Abstract
The first seven somites, the rhombomeres, and the pharyngeal arches were reassessed in 145 serially sectioned human embryos of stages 9–23, 22 of which were controlled by precise graphic reconstructions. Segmentation begins in the neuromeres, somites and aortic arches at stage 9. The following new observations are presented. (1) The first somite in the human, unlike that of the chick, is neither reduced in size nor different in structure, and it possesses sclerotome, somitocoel and dermatomyotome. (2) Somites 1–4, unlike those of the chick, are related to rhombomere 8 (rather than 7 and 8) and are caudal to pharyngeal arch 4 (rather than in line with 3 and 4). (3) Occipital segment 4 resembles a developing vertebra more than do segments 1–3. (4) The development of the basioccipital resembles that of the first two cervical vertebrae in that medial and lateral components arise in a manner that differs from that in the rest of the vertebral column. (5) The two groups of somites, occipital 1–4 and cervical 5–7, each form a median skeletal mass. (6) An ‘S-shaped head/trunk interface’, described for the chick and unjustifiably for the mouse, was not found because it is not compatible with the topographical development of the otic primordium and somite 1, between which neural crest migrates without hindrance in mammals. (7) Occipital segmentation and related features are documented by photomicrographs and graphic interpretations for the first time in the human. It is confirmed that the first somite, unlike that of the chick, is separated from the otic primordium by a distance, although the otic anlage undergoes a relative shift caudally. The important, although frequently neglected, distinction between lateral and medial components is emphasized. Laterally, sclerotomes 3 and 4 delineate the hypoglossal foramen, 4 gives rise to the exoccipital and participates in the occipital condyle, 5 forms the posterior arch of the atlas and 6 provides the neural arch of the axis, which is greater in height than the arches of the other cervical vertebrae. Medially, the perinotochord and migrated sclerotomic cells give rise to the basioccipital as well as to the vertebral centra, including the tripartite column of the axis. Registration between (1) the somites and (2) the occipital and cervical medial segments becomes interrupted by the special development of the axis, the three components of which come to occupy the height of only 2½ segments.
Keywords: head/trunk junction, human embryo, pharyngeal arches, rhombomeres, somites
Introduction
Segmentation, ‘one of the most crucial prerequisites’ in vertebrate development (according to Stolte et al. 2001), is particularly noticeable in the nervous system, including the neural crest and especially the rhombomeres, as well as in the somites, pharyngeal and aortic arches.
Although many studies of the various types of segmentation are available (e.g. Christ et al. 1988), it needs to be stressed that ‘mammalian embryos have important differences from other vertebrate embryos, and it is not always safe to extrapolate from the results of experimental morphogenetic studies in avian or amphibian embryos to make functional interpretation of mammalian development’ (Ruberte et al. 1997). Furthermore, ‘one cannot extrapolate murine ontogeny to human development’ (Gérard et al. 1995). Important differences occur, for example, with regard to: (1) the prechordal plate and the notochordal process (Müller & O'Rahilly, 2003); (2) the absence of neural crest in the human forebrain, although present in the rat (Bartelmez, 1960); and (3) the different pattern of closure of the neural folds (O'Rahilly & Müller, 2002). It is also important to keep in mind that until now ‘molecular genetics has not solved the problem of head segmentation. We still do not understand whether pharyngeal pouches, head muscles derived from paraxial mesoderm, neural-crest derived pharyngeal arches and hindbrain segmentation are all parts of a single segmental pattern or the result of two or more processes’ (Northcutt, 1996).
Hox genes confer segmental identity and their ‘expression starts sequentially in the primitive streak, and forms overlapping and time- and site-specific expression domains along the body axis’ (Li & Shiota, 1999). A high degree of conservation of the pharyngeal Hox code has been found in a study of various groups of Hox genes that have been shown to be expressed in human rhombomeres and pharyngeal arches (Vieille-Grosjean et al. 1997).
The present study was undertaken: (1) to elucidate further the major types of segmentation in the human embryo, particularly somitic, rhombomeric, and pharyngeal; (2) to clarify the relationships between them; (3) to re-examine the development of the occipital component and to assess the head/trunk junction in the human; (4) to provide examples of photomicrographs and graphic reconstructions from rarely seen sections of early human embryos; and (5) to compare the results with those available for other species.
Materials and methods
Serial sections of 145 human embryos and three fetuses from the Carnegie Collection were studied. They were in various planes and concerned stages 9 (five embryos), 10 (10), 11 (14), 12 (22), 13 (22), 14 (33), 15 (seven), 16 (eight), 17 (seven), 18 (two),19 (three), 20 (three) 22 (two), 23 (seven) and three fetuses of 32, 33 and 39 mm. Nineteen were impregnated with silver, and 23 graphic reconstructions were prepared by the authors, using the point-plotting technique (Gaunt & Gaunt, 1978). Sagittal planes proved to be particularly important for evaluating differences between central and peripheral areas of somitic derivatives. The internationally accepted Carnegie stages were used and have been described in detail elsewhere (O'Rahilly & Müller, 1987). It should be stressed, however, that neither ages nor embryonic lengths are stages, that the term ‘gestational age’ is devoid of scientific value (O'Rahilly & Müller, 2000) and that the Carnegie stages assigned to embryos in the literature are not always correct. The technical problems in counting somites have been studied already (O'Rahilly & Müller, 2003).
Definitions and nomenclature
The term ‘segment’ is used in this study for each of a series of structural units arranged repetitively, e.g. somites and neuromeres.
Somitomeres are clusters of cells in the presomitic mesenchyme of certain species, chiefly the mouse and the chick as studied by scanning electron microscopy (Meier & Tam, 1982). They represent a ‘segmental prepattern’ of the cranial mesenchyme (Trainor et al. 1994). In the absence of similar studies, their presence in the human can as yet be neither affirmed nor denied.
Occipital somites are those situated rostral to the first cervical nerve, and the first somite is defined by its position caudal to the vagal neural crest or ganglion (O'Rahilly & Müller, 1984). A sclerotome is the mesenchymal region between two intersegmental arteries, and this paraxial mesenchyme is situated laterally. Medially, the perinotochord (or perinotochordal cellular sheath) consists at first of axial mesenchyme into which sclerotomic mesenchyme then migrates. The segmented units of the perinotochord are called central segments in the occipital region, centra in the future vertebral column, and the first three cervical centra are designated X, Y and Z.
The basioccipital contains the notochord and reaches the spheno-occipital junction. Laterally, the exoccipital includes at least the greater portion of the occipital condyle.
Hypochordal bows (or arches) are mesenchymal (later cartilaginous and osseous) condensations ventral to the notochord.
The neuromeres of the human embryo have been described and illustrated in detail (Müller & O'Rahilly, 1997). Rhombomeres are the neuromeres at the level of the hindbrain, and initially they are four in number, designated A–D. These four rhombomeres serve as very useful landmarks in the human embryo. Rhombomere B was defined by Bartelmez (1923) as the neuroectoderm opposite the otic plates. In the mouse embryo corresponding to stage 9, it is more clearly delineated by a preotic sulcus rostrally and by a postotic sulcus caudally (prorhombomere B, Ruberte et al. 1997). In the human the four rhombomeres soon become subdivided into eight.
The term myelomere, used anatomically for a subdivision of the spinal cord to which a set of spinal roots is attached, refers developmentally to one of periodic undulations (in the chick) produced passively by segmentation in the somitic mesoderm adjacent to the spinal cord (Keynes & Lumsden, 1990; Lim et al. 1991).
The ectodermal ring, named by Blechschmidt (1948) and described in detail by O'Rahilly & Müller (1985), consists of thickened surface ectoderm that extends from the oropharyngeal membrane to the cloacal membrane.
The hypoglossal cell cord is composed of hypoglossal neural crest and myoblasts from the occipital somites (O'Rahilly & Müller, 1984).
Pharyngeal arches, which give rise to gills (branchiae) in aquatic vertebrates, develop into varied structures initially associated with the pharyngeal region in mammals, in which branchial structures do not appear and hence that term should not be used in mammalian development.
Results
Stage 9 (c. 1.5–2.5 mm)
This stage is defined by the first appearance of somites, of which 1–3 pairs are present. In the first somitic pair (Fig. 1A) the cells have a dorsoventral orientation of their longitudinal axes, and a somitocoel is about to begin its appearance. The neural plate already shows four primary rhombomeres (Fig. 2A). The first somite is related to rhombomere D, which corresponds to future Rh. 8. Between the otic plate (related to rhombomere B) and the first somite is rhombomere C, which corresponds to future Rh. 5, 6, 7, i.e. there is a gap between the otic disc and the first occipital somite (Figs 2A and 3A). Neural crest cells are present rostral to the otic primordium. The first and second aortic arches begin to develop, but intersegmental arteries are not yet present.
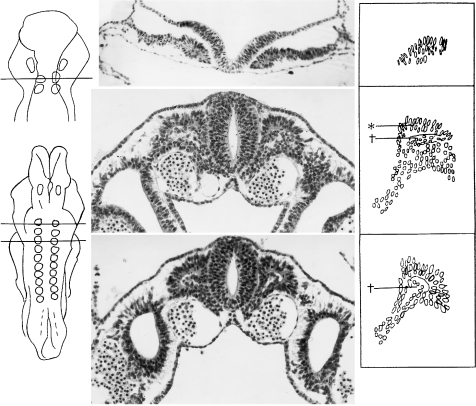
Fig. 1.
Early formation of somites. (A) Transverse section of a stage 9 embryo (Da 1, Ludwig, 1928) with two somitic pairs. Somite 1, shown here, is characterized by epithelized cells derived from the lateral plate. The longitudinal axes of the somitic cells are orientated dorsoventrally. The neural folds are widely open. (B) Transverse section at stage 10 in an embryo of 10 somites (No. 5074). The first somite is characterized by a dermatomyotome (asterisk), a somitocoel (dagger) containing rounded mesenchymal cells, and a long tail of sclerotomic cells. In areas such as that represented, the neural folds are fused and are forming the neural tube. Neural crest cells are visible between the surface ectoderm and the neural ectoderm. (C) Somite 3 of the same embryo as in B is similar in size to somite 1 and possesses similar features.
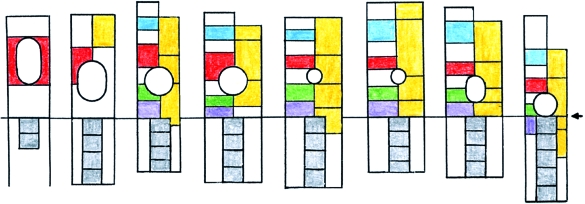
Fig. 2.
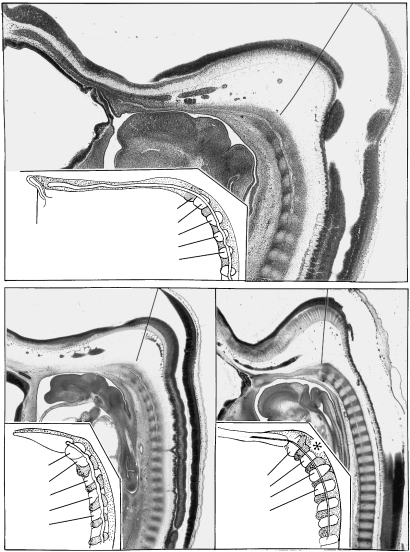
The relative caudal shift of the otic primordium (Ot.) in relation to the rhombomeres, at all times separated in A–G from the first somite. The base line is at the commencement of somite 1. A, B, D, E, F and G are at stages 9, 10, 11, 12, 13 and 14, respectively. The height of the rhombomeres is the mean found in 54 human embryos at these stages (Table 1). In the upper half of each column from B to H, rhombomeres are represented at the left and pharyngeal arches are indicated at the right. Colour scheme: Rhombomere 2, blue; B and 4, vermilion; 6, green; 7, mauve; 8 (beside the somites), white. Pharyngeal arches, yellow. Somites, grey. For comparison, C represents a mouse at approximately Carnegie stages 10, 11 (after data in Mahmood et al. 1996). H represents a chick embryo of HH 18 (after Lumsden, 1990), at approximately Carnegie stages 13, 14. Here, by contrast, no space is found between the otic primordium and somite 1 (arrow).
Fig. 3.
Reconstructions showing the interrelationship of the otic primordium and the first somite (both stippled) and the distance between them at stages 9–12 (A–E). Horizontal lines are shown connecting the caudal limit of the otic primordium in each drawing and the rostral limit of the first somite. These emphasize the developmental increase of the glossopharyngeal region in contrast with the situation in the chick (Fig. 2H). (A) Stage 9 (No. 1878, three somites on the left, two on the right). The first somite is separated from the otic disc by rhombomere C and the future glossopharyngeal-vagal area (between the horizontal lines). (B) Stage 10 (No. 4216, eight somites). Glossopharyngeal/vagal neural crest (not shown here) is present between the otic disc and the first somite. (C) Stage 10 (No. 5074, 10 somites). The oto-somitic distance is increasing. (D) Stage 11 (No. 6344, 13 somites). Rhombomeres 5, 6 and 7 become evident between the otic pit and the first somite. (E) Stage 12 (No. 5923, 28 somites). The first somite is still visible in some embryos. Trigeminal, facial-acoustic and vagal ganglia are shown.
Stage 10 (c. 2–3.5 mm; probably c. 4 post-fertilizational weeks)
The first somite is now approximately the same size as the following three. The dermatomyotome, which consists (in cross-section) of longitudinally arranged elements, has appeared (Fig. 1B,C). The somitocoel is accumulating rounded mesenchymal cells. Ventrally the sclerotomic cells appear to be spreading towards the notochord. The otic disc is related to both Rh. A and Rh. B. Either one or two pharyngeal arches have appeared (Fig. 2B) and consist mainly of paraxial mesenchyme. Trigeminal neural crest is beginning to spread into the maxillary part of pharyngeal arch 1. Facial, glossopharyngeal, vagal and hypoglossal neural crest is present. Intersegmental arteries are developing.
Stage 11 (c. 2.5–4.5 mm)
The first somite is now smaller than those following (Fig. 4B,C) and the sclerotomic material seems to be reduced. The somite lies immediately caudal to the vagal–accessory neural crest. A somitocoel is not evident. The appearance of the primordia of cranial ganglia establishes subunits in the four primary rhombomeres: Rh. A contains Rh. 1–3 with the trigeminal neural crest related to Rh. 2; Rh. B is associated with ganglia 7 and 8, and is at the level of Rh. 4; Rh. C corresponds to future Rh. 5, 6 and 7; and Rh. D is Rh. 8. The otic pit occupies part of Rh. 4 and the whole of Rh. 5 (Fig. 2D). In the more advanced embryos the first three pharyngeal arches are present, and the otic pit is related to arches 2 and 3 (Fig. 2D). The pharyngeal arches, especially 2 and 3, consist mainly of paraxial mesenchyme. Trigeminal neural crest continues to migrate and facial neural crest moves into pharyngeal arch 2. Hypoglossal neural crest is present at the level of somites 2–4. Spinal neural crest reaches to somite 9 in the least advanced embryos (No. 4529), and to somites 13 and 14 in the more advanced embryos (e.g. No. 2053). Evidence of epipharyngeal discs (neurogenic placodes), which at this stage are part of the ectodermal ring, can be observed at the ventral-most level of pharyngeal arches 2 and 3, although cells are not yet given off. Short, stumpy intersegmental arteries are present between the first four somites.
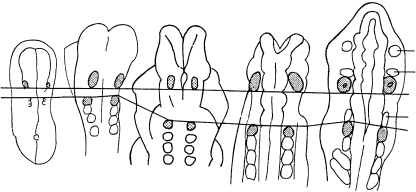
Fig. 4.
Changes in the proportions of occipital somites 1–4 in stages 10–13 shown in left lateral views. The first somites that possess completely typical features are stippled in A–C. (A) Stage 10 (No. 5074). The size of the first somite is comparable to that of the other occipital somites. (B) Stage 11 (Nos. 6784, 7665 and 2053). The first somite is the smallest of the group. Ganglia 9 and 10 are indicated. (C) Stage 12 (Nos. 7853, 8943 and 6097). The first somite continues to change and no longer lies close to the vagal ganglion, because of the developing neural crest of the accessory nerve. The glossopharyngeal ganglion (not shown here) precedes the vagal. (D) Stage 13 (Nos. 8002, 836 and 1954A). The dermatomes are hatched horizontally and the vagal and accessory nerves are represented.
Stage 12 (c. 3–5 mm)
The reduced size of the first somite is noticeable (Figs 4C and 5A). The dermatomyotomes of somites 1–4 are still clearly visible (Fig. 6). Innervation by the hypoglossal nerve is detectable in somites 2–4. The sclerotomic mesenchyme reaches the notochord in a relatively advanced embryo (Fig. 6). The total set of cranial ganglia is now present, as well as the complete set of rhombomeres 1–8 (Fig. 5) and pharyngeal arches 1–4 (Fig. 6). The otic vesicle is related to Rh. 5 (Fig. 2E). Neural crest cells are present in all pharyngeal arches. Differences in density make it possible to distinguish cranial ganglia, neural crest and paraxial mesenchyme of arches 1–4 (Figs 6 and 7). Activity in the epipharyngeal discs is particularly noticeable in pharyngeal arch 1 (Fig. 5B), and the cells of the discs join the neural crest of the trigeminal, facial and vagal ganglia, and form their distal portions. Glossopharyngeal and vagal neural crest occupy pharyngeal arch 3, and the emigrated material of that arch reaches into the conotruncal region of the heart. The neural crest cells in arch 4, which are mainly material of the hypoglossal cell cord, migrate to the laryngeal region and in stage 15 to the tongue. Intersegmental arteries are present between somites 2 and 3, and between 3 and 4.
Fig. 5.
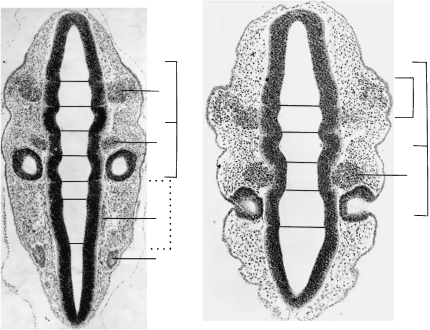
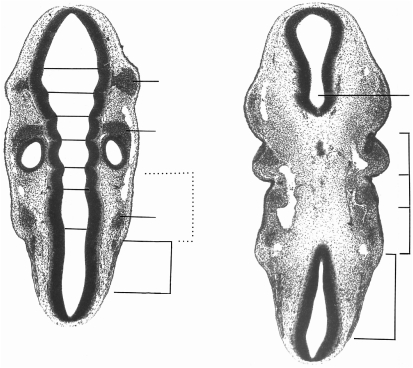
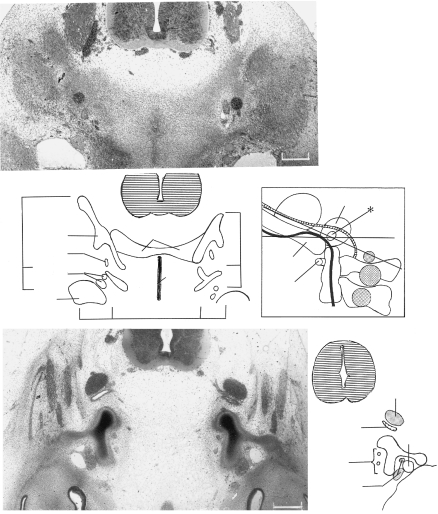
Sections through the rhombencephalon of two embryos at stage 12, each possessing 25 somites. The thickened surface ectoderm of an epipharyngeal disc is best visible in pharyngeal arch 1. (A) (No. 7852). Somite 1 is related to rhombomere 8 and is at some distance (d) from the otic vesicle. Cf. Fig. 3(D). (B) (No. 6097). As in A, the otic vesicle is seen to be related mainly to rhombomere 5. Ph., pharyngeal arches. Rh., rhombomere. The cranial ganglia are numbered.
Fig. 6.
Another section at stage 12 (No. 6097) at a more ventral level than that in Fig. 5(B). Three of the four pharyngeal arches with their contained aortic arches are evident. Sclerotomic mesenchyme appears to be spreading ventromedially from somite 2 towards the notochord (inset). A perinotochord is not yet clear. Hypoglossal neural crest is distinct between the hindbrain and the sclerotome. Ao., aortic arch. N.cr., neural crest. Ph., pharyngeal arches.
Fig. 7.
Two sections at stage 13 (No. 836). The dermatomes of somites 1–4 are still recognizable. (A) The first eight rhombomeres are distinct. (B) Four pharyngeal arches have developed and three are visible here. d, oto-somitic distance. M, mesencephalon. Ph., pharyngeal arches. Rh., rhombomeres.
Stage 13 (c. 4–6 mm; c. 4½ post-fertilizational weeks)
Some thin dermatomyotomes are still recognizable (Fig. 7), although they had disappeared in 12 of 18 embryos. Myotomes, which are present in all four somites, fuse but are separated by septa. They continue into the descending hypoglossal cell cord, and hypoglossal nerve roots can be followed to them. Dense and loose zones begin to develop in occipital sclerotome 4 and in the sclerotomes of the cervical region (Fig. 8). The perinotochord of the head is developing in a caudorostral direction, and that of the cervical region is appearing at the same time. Only a slight difference in density is noticeable between the rostral and caudal parts of the cervical segments. The otic vesicle is related to Rh. 5 and to pharyngeal arches 2 and 3 (Fig. 2F). A total of four pharyngeal arches is present, and they consist of (1) paraxial mesenchyme, (2) neural crest, (3) cells given off from the epipharyngeal discs and (4) hypoglossal cell cord in arch 4. Clear signs of the accessory nerve are present (Fig. 4D). All cervical ganglia are evident.
Fig. 8.
Sagittal section at stage 13 (No. 9297). The sclerotome of somite 4 and the hypoglossal artery mark the junction between the rhombencephalon and the spinal cord. Sclerotome 4 is the first in the occipital region to show a dense zone, although similarly appearing dense zones are visible also in some of the cervical sclerotomes. Spinal ganglia are developing in a rostrocaudal direction. Di., diencephalon. M, mesencephalon. Tel., telencephalon. Asterisk, cerebrospinal junction.
Stage 14 (c. 5–7 mm)
The dermatomyotomes have disappeared in 28 of 33 embryos. Dense zones appear in occipital sclerotomes 3 and 2, although that of 3 is not constant. The three rostral sclerotomes and the perinotochord begin to fuse and form a unit that is separated from sclerotome 4 by a cleft through which the caudal-most thick root of the hypoglossal nerve passes, together with the hypoglossal artery. The cervical perinotochord has thickened considerably (No. 8999). The central segments are detectable in transverse section (Nos. 6502 and 8553), but they are only faintly visible in median section. The otic vesicle is related to Rh. 5 and 6, and to pharyngeal arches 2 and 3 (Fig. 2G). Fibres of the accessory nerve are present, and the hypoglossal nerve bends rostrally towards the future tongue.
Stages 15–22 (c. 5–8 weeks) and early fetal period
In the central occipital region only the perinotochord of segment 4 has developed a dense zone (asterisk in Fig. 9A) and it reaches the median plane in stage 17 (Figs 9A and 10A). In the next two stages segments 1–4 form a unit (Figs 9C and 11A) that extends rostrally towards the adenohypophysis. Peripherally the sclerotomic dense zones can clearly be distinguished up to stage 17 (Fig. 10A). Later, that of occipital sclerotome 4 develops into the cartilaginous exoccipital (Fig. 10B). In stage 19 the dense material of the central segment ventral to the notochord represents a hypochordal bow (Fig. 11A), which, together with sclerotome 4, is soon transformed into cartilage and is visible from the ventral side of the basioccipital as a transverse bar. In one of the embryos a second bar-like protuberance was present on the ventral aspect of the basioccipital, in line with the paired peripheral dense zones between the rostral and caudal nerve roots of the hypoglossal nerve (Fig. 12A). This is at the level of central segment 3.
Fig. 9.
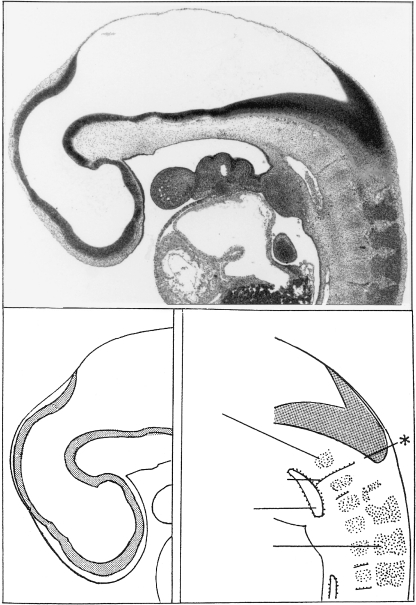
Sagittal sections of embryos of stages 17 and 18. (A) Nearly median section through the head at stage 17 (No. 8789). The notochord almost reaches the adenohypophysis, and it, as well as the occipital and cervical centra, is embedded in the perinotochord. (B) Sagittal section of another embryo of stage 17 (No. 1771) in which fusion within the occipital primordium has almost taken place, except (as shown in the drawing) between sclerotome 4 and the remainder of the basioccipital (at the left). (C) In stage 18 (No. 144) the central parts of the basioccipital are completely fused and appear as a morphological unit. Segment 4, the parts of the axis (X, Y, Z), and cervical vertebra 3 are indicated. A diagonal line in the upper part of each section represents the occipitocervical junction.
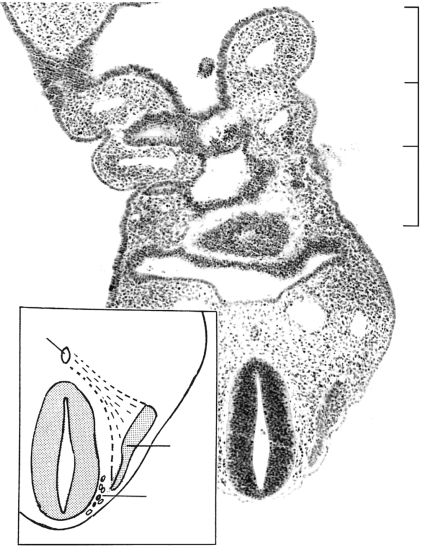
Fig. 10.
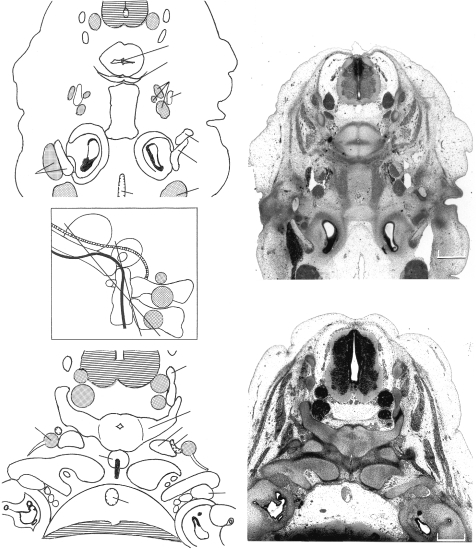
The contributions of sclerotomes 3 and 4 to the exoccipital. (A) In stage 17 (No. 8101) mesenchymal arches that developed in stage 15 are still present laterally. The dense zone caudal to the hypoglossal nerve belongs to sclerotome 4, whereas that rostral to that nerve pertains to sclerotome 3. Both will participate in the formation of the hypoglossal canal. The material of sclerotome 4 is continuous with the dense zone of central segment 4. Some of the lateral dense zones (not indicated in the key) represent muscular primordia. (B) Cartilaginous derivatives at stage 20 (No. 7274). The medially directed hypoglossal foramen (asterisk) and two stems of the hypoglossal nerve are visible within it. Rostrally, the exoccipital fuses with the otic capsule, thereby forming the jugular foramen for the internal jugular vein and cranial nerves 9 and 10. The posterior arch of the atlas and the vertebral artery are detectable. (C) The planes of sections A and B projected onto a reconstruction at stage 23. The notochord is shown in black and the spinal ganglia are stippled. Veins other than the internal jugular are omitted. The course of the basilar and vertebral arteries is shown as a cross-hatched band. Bars: A, 0.22 mm; B, 0.4 mm. Basiocc., basioccipital; CV1, cervical vertebra 1 (atlas); CV2, axis; Exocc., exoccipital; Ext.jug.v., external jugular vein; Int.jug.v., internal jugular vein; Lat., lateral, Not., notochord; Otic, otic capsule; Scl., sclerotomes; Vert.a., vertebral artery; X, Y, Z, components of column of axis; 7, 9, 10, 11, 12, cranial nerves; asterisk, hypoglossal foramen.
Fig. 11.
Derivatives of somites and central segments 3–7. (A) In stage 19 (No. 9113) a hypochordal arch at the level of central segment 5 can be seen. It represents the primordium of the anterior arch of the atlas. The cartilaginous elements Y and Z caudal to it are from central segments 6 and 7. Part of the basioccipital, including mainly central segments 4 and 3, is visible. (B) In stage 21 (No. 8553) the occipital condyles are seen to be derived mainly from central segment 4 and from the exoccipital. X, Y and Z are from central segments 5–7, and they form the central column of the axis. Moreover, the neural arch of that vertebra is derived from both Y and Z, and it is noticeably voluminous. (C) The planes of sections A and B projected onto a reconstruction at stage 23. The notochord is shown in black and the ganglia are stippled. Veins other than the internal jugular are omitted. The course of the basilar and vertebral arteries is shown as a cross-hatched band. Bars: A, 0.63 mm; B, 0.55 mm. Abbreviations in addition to those used in Fig. 10: Bas.a., basilar artery; Styloid, styloid process; 5, trigeminal ganglion; 7, geniculate ganglion.
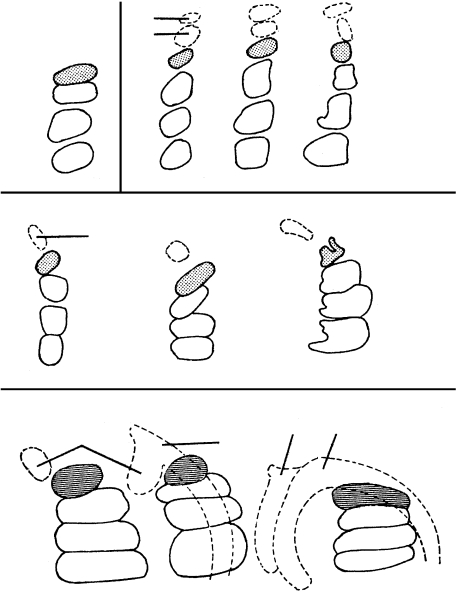
Fig. 12.
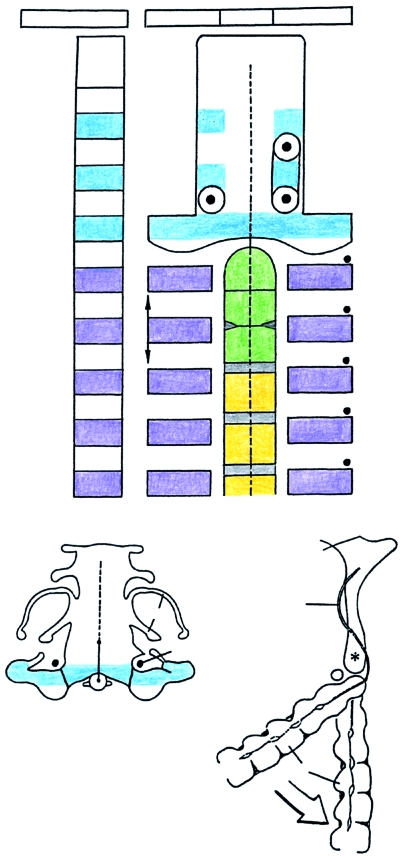
Summary of the development of the craniovertebral region. (A) Schematic view of the sclerotomes (left-hand column). To the right, the sclerotomic derivatives and the perinotochordal components are represented. The vertical arrows on segment 6 are to indicate the greater height of the neural arch of the axis. Colour scheme: dense zones of sclerotomes 2–4 and exoccipital, blue; dense zones of sclerotomes 5–9 and the corresponding neural arches, mauve; tripartite pillar (XYZ) of axis, green; cervical centra 3–5, yellow; intervertebral discs, grey; hypoglossal and cervical nerves 1–5, black dots. CV3, cervical vertebra 3. H, site of a hypochordal bow. (H) shows a variation. (B) The basioccipital cartilage and the dens at stage 23 (No. 9226) traced from a photomicrograph. The portion of the notochord that is not visible is shown by a dotted line. The exoccipital is in blue. Ot., otic capsule. 12, hypoglossal nerve. (C) A median section at stage 23 (Nos. 5422 and 9226 combined) showing the winding course of the notochord through the base of the skull. The cervical column, which contributes to the craniovertebral angle, has been opened (white arrow) merely to allow a better comparison with A. CV3, cervical vertebra 3. Asterisk shows the portion of the basioccipital corresponding to central segment 4. Based on graphic reconstructions by the authors.
In the central cervical region dense and loose zones are seen at first in regular succession (Fig. 9A–C). The dense zones between X, Y and Z, and between all the other centra begin to disappear at stage 20. They develop into two portions: (1) a central part surrounding the notochord and consisting of special embryonic cells that connect all the vertebrae into a continuous cellular column; (2) a peripheral part, the future anulus fibrosus, which is missing between X and Y (Figs 9 and 12A) and seems to be slightly reduced between Y and Z (Fig. 12A). Nevertheless, the three parts of the axial column of cervical vertebra 2 are clearly recognizable and of equal size well into the fetal period (Figs 9 and 12). The sclerotomic dense and loose zones follow a regular pattern (Fig. 12A). They develop into the posterior arch of the atlas at the level of segment 5, and into the neural arches of cervical vertebra 2 and of the following vertebrae. The neural arch of the axis is connected with both Y and Z (Fig. 11B). The anterior arch of the atlas seems to develop from the hypochordal bow of segment 5 (Fig. 11A).
Discussion
Previous investigations
The most significant study is that of Sensenig (1957) because it involved staged human embryos. Nevertheless, it did not include: (1) reconstructions and exact levels of sections, (2) segmentation of the basioccipital, (3) the important distinction between lateral and medial components, and (4) recognition of the first two intersegmental arteries. In addition, fundamental misinterpretations occur: (1) the intercalation of a half-segment between occipital and cervical regions, (2) in the development of the atlas and axis, (3) identification of ‘occipital’ or ‘hypoglossal’ ganglia (of Froriep) and (4) ‘a very short rib rudiment’ that is probably the exoccipital.
A landmark in research on the development of the vertebral column is the experimental study of thoracic vertebrae in the mouse by Dalgleish (1985), who showed the importance of the perinotochord in the formation of the centra, and its relationship to the lateral sclerotomic material in the establishment of the neural arches and ribs.
Development of segmentation and somitogenesis
Segmentation arises very suddenly in the elongated human embryo of stage 9, when primary neuromeres (including the primary rhombomeres), three pairs of somites and the first aortic arches appear, as does also the otic disc (Müller & O'Rahilly, 1983). At stage 10 the cranial neural crest (already presaged at the previous stage), the occipital sclerotomes, intersegmental arteries and the first pharyngeal arches make their entry. Within the next two stages the spinal neural crest, myotomes and the hypoglossal nerve are detectable. All four pharyngeal arches take little more than a day to appear, aortic arches 1–4 and 6 are noticeable within a week and the 16 neuromeres (Müller & O'Rahilly, 1997) are present within about 11 days.
The formation of somites has been shown in the chick to be an extremely complex process. It is not ‘a simple periodic slicing of tissue blocks’ but rather a process whereby cells move across a presumptive somitic frontier and ‘violate gene expression boundaries’ thought to be correlated with the site of the somitic boundary (Kulesa & Fraser, 2002).
Pharyngeal arches, occipital somites and the otic primordium
A relationship develops between the occipital somites and pharyngeal arches 3 and 4, as shown by the emigration of hypoglossal neural crest and cells of the hypoglossal cord into those arches, chiefly at stage 14 (O'Rahilly & Müller, 1984). In addition, migration of hypoglossal neural crest into the conotruncal region of the heart of the rat has been reported (Fukiishi & Morriss-Kay, 1993). Topographical relationships of the pharyngeal arches to the occipital somites, to the rhombomeres and to the otic primordium are related to developmental stages (Fig. 2). It can be shown (Table 1) that Rh. A increases in length, Rh. B decreases to approximately one-fifth, Rh. C increases less than A, and Rh. D becomes slightly shorter.
Table 1.
Lengths of rhombomeres as percentages of rhombencephalon in 54 embryos
| Stage | 9 | 10 | 11 | 12 | 13 | 14 |
|---|---|---|---|---|---|---|
| N | 1 | 10 | 14 | 15 | 10 | 4 |
| Rh. A | 12.5 | 12 | 15 | 15 | 21.8 | 20.8 |
| Rh. B | 16.7 | 12 | 13 | 5.9 | 4.4 | 3.8 |
| Rh. C | 14.6 | 13 | 21 | 18.9 | 17.8 | 16.4 |
| Rh. D | 25 | 24 | 29 | 27.5 | 21.5 | 17.9 |
| Rhombencephalon | ||||||
| as % of brain | 68.8 | 61 | 78 | 67.3 | 65.5 | 58.9 |
The otic disc of the human appears as a thickening in stage 9 (1–3 somites), and its relationship to the neuromeres and pharyngeal arches changes in subsequent stages (Fig. 2). In the mouse, however, the otic primordium is seen only when 8–10 somites have developed (Mahmood et al. 1996) and at that time it occupies a site, which, in the human, is reached only at stage 12. The characteristic and important distance between the otic primordium and somite 1 (Fig. 2) is found also in the mouse (Fukiishi & Morriss-Kay, 1993), whereas such a space is not present in the chick (Fig. 2G).
Somite 1
The first epithelized cellular group within the paraxial mesenchyme at stage 9 is somite 1 (Fig. 1A) because it is not preceded even in later stages by another somite. Its cells are orientated with their axes dorsoventrally and the formation of a somitocoel is imminent. At stage 10 the first somite in the human possesses all the characteristics of a fully developed somite, namely sclerotome, somitocoel with mesenchymal cells, and dermatomyotome (Fig. 1B,C). The claim that the first somite in stage 10 ‘is much smaller’ than the following somites (Sensenig, 1957) is incorrect (Fig. 4A). The myotomic elements begin to change during later stages (mainly 12 and 13) into material of the hypoglossal cell cord (Table 2), which moves ventrally and participates in the formation of the lingual muscles (O'Rahilly & Müller, 1984). After this so-called partial ‘disappearance’ (Arey, 1938) of the first somite, somites 2–4 are defined by the originally segmental roots of the hypoglossal nerve and by the intersegmental arteries.
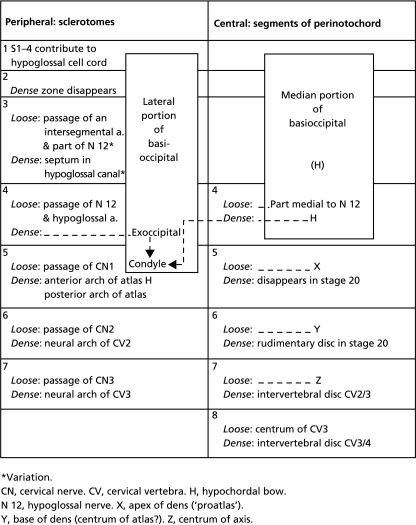
Table 2.
The derivatives of the loose and dense zones in the occipitocervical region
Variation.
CN, cervical nerve. CV, cervical vertebra. H, hypochordal bow.
N 12, hypoglossal nerve, X, apex of dens (‘proatlas’).
Y, base of dens (centrum of atlas?), Z, centrum of axis.
The first somite in the human shows a number of significant differences from that in the chick: (1) it develops at a distance from the otic primordium, in the chick close to it; (2) it lies caudal to the vagal crest/ganglion, whereas it is on the same plane in the chick (Fig. 2C); (3) it occupies the region caudal to pharyngeal arch 3, but to arches 3, 4 in the chick; (4) it is of the same size as the following somite and does not disappear, whereas in the chick it is unclear whether somite 1 disappears (Kuratani, 1997) or behaves as the other somites do (Huang et al. 1997); and (5) the derivatives of somite 1 in the human (O'Rahilly & Müller, 1984; Müller & O'Rahilly, 1994) seem to be less extensive than those proposed for the chick (Couly et al. 1993; Huang et al. 1997). The first sclerotome possesses no dense and loose zones in the human and the same is true also of the chick (Lim et al. 1987; Huang et al. 1997).
Furthermore, in studies of lectin-binding patterns in the paraxial mesenchyme it has been found that in the human ‘PNA receptors in contrast to those of the chick embryo, are in all probability not involved in the control or regulation of axonal outgrowth and neural crest migration’ (Götz et al. 1993). With regard to central segmentation, the ‘onset of gene expression for type II collagen in the human embryo takes place concomitantly with the first signs of mesodermal segmentation’ at stage 14 (Krengel et al. 1996).
Somites 1–4
Somite 4 possesses a complete dense zone caudally in its sclerotome, for the exoccipital, and the main stem of the hypoglossal nerve enters its loose zone (Table 2). Furthermore, up to stage 17, it possesses dense and loose zones also medially (Figs 9A and 10A). In these respects segment 4 resembles a developing vertebra more than do the three more rostrally situated occipital segments of the human (Fig. 12). A significant landmark is the hypoglossal artery, which accompanies the hypoglossal nerve and is situated between occipital somites/sclerotomes 3 and 4. Hence the number of occipital somites in the human, as shown in carefully prepared reconstructions, is four (Table 3).
Table 3.
Examples of the number of occipital somites given in the literature
Sensenig includes the literature from 1883 to 1944, in which ranges of 2–5 in the human and 3–6 in various mammals are claimed.
Reiter's interpretation is incorrect. See text.
Reiter (1944) listed five because he gave the number 1 merely to a group of cells that lacked a sclerotome, so that in reality his numbers 2–5 were numbers 1–4. Hence the statement in a study of avian species and the mouse that ‘in human embryos, the first 5 somites fuse to form the basioccipital bone’ (Wilting et al. 1995) is without foundation. Furthermore, our research is not based only ‘on relatively late embryos’, and the additional claim that such investigations were probably characterized by ‘overlooking the first transient somite’ (Wilting et al. 1995) is completely gratuitous.
Probably largely because of the difficulty in identifying the first somite, the number of occipital somites in the chick and the mouse is given variously as either four or five, and some authors have changed their opinion (Table 3).
Somites 5–7
A resemblance is noted between occipital somites 1–4 and cervical somites 5–7 in that each of these somitic groups gives rise to a median skeletal mass, namely the basioccipital and the atlanto-axial column (X, Y, Z), respectively.
The sclerotomes of somites 5–7 first develop loose and dense zones similar to those of the following cervical sclerotomes (Fig. 12A; Table 2). The central region of the future cervical vertebrae also shows loose and dense zones up to stage 18, but subsequently the dense part of segment 5 between X and Y disappears, and that between Y and Z becomes greatly reduced (Fig. 11). Nevertheless, the registration of the centra with the sclerotomes is retained up to stages 19 and 20.
The change from (1) registration between somites and occipital segments to (2) lack of registration between somites and cervical centra depends on the development of the axis (second cervical vertebra) from three segments (X, Y, Z) that occupy the extent of only 2½ somites (Fig. 12A). The change occurs chiefly in the transition from Y to Z. It is likely that the situation in the mouse resembles that in the human. In the chick, however, where five rather than four occipital somites may be found (Table 3), the change in level may be chiefly in the transition from somite 7 to somite 8, both of which may take part in the development of the axis (Christ & Ordahl, 1995; Fig. 5, left half).
The atlas and axis
A few hypochordal bows can usually be distinguished ventral to the notochord: (1) that of occipital segment 4, first evident in stage 19 (O'Rahilly & Müller, 1984, figure 14b′ and c′); (2) that of sclerotome 5; and (3) a third arch found as a variation in the presence of a bipartite hypoglossal canal in segment 3 (O'Rahilly & Müller, 1984). The hypochordal bow in segment 5 (Fig. 11A) is considered to give rise to the anterior arch of the atlas (Table 2; see also Sensenig, 1957; figure 3 in Mérida-Velasco et al. 1993). The posterior arch of the atlas develops from sclerotome 5. The controversial interpretations of the uppermost cervical region (e.g. proatlas and proatlantal arch) have been discussed elsewhere (O'Rahilly et al. 1983).
The axis is characterized developmentally by three median constituents that have been designated X, Y, Z in order to allow for varying interpretations (O'Rahilly et al. 1983). In all probability X is the apex of the dens (proatlas, Cave, 1938; Reiter, 1944; Sensenig, 1957; Frame, 1960), Y is the dens axis (centrum of the atlas, Cave, 1938; Kladetzky, 1955; Sensenig, 1957) and Z is the centrum of the axis. The connection of the neural arch of the axis with both Y and Z (Fig. 11B) makes this arch of greater height than the others, a point that appears not to have been recorded previously.
Comparison between occipital and cervical segments
The occipital segments (1–4) resemble those in the cervical region: (1) medial (central) and lateral (peripheral) components are found in both; (2) occipital sclerotomes 2–4 and sclerotomes 5–7 are in register with the original somites; (3) the medial parts of segments 1–4 and 5–7 each form a skeletal mass, as has been mentioned; (4) sclerotome 4, peripherally, possesses a dense exoccipital zone (corresponding to a neural arch), which continues centrally into the perinotochord, whereas sclerotome 5 forms the posterior arch of the atlas; and (5) somites 2–4 (in stages 12, 13) are innervated segmentally by roots of the hypoglossal nerve, as are the cervical somites by spinal nerves.
Hox genes are in part responsible for the specification of segmental identity along the rostrocaudal axis (Kessel & Gruss, 1991; Burke et al. 1995). Moreover, ectopic Hox-4.2 expression in mice is ‘limited to paraxial [somitic] mesoderm-derived structures’ rostral to the atlanto-axial complex, and absence of overt segmentation of the basioccipital suggests that ‘the spatial interactions affecting vertebral segmentation are independent for dorsolateral [lateral] and ventral [medial] components’ (Lufkin et al. 1992).
Rhombomeric segmentation
Rhombomeres A–C correspond to those described in the mouse (Ruberte et al. 1997) and give rise to rhombomeres 1–7.
Rhombomere 8 (from primary Rh. D), sometimes termed the occipital region of the hindbrain, is completely ignored by a number of authors (e.g. Bulfone et al. 1993). It differs from the more rostral rhombomeres in that it forms (1) the hypoglossal neural crest, which is part of the hypoglossal cell cord, and (2) hypoglossal fibres (in stages 12, 13), which show an obvious resemblance to a spinal nerve. However, sensory ganglia are lacking (as is sometimes true also of the first cervical nerve). Moreover, soon after their appearance in stage 12 (Müller & O'Rahilly, 1987), although the hypoglossal fibres have reached the somites, they are no longer strictly segmental (Müller & O'Rahilly, 1988a, figure 10). Finally, in stage 14 the multiple hypoglossal roots form two stems before uniting as the hypoglossal nerve (Müller & O'Rahilly, 1988b, figure 3), so that by that time resemblance to a spinal nerve is lost. A further feature of Rh. 8 is that it shows a higher level of nuclear retinoic acid in the mouse (Morriss-Kay, 1993).
The occipitocervical junction
The occipitocervical junction develops between segments 4 and 5, approximately caudal to pharyngeal arch 4, whereas the cerebrospinal junction occurs between rhombomere 8 and the spinal cord.
On the basis of studies of the chick embryo an ‘S-shaped head/trunk interface’ has been described (Kuratani, 1997). The original review needs to be consulted for details of this complicated proposal for ‘the plan of the vertebrate head’ and its ‘dual metamerism’. The S-shaped interface is related to migratory pathways of postotic neural crest, its dorsal border is a tract of ‘circumpharyngeal crest cells’ and its ventral boundary is a ‘horseshoe-shaped ridge’ containing crest cells.
The extension of this concept to mammals has not been justified. In brief: (1) pharyngeal arches and somites are not at the same axial level; (2) because of the interval between the otic primordium and somite 1 (Fig. 2) in mammals, postotic neural crest is not hindered by somites in its migration to the pharyngeal arches, and a circumpharyngeal route has not been observed (Müller & O'Rahilly, 1980; O'Rahilly & Müller, 1984); (3) the migration of postotic neural crest is largely completed by stage 12 in the human and stage 11 in the mouse (Jiang et al. 2002), so that a mouse embryo corresponding to stage 14 (Kuratani, 1997, figure 1a) shows motor fibres arising directly from the brain and not neural crest; (4) the relevant topography in the mouse (Morriss-Kay, 1992), pig (Van Straaten et al. 2000) and monkey (Peterson et al. 1996) appears to resemble that in the human; (5) glossopharyngeal and vagal neural crest are not ‘the caudalmost members of the cranial cell population’, because the hypoglossal nerve and its neural crest clearly belong to the head; and (6) the interface is based on migrating neural crest rather than on more reliable somitic and skeletal landmarks. In conclusion, in mammals the head is not derived exclusively from the pharyngeal arches, and the trunk from the somites, as supposed by ‘dual metamerisms’, meaning ‘somitomerism and so-called “branchiomerism”’ (Kuratani, 1997; based on Romer). In the human, the four occipital somites innervated by the hypoglossal nerve, as well as their relationship to rhombomere 8, indicate an integrative process of structures within the head that does not correspond to the above suggestion.
Occipitocervical malformations
Some well-known variations and malformations of the occipitocervical region (Prescher et al. 1996; Prescher, 1997) can be seen to have their origin during the embryonic period, e.g. bipartite hypoglossal canal (O'Rahilly & Müller, 1984, figure 14c) and paracondylar process (O'Rahilly & Müller, 1984, figure 14b′). A third occipital condyle, when seen in the adult, is in the region of a hypochordal bow, but its development and its variable relationship to the dens are unclear because during the embryonic and early fetal periods the dens is at a very high level and is almost continuous with the basioccipital (O'Rahilly et al. 1983, figure 1A).
Relevant occipitocervical anomalies have been recorded in mice, e.g. caudal or rostral vertebral transformations after exposure to retinoic acid, which may interfere with a combination of active Hox genes, a ‘Hox code’ (Kessel & Gruss, 1991). Similarly, expression of Hox-4.2 gene more rostrally than normal resulted in a transformation of the occipital bones into neural arches (Lufkin et al. 1992). Also in transgenic mice a caudal projection from the basioccipital may be fused with the dens (cf. the third occipital condyle), in addition to occipital transformation to neural arches (Charité et al. 1995), whereas rostral shifts have been recorded after heat exposure (Li & Shiota, 1999).
References
- Arey LB. The history of the first somite in human embryos. Contrib. Embryol. Carnegie Inst. 1938;27:233–269. [Google Scholar]
- Bardeen CR. Early development of the cervical vertebrae and the base of the occipital bone in man. Am. J. Anat. 1908;8:181–186. [Google Scholar]
- Bartelmez GW. The subdivisions of the neural folds in man. J. Comp. Neurol. 1923;35:231–247. [Google Scholar]
- Bartelmez GW. Neural crest from the forebrain in mammals. Anat. Rec. 1960;138:269–281. doi: 10.1002/ar.1091380308. [DOI] [PubMed] [Google Scholar]
- Blechschmidt E. I. Mechanische Genwirkungen. Göttingen: Musterschmidt; 1948. [Google Scholar]
- Bulfone A, Puelles L, Porteus MH, Frohman MA, Martin GR, Rubenstein RJLR. Spatially restricted expression of Dlx-1, Dlx-2 (Test-1), Gbx-2, and Wnt-3 in the embryonic day 12.5 mouse forebrain defines potential transverse and longitudinal segmental boundaries. J. Neurosci. 1993;13:3155–3172. doi: 10.1523/JNEUROSCI.13-07-03155.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke AC, Nelson CE, Morgan BA, Tabin C. Hox genes and the evolution of vertebrate axial morphology. Development. 1995;121:333–346. doi: 10.1242/dev.121.2.333. [DOI] [PubMed] [Google Scholar]
- Cave AJE. The morphological constitution of the odontoid process. J. Anat. 1938;72:621. [Google Scholar]
- Charité J, de Graaff W, Deschaps J. Specification of multiple vertebral identities by ectopically expressed Hoxb-8. Devel. Dynamics. 1995;204:13–21. doi: 10.1002/aja.1002040103. [DOI] [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Seifert R. Über die Entwicklung der kraniozervikalen Übergangsregion. Neuroorthopädie. 1988;4:13–22. [Google Scholar]
- Christ B, Wilting J. From somites to vertebral column. Ann. Anat. 1992;174:23–32. doi: 10.1016/s0940-9602(11)80337-7. [DOI] [PubMed] [Google Scholar]
- Christ B, Ordahl CP. Early stages of chick somite development. Anat. Embryol. 1995;191:381–396. doi: 10.1007/BF00304424. [DOI] [PubMed] [Google Scholar]
- Couly GF, Coltey PM, Le Douarin NM. The triple origin of skull in higher vertebrates: a study in quail-chick chimeras. Development. 1993;11:409–429. doi: 10.1242/dev.117.2.409. [DOI] [PubMed] [Google Scholar]
- Dalgleish AE. A study of the development of thoracic vertebrae in the mouse assisted by autoradiography. Acta Anat. 1985;122:91–98. doi: 10.1159/000145988. [DOI] [PubMed] [Google Scholar]
- Frame J. Some observations on the development of the cranio-vertebral region. J. Royal Coll Surg. Edinb. 1960;5:320–324. [Google Scholar]
- Fukiishi Y, Morriss-Kay GM. Migration of cranial neural crest cells to the pharyngeal arches and heart in rat embryos. Cell Tissue Res. 1992;268:1–8. doi: 10.1007/BF00338048. [DOI] [PubMed] [Google Scholar]
- Gaunt PN, Gaunt WA. Three Dimensional Reconstruction in Biology. Tunbridge Wells, UK: Pitman Medical; 1978. [Google Scholar]
- Gérard M, Abitbol M, Delezoide A-L, Dufier J-L, Mallet J, Vekemans M. PAX-genes expression during human embryonic development, a preliminary report. S. C. Acad. Sci. 1995;318:57–66. [PubMed] [Google Scholar]
- Götz W, Frisch D, Osmers R, Herken R. Lectin-binding patterns in the embryonic human paraxial mesenchyme. Anat. Embryol. 1993;188:579–585. doi: 10.1007/BF00187013. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhi Q, Ordahl CP, Christ B. The fate of the first avian somite. Anat. Embryol. 1997;195:435–449. doi: 10.1007/s004290050063. [DOI] [PubMed] [Google Scholar]
- Jiang X, Iseki S, Maxson RE, Sucov HM, Morriss-Kay GM. Tissue origins and interactions in the mammalian skull vault. Dev. Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Kessel M, Gruss P. Homeotic transformations of murine vertebrae and concomitant alteration of Hox codes induced by retinoic acid. Cell. 1991;67:89–104. doi: 10.1016/0092-8674(91)90574-i. [DOI] [PubMed] [Google Scholar]
- Keynes R, Lumsden A. Segmentation and the origin of regional diversity in the vertebrate central nervous system. Neuron. 1990;2:1–9. doi: 10.1016/0896-6273(90)90438-l. [DOI] [PubMed] [Google Scholar]
- Kladetzky J. Zur Entwicklung des Dens epistrophei. Morph. Jb. 1955;94:520–554. [Google Scholar]
- Krengel S, Götz W, Herken R. Expression pattern of type II collagen mRNA during early vertebral development in the human embryo. Anat. Embryol. 1996;193:43–51. doi: 10.1007/BF00186832. [DOI] [PubMed] [Google Scholar]
- Kulesa PM, Fraser SE. Cell dynamics during somite boundary formation revealed by time-lapse analysis. Science. 2002;298:991–995. doi: 10.1126/science.1075544. [DOI] [PubMed] [Google Scholar]
- Kuratani S. Spatial distribution of postotic crest cells defines the head/trunk interface of the vertebrate body: embryological interpretation of peripheral nerve morphology and evolution of the vertebrae head. Anat. Embryol. 1997;195:1–13. doi: 10.1007/s004290050020. [DOI] [PubMed] [Google Scholar]
- Li Z-L, Shiota K. Stage-specific homeotic vertebral transformations in mouse fetuses induced by maternal hyperthermia during somitogenesis. Dev. Dynamics. 1999;216:336–348. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<336::AID-DVDY3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Lim TM, Lunn ER, Keynes RJ, Stern CD. The differing effects of occipital and trunk somites on neural development in the chick embryo. Development. 1987;100:525–533. doi: 10.1242/dev.100.3.525. [DOI] [PubMed] [Google Scholar]
- Lim TM, Jaques KF, Stern CD, Keynes RJ. An evaluation of myelomeres and segmentation of the chick embryo spinal cord. Development. 1991;113:227–238. doi: 10.1242/dev.113.1.227. [DOI] [PubMed] [Google Scholar]
- Ludwig E. Über einen operativ gewonnenen menschlichen Embryo mit einem Ursegmente (Embryo Da 1) Morph. Jb. 1928;59:41–104. [Google Scholar]
- Lufkin T, Mark M, Hart ChP, Dollé P, LeMeur M, Chambon P. Homeotic transformation of the occipital bones of the skull by ectopic expression of a homeobox gene. Lett. Nature. 1992;359:835–841. doi: 10.1038/359835a0. [DOI] [PubMed] [Google Scholar]
- Lumsden A. The cellular basis of segmentation in the developing hindbrain. Trends Neurosci. 1990;13:413–429. doi: 10.1016/0166-2236(90)90144-y. [DOI] [PubMed] [Google Scholar]
- Mahmood R, Mason IJ, Morriss-Kay GM. Expression of Fgf-3 in relation to hindbrain segmentation, otic pit position and pharyngeal arch morphology in normal and retinoic acid-exposed mouse embryos. Anat. Embryol. 1996;184:1–10. doi: 10.1007/BF00196311. [DOI] [PubMed] [Google Scholar]
- Meier S, Tam PPL. Metameric pattern development in the embryonic axis of the mouse. I. Differentiation of the cranial segments. Differentiation. 1982;21:95–108. doi: 10.1111/j.1432-0436.1982.tb01202.x. [DOI] [PubMed] [Google Scholar]
- Mérida-Velasco JA, Espin-Ferra J, Sanchez-Montesinos I, Garcia-Garcia JD, Roldan-Schilling V. Development of the human craniovertebral joints. Eur. Arch. Biol. 1993;104:125–133. [Google Scholar]
- Morriss-Kay G. Retinoic acid receptors in normal growth and development. Cancer Surveys. 1992;14:181–193. [PubMed] [Google Scholar]
- Morriss-Kay G. Retinoic acid and craniofacial development: molecules and morphogenesis. Bioessays. 1993;15:9–15. doi: 10.1002/bies.950150103. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The early development of the nervous system in staged insectivore and primate embryos. J. Comp. Neurol. 1980;193:741–751. doi: 10.1002/cne.901930311. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The first appearance of the major divisions of the human brain at stage 9. Anat. Embryol. 1983;168:419–432. doi: 10.1007/BF00304278. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage 12. Anat. Embryol. 1987;176:413–430. doi: 10.1007/BF00310083. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The development of the human brain from a closed neural tube at stage 13. Anat. Embryol. 1988a;177:203–224. doi: 10.1007/BF00321132. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The first appearance of the future cerebral hemispheres in the human embryo at stage 14. Anat. Embryol. 1988b;177:495–511. doi: 10.1007/BF00305137. [DOI] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. Occipitocervical segmentation in staged human embryos. J. Anat. 1994;185:251–258. [PMC free article] [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The timing and sequence of appearance of neuromeres and their derivatives in staged human embryos. Acta Anat. 1997;158:83–99. [PubMed] [Google Scholar]
- Müller F, O'Rahilly R. The prechordal plate, the rostral end of the notochord and nearby median features in staged human embryos. Cells Tissues Organs. 2003;678:1–19. doi: 10.1159/000068214. [DOI] [PubMed] [Google Scholar]
- Noden DM. The embryonic origins of avian cephalic and cervical muscles and associated connective tissues. Am. J. Anat. 1983;168:257–276. doi: 10.1002/aja.1001680302. [DOI] [PubMed] [Google Scholar]
- Northcutt RG. Heads and tails. Science. 1996;274:1629. [Google Scholar]
- O'Rahilly R, Müller F, Meyer DB. The human vertebral column at the end of the embryonic period proper. 2. The occipitocervical region. J. Anat. 1983;136:181–195. [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. The early development of the hypoglossal nerve and occipital somites in staged human embryos. Am. J. Anat. 1984;169:237–257. doi: 10.1002/aja.1001690302. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. The origin of the ectodermal ring in staged human embryos of the first 5 weeks. Acta Anat. 1985;122:145–157. [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. Developmental Stages in Human Embryos Including a Revision of Streeter's ‘Horizons’ and a Survey of the Carnegie Collection. Washington, DC: Carnegie Institution of Washington; 1987. [Google Scholar]
- O'Rahilly R, Müller F. Prenatal ages and stages: measures and errors. Teratol. 2000;61:382–384. doi: 10.1002/(SICI)1096-9926(200005)61:5<382::AID-TERA10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. The two sites of fusion of the neural folds and the two neuropores in the human embryo. Teratol. 2002;65:162–170. doi: 10.1002/tera.10007. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. Somites, spinal ganglia, and centra. Enumeration and interrelationships in staged human embryos, and implications for neural tube defects. Cells Tissues Organs. 2003;173:75–92. doi: 10.1159/000068948. [DOI] [PubMed] [Google Scholar]
- Padget DH. Designation of the embryonic intersegmental arteries in reference to the vertebral artery and subclavian stem. Anat. Rec. 1954;119:349–356. doi: 10.1002/ar.1091190306. [DOI] [PubMed] [Google Scholar]
- Peterson PE, Blankenship TN, Wilson DB, Hendrickx AG. Analysis of hindbrain neural crest migration in the long-tailed monkey (Macaca fascicularis) Anat. Embryol. 1996;194:235–246. doi: 10.1007/BF00187134. [DOI] [PubMed] [Google Scholar]
- Prescher A. The craniocervical junction in man, the osseous variations, their significance and differential diagnosis. Ann. Anat. 1997;179:1–19. doi: 10.1016/S0940-9602(97)80126-4. [DOI] [PubMed] [Google Scholar]
- Prescher A, Brors D, Adam G. Anatomic and radiologic appearance of several variants of the craniocervical junction. Skull Base Surg. 1996;6:83–94. doi: 10.1055/s-2008-1058649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter A. Die Frühentwicklung der menschlichen Wirbelsäule. II. Mitteilung: Die Entwicklung der Occipitalsegmente und der Halswirbelsäule. Z. Anat. Entwickl. 1944;113:66–104. [Google Scholar]
- Ruberte E, Wood HB, Morriss-Kay GM. Prorhombomeric subdivision of the mammalian embryonic hindbrain: is it functionally meaningful? Int. J. Dev Biol. 1997;41:213–222. [PubMed] [Google Scholar]
- Sensenig EC. The origin of the vertebral column in the deer-mouse, Peromyscus maniculatus rufinus. Anat. Rec. 1943;86:123–141. [Google Scholar]
- Sensenig EC. The development of the occipital and cervical segments and their associated structures in human embryos. Contrib. Embryol. Carnegie Inst. 1957;36:141–151. [Google Scholar]
- Stolte D, Huan K, Patel G, Cann GM, Stockdale FE, Christ B. FGF-8 expression in the somites is regulated by adjacent tissues and stimulates chondrogenesis in the chick embryo. Verhandl. Anat. Gesellsch. 2001;184:154–155. [Google Scholar]
- Trainor PA, Tan S-S, Tam PPL. Cranial paraxial mesoderm: regionalisation of cell fate and impact on craniofacial development in mouse embryos. Development. 1994;120:2397–2408. doi: 10.1242/dev.120.9.2397. [DOI] [PubMed] [Google Scholar]
- Van Straaten HWM, Peeters MCE, Hekking JWM, van der Lende T. Neurulation in the pig embryo. Anat. Embryol. 2000;202:75–84. doi: 10.1007/s004290000088. [DOI] [PubMed] [Google Scholar]
- Vieille-Grosjean I, Hunt P, Gulisano M, Boncinelli E, Thorogood P. Branchial HOX gene expression and human craniofacial development. Dev. Biol. 1997;183:49–60. doi: 10.1006/dbio.1996.8450. [DOI] [PubMed] [Google Scholar]
- Wilting J, Ebensperger C, Müller TS, Koseki H, Wallin J, Christ B. Pax-1 in the development of the cervico-occipital transitional zone. Anat. Embryol. 1995;192:221–227. doi: 10.1007/BF00184746. [DOI] [PubMed] [Google Scholar]