Abstract
Post-implantation mouse and rat embryos are usually cultured in sera obtained from various rodents and from humans. We describe here a simple and inexpensive culture medium – Dulbecco's minimal essential medium/Ham's F12 (DMEM/F12) supplemented with 20% fetal bovine serum (FBS) – in which E11.5 rat embryos of the Hebrew University Sabra strain grow for 28 h slightly better than in heat-inactivated 90% rat serum with 10% distilled water and addition of 1 mg mL−1 glucose. They have good growth and development and a low rate of anomalies, similar to that observed in embryos cultured on rat serum. Their size, total score and olfactory system development is slightly higher than in rat embryos cultured in rat serum. About 7.2% of these embryos exhibit subcutaneous haemorrhages in various areas, but these do not seem to interfere with their growth and development. This method is not appropriate for younger embryos; in E10.5 embryos cultured for 28 h we found increased embryonic death and anomalies.
Keywords: culture; DMEM/F12; FBS, growth; rat embryos; rat serum
Introduction
Whole embryo cultures have been used for more than 30 years to study various aspects of embryology and teratology. Mouse and rat embryos at different developmental stages are used more often than embryos of other species. For the mouse embryo, the more commonly used days for culture are E8.5–10.5, the culture lasting for 28–48 h. For rats, it is E9.5–11.5. Usually the embryos are cultured for 28–48 h. However, culture is possible in other gestational days as well (Brown & Fabro 1981; Copp & Cockroft 1990; Matalon et al. 2002).
Although pre-implantation embryos are often cultured in chemically defined media such as F10, F12 or Dulbecco's minimal essential medium (DMEM) (Zusman et al. 1984; Schramm & Bavister 1996; Tucker et al. 1996; Goh et al. 2000), there are very few studies describing successful culture of post-implantation embryos in chemically defined media.
Since the first description by New (1966), the most commonly used medium for the culture of post-implantation embryos is rat or mouse serum. In the last two decades embryos have also been cultured in human serum (Chatot et al. 1980; Zusman et al. 1989), in monkey serum (Klein et al. 1982), in bovine serum with or without the addition of 15% Tyrodes solution (Klug et al. 1985 1990), in rabbit serum (Nagajima et al. 1997) and in canine serum (Flynn et al. 1997), with a pattern of growth and development similar to that existing when cultured in rat or mouse serum.
Growth of post-implantation embryos can also be achieved in dialysed serum if DMEM, vitamins and glucose are added. In addition, rat serum can be diluted with 75% Tyrodes solution or DMEM and still, respectively, enable growth of post-implantation rat embryos cultured with open yolk sacs, or of prehead-fold stage mouse embryos (Copp & Cockroft 1990). Barber et al. (1993) successfully cultured 14-day-old rat embryos for 26 h in a chemically defined medium of Earle's balanced salt solution and 0.7% bovine serum albumin.
It is often difficult to obtain sufficient amounts of rat, mouse or human serum for culture. In most cultures of cells or organs, chemically defined media with or without fetal calf serum (FCS) or fetal bovine serum (FBS) are used with greater simplicity and success. It is therefore important to find a simple culture medium for whole embryo cultures. In the present investigation we report on the successful culture of 11.5-day-old rat embryos in a partially ‘chemically defined medium’, and compare the results to the cultures of embryos on 90% heat-inactivated rat serum, which is routinely used in most laboratories.
Materials and methods
Rat embryos of the Hebrew University Sabra strain (derived from Wistar) at the age of E11.5 (day of sperm finding is E0) were removed from the uteri as reported by us elsewhere (Zusman et al. 1989; Matalon et al. 2002). The embryos from each dam were randomly assigned to two groups:
69 embryos were cultured on 90% heat-inactivated rat serum with 10% double distilled water and additional 1 mg mL−1 glucose (total of 2 mg mL−1);
85 embryos cultured on DMEM/F12 (50%/50%) supplemented with 20% FBS (all purchased from Biological Industries, Beth Haemek, Israel). The study was performed with two different batches.
All embryos were cultured for 28 h in 50-mL bottles, 4–5 embryos in each bottle with 1 mL medium for each embryo. Gas mixtures were as follows: 20% oxygen for 24 h and then 40% oxygen for a further 4 h. The bottles were rotated 30 r.p.m. and kept at 37 °C as described by us elsewhere (Zusman et al. 1989; Matalon et al. 2002). Both the rat serum and the FBS were heat-inactivated at 56 °C for 30 min.
To evaluate the size of the embryos at the beginning of culture we measured embryonic and yolk sac length and number of somites in 32 E11.5 live embryos. These embryos were not cultured. The average number of somites was 13.4 ± 3.6 pairs, ranging from eight to 20. The average yolk sac diameter was 4.24 ± 0.06 mm and the embryonic crown rump (C-R) length was 1.66 ± 0.19 mm (Table 1). In addition, we studied the osmolarity of the culture medium at the beginning of culture by a vapour pressure Osmometer 5500 Wescor USA. The osmolarity of the DMEM/F12 and 20% FBS in five samples was 295 + 8.9 mmol kg−1, and in five samples of the rat serum (after the addition of 10% water and glucose) it was 282 + 5.0 mmol kg−1, implying that there is no difference in osmolarity between the media.
Table 1.
Yolk sac diameter, embryonic size and embryonic scores in embryos following 28 h of culture in 90% rat serum and in DMEM/F12 supplemented with 20% FBS. Values are mean ± SD with number of live embros examined for each parameter in parentheses
| Embryos cultured on DMEM/F12 and 20% FBS | Embryos cultured on rat serum | |
|---|---|---|
| Embryonic C-R length (mm) at start of exp. | 1.66 ± 0.19 (32)* | 1.66 ± 0.19 (32)* |
| Embryonic CR length (mm) at end of exp. | 4.22 ± 0.58 † (67) | 3.97 ± 0.42 (49) |
| Yolk sac diameter (mm) at start of exp. | 4.62 ± 0.06 (32)* | 4.62 ± 0.06 (32)* |
| Yolk sac diameter (mm) at end of exp. | 5.72 ± 1.01 (67) | 5.66 ± 1.14 (49) |
| Embryonic score at the end of exp. | 40.17 ± 12.23† (67) | 39.05 ± 20.28 (49) |
| Embryonic anomalies | 4/67 (5.97%) | 3/50 (6.00%) |
These embryos were not cultured.
Significantly different from embryos cultured on rat serum: P < 0.05.
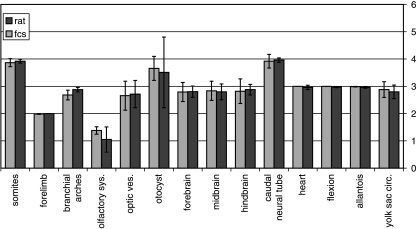
At the end of the experimental period, all cultured embryos were removed and examined for yolk sac circulation and beating hearts. The C-R length of the viable embryos (52 viable – 75%) of those cultured on rat serum and of those cultured on DMEM/F12 (67 viable – 79%) was measured and the number of somites counted. Embryos were evaluated for growth retardation, abnormal turning and for major anomalies. In addition, they were scored according to the method described by Brown & Fabro (1981) with modifications, i.e. the hindlimbs were not scored because they were minimally developed in all embryos. According to that scoring system, each of the developing organs and systems visualized externally (17 items in total) is given a score from 0 to 5. Zero is given if the organ did not start to develop and 4 or 5 when the organ is fully developed. For example, the number of somites: a score of 0 is given when there are only 0–6 pairs of somites, a score of 1 for 7–13 pairs, a score of 2 for 14–20 somites, a score of 3 for 21–27 pairs, a score of 4 for 28–34 somites and a score of 5 for 35–41 somites. We specifically examined yolk sac circulation, body size, axial rotation, presence of telencephalic hemispheres, presence of different parts of the brain, the presence of the neural tube, optic and otic vesicles, number of somites, number of branchial arches, fore- and hindlimb buds, etc., as outlined in Fig. 1. We scored all 67 live embryos cultured in DMEM/F12 and 50 embryos cultured on rat serum.
Fig. 1.
Embryonic scores in both groups according to Brown & Fabro (1981).
Statistical evaluation
The results are given as mean and SD. Differences between the two groups were assessed by the Mann–Whitney rank sum test.
Results
Embryonic death rate at the end of the experiment was similar in both groups as stated in the Methods. Only living embryos with a beating heart were evaluated.
Table 1 describes the yolk sac diameter and embryonic C-R length at the beginning and end of the experiment, and the embryonic score and rate of malformations at the end of the experimental period.
As observed, embryos cultured in DMEM/F12 and 20% FBS were slightly better developed when compared with embryos cultured on rat serum (Table 1, Fig 1–3). The diameter of the yolk sac and embryonic scores were significantly higher in the embryos cultured in 20% FBS (Table 1). Embryonic C-R length was similar in both groups. The total score of the embryos at the end of the experiment was slightly but significantly higher in the DMEM/F12 and 20% FBS group (Table 1). The scores assigned to embryonic structures were generally similar in both groups. However, a significantly higher score for the olfactory system vesicles was observed among the embryos cultured on DMEM/F12 and 20% FBS and a lower score on the branchial bars (Fig. 1). There was no difference between the batches of FBS.
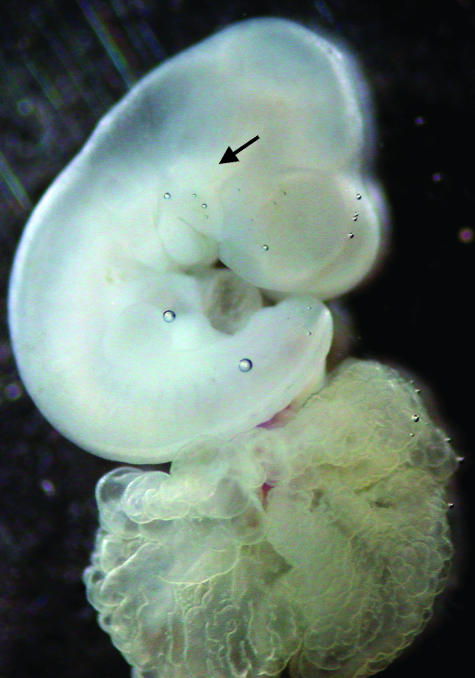
Fig. 3.
Photograph of an E12.5 embryo following culture for 28 h in DMEM/F12 and 20% FBS. Notice the head, divided brain vesicles, right forelimb bud and somites marked by arrows. Also note subcutaneous haemorrhage in the head region.
There were subcutaneous haemorrhages in the limbs, head and trunk in 7.2% of embryos cultured in DMEM/F12 and 20% FBS (Fig. 3), but these embryos grew normally and had no anomalies. The rate of anomalies in embryos cultured in DMEM/F12 and 20% FBS was 5.97%, similar to the 6.00% anomalies observed in embryos cultured on rat serum (Table 1). The type of anomalies was also similar between the two groups. The main anomalies were abnormal rotation, undivided brain vesicles, missing optic vesicle, open neural tube and abnormally enlarged heart.
We also cultured 36 E10.5 embryos in DMEM/F12 and 20% FBS for 28 h and found 19.5% embryonic death, and in the living 29 embryos we observed 15 embryos (52%) with various anomalies, implying that this culture medium does not support normal growth and development of these young embryos.
Discussion
We present here a simple method for the culture of E11.5 rat embryos in a chemically defined medium: DMEM/F12 supplemented with 20% FBS. Embryonic growth and development are generally not different from that found in embryos cultured on 90% inactivated rat serum. Moreover, the total score of the embryos cultured on DMEM/F12 and 20% FBS and the score for the olfactory system were even higher than in the embryos cultured on rat serum. This medium has certain advantages over rat serum as it is commercially available, relatively inexpensive and enables better growth than rat serum. The use of this culture medium avoids the need to use many animals just for the purpose of serum removal for culture. We do not know whether this culture medium will sustain culture for longer periods of time or enable culture of younger (or older) embryos. The entire score of the embryos in both groups at the beginning and end of culture was lower than that described by Brown & Fabro (1981) for E11.5 and E12.5 rat embryos of the Sprague–Dawley strain and corresponded to the developmental stage of E10.5 and E11.5 embryos, respectively. We do not have an explanation for the reduced developmental age of these embryos, but it is probably a result of strain differences as our strain is derived from Wistar rats. In this context, we should add that in a different strain of inbred animals of the Cohen diabetic sensitive rats (these animals develop diabetes when fed a high-sucrose, low-copper diet), the E12.5 rat embryos are even less developed than those of the Sabra strain used in the present study (Weksler-Zangen et al. 2003). The fact that our embryos are slower to develop than embryos of Sprague–Dawley rats may explain the findings that it was sufficient to use relatively low oxygen (only 20%) for the first 24 h of culture, whereas at that age it is recommended to use 40% oxygen (Copp & Cockroft 1990).
We currently use this culture medium under the same conditions for our whole embryo cultures, with only about 5% anomalies and embryonic growth and development, which is better than that obtained in embryos cultured on rat or human serum (Matalon et al. 2002). In recent studies performed on E11.5 rat embryos using DMEM/F12 + 20% FBS as culture media, the addition of various anti-phospholipid antibodies decreased embryonic and yolk sac growth, implying that the deleterious effects of teratogens can be assessed in this culture medium to the same extent as in rat serum (Matalon et al. 2002).
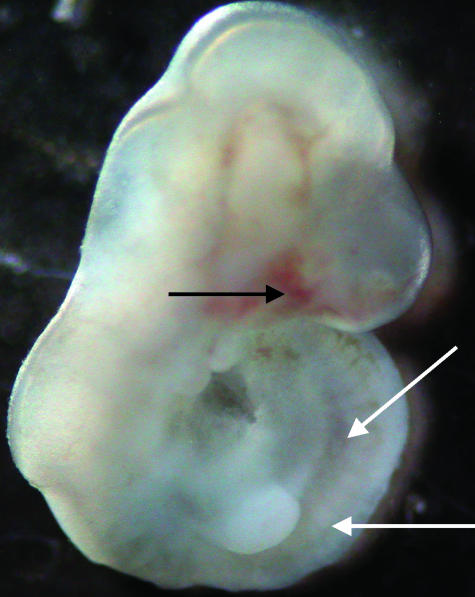
Fig. 2.
Photograph of an E12.5 embryo following culture for 28 h in DMEM/F12 and 20% FBS. Notice the head, divided brain vesicles, two branchial arches, forelimb bud, cardiac prominence and the attached open yolk sac. The arrowhead points to the optic vesicle.
Acknowledgments
This study was supported by grant no. 032-5196 from the Israel Science Foundation and by grant 037-4264 from the Binational American Israeli Science Foundation.
References
- Barber CV, Carda MB, Fantel AG. A new technique for culturing rat embryos between gestation days 14 and 15. Toxicol. In Vitro. 1993;7:695–700. doi: 10.1016/0887-2333(93)90070-l. [DOI] [PubMed] [Google Scholar]
- Brown NA, Fabro S. Quantitation of rat embryonic development in-vitro: a morphological scoring system. Teratology. 1981;24:65–78. doi: 10.1002/tera.1420240108. [DOI] [PubMed] [Google Scholar]
- Chatot CA, Klein NW, Piatek J, Pierro LI. Successful culture of rat embryos on human serum: use in the detection of teratogens. Science. 1980;207:1471–1473. doi: 10.1126/science.7361097. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Cockroft DL. Postimplantation Mammalian Embryos. A Practical Approach. Oxford: Oxford University Press; 1990. [Google Scholar]
- Flynn TJ, Friedman L, Black TN, Klein NW. Methionine and iron as growth factors for rat embryos cultured in canine serum. J. Exp. Zool. 1997;244:319–324. doi: 10.1002/jez.1402440216. [DOI] [PubMed] [Google Scholar]
- Goh VH, Adiga SK, Tain CF, Tong TY. Successful in vitro growth of rat two-cell embryos to blastocysts using a simple chemically defined medium. J. Pharmacol. Toxicol. Meth. 2000;43:171–175. doi: 10.1016/s1056-8719(00)00084-8. [DOI] [PubMed] [Google Scholar]
- Klein NW, Plenefisch JD, Carey SW, Fredrickson WT, Sackett GP, Burbacher TM, et al. Serum from monkeys with histories of fetal wastage causes abnormalities in cultured rat embryos. Science. 1982;215:66–69. doi: 10.1126/science.7053560. [DOI] [PubMed] [Google Scholar]
- Klug S, Lewandowski C, Neubert D. Modification and standardization of the culture of early postimplantation embryos for toxicological studies. Arch. Toxicol. 1985;58:84–88. doi: 10.1007/BF00348314. [DOI] [PubMed] [Google Scholar]
- Klug S, Lewandowski C, Neubert D. Bovine serum: an alterative to serum as a culture medium for the rat whole embryo culture. Toxicol. In Vitro. 1990;4:598–601. doi: 10.1016/0887-2333(90)90123-b. [DOI] [PubMed] [Google Scholar]
- Matalon S, Blank M, Shoenfeld Y, Blank M, Yacobi S, Blumenfeld Z, et al. The effects of IgG purified from women with SLE and associated pregnancy loss on rat embryos in culture. Am. J. Reprod. Immunol. 2002;48:296–303. doi: 10.1034/j.1600-0897.2002.01084.x. [DOI] [PubMed] [Google Scholar]
- Nagajima M, Sasaki M, Kobayashi Y, Ohno Y, Usami M. Rat embryo culture using rabbit serum as a medium for developmental toxicity studies. J. Appl. Toxicol. 1997;17:185–188. doi: 10.1002/(sici)1099-1263(199705)17:3<185::aid-jat428>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- New DAT. Development of rat embryos cultured in blood sera. J. Reprod. Fertil. 1966;12:509–522. doi: 10.1530/jrf.0.0120509. [DOI] [PubMed] [Google Scholar]
- Schramm RD, Bavister BD. Development of in-vitro fertilized primate embryo into blastocyst in a chemically defined, protein-free culture medium. Hum. Reprod. 1996;11:1690–1697. doi: 10.1093/oxfordjournals.humrep.a019471. [DOI] [PubMed] [Google Scholar]
- Tucker KE, Hurst BS, Guadagnoli S, Dymecki C, Mendelsberg B, Awoniyi CA, et al. Evaluation of synthetic serum substitute versus serum as protein supplementation for mouse and human embryo culture. J. Assist. Reprod. Genet. 1996;13:32–37. doi: 10.1007/BF02068866. [DOI] [PubMed] [Google Scholar]
- Weksler-Zangen S, Yaffe P, Ornoy A. Reduced SOD activity and increased neural tube defects in embryos of the sensitive but not of the resistant Cohen diabetic rats cultured under diabetic conditions. Birth Defects Res. 2003;67:429–437. doi: 10.1002/bdra.10043. [DOI] [PubMed] [Google Scholar]
- Zusman I, Engelhard D, Yaffe P, Ron A, Panet A, Ornoy A. Effects of interferon and encephalomyocarditis virus on in vitro development of preimplantation mice embryos with and without the zona pellucida. Teratology. 1984;29:405–409. doi: 10.1002/tera.1420290311. [DOI] [PubMed] [Google Scholar]
- Zusman I, Yaffe P, Raz I, Baron H, Ornoy A. The effects of human diabetic serum on the in vitro development of early somite rat embryos. Teratology. 1989;39:85–92. doi: 10.1002/tera.1420390110. [DOI] [PubMed] [Google Scholar]