Abstract
We investigated the expression pattern of versican, aggrecan, link protein and hyaluronan in the developing limb bud cartilage of the fetal mouse using in situ hybridization and/or immunohistochemistry. Versican mRNA and immunostaining were detected in the mesenchymal cell condensation of the future digital bone at E13. Versican mRNA expression rapidly disappeared from the tibial cartilage, as cartilage formation progressed during E13–15, but the immunostaining was gradually replaced by aggrecan immunostaining from the diaphysis. Immunostaining for both molecules thus had a ‘nega-posi’ pattern and consequently versican immunostaining was still detected at the epiphyseal end at E15. This result indicated that versican functions as a temporary framework in newly formed cartilage matrix. An aggrecan-positive region within the cartilage invariably had intense hyaluronan staining, whereas a versican-positive region also had affinity for hyaluronan within the cartilage, but not in the mesenchymal cell condensation. Therefore, the presence of versican aggregates was not confirmed in the developing limb bud cartilage. Furthermore, although link protein was more closely related with aggrecan than versican during limb bud cartilage formation, there was a discrepancy between the expression of aggrecan and link protein in tibial cartilage at E15. In particular, only a link protein-positive region was present in the marginal area of the metaphysis and the epiphysis at this stage. This finding may indicate a novel role for link protein.
Keywords: aggrecan, cartilage formation, hyaluronan, link protein, versican
Introduction
Versican (also known as PG-M in chick) and aggrecan belong to the family of large aggregating proteoglycans named the hyalectans or lecticans. Hyalectans share highly similar N- and C-terminal globular domains that are separated by the glycosaminoglycan-attached domain (Zimmermann, 2000). Versican/PG-M localizes in various tissues both in fetal and adult animals (Zimmermann et al. 1994; Landolt et al. 1995; Bode-Lesniewska et al. 1996; Shibata et al. 1999). In particular, it is expressed in prechondrogenic mesenchyme and during the transition to cartilage its expression becomes restricted to the periphery of the newly formed cartilage that is predominantly occupied by aggrecan (Kimata et al. 1986; Shinomura et al. 1990; Bignami et al. 1993; Yamagata et al. 1993; Landolt et al. 1995). The detailed transitional pattern of expression from versican to aggrecan has rarely been studied, although immunostaining for both molecules transiently co-localizes in the newly formed rat mandibular condylar cartilage (Shibata et al. 2001). Furthermore, the relation of mRNA expression and protein accumulation to the matrix for both proteoglycans has not been studied. The main purpose of this study was to investigate the transitional pattern of expression of both proteoglycan in developing mouse limb bud cartilage from the viewpoint of mRNA expression and protein accumulation to the matrix.
In addition, versican or corresponding proteoglycan has a peptide domain homologous to the hyaluronan (HA)-binding domain of aggrecan, and is also believed to bind with HA (Chang et al. 1983; LeBaron et al. 1992) and a ternary aggregate consisting of versican, HA and link protein is practically isolated from rat dental pulp (Shibata et al. 2000) and smooth muscle cells (Evanko et al. 2001), which leads to the suggestion that they are co-localized in vivo. Link protein gene is transcribed simultaneously with aggrecan gene in the developing limb bud cartilage in chick (Stirpe & Goetinck, 1989) and human (Mundlos et al. 1991). In addition, versican is co-localized with link protein in the mouse central nervous system (Oohashi et al. 2002), in bovine dental pulp (Yamauchi et al. 1997) and in rat periodontium (Sato et al. 2002). Versican and HA are often co-localized, but not always in rat dental pulp (Shibata et al. 1999), periodontium (Sato et al. 2002) or developing mandibular condylar cartilage (Shibata et al. 2001). These results further suggest that distribution of these molecules varies among tissues. Therefore, another aim of this study was to compare the distribution of versican and/or aggrecan with that of link protein and HA in developing limb bud cartilage.
Materials and methods
Tissue preparation
All animals were housed in facilities approved by the Tokyo Medical and Dental University. The animal-use protocol conformed to the NIH guidelines as stated in the ‘Principles of Laboratory Animal Care’ (NIH publication no. 86-23, revised 1985) and was reviewed and approved by the Screening Committee for Animal Research of the Tokyo Medical and Dental University.
Nine pregnant ICR mice, gestational days 13–15 (08:00 h on the day of the vaginal plug was designated as gestational d0), were used for this study. At each time point, the pregnant mice were killed by cervical dislocation under ether anesthesia, after which each fetal mouse was killed by cervical dislocation. The lower limbs were then taken and immersed in 4% paraformaldehyde (0.1 mol L−1 phosphate buffer, pH 7.4) for 1 day at 4 °C. The specimens were decalcified with 10% EDTA for 7 days at 4 °C then embedded in paraffin. Sections (5 µm) were cut longitudinally to the lower limbs and stained with 0.1% toluidine blue (0.1 mol L−1 phosphate buffer, pH 7.4) for histological observation.
Digoxigenin-labelled RNA probes and in situ hybridization
The probe used for aggrecan was reported in a previous in situ hybridization study (Fukada et al. 1999). Total RNA was extracted from the rib cartilage of newborn mice and cDNAs for versican and link protein were synthesized by reverse transcription-polymerase chain reaction (RT-PCR) using a first-strand cDNA synthesis kit (Amersham Pharmacia Biotech, Tokyo, Japan). Primers used were as follows: versican (PG-M), forward, 5′(6394)-TTTCCTGATTGGCATTAATGAAGA-3′(6417), reverse, 5′(7117)-TCCAAAGGTCTTGGCATTTTCTAC-3′(7094) amplified length, 724 bp (Ito et al. 1995); link protein, forward, 5′(1081)-CAAGGTCTTCTCTCACCGAG-3′(1100), reverse, 5′(1750)-GAGTTTGGTGGGGTGGATCA-3′, amplified length, 670 bp (Kitaoka et al. 2001). Recently, Spicer et al. (2003) indicated the association of four vertebrate link proteins including cartilage link protein (HAPLN1), brain-specific link proteins (HAPLN2 and 4) and a widely expressed link protein (HAPLN3). The probe used in this study corresponds to the portion of mouse HAPLN1 and has about 48% amino acid similarity to mouse HAPLN3. Therefore, this probe seems to have low sensitivity that reacts with mouse HAPLN3 mRNA by in situ hybridization.
After identification of each homology by sequencing, these PCR products were subcloned into pCRII vectors (Strategene, La Jolla, CA, USA), and digoxigenin-labelled antisense and sense RNA probes were synthesized using a DIG-labelled Kit (Roche Diagnostics, Mannheim, Germany).
In situ hybridization using a nucleic acid detection kit (Roche Diagnostics) was performed as previously described (Fukada et al. 1999; Shibata et al. 2002). Sections were observed after counterstaining with nuclear fast red. Sense probes were used as negative controls.
Antibodies
A rabbit polyclonal antibody against versican (chondroitin sulphate attaching region b) was kindly supplied by Dr Tamayuki Shinomura (Tissue Regeneration, Department of Hard Tissue Engineering, Graduate School, Tokyo Medical and Dental University, Tokyo, Japan) and has been characterized in a previous study (Zou et al. 2000).
Monoclonal antibodies 12/21/1C6 (anti-reduced, alkylated aggrecan core protein) and 9/30/8A4 (anti-link protein) were obtained from Developmental Studies Hybridoma Bank (Iowa City, IA, USA). Antibody 12/21/1C6 has been well characterized and slightly cross-reacts with non-cartilaginous proteoglycans (Calabro et al. 1992). Because tissues other than cartilage do not produce a positive reaction using immunohistochemistry (Shibata et al. 2001), we utilized this antibody for aggrecan immunohistochemistry. Antibody 9/30/8A4 (anti-cartilage link protein) has also been characterized (Caterson et al. 1985) and utilized for immunohistochemistry (Yamauchi et al. 1997; Sato et al. 2002). Biotin-labelled HA binding protein from Seikagaku Corporation (Tokyo, Japan) was used as a probe to detect HA localization.
Immunohistochemitry and histochemical staining for HA
A HISTOFINE SAB kit (Nichirei, Tokyo, Japan) and an MOM kit (Vector Laboratories, Burlingame, CA, USA) were used for immunohistochemistry. After deparaffinization, sections were digested with testicular hyaluronidase (Sigma, St Louis, MO, USA; 25 mg mL−1 in phosphate-buffered saline for 30 min at 37 °C) for versican and link protein immunohistochemistry or with chondroitinase ABC protease-free (Seikagaku Corporation, Tokyo, Japan; 1 U mL−1 in 0.1 mol L−1 Tris-acetate, pH 7.3) for aggrecan immunohistochemistry. Sections for aggrecan immunohistochemistry were further reduced and alkylated as previously described (Shibata et al. 2000). All sections were then immersed in methanol containing 1% hydrogen peroxide to block endogenous peroxidase activity and sections for aggrecan and link protein analysis were further immersed in mouse IgG blocking reagent in the MOM kit. All sections were then reacted with primary antibodies diluted with phosphate-buffered saline containing 1% bovine serum albumin (1 : 2000 for versican, 1 : 100 for aggrecan and link protein). The streptavidin–biotin method was then applied to the sections using the HISTOFINE SAB kit, as previously described (Shibata et al. 1997, 2001). Finally, sections were treated with 3-amino-9-ethylcarbazole (Nichirei, Tokyo, Japan) to reveal any reaction. Negative control sections were incubated with normal rabbit IgG (for versican) or mouse IgG (for aggrecan and link protein) instead of the primary antibodies. Histochemical staining for HA was performed as previously described (Shibata et al. 1999, 2001). Sections were pretreated with 0.1% trypsin (Wako Chemicals, Tokyo, Japan) at 37 °C for 30 min and chondroitinase ABC protease-free (0.1 mol L−1 phosphate buffer, pH 8.0) at 37 °C for 3 h to unmask chondroitin sulphate chains as previously described (Asari et al. 1992). All sections were observed after counterstaining with haematoxylin.
Results
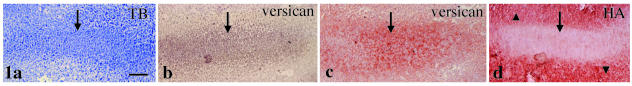
At E13, the anlage of the future digital bone consisted of a mesenchymal cell condensation with no matrix metachromasia (Fig. 1a). Versican mRNA was expressed in this mesenchymal cell condensation (Fig. 1b), but aggrecan and link protein mRNAs were not expressed in the condensation (data not shown). There was clear versican immunostaining in the mesenchymal cell condensation (Fig. 1c), but no aggrecan and link protein immunostaining (data not shown). There was strong HA staining in the mesenchyme around the condensation but not in the condensation (Fig. 1d).
Fig. 1.
The anlage of the future digital bone at E13. Mesenchymal cell condensation (arrow in a) with no matrix metachromasia is seen by toluidine blue staining (TB). Versican mRNA (arrow in b) as well as immunostaining (arrow in c) are expressed in this mesenchymal cell condensation. There is strong HA staining in the mesenchyme around the condensation (arrowheads in d) but not in the mesenchymal condensation (arrow in d). Width of view = 100 µm.
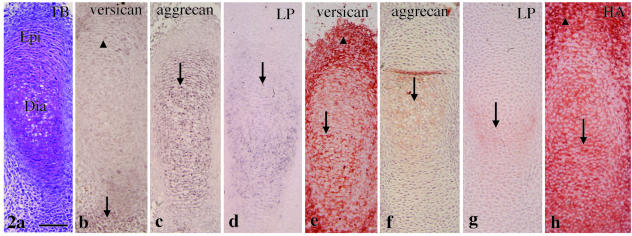
A metachromatically stained matrix was first observed in the cartilaginous anlage of the future tibia (termed the tibial cartilage) at E13 (Fig. 2a). Versican mRNA was strongly expressed in the mesenchyme around the epiphysis and only slightly in flattened chondrocytes of the epiphysis (Fig. 2b). Aggrecan and link protein mRNAs were expressed throughout the tibial cartilage (Fig. 2c,d). There was clear versican immunostaining in the tibial cartilage and in the mesenchyme around it (Fig. 2e). There was weak aggrecan and link protein immunostaining in the diaphysis of this cartilage (Fig. 2f,g). There was HA staining within the tibial cartilage and in the mesenchyme around it (Fig. 2h).
Fig. 2.
Tibial cartilage at E13. (a) A metachromatically stained matrix with toluidine blue staining is seen. The diaphysis (Dia) and the epiphysis (Epi) are distinguishable. (b) Versican mRNA is strongly expressed in the mesenchyme around the epiphysis (arrow) and only slightly in flattened chondrocytes of the epiphysis (arrowhead). (c,d) Aggrecan (c) and link protein (d) mRNA is expressed throughout the tibial cartilage (arrows). (e) There is clear versican immunostaining in the tibial cartilage (arrow) and in the mesenchyme around it (arrowhead). (f,g) There is weak aggrecan (arrow in f) and link protein (arrow in g) immunostaining in the diaphysis of this cartilage. (h) There is HA staining within the tibial cartilage (arrow) and mesenchyme around it (arrowhead). Width of view = 100 µm.
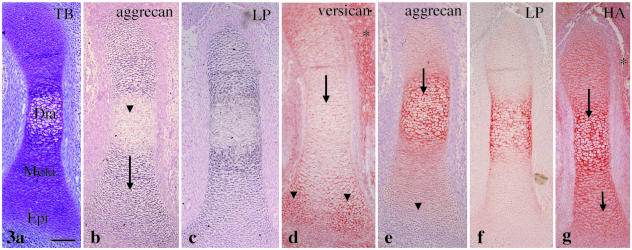
At E14, tibial cartilage increased in length and could be classified into three parts: diaphysis, metaphysis and epiphysis, and hypertrophic chondrocytes appeared in the diaphysis (Fig. 3a). No versican mRNA was detected within the cartilage in this and later stages (data not shown). Aggrecan mRNA expression was detected in the chondrocytes of the metaphysis and the epiphysis but reduced in the hypertrophic chondrocytes of the diaphysis (Fig. 3b). Link protein mRNA had an expression pattern similar to aggrecan (Fig. 3c). There was clear versican immunostaining in the mesenchyme around the cartilage and in the epiphysis, but there was less toward the diaphysis (Fig. 3d). By contrast, there was strong aggrecan immunostaining in the diaphysis with less toward the epiphysis (Fig. 3e). Therefore, versican and aggrecan immunostaining had a ‘nega-posi’ pattern in the cartilage. Link protein immunostaining had a staining pattern similar to that of aggrecan at this stage (Fig. 3f). HA staining was detected throughout the cartilage and in the mesenchyme around it (Fig. 3g).
Fig. 3.
Tibial cartilage at E14. (a) Diaphysis (Dia), metaphysis (Meta) and epiphysis (Epi) are distinguishable. Toluidine blue staining. (b) Aggrecan mRNA expression is detected in the metaphysis and the epiphysis (arrow), but reduced in the diaphysis (arrowhead). (c) Link protein mRNA shows an expression pattern similar to aggrecan. (d) There is clear versican immunostaining in the mesenchyme around the cartilage (*) and in the epiphysis (arrowheads), with less toward the diaphysis (arrow). (e) There is strong aggrecan immunostaining in the diaphysis (arrow) with less toward the epiphysis (arrowhead). (f) Link protein immunostaining shows an expression pattern similar to aggrecan. (g) HA staining is detected throughout the cartilage (arrows) and mesenchyme around it (*). Width of view = 100 µm.
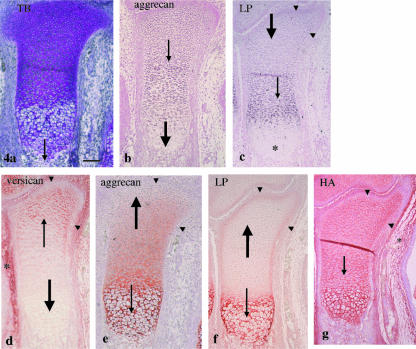
At E15, endochondral bone formation started from the diaphysis (Fig. 4a). Aggrecan mRNA was expressed in the chondrocytes of the epiphysis and metaphysis but not in the hypertrophic chondrocytes of the diaphysis (Fig. 4b). Link protein mRNA was strongly expressed in the chondrocytes of the metaphysis, weakly in those of the epiphysis, but not in the hypertrophic chondrocytes of the diaphysis. In the epiphysis, however, it was more strongly expressed in chondrocytes of the marginal area than in chondrocytes of the remaining areas (Fig. 4c). Versican immunostaining was seen in the mesenchyme around the tibial cartilage and still in the epiphyseal end of this cartilage, but not in the metaphysis and diaphysis (Fig. 4d). There was strong aggrecan immunostaining in the diaphysis but there was less toward the epiphysis and none at the epiphyseal end (Fig. 4e). Therefore, versican and aggrecan immunostaining still had a ‘nega-posi’ pattern in the cartilage. There was strong link protein immunostaining in the diaphysis, but it was very weak in the metaphysis and epiphysis. The marginal area of the diaphysis and epiphysis, however, had moderate immunostaining (Fig. 4f). HA staining had a similar staining pattern as at E14 (Fig. 4g). Note that in the marginal area of the metaphysis and epiphysis, there was no versican or aggrecan immunostaining and no HA staining (see Fig. 4d,e,g). None of the negative control sections for in situ hybridization, immunostaining and HA staining had a positive reaction at any time point (data not shown), as previously described (Fukada et al. 1999; Shibata et al. 1999, 2001).
Fig. 4.
Tibial cartilage at E15. (a) Endochondral bone formation has started from the diaphysis (thin arrow). Toluidine blue staining. (b) Aggrecan mRNA is expressed in the epiphysis and metaphysis (thin arrow) but not in the diaphysis (thick arrow). (c) Link protein mRNA is strongly expressed in the metaphysis (thin arrow), weakly in the epiphysis (thick arrow) but not in the diaphysis (*). The marginal area of metaphysis and epiphysis shows relatively strong expression (arrowheads). (d) There is versican immunostaining in the mesenchyme around the tibial cartilage (*) and still in the epiphyseal end of this cartilage (thin arrow), but not in the metaphysis and diaphysis (thick arrow). (e) There is strong aggrecan immunostaining in the diaphysis (thin arrow) but there is less toward the epiphysis and none at the epiphyseal end (thick arrow). (f) There is strong link protein immunostaining in the diaphysis (thin arrow), but very weak in the metaphysis and epiphysis (thick arrow), but the marginal area of this region has moderate immunostaining (arrowheads). (g) HA staining is detected in the mesenchyme around the cartilage (*) and throughout the cartilage (thin arrow). Note that the marginal area shows no versican and aggrecan immunostainings (arrowheads in d and e, respectively) and HA staining (arrowheads in g). Width of view = 100 µm.
Discussion
Versican mRNA and immunostaining were clearly detected in the mesenchymal cell condensation in the future digital bone at E13, as previously described in chick studies (Kimata et al. 1986; Shinomura et al. 1990; Yamagata et al. 1993; Landolt et al. 1995). In the initially formed tibial cartilage at E13, aggrecan mRNA was expressed throughout the cartilage, whereas versican mRNA expression was remarkably reduced and only slightly detected in the flattened chondrocytes of the epiphysis. This transitional pattern is consistent with previous studies (Kimata et al. 1986; Shinomura et al. 1990; Bignami et al. 1993; Yamagata et al. 1993; Landolt et al. 1995). The protein accumulation, however, did not synchronize with mRNA expression, i.e. aggrecan immunostaining was only slightly detected in the diaphysis whereas versican immunostaining was still predominantly detected throughout the cartilage at this stage. This result is slightly different from that of a previous immunohistochemical study in which versican and aggrecan were evenly co-localized in newly formed rat condylar cartilage (Shibata et al. 2001). Because differentiation of the mandibular condylar cartilage progresses rapidly (Shibata et al. 1997; Fukada et al. 1999), this slight difference is probably due to tissue differences.
Versican mRNA completely disappeared from the tibial cartilage at E14 and 15, whereas its immunostaining was gradually displaced by aggrecan from the diaphysis but still remained at the epiphyseal end at these stages. This is the first report to indicate such a transitional pattern of immunostaining for both proteoglycans and it is clear that versican functioned as a temporary framework in the newly formed cartilage matrix. Furthermore, because aggrecan mRNA expression was reduced in the diaphysis (hypertrophic cell zone) at E14–15, there is also a discrepancy between mRNA expression and protein accumulation at this stage.
HA was barely detected in the mesenchymal cell condensation but was detected in the mesenchyme around it. As cartilage formed, HA became detected in the expanded intercellular matrix. Based on in vivo and in vitro studies, Toole (2000) suggested that early mesodermal cells are surrounded by an HA-rich matrix, and during cell condensation, much HA is removed from the intercellular matrix by receptor-mediated endocytosis, and further differentiation to cartilage is accompanied by extensive matrix formation and recovery of the ability to form hyaluronan-dependent pericellular matrices. Our results entirely support this hypothesis.
As cartilage formation progressed further, HA staining appeared throughout the cartilage. In addition, because previous in situ hybridization studies in growing mouse tibial cartilage indicated that late hypertrophic chondrocytes still express HA synthase mRNAs (Takada et al. 1999), HA seems to be constantly synthesized by chondrocytes at all stages.
Like aggrecan, versican or corresponding proteoglycan is believed to bind HA (Chang et al. 1983; Lebaron et al. 1992) and recently versican aggregates were isolated from rat dental pulp (Shibata et al. 2000) and smooth muscle cells (Evanko et al. 2001), but it is still unclear whether versican forms an aggregate structure in other tissues. Versican and HA are often co-localized, but not always in rat dental pulp (Shibata et al. 1999), periodontium (Sato et al. 2002) or developing rat mandibular condylar cartilage (Shibata et al. 2001). In the present study, although an aggrecan-positive area within cartilage invariably had intense HA staining, the versican-positive area also had an affinity for HA within the cartilage, but not in the mesenchymal cell condensation. Therefore, we could not confirm the presence of versican aggregates in the developing limb bud cartilage that we examined. Even if it forms an aggregate, the ratio of versican to HA might vary among tissues. In particular, it is clear that there is a large decrease in the ratio of HA and versican produced by mesenchymal cell condensation as compared with precondensation mesenchyme, as previously reported (Knudson & Toole, 1987).
In the early stage of cartilage formation (E13–14), link protein mRNA expression and immunostaining were simultaneously detected with aggrecan mRNA expression and immunostaining, respectively. This result agrees with results from a previous study in the developing chick limb bud (Stirpe & Goetinck, 1989) and in human embryo (Mundlos et al. 1991). There was a discrepancy between the expression of both molecules, however, in the advanced stage (E15), i.e. mRNA expression and immunostaining for link protein were reduced in the epiphysis and metaphysis, but still intense for aggrecan. This is the first report of a discrepancy in the expression for both molecules in cartilage, but it is not surprising because link protein mRNA is expressed in non-cartilaginous tissues and is not necessarily regulated together with aggrecan mRNA (Stirpe et al. 1990; Binette et al. 1994). There was only a link protein-positive region in the marginal area of the metaphysis and the epiphysis in the tibial cartilage at E15. Link protein mRNA was strongly expressed in the marginal area, indicating that this finding is not an artefact and might indicate a novel role of link protein, e.g. moulding the cartilage.
Finally, link protein is believed to stabilize the interaction of non-cartilaginous proteoglycans as well as aggrecan with HA (Chang et al. 1983; Binette et al. 1994). Furthermore, link protein is co-localized with versican in rat brain (Oohashi et al. 2002), bovine dental pulp (Yamauchi et al. 1997) and rat periodontium (Sato et al. 2002). Co-localization of both molecules, however, was only observed in the newly formed tibial cartilage at E13 (see Fig. 2) in the present study. Therefore, link protein is more closely related to aggrecan than versican during limb bud cartilage formation. Recently, however, four link proteins have been cloned and characterized (Spicer et al. 2003). Because the probe and the antibody used seem to react more intensely with cartilage link protein (HAPLN1) than with other link proteins (HAPLN2-4), the present findings may have been dependent on their characteristics. Probes and/or antibodies that can distinguish four link proteins are required to clarify the relationship between link protein and hyalectans.
Acknowledgments
The monoclonal antibodies 12/21/1C6 and 9/30/8A4 developed by B. Caterson (Connective Tissue Biology Laboratory, School of Molecular and Medical Biosciences) were obtained from the Developmental Studies Hybridoma Bank maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA, USA, under contracts NO1-HD- and -3263 from the NICHD. This work was supported by a Grant-in-Aid for Scientific Research (No. 12671762) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
References
- Asari A, Miyauchi S, Miyazaki K, Hamai A, Horie K, Takahashi T, et al. Intra- and extracellular localization of hyaluronic acid and proteoglycan constituents (chondroitin sulfate, keratan sulfate, and protein core) in articular cartilage of rabbit tibia. J. Histochem. Cytochem. 1992;40:1693–1703. doi: 10.1177/40.11.1431058. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Bignami A, Perides G, Rahemtulla F. Versican, a hyaluronate-binding proteoglycan of embryonal precartilaginous mesenchyma, is mainly expressed postnatally in rat brain. J. Neurosci. Res. 1993;34:97–106. doi: 10.1002/jnr.490340110. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Binette F, Cravens J, Kahoussi B, Haudenschild DR, Goetinck PF. Link protein is ubiquitously expressed in non-cartilaginous tissues where it enhances and stabilizes the interaction of proteoglycans with hyaluronic acid. J. Biol. Chem. 1994;269:19116–19122. 10.1046/j.1469-7580.2003.00226.x. [PubMed] [Google Scholar]
- Bode-Lesniewska B, Dours-Zimmermann MT, Odermatt BF, Briner J, Heintz PU, Zimmermann DR. Distribution of the large aggregating proteoglycan versican in adult human tissues. J. Histochem. Cytochem. 1996;44:303–312. doi: 10.1177/44.4.8601689. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Calabro A, Hascall VC, Caterson B. Monoclonal antibodies directed against epitopes within the core protein structure of the large aggregated proteoglycan (aggrecan) from the Swarm rat chondrosarcoma. Arch. Biochem. Biophys. 1992;298:349–360. doi: 10.1016/0003-9861(92)90421-r. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Caterson B, Baker JR, Christner JE, Lee Y, Lentz M. Monoclonal antibodies as probes for determining the microheterogeneity of the link proteins of cartilage proteoglycan. J. Biol. Chem. 1985;260:11348–11356. 10.1046/j.1469-7580.2003.00226.x. [PubMed] [Google Scholar]
- Chang Y, Yanagishita M, Hascall VC, Wight TN. Proteoglycans synthesized by smooth muscle cells derived from monkey (Macaca nemestrina) aorta. J. Biol. Chem. 1983;258:5679–5688. 10.1046/j.1469-7580.2003.00226.x. [PubMed] [Google Scholar]
- Evanko SP, Johnson PY, Braun KR, Underhill CB, Duchi J, Wight TN. Platelet-derived growth factor stimulates the formation of versican–hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch. Biochem. Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Fukada K, Shibata S, Suzuki S, Ohya K, Kuroda T. In situ hybridization study of type I, II, X collagens and aggrecan mRNAs in the developing condylar cartilage of fetal mouse mandible. J. Anat. 1999;195:321–329. doi: 10.1046/j.1469-7580.1999.19530321.x. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Shinomura T, Zako M, Ujita M, Kimata K. Multiple forms of mouse PG-M, a large chondroitin sulfate proteoglycan generated by alternative splicing. J. Biol. Chem. 1995;270:958–965. doi: 10.1074/jbc.270.2.958. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Kimata K, Oike Y, Tani K, Shinomura T, Yamagata M, Uritani M, et al. A large chondroitin sulfate proteoglycan (PG-M) synthesized before chondrogenesis in limb bud of chick embryo. J. Biol. Chem. 1986;261:13517–13525. 10.1046/j.1469-7580.2003.00226.x. [PubMed] [Google Scholar]
- Kitaoka E, Satomura K, Hayashi E, Yamanouchi K, Tobiume S, Kume K, et al. Establishment and characterization of chondrocyte cell lines from the costal cartilage of SV40 large T antigen transgenic mice. J. Cell. Biochem. 2001;81:571–582. doi: 10.1002/jcb.1075. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Knudson CB, Toole BP. Hyaluronate–cell interactions during differentiation of chick embryo limb mesoderm. Dev. Biol. 1987;124:82–90. doi: 10.1016/0012-1606(87)90462-3. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Landolt RM, Vaughan L, Winterhalter KH, Zimmermann DR. Versican is selectively expressed in embryonic tissues that act as barriers to neural crest cell migration and axon outgrowth. Development. 1995;121:2303–2312. doi: 10.1242/dev.121.8.2303. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J. Biol. Chem. 1992;267:10003–10010. 10.1046/j.1469-7580.2003.00226.x. [PubMed] [Google Scholar]
- Mundlos S, Meyer R, Yamada Y, Zabel B. Distribution of cartilage proteoglycan (aggrecan) core protein and link protein gene expression during human skeletal development. Matrix. 1991;11:339–346. doi: 10.1016/s0934-8832(11)80205-2. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Oohashi T, Hirakawa S, Bekku Y, Rauch U, Zimmermann DR, Su WD, et al. Bral 1, a brain-specific link protein, colocalizing with the versican V2 isoform at the nodes of Ranvier in developing and adult mouse central nerve systems. Mol. Cell. Neurosci. 2002;19:43–57. doi: 10.1006/mcne.2001.1061. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Sato R, Yamamoto H, Kasai K, Yamauchi M. Distribution pattern of versican, link protein and hyaluronic acid in the rat periodontal ligament during experimental tooth movement. J. Periodont. Res. 2002;37:15–22. doi: 10.1034/j.1600-0765.2002.90770.x. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Yamashita Y. Immunohistochemistry of collagen types II and X, and enzyme-histochemistry of alkaline phosphatase in the developing condylar cartilage of the fetal mouse mandible. J. Anat. 1997;191:561–570. doi: 10.1046/j.1469-7580.1997.19140561.x. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Kaneko S, Yanagishita M, Yamashita Y. Histochemical localization of hyaluronan and versican in the rat molar dental pulp. Arch. Oral Biol. 1999;44:373–376. doi: 10.1016/s0003-9969(98)00110-1. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Shibata S, Yoneda S, Yanagishita M, Yamashita Y. Isolation of proteoglycan (versican) aggregate from rat dental pulp. Arch. Oral Biol. 2000;45:563–568. doi: 10.1016/s0003-9969(00)00023-6. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y. Histochemical localization of versican, aggrecan and hyaluronan in the developing condylar cartilage of the fetal rat mandible. J. Anat. 2001;198:129–135. doi: 10.1046/j.1469-7580.2001.19820129.x. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata S, Fukada K, Suzuki S, Ogawa T, Yamashita Y. In situ hybridization and immunohistochemistry of bone sialoprotein and secreted phosphoprotein 1 (osteopontin) in the developing mouse mandibular condylar cartilage compared with limb bud cartilage. J. Anat. 2002;200:309–320. doi: 10.1046/j.1469-7580.2002.00033.x. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomura T, Jensen KL, Yamagata M, Kimata K, Solursh M. The distribution of mesenchyme proteoglycan (PG-M) during wing bud outgrowth. Anat. Embryol. 1990;181:227–233. doi: 10.1007/BF00174617. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Spicer AP, Joo A, Bowling RA. A hyaluronan binding link protein gene family whose members are physically linked adjacent to chondroitin sulfate proteoglycan core protein genes. J. Biol. Chem. 2003;278:21083–21091. doi: 10.1074/jbc.M213100200. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Stirpe NS, Goetinck PF. Gene regulation during cartilage differentiation: temporal and spatial expression of link protein and cartilage matrix protein in the developing limb. Development. 1989;107:23–33. doi: 10.1242/dev.107.1.23. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Stirpe NS, Dickerson KT, Goetinck PF. The chicken embryonic mesonephros synthesizes link protein, an extracellular matrix molecule usually found in cartilage. Dev. Biol. 1990;137:419–424. doi: 10.1016/0012-1606(90)90266-l. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Takada Y, Sakiyama H, Kuriiwa K, Masuda R, Inoue N, Nakagawa K, et al. Metabolic activities of partially degenerated hypertrophic chondrocytes: gene expression of hyaluronan synthases. Cell Tissue Res. 1999;298:317–325. doi: 10.1007/s004419900082. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan. In: Iozzo RV, editor. Proteoglycans: Structure, Biology, and Molecular Interactions. New York: Marcel Dekker, Inc.; 2000. pp. 61–92. 10.1046/j.1469-7580.2003.00226.x. [Google Scholar]
- Yamagata M, Shinomura T, Kimata K. Tissue variation of two large chindroitin sulfate proteoglycans (PG-M/versican and PG-H/aggrecan) in chick embryos. Anat. Embryol. 1993;187:433–444. doi: 10.1007/BF00174419. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi S, Cheng H, Neame P, Caterson B, Yamauchi M. Identification, partial characterization, and distribution of versican and link protein in bovine dental pulp. J. Dent. Res. 1997;76:1730–1736. doi: 10.1177/00220345970760110301. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann DR, Dours-Zimmermann MT, Schubert M, Bruckner-Tuderman L. Versican is expressed in the proliferating zone in the epidermis and in association with the elastic network of the dermis. J. Cell Biol. 1994;124:817–825. doi: 10.1083/jcb.124.5.817. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann DR. Versican. In: Iozzo RV, editor. Proteoglycans: Structure, Biology, and Molecular Interactions. New York: Marcel Dekker, Inc.; 2000. pp. 327–341. 10.1046/j.1469-7580.2003.00226.x. [Google Scholar]
- Zou K, Muramatsu H, Ikematsu S, Sakuma S, Salama RHM, Shinomura T, et al. A heparin-binding growth factor, midkine, binds to a chondroitin sulfate proteoglycan, PG-M/versican. Eur. J. Biochem. 2000;267:4046–4053. doi: 10.1046/j.1432-1327.2000.01440.x. 10.1046/j.1469-7580.2003.00226.x. [DOI] [PubMed] [Google Scholar]