Abstract
In this study, we set out to make a fine characterization of the angiogenic response induced by plasma cells obtained from patients with active-multiple myeloma (MM), in comparison with cells obtained from patients with non-active MM and benign lesions such as monoclonal gammopathy of undetermined significance (MGUS), in the chick embryo chorioallantoic membrane (CAM) assay. To achieve this we investigated the time-course of the angiogenic response induced by gelatin sponges soaked in the cell suspensions and implanted on the CAM surface from day 8 to day 12 of incubation by evaluating the number of vessels, of the vessel bifurcation and the intervascular distance at 24, 48, 72 and 96 h after the implants. The results show that plasma cell suspensions obtained from patients with active MM induce a vasoproliferative response that was significantly higher than that induced by cell suspensions obtained from patients with non-active MM or with MGUS, which is also a function of the day of implantation. In fact, implants made from day 8 to day 10 induce a strong angiogenic response, whereas those made from day 11 to day 12 do not. This finding might depend on the fact that CAM endothelium exhibits an intrinsically high mitotic rate until day 10. Thereafter, the endothelial mitotic index declines rapidly, and consequently cell suspensions implanted on the CAM of successively older embryos are not able to induce a vasoproliferative response in parallel with the reduced rates of growth of the CAM's endothelial cells.
Keywords: angiogenesis, chorioallantoic membrane, multiple myeloma, plasma cells
Introduction
Angiogenesis is a mandatory step in the progression of tumour growth, invasion and metastasis (Folkman, 1995). Yet whereas the role of angiogenesis in the growth, progression and metastatic spread of solid tumours is well established (Folkman, 1995), its potential role in haematological tumours has only been investigated in recent years (Ribatti et al. 2003).
In patients with monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma (MM), angiogenesis correlates with plasma cell growth (S-phase fraction) (Vacca et al. 1994). Moreover, angiogenesis is paralleled by an increased angiogenic ability of bone marrow plasma cell conditioned medium (CM) in patients with active MM as compared with those with non-active MM and MGUS, and is partly dependent on fibroblast growth factor-2 (FGF-2) production (Vacca et al. 1999).
The chick embryo chorioallantoic membrane (CAM) assay is a well-established method to study angiogenesis and anti-angiogenesis (Ribatti et al. 2001b). Plasma cell CM was tested for its ability to induce angiogenesis in the CAM (Vacca et al. 1999). The CM of 20 of 26 (77%) active MM patients induced an angiogenic response; by contrast, only 33% and 20% of CM from non-active MM and MGUS patients, respectively, induced the response. Anti-FGF-2 antibody partly inhibited the CM angiogenic response.
Our modification of the CAM assay enables the delivery of a very small number of tumour cells soaked onto gelatin sponges implanted on top of a growing CAM, and the assessment of their angiogenic capacity (Ribatti et al. 1997, 1999, 2001b).
On this basis, we have attempted a fine characterization of the angiogenic response induced by plasma cells obtained from patients with active MM, as compared with those obtained from patients either with non-active MM or with MGUS, in the CAM assay. We investigated the time-course of the angiogenic response induced by gelatin sponges soaked with the cell suspensions and implanted on the CAM surface from day 8 to day 12 of incubation by evaluating the number of vessels, the vessel bifurcation and the intervascular distance at 24, 48, 72 and 96 h after the implants.
Materials and methods
Cell cultures
Plasma cells were obtained by Ficoll-Hypaque (Pharmacia Biotech, Uppsala, Sweden) separation from bone marrow aspirates close to the biopsy site and B-cell enrichment with magnetic microbeads (Dynal, Oslo, Norway) coated with a monoclonal antibody (MoAb) to the plasma cell marker CD38 (Becton Dickinson, Mountain View, CA, USA), as described previously (Vacca et al. 1999). Cells consisted of > 95% tumour plasma cells and their clonally related cells (Billedeau et al. 1993) as assessed by morphology in May–Grunwald–Giemsa and flow cytometry with the anti-CD38 MoAb (FACSscan, Becton Dickinson), or by immunocytochemical staining with anti-κ or anti-γ polyclonal antibodies (Dako, Glostrup, Denmark) according to the light chain of the M-component. Cells were cultured in serum-free RPMI-1640 medium (SFM) at 1 × 107 per 25-cm2 flask, with 6 mL medium supplemented with 1% glutamine for 24 h in a 5% CO2 humidified atmosphere at 37 °C. If the number of harvested plasma cells was lower (as in MGUS or control subjects), cells were cultured in a smaller quantity of the medium to preserve the same cell number/medium volume ratio. Cell viability, assessed by trypan blue exclusion, was > 90%.
In vivo CAM assay
The chicken embryos hatches 21 days after fertilization. The CAM emerges on day 4–5 and its vessels subsequently spread over the surface of the yolk sac, totally covering it (Ribatti et al. 2001b). Fertilized White Leghorn chicken eggs were incubated at 37 °C at constant humidity. On day 3 of incubation a square window was opened in the eggshell after removal of 2–3 mL of albumen so as to detach the developing CAM from the shell. The window was sealed with a glass and the eggs were returned to the incubator. Gelatin sponges (Gelfoam, Upjohn Company, Kalamazoo, MI, USA) were cut to a size of 1 mm3 and placed over a growing CAM at days 8, 9, 10, 11 and 12 of incubation under sterile conditions (Ribatti et al. 1997). The sponges were then adsorbed with 2 µL of cell suspension (18 000 cells per sponge) of plasma cells obtained from 10 patients with active MM, 10 patients with non-active MM and 10 patients with MGUS.
Quantification of the angiogenic response
The angiogenic response was evaluated 24, 48, 72 and 96 h after implant by means of a stereomicroscope connected to an image analyser system (Olympus Italia, Italy). The following paramenters were evaluated: number of vessels converging toward the sponge and tangent to a ring encircling the sponge; number of vessel bifurcations; and intervascular distance in the space between two rings, the inner encircling the sponge and the outer traced at a distance equal to one-quarter of the diameter of the inner ring. Means ±1 standard deviation (SD) were evaluated for all the parameters and the statistical significance of the differences between the counts was determined using Student's t-test for unpaired data.
The CAMs were fixed in ovo in Bouin's fluid, deydrated in graded ethanol, embedded in paraffin, serially sectioned at 7 µm according to a plane perpendicular to their free surface, and stained with a 0.5% acqueous solution of toluidine blue (Merck, Darmstadt, Germany).
Results
Daily measurements were made on plasma cell suspensions implanted on the CAM (aged 9–16 days) in a total of 330 eggs. Plasma cells obtained from patients with active MM induced a strong angiogenic activity in 75% of implants, compared with only 20% and 10% of implants of plasma cells from non-active MM and MGUS, respectively. These results confirm our previous data obtained by testing plasma cell CM in the same assay (Vacca et al. 1999).
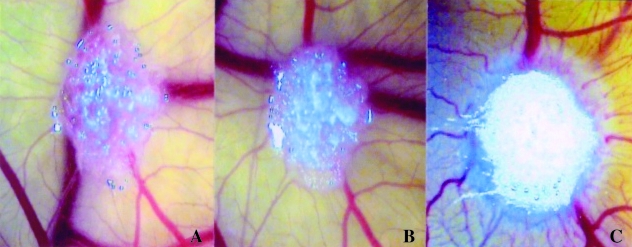
When we analysed the time-course of the angiogenic response, we observed that implants made from day 8 to day 10 induced a vasoproliferative response, when the CAM was observed in the next 4 days. Changes in the vasculature of the CAM in response to the implants were not grossly observable in the first 24–36 h (Fig. 1A,B). Later, after 72–96 h, the CAM showed a positive response, with numerous allantoic vessels developing radially toward the implant in a ‘spoked-wheel’ pattern (Fig. 1C), similar to that in important angiogenic cytokines such as FGF-2 and vascular endothelial growth factor (VEGF) (Ribatti et al. 1995, 1997, 2001a). The number of vessels and of bifurcations was higher, whereas the intervascular distance was lower in active than in non-active MM, and also in MGUS specimens (Tables 1–3).
Fig. 1.
Time-course of the macroscopic appearance of a CAM implanted at day 8 (in A) with a sponge loaded with 18 000 plasma cells of an active MM patient. Note that, whereas on day 9 (in B) no vascular reaction is detectable, on day 12 (in C) numerous allantoic vessels develop radially towards the implant in a ‘spoked-wheel’ pattern. Original magnification, ×50.
Table 1.
Grading of the response of CAM blood vessels to plasma cells from active MM (A-MM), non-active MM (NA-MM) and MGUS patients in terms of number of vessels converging toward the sponge
| Day of reading | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day of implantation | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| 8 | A-MM | 7 ± 2 | 10 ± 4 | 15 ± 4 | 26 ± 7 | 34 ± 5* | ||||
| NA-MM | 8 ± 2 | 11 ± 3 | 14 ± 5 | 17 ± 3 | 21 ± 4 | |||||
| MGUS | 7 ± 1 | 9 ± 2 | 12 ± 3 | 14 ± 4 | 16 ± 5 | |||||
| 9 | A-MM | 8 ± 2 | 10 ± 3 | 14 ± 6 | 25 ± 7 | 30 ± 4* | ||||
| NA-MM | 9 ± 3 | 11 ± 2 | 13 ± 3 | 17 ± 5 | 19 ± 5 | |||||
| MGUS | 8 ± 3 | 10 ± 3 | 12 ± 4 | 12 ± 5 | 13 ± 4 | |||||
| 10 | A-MM | 10 ± 3 | 15 ± 6 | 18 ± 5 | 25 ± 5 | 32 ± 4* | ||||
| NA-MM | 11 ± 2 | 12 ± 3 | 15 ± 6 | 20 ± 4 | 22 ± 2 | |||||
| MGUS | 9 ± 3 | 11 ± 4 | 12 ± 5 | 13 ± 4 | 15 ± 3 | |||||
| 11 | A-MM | 12 ± 4 | 12 ± 5 | 14 ± 3 | 15 ± 4 | 16 ± 5 | ||||
| NA-MM | 11 ± 3 | 13 ± 2 | 14 ± 5 | 14 ± 5 | 15 ± 4 | |||||
| MGUS | 10 ± 3 | 12 ± 4 | 14 ± 6 | 15 ± 3 | 15 ± 4 | |||||
| 12 | A-MM | 12 ± 3 | 14 ± 5 | 14 ± 6 | 15 ± 5 | 16 ± 4 | ||||
| NA-MM | 11 ± 4 | 13 ± 2 | 14 ± 3 | 14 ± 5 | 14 ± 6 | |||||
| MGUS | 10 ± 4 | 11 ± 3 | 13 ± 4 | 15 ± 4 | 15 ± 5 | |||||
P < 0.001 vs. NA-MM and MGUS.
Table 3.
Grading of the response of CAM blood vessels to plasma cells from active MM (A-MM), non-active MM (NA-MM) and MGUS patients in terms of terms of intervascular distance (μm)
| Day of reading | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day of implantation | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| 8 | A-MM | 17 ± 3 | 17 ± 4 | 15 ± 2 | 13 ± 3 | 10 ± 4* | ||||
| NA-MM | 18 ± 4 | 18 ± 5 | 16 ± 4 | 15 ± 3 | 14 ± 3 | |||||
| MGUS | 16 ± 4 | 16 ± 5 | 15 ± 4 | 14 ± 3 | 14 ± 4 | |||||
| 9 | A-MM | 16 ± 3 | 14 ± 4 | 12 ± 3 | 11 ± 2 | 10 ± 3* | ||||
| NA-MM | 15 ± 3 | 14 ± 3 | 14 ± 4 | 13 ± 2 | 13 ± 4 | |||||
| MGUS | 16 ± 5 | 15 ± 4 | 15 ± 4 | 14 ± 3 | 14 ± 2 | |||||
| 10 | A-MM | 15 ± 4 | 13 ± 2 | 11 ± 2 | 11 ± 3 | 9 ± 2* | ||||
| NA-MM | 14 ± 3 | 14 ± 4 | 13 ± 3 | 13 ± 3 | 12 ± 2 | |||||
| MGUS | 15 ± 4 | 15 ± 5 | 16 ± 4 | 15 ± 3 | 14 ± 2 | |||||
| 11 | A-MM | 14 ± 4 | 14 ± 5 | 13 ± 4 | 13 ± 5 | 13 ± 4 | ||||
| NA-MM | 15 ± 3 | 14 ± 5 | 14 ± 4 | 13 ± 3 | 13 ± 4 | |||||
| MGUS | 16 ± 4 | 15 ± 5 | 15 ± 4 | 14 ± 3 | 14 ± 4 | |||||
| 12 | A-MM | 14 ± 4 | 14 ± 5 | 14 ± 5 | 13 ± 5 | 13 ± 5 | ||||
| NA-MM | 15 ± 5 | 14 ± 6 | 14 ± 4 | 14 ± 4 | 14 ± 5 | |||||
| MGUS | 16 ± 4 | 15 ± 4 | 15 ± 5 | 15 ± 5 | 15 ± 4 | |||||
P < 0.001 vs. NA-MM and MGUS.
By contrast, implants of all specimens made from day 11 to day 12 did not induce a significant response, in terms of number of vessels, bifurcations or intervascular distance, when the CAMs were observed over the next four days (Tables 1–3).
Microscopically, no significant increase of the mononuclear cell infiltrate was observed in the gelatin sponges treated with plasma cell CM obtained from all patients, ruling out the possibility that the angiogenic response could have been due to the triggering of an inflammatory response.
Discussion
The CAM is a well-established assay for studying the effects of growth factors on blood vessel formation (Ribatti et al. 2001b). We have developed a modification of the CAM assay, which enables the delivery of a very small number of tumour cells onto the CAM and the assessment of their angiogenic capacity (Ribatti et al. 1997, 1999, 2001a). This experimental condition allows the slow, continuous delivery of growth factor(s) released by a few implanted tumour cells, closely resembling the initial stages of tumour-cell-induced angiogenesis (Li et al. 2000). As compared with the application on the CAM of large amounts of a pure recombinant angiogenic cytokine in a single bolus, implants of cells overexperessing angiogenic cytokines enable the continuous delivery of growth factors, following a more ‘physiological’ mode of interaction with the CAM vasculature.
In this study, we have shown that plasma cell suspensions obtained from patients with active MM and implanted onto the CAM induce a vasoproliferative response, significantly higher than that induced by cell suspensions obtained from patients either with non-active MM or with MGUS. Moreover, the higher number of vascular bifurcations, as well as the reduction of the intervascular distance, observed in the CAM treated with plasma cells obtained from patients with active MM is consistent with the concept that angiogenesis within the CAM is the result of, in part, continuous neoformation of first- and second-order vessels associated with a reduction of the intervascular distance.
These responses are a function of the day of implantation. In fact, implants made from day 8 to day 10 are strongly angiogenic, whereas those made from day 11 to day 12 are not. This finding might depend on the fact that CAM endothelium exhibits an intrinsically high mitotic rate until day 10 (Ausprunk et al. 1974). Thereafter, the endothelial mitotic index declines rapidly, and the vascular system attains its final arrangement on day 18, just before hatching (Ausprunk et al. 1974). Consequently, cell suspensions implanted on the CAM of successively older embryos are not able to induce a vasoproliferative response in parallel with the reduced rates of growth of the CAM's endothelial cells.
Similar results were obtained by Knighton et al. (1977), who implanted Walker 256 carcinoma specimens on the CAM aged 5–16 days. Tumours remained avascular for 72 h, after which they were penetrated by new blood vessels and began a phase of rapid growth. The rate of growth during this vascular phase was greater for implants on days 5 and 6, and decreased for later days of implantation.
The time of onset of the angiogenic response seems to be independent of the immunological status of the embryo. Early lymphoid cells deriving from the yolk sac and spleen are usually recognizable in the thymus on day 8 and in the bursa of Fabricius on day 11 (Leene et al. 1973). Thymus cells are present by day 11 and cell-mediated immunity has been demonstrated by days 13–14 (Solomon, 1971). Moreover, we did not observe a significant increase in the mononuclear cell infiltrate, ruling out the possibility of the induction of a non-specific reaction around the sponge capable of stimulating a secondary angiogenesis.
One hypothesis may be that MM plasma cells exert their angiogenic activity through the release of angiogenic cytokine, such as FGF-2, VEGF and interleukin-6 (IL-6) (Vacca et al. 1999; Dankbar et al. 2000), and the activation of endogenous angiogenic cytokines, such as FGF-2 and VEGF, normally expressed during the development of the vascular system in the CAM (Nico et al. 2001; Ribatti et al. 1995, 2002). FGF-2 and VEGF released by MM plasma cells may act both directly and indirectly in the CAM via a paracrine pathway through the activation of proteolytic enzymes, such as interstitial collagenase and urokinase-type plasminogen activator. This enhanced proteolyitic activity may induce the release of endogenous angiogenic factors such as FGF-2 and VEGF from the CAM extracellular matrix. Clearly, in an in vivo system such as the CAM, evaluation of the synergistic activity between cytokines expressed in different amounts and discrimination of their specific activity are more difficult than in an in vitro system. Moreover, these observations highlight the notion of context, i.e. that the activity of an angiogenic cytokine depends on the presence and concentration of other cytokines around the responding endothelial cell (Pepper et al. 1998).
In summary, our data are in line with the higher serum FGF-2 and VEGF levels in patients with MM than in those with MGUS, and the correlation of VEGF with worse prognosis (Sezer et al. 2001; Iwasaki et al. 2002), and suggest that VEGF and its signalling proteins may be appropiate targets in the management of MM (Hideshima et al. 2001). The VEGF signalling pathway can be inhibited at various levels, i.e. by blocking the activity of VEGF with monoclonal antibodies (Jung et al. 2002), blocking the VEGF receptors (VEGFR) with specific antibodies (Kim et al. 2002) or interfering with the tyrosine-kinases activated by the VEGF/VEGFR interaction (Wedge et al. 2002).
Table 2.
Grading of the response of CAM blood vessels to plasma cells from active MM (A-MM), non-active MM (NA-MM) and MGUS patients in terms of number of vessel bifurcations
| Day of reading | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Day of implantation | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | |
| 8 | A-MM | 1 ± 1 | 2 ± 1 | 3 ± 1 | 4 ± 2 | 5 ± 2* | ||||
| NA-MM | 2 ± 1 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | |||||
| MGUS | 1 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | |||||
| 9 | A-MM | 1 ± 1 | 2 ± 1 | 3 ± 2 | 4 ± 2 | 5 ± 3* | ||||
| NA-MM | 1 ± 1 | 1 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | |||||
| MGUS | 1 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | |||||
| 10 | A-MM | 2 ± 1 | 2 ± 1 | 3 ± 1 | 4 ± 2 | 6 ± 2* | ||||
| NA-MM | 1 ± 1 | 1 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | |||||
| MGUS | 1 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | |||||
| 11 | A-MM | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | ||||
| NA-MM | 1 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | |||||
| MGUS | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 2 ± 1 | |||||
| 12 | A-MM | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | ||||
| NA-MM | 1 ± 1 | 1 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | |||||
| MGUS | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | |||||
P < 0.001 vs. NA-MM and MGUS.
Acknowledgments
This work was supported in part by grants from the Fondazione italiana per la Lotta al Neuroblastoma, Genoa, and Ministero dell’Istruzione, dell’Università e della Ricerca (FIRB and local funds), Rome, Italy, to D.R.
References
- Ausprunk DH, Knighton DR, Folkman J. Differentiation of the vascular endothelium in the chick chorioallantois: a structural and autoradiographic study. Dev. Biol. 1974;38:237–247. doi: 10.1016/0012-1606(74)90004-9. [DOI] [PubMed] [Google Scholar]
- Billadeau D, Ahmann G, Greippe P, Van Ness B. The bone marrow of multiple myeloma patients contains B cell populations at different stages of differentiation that are clonally related to malignant plasma cells. J. Exp. Med. 1993;178:1023–1033. doi: 10.1084/jem.178.3.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbar B, Padro T, Leo R, Feldmann B, Kropff M, Mesters RM, et al. Vascular endothelial growth factor and interleukin-6 in paracrine tumor–stromal cell interactions in multiple myeloma. Blood. 2000;95:2630–2636. [PubMed] [Google Scholar]
- Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nature Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- Hideshima T, Chauhan D, Podar K, Schlossman RL, Richardson P, Anderson KC. Novel therapies targeting the myeloma cells and its bone marrow microenvironment. Semin. Oncol. 2001;28:607–612. doi: 10.1016/s0093-7754(01)90033-8. [DOI] [PubMed] [Google Scholar]
- Iwasaki T, Hamano T, Ogata A, Hashimoto N, Kitano M, Kakishita E. Clinical significance of vascular endothelial growth factor and hepatocyte growth factor in multiple myeloma. Br. J. Haematol. 2002;116:796–802. doi: 10.1046/j.0007-1048.2002.03364.x. [DOI] [PubMed] [Google Scholar]
- Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, et al. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur. J. Cancer. 2002;38:1133–1140. doi: 10.1016/s0959-8049(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kim ES, Serur A, Huang J, Manley CA, McCrudden KW, Frischer JS. Potent VEGF blockade causes regression of coopeted vessels in a model of neuroblastoma. Proc. Natl Acad. Sci. USA. 2002;99:11399–11404. doi: 10.1073/pnas.172398399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knighton D, Ausprunk D, Tapper D, Folkman J. Avascular and vascular phases of tumour growth in the chick embryo. Br. J. Cancer. 1977;35:347–356. doi: 10.1038/bjc.1977.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leene W, Duyzings MJM, Von Steeg G. Lymphoid stem cell identification in the developing thymus and bursa of Fabricius of the chick. Z. Zellforsch. 1973;136:521–533. doi: 10.1007/BF00307368. [DOI] [PubMed] [Google Scholar]
- Li CY, Shan S, Huang Q, Braun RD, Lanzen J, Hu K, et al. Initial stages of tumor cell-induced angiogenesis: evaluation via skin window chambers in rodent models. J. Natl Cancer Inst. 2000;92:143–147. doi: 10.1093/jnci/92.2.143. [DOI] [PubMed] [Google Scholar]
- Nico B, De Falco G, Vacca A, Roncali L, Ribatti D. In vivo absence of synergism between fibroblast growth factor-2 and vascular endothelial growth factor. J. Hematother. Stem Cell Res. 2001;10:905–912. doi: 10.1089/152581601317211006. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Mandriota SJ, Jeltsch M, Kumar M, Alitalo K. Vascular endothelial growth factor (VEGF)-C synergizes with basic fibroblast growth factor and VEGF in the induction of angiogenesis in vitro and alters endothelial cell extracellular proteolytic activity. J. Cell Physiol. 1998;177:439–452. doi: 10.1002/(SICI)1097-4652(199812)177:3<439::AID-JCP7>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Urbinati C, Nico B, Rusnati M, Roncali L, Presta M. Endogenous basic fibroblast growth factor is implicated in the vascularization of the chick embryo chorioallantoic membrane. Dev. Biol. 1995;170:39–49. doi: 10.1006/dbio.1995.1193. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Gualandris A, Bastaki M, Vacca A, Iurlaro M, Roncali L, et al. A new model for the study of angiogenesis and antiangiogenesis in the chick embryo chorioallantoic membrane (CAM): the gelatin sponge/CAM assay. J. Vasc. Res. 1997;34:455–463. doi: 10.1159/000159256. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Gualandris A, Belleri M, Massardi L, Nico B, Rusnati M, et al. Alterations of blood vessel development by endothelial cells overexpressing fibroblast growth factor-2. J. Pathol. 1999;189:590–599. doi: 10.1002/(SICI)1096-9896(199912)189:4<590::AID-PATH461>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Morbidelli L, Donnini S, Ziche M, Vacca A, et al. Cell-mediated delivery of fibroblast growth factor-2 and vascular endothelial growth factor onto the chick chorioallantoic membrane: endothelial fenestrations and angiogenesis. J. Vasc. Res. 2001a;38:389–397. doi: 10.1159/000051070. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Nico B, Vacca A, Roncali L, Burri PH, Djonov V. Chorioallantoic membrane capillary bed: a useful target for studying angiogenesis and anti-angiogenesis in vivo. Anat. Rec. 2001b;264:317–324. doi: 10.1002/ar.10021. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Cruz A, Nico B, Favier J, Vacca A, Corsi P, et al. In situ hybridization and immunogold localization of vascular endothelial growth factor receptor-2 in the pericytes of the chick embryo chorioallantoic membrane. Cytokine. 2002;17:262–265. doi: 10.1006/cyto.2002.1002. [DOI] [PubMed] [Google Scholar]
- Ribatti D, Vacca A, Dammacco F, English D. Angiogenesis and anti-angiogenesis in haematological malignancies. J. Hematother. Stem Cell Res. 2003;12:11–22. doi: 10.1089/152581603321210091. [DOI] [PubMed] [Google Scholar]
- Sezer O, Jacob C, Eucker J, Niemoller K, Gatz F, Wernecke K, et al. Serum levels of the angiogenic cytokines basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF) and hepatocyte growth factor (HGF) in multiple myeloma. Eur. J. Haematol. 2001;66:83–88. doi: 10.1034/j.1600-0609.2001.00348.x. [DOI] [PubMed] [Google Scholar]
- Solomon JB. Fetal and Neonatal Immunology. New York: Frontiers of Biology; 1971. Lymphocytopoiesis and ontogeny of defined immunity in birds. Monograph 20. [Google Scholar]
- Vacca A, Ribatti D, Roncali L, Ranieri G, Serio G, Silvestris F, et al. Bone marrow angiogenesis and progression in multiple myeloma. Br. J. Haematol. 1994;87:503–508. doi: 10.1111/j.1365-2141.1994.tb08304.x. [DOI] [PubMed] [Google Scholar]
- Vacca A, Ribatti D, Presta M, Minischetti M, Iurlaro M, Ria R, Albini A, Bussolino F, Dammacco F. Bone marrow neovascularization, plasma cell angiogenic potential and matrix metalloproteinase-2 secretion parallel progression of human multiple myeloma. Blood. 1999;93:3064–3073. [PubMed] [Google Scholar]
- Wedge SR, Ogilvie DJ, Dukes M, Kenderw J, Chester R, Jackson JA, et al. ZD 6474 inhibits vascular endothelial growth factor signaling, angiogenesis, and tumor growth following oral administration. Cancer Res. 2002;62:4645–4655. [PubMed] [Google Scholar]