti pontis to the cerebellar cortex of the rat. Eur. J. Neurosci. 2002;15:1854–1858. doi: 10.1046/j.1460-9568.2002.02029.x. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Hawkes R. Whole-mount immunohistochemistry: a high throughput screen for patterning defects in the mouse cerebellum. J. Histochem. Cytochem. 2002;50:235–244. doi: 10.1177/002215540205000211. [DOI] [PubMed] [Google Scholar]
- Sillitoe RV, Hulliger M, Dyck R, Hawkes R. Antigenic compartmentation of the cat cerebellar cortex. Brain Res. 2003;977:1–15. doi: 10.1016/s0006-8993(03)02569-1. [DOI] [PubMed] [Google Scholar]
- Sotelo C, Wassef M. Cerebellar development: afferent organization and Purkinje cell heterogeneity. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1991;331:307–313. doi: 10.1098/rstb.1991.0022. [DOI] [PubMed] [Google Scholar]
- Stanhope MJ, Waddell VG, Madsen O, de Jong W, Hedges SB, Cleven GC, et al. Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals. Proc. Natl Acad. Sci. USA. 1998;95:9967–9972. doi: 10.1073/pnas.95.17.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan H, Baron G, Frahm HD, editors. Comparative Brain Research in Mammals. Vol. 1. New York: Springer-Verlag; 1991. Insectivora with a stereotaxic atlas of the hedgehog brain; pp. 29–47. [Google Scholar]
- Voogd J. The importance of fiber connections in the comparative anatomy of the mammalian cerebellum. In: Llinás R, editor. Neurobiology of Cerebellar Evolution and Development. Chicago: A.M.A.; 1969. pp. 493–541. [Google Scholar]
- Voogd J, Jaarsma D, Marani E. The cerebellum: chemoarchitecture and anatomy. In: Swanson LW, Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy. Amsterdam: Elsevier Science; 1996. pp. 1–369. [Google Scholar]
- Voogd J, Glickstein M. The anatomy of the cerebellum. Trends Neurosci. 1998;21:370–375. doi: 10.1016/s0166-2236(98)01318-6. [DOI] [PubMed] [Google Scholar]
- Walther EU, Dichgans M, Maricich SM, Romito RR, Yang F, Dziennis S, et al. Genomic sequences of aldolase C (zebrin II) direct lacZ. expression exclusively in non-neuronal cells of transgenic mice. Proc. Natl Acad. Sci, USA. 1998;95:2615–2620. doi: 10.1073/pnas.95.5.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]