Abstract
We have examined the role of the signalling molecule, retinoic acid, in the process of neurulation and the subsequent growth and differentiation of the central nervous system using quail embryos that have developed in the absence of retinoic acid. Such retinoic acid-free embryos undergo abnormal neural tube formation in terms of its shape and structure, but the embryos do not display spina bifida or exencephaly. The neural tubes have a wider floor plate, a thicker roof plate and a different dorsoventral shape. Phalloidin staining and electron microscopy revealed alterations in the actin filaments and the junctional complexes of the cell layer lining the lumen. Initially the neural tubes proliferated at the same rate as normal, but later the proliferation rate declined drastically and neuronal differentiation was highly deficient. There were very few motoneurons extending neurites into the periphery, and within the neural tube axon trajectories were chaotic. These results reveal several functions for retinoic acid in the morphogenesis and growth of the neural tube, many of which can be explained by defective notochord signalling, but they do not suggest that this molecule plays a role in neural tube closure.
Keywords: neural tube, neurulation, quail embryo, retinoic acid, retinoids
Introduction
Neurulation and the subsequent growth and patterning of the neural tube is an immensely complex process. During neurulation the flat neural plate undergoes mediolateral narrowing and rostrocaudal lengthening as the neural folds elevate and fuse in the dorsal midline to form the neural tube. Bending in the transverse axis of the neural plate occurs at two locations, the ventral midline (median hinge point) and the dorsolateral hinge point on either side of the midline (Schoenwolf & Smith, 1990; Smith & Schoenwolf, 1997). The neural tube thus formed is initially a single layer of pseudostatified epithelium which then proliferates rapidly and differentiation of neurons takes place laterally in the mantle layer. Patterning of the growing neural tube occurs in both the rostrocaudal axis and the dorsoventral axis under the influence of extracellular morphogens such as fibroblast growth factors, retinoic acid, Wnts, sonic hedgehog and bone morphogenetic proteins (Lee & Jessell, 1999; Jessell, 2000; Briscoe & Ericson, 2001; Helms & Johnson, 2003).
Failure to complete closure of the neural tube at the rostral or caudal end leads to anencephaly and spina bifida (Copp et al. 1990). Together these abnormalities comprise neural tube defects (NTDs), which, being the second most common congenital defect in humans, are clinically very important. An understanding of the aetiology of NTDs may lead to a decrease in their incidence. Indeed, great success has been achieved in reducing the incidence of NTDs by the administration of folic acid, one of the B complex of vitamins (MRC Vitamin Study Research Group, 1991; Czeizel & Dudas, 1992).
Another dietary component, vitamin A, and its biologically active metabolite retinoic acid (RA), is also thought to be involved in neurulation and subsequent neural tube patterning. This might be expected because the developing neural tube is the part of the embryo that contains the highest levels of endogenous RA (Maden et al. 1998b). A role for RA in rostrocaudal patterning of the nervous system has been well established (review in Maden, 2002) and in dorsoventral patterning we have recently provided evidence that RA sets the dorsal, interneuron and ventral neuron boundaries within the rostral spinal cord (Wilson et al. 2003). Here we investigate in what way RA is involved in neurulation, shaping and growth of the neural tube by examining the morphology of the quail neural tube that has developed in the absence of RA.
RA is a well-know teratogen when administered to embryos and one of its many effects is to induce NTDs including spina bifida, exencephaly and anencephaly in several different species (Shenefelt, 1972; Yasuda et al. 1986; Tibbles & Wiley, 1988). A variety of cellular effects have been suggested as being responsible for the appearance of spina bifida following RA administration. These include vascular damage, malformation of the notochord, distortion of the neural folds, cell death in the neural tube, delayed posterior neuropore closure and cell death in the tail gut endoderm and mesenchyme (Tibbles & Wiley, 1988; Kapron-Bras & Trasler, 1988a, b; Alles & Sulik, 1990; Padmanabhan, 1998). However, because these teratogenic effects are generated at excessive, non-physiological levels of RA, they may not tell us about an endogenous role for RA in neural tube formation.
Evidence in favour of such a role comes from experiments involving the retinoic acid receptors (RARs), the nuclear transcription factors through which RA acts in the nucleus, and the RA-synthesizing enzymes. Thus RARγ is expressed in the open neural tube and RARβ and RARα are expressed in the closed neural tube (Ruberte et al. 1991; Smith & Eichele, 1991; Smith, 1994; Chen et al. 1995). There are high levels of exencephaly in RARα/γ double null mouse mutants (Lohnes et al. 1994) and RARγ–/– mutant embryos are resistant to spina bifida, exencephaly and anencephaly caused by RA (Lohnes et al. 1993; Iulianella & Lohnes, 1997). The Cyp26A1–/– mutant embryos mimic the effects of excess RA by exhibiting exencephaly and spina bifida (Abu-Abed et al. 2001; Sakai et al. 2001) and this phenotype is rescued in the Cyp26A1/RARγ double mutant (Abu-Abed et al. 2003). The Raldh2–/– mutant mouse embryos that have lost the major RA-synthesizing enzyme also exhibit spina bifida (Niederreither et al. 1999).
In the curly tail mouse mutant, which is used as a model of human NTDs, the incidence of spina bifida is reduced by the administration of low does of RA at a specific time in development (Seller & Perkins, 1982), an effect which is though to involve the straightening of the tail bud, thereby decreasing the length of the posterior neuropore and normalizing posterior neuropore closure (Chen et al. 1994). In this mutant the spina bifida itself is though to involve the down-regulation of RARγ and RARβ transcripts in the posterior neuropore and hindgut (Chen et al. 1995).
Here we investigate the role of RA in neurulation and subsequent morphogenesis of the neural tube by examining its structure and subsequent growth in RA-deficient quail embryos. These embryos are derived from adult birds fed a vitamin-A-deficient diet and there are no detectable retinoids either in the yolk or in the embryo itself (Dong & Zile, 1995). We find that despite a disruption in the formation of the notochord there is no spina bifida or exencepahy in these embryos. The neural tube that completes neurulation successfully has, however, a highly abnormal structure – the floor plate is wider, the roof plate is thicker, the distribution of junctional complexes is dorsoventrally reversed and the neural tube fails to expand owing to a sharp decline in cell division. RA is thus required for generating the correct anatomy of the spinal cord and for its subsequent proliferative expansion, but not for its closure.
Materials and methods
Quail embryos
Normal Japanese quail (Coturnix coturnix) embryos were obtained from a local farm stock. RA-free embryos were produced at King's College from a stock of adults fed on a defined retinoid-free diet to which 10 mg kg−1 all-trans-RA had been added. Eggs were incubated in a humidified incubator at 37 °C until the required stage. Embryos were collected in phosphate-buffered saline (PBS), staged according to the Hamburger and Hamilton chick stage series (Hamburger & Hamilton, 1951) then fixed and stored in 4% paraformaldehyde (PFA) for further analysis.
In situ hybridization
Wholemount in situ hybridization was performed according to standard protocols (Wilkinson, 1992) using digoxygenin-labelled probes colour reacted with BMP purple to give a purple colour reaction.
Electron microscopy
Embryos were collected in PBS and fixed overnight at 4 °C in 2.5% (v/v) glutaraldehyde fixative. Embryos were post-fixed in osmium tetroxide fixative with Millonigs buffer (Sigma) for 90 min at 4 °C and dehydrated through to 100% ethanol and then placed in propylene oxide (Sigma) for 10 min. Embryos were then put into a resin (TAAB Premix Medium Resin Kit)/propylene oxide mixture of 50% of each for 90 min before being placed into 100% resin for 6 h. Five drops of resin was pipetted into each coffin mould and the embryo carefully placed and orientated to the desired cutting edge. The mould was then left to polymerize for 24 h. Resin blocks were cut on a Leica Ultra-cut machine to semithin thickness (0.75–2 µm) and stained with toludine blue (Sigma) to enable selection of the region to be viewed with the transmission electron microscope (TEM). Ultrathin sections were then cut of the selected viewing area and picked up on to a 200-mesh Guilder grid with a support film Pioloform. Grids were then stained with uranyl acetate (Sigma) and lead citrate (Sigma) and viewed on a Hitachi H7600 TEM.
Phalloidin staining
Fixed embryos were washed thoroughly in PBS-Triton (PBST) seven times for 1 h each at room temperature before addition of Alexa Fluor 568 phalloidin (Molecular Probes) in PBST at a concentration of 6.6 nm. Embryos were left overnight in dark conditions at 4 °C. This step was followed by three 60-min washes in PBST prior to washing in PBS for 5 min and then fixing in 4% PFA. Embryos were then embedded in 20% gelatin, sectioned and visualized using an Olympus confocal microscope.
Proliferation
Paraffin-wax-embedded tissue sections were processed and mounted on glass slides. A rabbit polyclonal antibody to the phosphorylated serine 10 residue of histone H3 (anti-phospho-H3) was used to label proliferating cells. The characterization of this antibody is described by Clayton et al. (2000) and it is used as a marker of cells preparing to enter mitosis. The antibody was used at a concentration of 1 : 8000 followed by a Vector ABC Elite kit (Vector Laboratories) to visualize the immunoreactivity.
Apoptosis
TdT-mediated dUTP Nick End Labelling (TUNEL) staining was performed using the ApopTag Peroxidase In Situ Apoptosis Detection Kit (Intergen) on paraffin-embedded tissue. Briefly, tissue was embedded and sectioned in wax. Tissue sections were deparaffinized and pretreated using proteinase K (20 µg mL−1). Endogenous peroxidase activity was quenched by washing in 0.3% hydrogen peroxidase and the sections were then placed in equilibration buffer before addition of working-strength TdT enzyme. Stop/wash buffer was applied after 1 h and anti-DIG conjugate added before colour was developed using peroxidase substrate. Specimens were dehydrated and mounted in XAM.
Neurofilament wholemount staining
Fixed embryos were washed three times for 1 h in 1% PBST and then blocked using 0.3% hydrogen peroxide/PBST overnight at 4 °C. This step was followed by three 1-h washes prior to addition of the primary neurofilament monoclonal antibody (concentration of 1 : 5000; RMO270, Zymed Laboratories). The primary antibody mix consisted of 10% goat serum (Sigma) in PBST. Embryos were left incubating at 4 °C for 3–5 days before undergoing thorough washing with PBST for several 1-h washes. This was followed by the addition of secondary antibody, anti-mouse IgG peroxidase conjugate (Sigma) (dilution 1 : 200) with 10% goat serum in PBST for 2 days at 4 °C. This was followed by two 5-min washes and three 1-h washes in PBST. Prior to developing the colour reaction, embryos were equilibrated with 1 mg mL−1 diaminobenzidine (DAB) (Sigma) with PBST solution for 1 h and then transferred to the colour reaction consisting of 1 µL 30% hydrogen peroxide/250 µL DAB-PBST. Both of these steps require dark conditions. After specific staining was attained, embryos were washed in PBS, fixed and stored in 4% PFA. Selected embryos were embedded in 20% gelatin and sectioned.
Results
External morphology
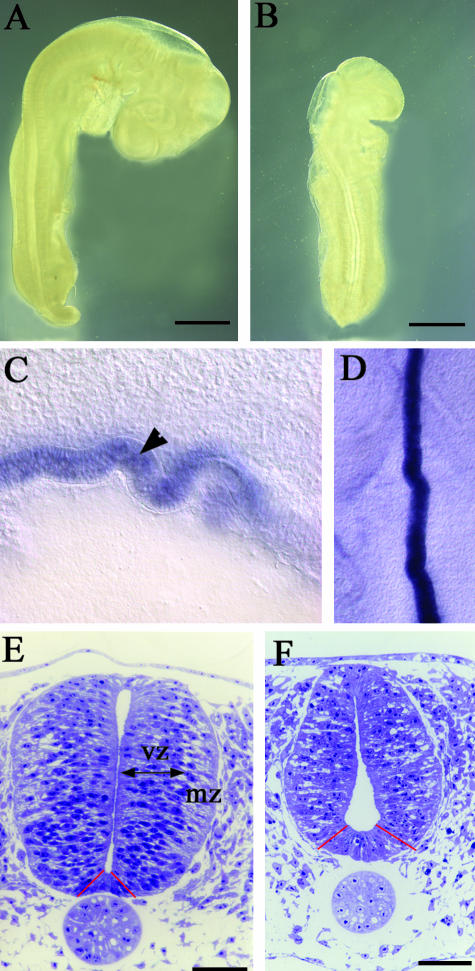
RA-free embryos show a range of embryonic defects by stage 20. These include abnormally small somites, disrupted heart looping, defective vitelline veins and hypoplastic limb buds (Heine et al. 1985; Dersch & Zile, 1993; Maden et al. 2000). Within the nervous system the posterior hindbrain fails to develop (Gale et al. 1999), and the neural crest cells undergo apoptosis and only extend a few neurites into the periphery from the CNS (Maden et al. 1996). Morpholgocially, however, the CNS looks reasonably normal except for a failure of the cervical flexure in the RA-free embryo, resulting in the embryo remaining in a straighter position than is normal together with the fact that it is significantly shorter (Fig. 1A,B). Most surprising is the complete absence of spina bifida or exencephaly and all transverse sections revealed complete closure of the neural tube (see below). No spina bifida or exencephaly occurred even though there was a highly abnormal notochord: instead of being a straight rod, the notochord in the RA-free embryos became zig-zagged in both the dorsoventral axis and the mediolateral axis. This abnormality could be readily observed in sections or when embryos had been processed for in situ hybridization with a probe such as shh, which highlights the notochord (Fig. 1C,D).
Fig. 1.
(A,B) Unstained whole embryos at stage 17 normal (A) and RA-free (B). The RA-free embryos are significantly smaller and as the posterior hindbrain cervical flexure is missing the embryos are also straighter. (C,D) Sections of shh wholemount in situ hybridizations to show the abnormal zig-zag shape of the notochord. A sagittal section is shown in C with the notochord marked with an arrowhead and a horizontal section shown in D. (E,F) Semithin (2 µm) transverse sections of stage 18 normal (E) and RA-free (F) spinal cords at the level of the forelimbs. In the normal embryo dividing cells of the ventricular zone (vz) and differentiating cells of the mantle zone (mz) are clearly distinguished and the ventral floor plate (between the red lines) is quite narrow. By contrast, the RA-free cord has many more intercellular spaces with no real distinction between the ventricular and mantle zones, the lumenal space is expanded ventrally, the floor plate is wider (between the red lines) and there is an overall decrease in size. The roof plate is also thicker (see Fig. 2). Bars in A,B = 1 mm; bars in E,F = 50 µm.
Microscopic morphology
Semithin transverse sections were taken at forelimb levels and stained with toludine blue. This revealed obvious differences between the normal and RA-free phenotype. In the normal embryo, neuroepithelial cells are radially aligned and their nuclei are located at characteristic medio-lateral positions within the neural tube depending on their stage of the cell cycle (Sauer, 1935, 1936). There is a clear distinction between the densely packed proliferating cells of the ventricular zone and the more loosely arranged post-mitotic cells of the differentiating mantle zone that are interspersed with intercellular spaces (Fig. 1E). In the RA-free embryos, however, there are fewer cells, interspersed with many more prominent intercellular spaces in both the ventricular zone and the mantle zone (Fig. 1F), and this irregular arrangement of cells makes it difficult to distinguish between the two zones of the cord. The overall decrease in cell number and mitoses in the ventricular zone (see below) makes the RA-free cord considerably smaller.
One of the most noticeable observations from these sections is the abnormal shape of the RA-free neural tube. In the normal embryo, the spinal cord shape is such that it tapers very slightly in the ventral half and in the intermediate region the left and right halves of the cord are closely associated so that the lumen has disappeared. There are two enlargements of the lumen at the dorsal and ventral poles where the roof plate and the floor plate can be readily located (Fig. 1E). The RA-free embryo, however, exhibits a striking medio-lateral broadening of the ventral half of the neural tube in comparison with the dorsal domain (Fig. 1F), a phenotype that is opposite to that observed with the ventral narrowing in the normal embryo. The lumen in the RA-free spinal cord is laterally expanded in the ventral half adjacent to the floor plate and considerably narrower in the dorsal region compared with the normal. The expansion of the ventral lumen is accompanied by a widening of the floor plate region (Fig. 1F). The roof plate, by contrast, is narrower and thicker (see below).
Electron microscopy
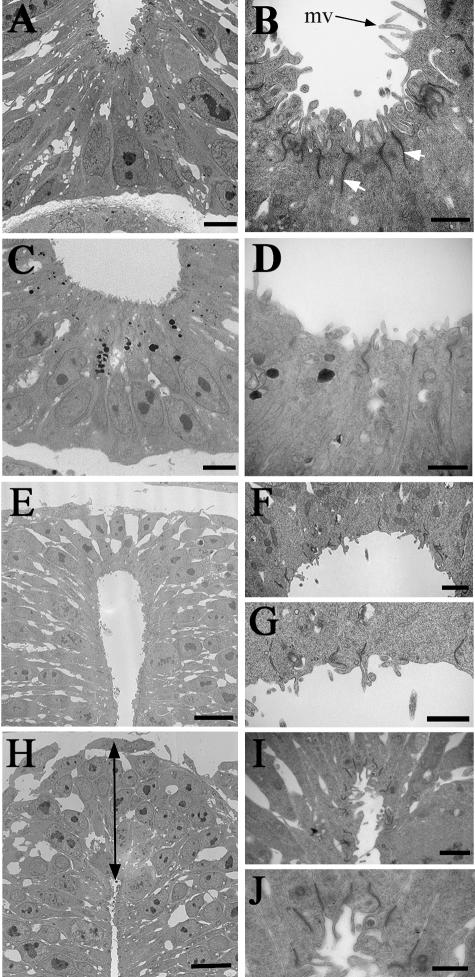
In the normal ventral spinal cord electron microscopy reveals the presence of cell junctional complexes at the apical surface of the cells lining the lumen of the floor plate (Fig. 2A,B). In the RA-free ventral spinal cord (Fig. 2C,D) there is a considerable decrease in the number of cell junctional complexes in this region as well as individual complexes being less well stained, perhaps weaker. This same decrease in electron density was apparent in the lumenal cytoplasm, perhaps reflecting a change in the actin organization (see Actin localization below). These cellular effects could be responsible, in part, for the ventro-lateral expansion of the floor plate as visualized here in the widening of the floor plate lumenal surface (Fig. 2C,D). Also, there is a reduction in the number of microvilli projecting into the central canal of the spinal cord in comparison with the normal embryo (Fig. 2B vs. Fig. 2D).
Fig. 2.
Transmission electron micrographs of the normal and RA-free quail spinal cord at the forelimb level. (A,B) The floor plate region of the normal spinal cord showing numerous microvilli (mv) projecting into the lumen and many cell junctional complexes at the apical surfaces of the cells lining the lumen (white arrows). (C,D) The floor plate region of the RA-free spinal cord showing a much wider lumenal surface and a decrease in the number of microvilli and junctional complexes. (E,F,G) The roof plate region of the normal spinal cord, which is about two cells in height with few microvilli and junctional complexes at the lumenal surface. (H,I,J) The roof plate region of the RA-free spinal cord showing an increased height (arrowed line) accompanied by a very narrow lumen with an increased number of microvilli and junctional complexes present at the lumenal surfaces of the ependymal cells. Bars in A,C = 2 mm; bars in B,D,G,J = 500 nm; bars in E,H = 5 µm; bars in F,I = 1 µm.
The dorsal region of the neural tube in the normal embryo showed that the roof plate consists of a region two cell layers thick with fewer microvilli projections into the central canal than in the normal floor plate region (Fig. 2E–G). In addition, there are far fewer cell junction complexes present (Fig. 2G). By contrast, the RA-free embryo exhibited a much deeper cell layer stretching 5–6 cells thick in the roof plate and the actual shape of the roof plate lumen was significantly constricted compared with the normal (Fig. 2H–J). This was accompanied by an increase in cell junction complexes and microvilli projections at the apical edge of the neuroepithelial cells, the complete opposite of the arrangement seen in the ventral cord.
Actin localization
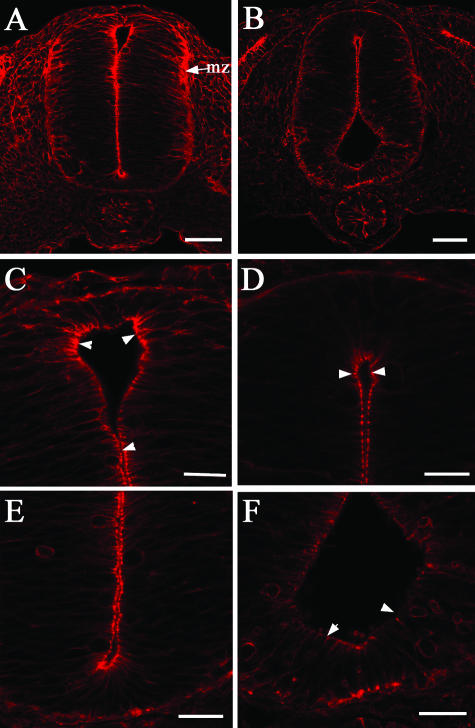
Actin microfilaments in the neuroepithelia of the developing spinal cord have been shown to play a role in the process of neurulation (Nagele & Lee, 1980; Schoenwolf et al. 1988; Ybot-Gonzalez & Copp, 1999) and so it was of interest to study actin localization in the RA-free neural tube, particularly in light of the changes in junctional complexes noted above. Phalloidin staining followed by confocal microscopy was used to investigate this aspect of normal and RA-free neural tubes.
In the normal embryo phalloidin was observed at the lumenal surface of the cells lining the neural canal (Fig. 3A,C,E) as well as in the neuroepithelial cells in the mantle zone of the dorsal neural tube, and less intensely in the ventral mantle zone (Fig. 3A). In the RA-free embryo there was a significant decrease in the intensity of cell labelling both at the lumenal surface and in the mantle zone (Fig. 3B,D,F), suggesting a strong reduction in the number of actin microfilaments.
Fig. 3.
Fluorescent (red) phalloidin antibody staining in transverse sections at the forelimb level of normal (A,C,E) and RA-free (B,D,F) stage 18 quail embryos. (A,C,E) Localization of phalloidin to the apical domain of all the neuroepithelial cells lining the lumen is strongly present in the normal embryo (arrowheads in C) as well a staining in the mantle zone of the neural tube (mz in A) and focal localizations (E). (B,D,F) The RA-free embryo exhibits a decrease in phalloidin localization most visible in the apical domain of the cells lining the lumen (arrowheads in D) especially in the cells of the floor plate region (arrowheads in F). Focal localizations of phalloidin also stain far less intensely. Bars in A,B = 25 µm; bars in C–F = 10 µm.
Proliferation and apoptosis
To investigate why there are fewer cells in the spinal cord of the RA-free embryo we asked whether this was due to a decrease in proliferation or an increase in apoptotic cell death.
Mitotic counts were performed using anti-phospho-H3, an antibody that recognizes phosphorylated histone protein in those cells in the late G2/early mitotic phase of cell division, on embryos in the early stages of neural development (stages 8 and 10) when the initial stages of dorsoventral (DV) patterning and neurulation are occurring, and also at later stages (stages 14 and 18) when neuronal differentiation takes place. Two embryos at each stage and ten consecutive sections from each embryo were counted at the level of the anterior spinal cord.
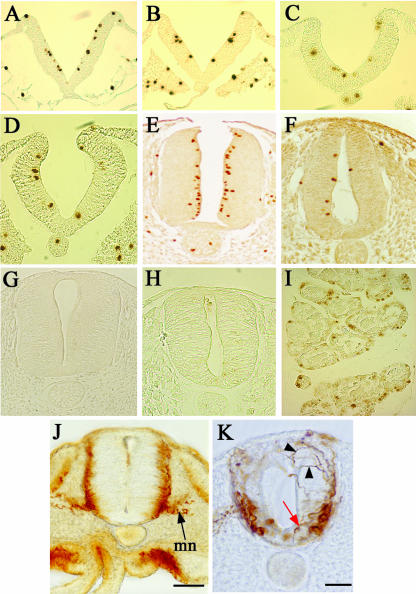
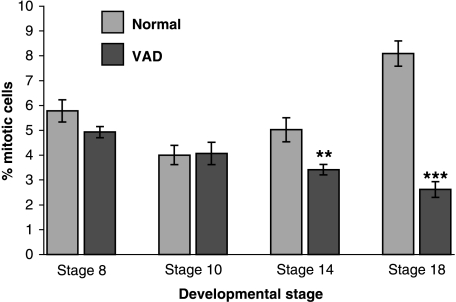
At stage 8 dividing cells were detected in similar numbers in the ventricular zone of the normal and RA-free neural tubes (Figs 4A,B and 5). The same was true at stage10 (Figs 4C,D and 5) and there was a significant drop in proliferation between stages 8 and 10 (P < 0.05). At stage 14 mitoses have started to rise again in normal neural tubes and for the first time there was a significant difference between normal and RA-free embryos (P < 0.005) (Fig. 5). In stage 18 normal embryos there was a near doubling of mitoses in the ventricular zone (Figs 4E and 5) whereas mitoses declined even further in the RA-free embryos (Figs 4F and 5). This indicates that the level of cell division in the RA-free spinal cord is initially the same as normal and does not decline until the stages of neural differentiation.
Fig. 4.
Cell proliferation (A–F) and apoptosis (G,H) in the normal and RA-free quail embryo spinal cord. Cell proliferation is assessed by an anti-phosphohistone H3 antibody, which marks the nuclei brown. (A,B) Sections of a stage 8 normal (A) and RA-free (B) embryo at the presumptive anterior spinal cord level. Equivalent levels of proliferation are apparent. (C,D) Sections of a stage 10 normal (C) and RA-free (D) embryo at the presumptive anterior spinal cord level. Equivalent levels of proliferation are apparent. (E,F) Sections of a stage 18 normal (E) and RA-free (F) embryo at the level of the forelimb. In E the proliferating cells are concentrated at the ventricular surface, but in F the numbers of proliferating cells are vastly reduced. (G,H) Absence of any apoptotic cells in a normal (G) and RA-free (H) stage 18 spinal cord at the forelimb level as assessed by TUNEL staining. (I) Control section of developing rat mammary gland run at the same time as G and H to show that apoptotic cells can readily be detected by this method when they are present. (J,K) Sections of wholemount neurofilament stained stage 18 embryos. The normal embryo (J) shows strong staining in the mantle zone and in the motor neurons (mn). The RA-free embryo (K) shows a decrease in staining with no real mantle zone, fewer motor neurons and completely chaotic axonal projections. Some motor neurons seem to be displaced into the floor plate with axons growing towards the lumen rather than out into the periphery (red arrow) and other axons wander dorsally (black arrowheads).
Fig. 5.
Graph showing cell proliferation counts (% mitoses) in the normal (blue bars) and RA-free neural tube (red bars) at four stages of development. Ten counts were taken from duplicate embryos at each stage. Error bars represent ± standard error of the means. Significant differences in proliferation between normal and RA-free neural tubes begin at stage 14 (**P < 0.005 in comparison with normal at the same stage) and increase greatly at stage 18 (***P < 0.0001 in comparison with normal at the same stage).
TUNEL staining was used to investigate the level of apoptosis at stage 18 of development when proliferation is declining, using wax sections taken through the forelimb level of the spinal cord. Cell counts of apoptotic cells were based on the analysis of ten consecutive sections in five normal and five RA-free embryos. In the normal embryo a very low level of apoptosis was observed (Fig. 4G) with a total of eight apoptotic cells. Similarly in the RA-free embryos a total of only ten apoptotic cells were detected (Fig. 4H). Control slides of developing rat mammary gland confirmed that apoptosis could be detected by this method (Fig. 4I). Thus it seems that the decrease in cell number in the RA-free neural tube is due to a decrease in proliferation, not an increase in apoptosis.
Neuronal differentiation
Finally we investigated whether the decline in cell number in the neural tube led to a decline in the number of differentiating cells using neurofilament staining. At stage 18 neurofilament immunoreactivity was observed in the mantle zone containing post-mitotic neuronal cells and in the axons of the motor neurons and dorsal root ganglia (Fig. 4J). Neurofilament staining in the RA-free embryo reveals a major disorganization in the neuronal arrangement in the spinal cord. The axonal growth and direction of the majority of neuronal groups from the mantle zone exhibit irregular axonal projections (Fig. 4K, black arrowheads), including motorneurons (MNs) that show decreased projection from the ventral spinal cord into the mesenchymal periphery and instead appear to be growing medially towards the central canal (Fig. 4K, red arrow). There is also some cell displacement such as motoneurons in the floor plate region (Fig. 4K, red arrow). The RA-free embryos also display a decrease in the overall number of differentiating neurons in the interneuronal regions of both the dorsal and the ventral mantle zone. These results suggest that neurons are still able to differentiate in the RA-free spinal cord, but that this process occurs at greatly reduced levels, and that the arrangement and projections of differentiated neurons becomes highly disrupted in the absence of RA.
Discussion
The results described above suggest that in the absence of RA several aspects of the morphological organization and cellular behaviour of the developing spinal cord are affected. Firstly, the morphological appearance of the RA-free spinal cord is abnormal in terms of its general shape, the shape of the lumen and in the spatial arrangement of the cell populations of the floor plate and the roof plate. Secondly, there is an alteration in the arrangement of cell junctional complexes of the cells lining the lumen, particularly in the floor plate and roof plate regions, and phalloidin demonstrated a decrease in the localization of actin microfilaments in these cells. Thirdly, levels of cell proliferation in the early RA-free embryo are normal, but have dramatically decreased by stage 18, whereas apoptotic cell death as detected through TUNEL staining was not altered. Finally, detection of differentiated neurons using a neurofilament antibody showed that there is a general decrease in the number of differentiated neurons as well as disrupted and irregular projection of axons. These alterations are associated with an abnormal notochord that becomes zig-zagged in the absence of RA. Surprisingly, what is not altered in the RA-free embryo is closure of the neural tube as there was no spina bifida or exencephaly.
Neurulation and the median hinge point
A major step in neurulation is the formation of the median hinge point (MHP), which is initiated through furrowing along the central neural groove in the neural plate. Furrowing in the MHP is driven by changes in the ventral neuroepithelial cells whereby they become wedge shaped (Schoenwolf & Franks, 1984; Schoenwolf, 1985; Smith et al. 1994) and an inductive signal coming from the underlying notochord whose cells share a common lineage with the floor plate cells (van Straaten et al. 1988; Smith & Schoenwolf, 1989; Placzek et al. 1990; Ybot-Gonzalez & Copp, 1999; Colas & Schoenwolf, 2001; Ybot-Gonzalez et al. 2002). However, the molecular nature of this interaction remains unclear, with recent studies showing that the signalling molecule Shh, which is expressed in the notochord and cells of the floor plate, is neither necessary nor sufficient for the induction of midline bending in mouse (Ybot-Gonzalez et al. 2002). Thus the formation of the MHP is likely to be regulated by another notochordal factor. However, embryos in which floor plate development has been suppressed as a result of notochord suppression (Smith & Schoenwolf, 1989; Davidson et al. 1999) or which do not have a floor plate owing to gene knockouts including null mutants for HNF3-β and Shh (Ang & Rossant, 1994; Weinstein et al. 1994; Chiang et al. 1996) do not exhibit spinal neural tube tube closure defects. These findings suggest the existence of a ‘default mechanism’ of neurulation that ensures successful neural tube closure even in the absence of a notochord and MHP (Ybot-Gonzalez et al. 2002). So perhaps it is to be expected that the abnormally shaped notochord seen here in RA-free embryos had no effect on neural tube closure.
However, the induction and shaping of the MHP cells and the bending and closure of the neural groove do ultimately affect the cross-sectional shape of the neural tube and its contained lumen (Colas & Schoenwolf, 2001). Thus in the RA-free spinal cord the broadened ventral lumenal space and wider floor plate region observed may be due to an increase in the production of floor plate cells. Indeed, in other experiments (Wilson et al. 2003) we have observed an increase in the expression of Shh in the RA-free neural tube, suggesting a potential over-production of floor plate cells. Alternatively, a wider floor plate could be achieved by a decrease in the presence of actin microfilaments resulting in a larger apical cell surface, as was indeed observed in the RA-free embryos using phalloidin staining. This suggests a causal relationship between actin apical constriction and the shaping and folding of neural cells. In support of this, recent studies have shown that actin is involved in the formation of the MHP, the adhesion of the dorsal neural folds and the shape of the lumen, because cytochalasin-D adminstration disturbs these processes (van Straaten et al. 2002). Thus lack of RA could result in a decreased synthesis of actin within cells, which could cause the observed structural changes.
Proliferation and differentiation
It was clear that in the RA-free neural tube the rate of proliferation was equivalent to normal during the early specification phase, but subsequently, betweeen stages 14 and 18, proliferation declined dramatically. A very low level of apoptosis was present in both normal and RA-free embryos and therefore proliferation rather than an increase in apoptosis was responsible for the decline in cell number. This absence of apoptosis in the neural tube contrasts with other areas of the RA-free embryos that exhibit dramatic levevls of apoptosis such as the lateral halves of the somites (Maden et al. 2000). The result of a decreased cell number in the spinal cord was that the neat radial distribution of cells into ventricular and mantle layers, as seen in the normal embryo, was untidy and jumbled in the RA-free cord and was interspersed by large intercellular gaps. This observation confirms previous studies in rat embryos that were deprived of retinoids by dietary means during a selected gestational window and then developed several CNS defects including reduced proliferation and differentiating neuronal population size, an underdeveloped hindbrain, and a neural tube that was reduced in size and cellularity (Dickman et al. 1997).
RA has a positive effect on cell proliferation in many types of cultured cells so we might expect that the lack of RA would result in decreased proliferation. Certainly, RA increases the expression of several cyclins (e.g. Crowe et al. 2003), but another possible mode of action in the neural tube is via the Wnts. Two proposed effectors of mitosis in the developing spinal cord are WNT1 and WNT3a proteins (Megason & McMahon, 2002) that are co-expressed at the dorsal midline of the developing neural tube (Hollyday et al. 1995). WNT1 and 3a are believed to function in a mitogen gradient fashion to organize cell growth and differentiation in the dorsal spinal cord as well as patterning some of the cell types in the dorsal neural tube (Megason & McMahon, 2002; Muroyama et al. 2002). In other experiments (Wilson et al. unpubl. obs.) we have shown that there is a decrease in the dorsal expression of these genes in the RA-free neural tube. This could lead to a decrease in the mitogenic effect of the Wnts in the spinal cord resulting in a decrease in cell number.
The use of neurofilament antibody staining in the spinal cord showed that neurons do differentiate in the neural tube, but that there is a drastic decrease in the numbers of differentiated neurons in the mantle zone. This accords with the well-established role of RA in promoting neuronal differentiation both in vitro (Maden, 2001) and in vivo (Franco et al. 1999; Sharpe &Goldstone, 2000a,b). Additionally, the few neurons that do express neurofilament proteins within the spinal cord have chaotic and highly abnormal trajectories. Perhaps RA also serves to guide axons by chemotaxis (Maden et al. 1998a) or RA could be involved in the control of expression of chemotactic or chemorepulsive molecules expressed within the neural tube such as netrin or the sempahorins.
The notochord: a primary role in these defects?
It is noteworthy that all of the alterations seen in the neural tube: widened floor plate, change in cell shape, decreased proliferation, fewer neurons, decreased motoneuron differentiation and lack of correct axon guidance, are all functions of the action of the notochord on the neural tube. Thus the notochord induces the wedge shape of the MHP cells (van Straaten et al. 1985; Smith & Schoenwolf, 1989), it induces proliferation of the neural tube (van Straaten et al. 1989; van Straaten & Hekking, 1991; Placzek et al. 1993) and notochordless embryos have smaller neural tubes, it induces motoneuron differentiation via the production of Sonic hedgehog (Ericson et al. 1997) and the induced floor plate directs the trajectories of commissural and motor axons via the secretion of netrin-1 (Colamarino & Tessier-Lavigne, 1995).
Thus deficient signalling from the notochord could have generated the observed RA-free phenotypes and the notochord was structurally abnormal in that it took on a zig-zagged shape (Fig. 1C). The classical molecule with which the notochord is said to perform these functions is Sonic hedgehog. But in other experiments (Wilson et al. unpubl. obs.) we have observed not a decrease but an increase in Shh expression in the RA-free neural tube. This rather paradoxical result implies either that there is another completely different inducing molecule originating in the notochord or that the increased expression of Shh leads to a down-regulation of Shh target genes perhaps via the transcriptional repressor Gli3 (Wang et al. 2000). These interesting possibilities centred on the notochord will have to be answered in future experimentation on this valuable model system.
Acknowledgments
We thank the BBSRC for the award of a grant to M.M. and E.G. L.W. was supported by a King's College studentship. We thank Professor L. Mahadevan for the gift of the anti-phospho H3 antibody.
References
- Abu-Abed S, Dolle P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Abed S, Dolle P, Metzger D, Wood C, MacLean G, Chambon P, et al. Developing with lethal RA levels: genetic ablation of Rarg can restore the viability of mioce lacking Cyp26a1. Development. 2003;130:1449–1459. doi: 10.1242/dev.00357. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Alles AJ, Sulik KK. Retinoic acid-induced spina bifida: evidence for a pathogenetic mechanism. Development. 1990;108:73–81. doi: 10.1242/dev.108.Supplement.73. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Ang SL, Rossant J. HNF-3 beta is essential for node and notochord formation in mouse development. Cell. 1994;78:561–574. doi: 10.1016/0092-8674(94)90522-3. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Ericson J. Specification of neuronal fates in the ventral neural tube. Curr. Op. Neurobiol. 2001;11:43–49. doi: 10.1016/s0959-4388(00)00172-0. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Chen W-H, Morriss-Kay GM, Copp AJ. Prevention of spinal neural tube defects in the curly tail mouse mutant by a specific effect of retinoic acid. Dev. Dynam. 1994;199:93–102. doi: 10.1002/aja.1001990203. [DOI] [PubMed] [Google Scholar]
- Chen WH, Morriss-Kay GM, Copp AJ. Genesis and prevention of spinal neural tube defects in the curly tail mutant mouse: involvement of retinoic acid and its nuclear receptors RAR-beta and RAR-gamma. Development. 1995;121:681–691. doi: 10.1242/dev.121.3.681. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Lee E, Young KE, Corden JL, Westphal H, et al. Cyclopia and defective axial patterning in mice lacking Sonic hedgehog gene function. Nature. 1996;383:407–413. doi: 10.1038/383407a0. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Clayton AL, Rose S, Barratt J, Mahadevan LC. Phosphoacetylation of histone H3 on c-fos and c-jun-associated nucleosomes upon gene activation. EMBO J. 2000;19:3714–3726. doi: 10.1093/emboj/19.14.3714. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colamarino SA, Tessier-Lavigne M. The role of the floor plate in axon guidance. Annu.Rev. Neurosci. 1995;18:497–529. doi: 10.1146/annurev.ne.18.030195.002433. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Colas JF, Schoenwolf GC. Towards a cellular and molecular understanding of neurulation. Dev. Dynam. 2001;221:117–145. doi: 10.1002/dvdy.1144. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Copp AJ, Brook FA, Estibeiro JP, Shum ASW, Cockcroft DL. The embryonic development of mammalian neural tube defects. Prog. Neurobiol. 1990;35:363–403. doi: 10.1016/0301-0082(90)90037-h. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Crowe DL, Kim R, Chandraratna RA. Retinoic acid differentially regulates cancer cell proliferation via dose-dependent modulation of the mitogen activated protein kinase pathway. Mol. Cancer Res. 2003;1:532–540. 10.1046/j.1469-7580.2003.00230.x. [PubMed] [Google Scholar]
- Czeizel AE, Dudas I. Prevention of the first occurrence of neural-tube defects by periconceptional vitamin supplementation. N. Engl. J. Med. 1992;24:1832–1835. doi: 10.1056/NEJM199212243272602. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Davidson BP, Kinder SJ, Steiner K, Schoenwolf GC, Tam PP. Impact of node ablation on the morphogenesis of the body axis and the lateral asymmetry of the mouse embryo during early organogenesis. Dev. Biol. 1999;211:11–26. doi: 10.1006/dbio.1999.9276. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Dersch H, Zile MH. Induction of normal cardiovascular development in the vitamin A-deprived quail embryo by natural retinoids. Dev. Biol. 1993;160:424–433. doi: 10.1006/dbio.1993.1318. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Dickman ED, Thaller C, Smith SM. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development. 1997;124:3111–3121. doi: 10.1242/dev.124.16.3111. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Dong D, Zile MH. Endogenous retinoids in the early avian embryo. Biochem. Biophys. Res.Commun. 1995;217:1026–1031. doi: 10.1006/bbrc.1995.2872. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Ericson J, Briscoe J, Rashbass P, van Heyningen V, Jessell TM. Graded sonic hedgehog signaling and the specification of cell fate in the ventral neural tube. Cold Spring Harb. Symp. Quant. Biol. 1997;62:451–466. 10.1046/j.1469-7580.2003.00230.x. [PubMed] [Google Scholar]
- Franco PG, Paganeli AR, Lopez SL, Carrasco AE. Functional association of retinoic acid and hedgehog signalling in Xenopus primary neurogenesis. Development. 1999;126:4257–4265. doi: 10.1242/dev.126.19.4257. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Gale E, Zile M, Maden M. Hindbrain respecification in the retinoid-deficient quail. Mech. Dev. 1999;89:43–54. doi: 10.1016/s0925-4773(99)00202-6. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morph. 1951;88:49–92. [PubMed] [Google Scholar]
- Heine UI, Roberts AB, Munoz EF, Roche NS, Sporn MB. Effects of retinoid deficiency on the development of the heart and vascular system of the quail embryo. Virchow's Arch. B [Cell Pathol] 1985;50:135–152. doi: 10.1007/BF02889897. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr. Op. Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Hollyday M, McMahon JA, McMahon AP. Wnt expression patterns in chick embryo nervous system. Mech. Dev. 1995;52:9–25. doi: 10.1016/0925-4773(95)00385-e. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Iulianella A, Lohnes D. Contribution of retinoic acid receptor gamma to retinoid-induced craniofacial and axial defects. Dev. Dynam. 1997;209:92–104. doi: 10.1002/(SICI)1097-0177(199705)209:1<92::AID-AJA9>3.0.CO;2-S. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Jessell TM. Neuronal specification in the spinal cord: inductive signals and transcriptional codes. Nature Rev. Genet. 2000;1:20–29. doi: 10.1038/35049541. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Kapron-Bras CM, Trasler DG. Histological comparison of the effects of the splotch gene and retinoic acid on the closure of the mouse neural tube. Teratology. 1988a;37:389–399. doi: 10.1002/tera.1420370412. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Kapron-Bras CM, Trasler DG. Interaction between the splotch mutation and retinoic acid in mouse neural tube defects in vitro. Teratology. 1988b;38:165–173. doi: 10.1002/tera.1420380209. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu. Rev. Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Kastner P, Dierich A, Mark M, LeMeur M, Chambon P. Function of retinoic acid γ in the mouse. Cell. 1993;73:643–658. doi: 10.1016/0092-8674(93)90246-m. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Lohnes D, Mark M, Mendelsohn C, Dolle P, Dierich A, Gorry P, et al. Function of the retinoic acid receptors (RARs) during development (I). Craniofacial and skeletal abnormalities in RAR double mutants. Development. 1994;120:2723–2748. doi: 10.1242/dev.120.10.2723. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Maden M, Gale E, Kostetskii I, Zile M. Vitamin A-deficient quail embryos have half a hindbrain and other neural defects. Curr. Biol. 1996;6:417–426. doi: 10.1016/s0960-9822(02)00509-2. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Maden M, Keen G, Jones GE. Retinoic acid as a chemotactic molecule in neuronal development. Int. J. Dev. Neurosci. 1998a;16:317–322. doi: 10.1016/s0736-5748(98)00046-x. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Maden M, Sonneveld E, van der Saag PT, Gale E. The distribution of endogenous retinoic acid in the chick embryo: implications for developmental mechanisms. Development. 1998b;125:4133–4144. doi: 10.1242/dev.125.21.4133. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Maden M, Graham A, Zile M, Gale E. Abnormalities of somite development in the absence of retinoic acid. Int. J. Dev. Biol. 2000;44:151–159. 10.1046/j.1469-7580.2003.00230.x. [PubMed] [Google Scholar]
- Maden M. The role and distribution of retinoic acid during central nervous system development. Int. Rev. Cytol. 2001;209:1–77. doi: 10.1016/s0074-7696(01)09010-6. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Megason SG, McMahon AP. A mitogen gradient of dorsal midline Wnts organizes growth in the CNS. Development. 2002;129:2087–2098. doi: 10.1242/dev.129.9.2087. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- MRC Vitamin Study Research Group. Prevention of neural tube defects: results of the medical research council vitamin study. Lancet. 1991;338:131–137. 10.1046/j.1469-7580.2003.00230.x. [PubMed] [Google Scholar]
- Muroyama Y, Fujihara M, Ikeya M, Kondoh H, Takada S. Wnt signaling plays an essential role in neuronal specification of the dorsal spinal cord. Genes Dev. 2002;16:548–553. doi: 10.1101/gad.937102. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele RG, Lee H. Studies on the mechanisms of neurulation in the chick: microfilament-mediated changes in cell shape during uplifting of neural folds. J. Exp. Zool. 1980;213:391–398. 10.1046/j.1469-7580.2003.00230.x. [Google Scholar]
- Niederreither K, Subbarayan V, Dolle P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21:444–448. doi: 10.1038/7788. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Padmanabhan R. Retinoic acid-induced caudal regression syndrome in the mouse fetus. Reprod. Toxicol. 1998;12:139–151. doi: 10.1016/s0890-6238(97)00153-6. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Placzek M, Tessier-Lavigne M, Yamada T, Jessell T, Dodd J. Mesodermal control of neural cell identity: floor plate induction by the notochord. Science. 1990;250:985–988. doi: 10.1126/science.2237443. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Placzek M, Jessell T, Dodd J. Induction of floor plate differentiation by contact-dependent, homeogenetic signals. Development. 1993;117:205–218. doi: 10.1242/dev.117.1.205. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Ruberte E, Dolle P, Chambon P, Morriss-Kay G. Retinoic acid receptors and cellular retinoid binding proteins. II. Their differential pattern of transcription during early morphogenesis in mouse embryos. Development. 1991;111:45–60. doi: 10.1242/dev.111.1.45. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, et al. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–225. doi: 10.1101/gad.851501. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer F. Mitosis in the neural tube. J. Comp. Neurol. 1935;62:377–405. 10.1046/j.1469-7580.2003.00230.x. [Google Scholar]
- Sauer F. The interkinetic migration of embryonic epithelial nuclei. J. Morph. 1936;60:1–11. 10.1046/j.1469-7580.2003.00230.x. [Google Scholar]
- Schoenwolf GC, Franks MV. Quantitative analyses of changes in cell shapes during bending of the avian neural plate. Dev. Biol. 1984;105:257–272. doi: 10.1016/0012-1606(84)90284-7. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC. Shaping and bending of the avian neuroepithelium: morphometric analyses. Dev. Biol. 1985;109:127–139. doi: 10.1016/0012-1606(85)90353-7. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Folsom D, Moe A. A reexamination of the role of microfilaments in neurulation in the chick embryo. Anat. Rec. 1988;220:87–102. doi: 10.1002/ar.1092200111. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990;109:243–270. doi: 10.1242/dev.109.2.243. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Seller MJ, Perkins KJ. Prevention of neural tube defects in curly-tail mice by maternal administration of vitamin A. Prenatal Diag. 1982;2:297–300. doi: 10.1002/pd.1970020409. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Sharpe C, Goldstone K. The control of Xenopus embryonic primary neurogenesis is mediated by retinoid signalling in the neurectoderm. Mech. Dev. 2000a;91:69–80. doi: 10.1016/s0925-4773(99)00273-7. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Sharpe C, Goldstone K. Retinoid signalling acts during the gastrula stages to promote primary neurogenesis. Int. J. Dev. Biol. 2000b;44:463–470. 10.1046/j.1469-7580.2003.00230.x. [PubMed] [Google Scholar]
- Shenefelt RE. Morphogenesis of malformations in hamsters caused by retinoic acid: relation to dose and stage at treatment. Teratology. 1972;5:103–118. doi: 10.1002/tera.1420050115. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Notochordal induction of cell wedging in the chick neural plate and its role in neural tube formation. J. Exp. Zool. 1989;250:49–62. doi: 10.1002/jez.1402500107. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC, Quan J. Quantitative analyses of neuroepithelial cell shapes during bending of the mouse neural plate. J. Comp. Neurol. 1994;342:144–151. doi: 10.1002/cne.903420113. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Smith JL, Schoenwolf GC. Neurulation: coming to a closure. Trends Neurosci. 1997;20:510–517. doi: 10.1016/s0166-2236(97)01121-1. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Smith SM, Eichele G. Temporal and regional differences in the expression pattern of distinct retinoic acid receptor-beta transcripts in the chick embryo. Development. 1991;111:245–252. doi: 10.1242/dev.111.1.245. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Smith SM. Retinoic acid receptor isoform beta 2 is an early marker for alimentary tract and central nervous system positional specification in the chicken. Dev. Dyn. 1994;200:14–25. doi: 10.1002/aja.1002000103. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, Hekking JW, Thors F, Wiertz-Hoessels EL, Drukker J. Induction of an additional floor plate in the neural tube. Acta Morph. Neerl. Scand. 1985;23:91–97. 10.1046/j.1469-7580.2003.00230.x. [PubMed] [Google Scholar]
- van Straaten HW, Hekking JW, Wiertz-Hoessels EL, Thors F, Drukker J. Effect of the notochord on the differentiation of a floor plate area in the neural tube of the chick embryo. Anat. Embryol. (Berlin) 1988;177:317–324. doi: 10.1007/BF00315839. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, Hekking JW, Beursgens JP, Terwindt-Rouwenhorst E, Drukker J. Effect of the notochord on proliferation and differentiation in the neural tube of the chick embryo. Development. 1989;107:793–803. doi: 10.1242/dev.107.4.793. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- van Straaten HW, Hekking JW. Development of floor plate, neurons and axonal outgrowth pattern in the early spinal cord of the notochord-deficient chick embryo. Anat. Embryol. (Berlin) 1991;184:55–63. doi: 10.1007/BF01744261. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- van Straaten HWM, Sieben I, Hekking JWM. Multistep role for actin in initial closure of the mesencephalic neural groove in the chick embryo. Dev. Dynam. 2002;224:102–108. doi: 10.1002/dvdy.10078. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Tibbles L, Wiley MJ. A comparative study of the effects of retinoic acid given during the critical period for inducing spina bifida in mice and hamsters. Teratology. 1988;37:113–125. doi: 10.1002/tera.1420370204. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Wang B, Fallon JF, Beachy PA. Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell. 2000;100:423–434. doi: 10.1016/s0092-8674(00)80678-9. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Weinstein DC, Altaba A, Chen WS, Hoodless P, Prezioso VR, Jessell TM, et al. The winged-helix transcription factor HNF-3 beta is required for notochord development in the mouse embryo. Cell. 1994;78:575–588. doi: 10.1016/0092-8674(94)90523-1. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson DGE. In Situ Hybridisation. Oxford: Oxford University Press; 1992. 10.1046/j.1469-7580.2003.00230.x. [Google Scholar]
- Yasuda Y, Konishi H, Matsuo T, Kihara T, Tanimura T. Aberrant differentiation of neuroepithelial cells in developing mouse brains subsequent to retinoic acid exposure in utero. Am. J. Anat. 1986;186:271–284. doi: 10.1002/aja.1001860304. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Copp AJ. Bending of the neural plate during mouse spinal neurulation is independent of actin microfilaments. Dev. Dynam. 1999;215:273–283. doi: 10.1002/(SICI)1097-0177(199907)215:3<273::AID-AJA9>3.0.CO;2-H. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]
- Ybot-Gonzalez P, Cogram P, Gerrelli D, Copp AJ. Sonic hedgehog and the molecular regulation of mouse neural tube closure. Development. 2002;129:2507–2517. doi: 10.1242/dev.129.10.2507. 10.1046/j.1469-7580.2003.00230.x. [DOI] [PubMed] [Google Scholar]