Abstract
The entire head and neck of a wild adult male Mongolian gazelle (Procapra gutturosa) was dissected with special reference to its enlarged larynx. Two additional adult male specimens taken from the wild were analysed by computer tomography. The sternomandibularis, omohyoideus, thyrohyoideus and hyoepiglotticus muscles are particularly enlarged and improve laryngeal suspension and stabilization. The epiglottis is exceptionally large. A permanent laryngeal descent is associated with the evolution of an unpaired palatinal pharyngeal pouch. A certain momentary descent seems to occur during vocalization. The high lateral walls of the thyroid cartilage are ventrally connected by a broad keel. The large thyroarytenoid muscle is divided into two portions: a rostral ventricularis and a caudal vocalis muscle. A paired lateral laryngeal ventricle projects between these two muscles. The massive vocal fold is large and lacks any rostrally directed flexible structures. It is supported by a large cymbal-like fibroelastic pad. Vocal tract length was measured in the course of dissection and in computer tomographic images. Two representative spectrograms, one of an adult male and one of a juvenile, recorded in the natural habitat of the Mongolian gazelle are presented. In the spectrograms, the centre frequency of the lowest band is about 500 Hz in the adult male and about 790 Hz in the juvenile. The low pitch of the adult male's call is ascribed to the evolutionary mass increase and elongation of the vocal folds. In the habitat of P. gutturosa a call with a low pitch and, thus, with an almost homogeneous directivity around the head of the vocalizing animal may be optimally suited for multidirectional advertisement calls during the rut. The signal range of an adult male's call in its natural habitat can therefore be expected to be larger than the high-pitched call of a juvenile.
Keywords: acoustic communication, comparative anatomy, computer tomography, evolution, laryngeal sound production
Introduction
Among the 14 ungulate species of Mongolia, the Mongolian gazelle (Procapra gutturosa) has the largest population, today occurring mainly in the eastern provinces of the Republic of Mongolia. A distinguishing feature of the Mongolian gazelle (or Dzeren) is its permanent nomadism. Only in the birth and rutting seasons do they stay in particular places for a short time. Mongolian gazelles were formerly distributed across the whole area of the Republic of Mongolia. However, the range of this species, between the 1940s and 1960s, was reduced by 70% owing to excessive official hunting and poaching (Sokolov et al. 1982; Lushchekina, 1990; Lhagvasuren & Milner-Gulland, 1997; Lhagvasuren et al. 2001).
The Mongolian gazelle's optimal habitat is level or undulating arid steppes and semi-desert regions at altitudes of 800–1000 m, with an average annual precipitation of 200–300 mm m−2 (www.wetter.com: Dalanzadgad, Mongolia) and low levels of human disturbance. Mongolian gazelles avoid rocky or broken terrain, narrow valleys, forests, shrub thickets, sand dunes or cultivated fields, unless driven there by exceptional circumstances. The vegetation of the dry steppes of eastern Mongolia is typically dominated by feather grasses (Stipa spp.), together with sagebrush (Artemisia spp.), Anabasis brevifolia, Nanophyton erinaceum and Allium polyrrhizum. Usually the plant cover of the Mongolian gazelle's habitat is extremely sparse, generally 5–20%, rarely reaching 30–40% (Heptner et al. 1989, p. 644).
Intra- and interspecific comparisons suggest that the larynx of the male P. gutturosa is evolutionarily specialized and allometrically enlarged to an extent that it forms a bulge in the ventral neck region (Fig. 1). This specialization has resulted in the occurrence of a paired lateral laryngeal ventricle, lacking in other ruminants, and the size of the male larynx being about double the size that would be predicted from male-to-female body mass proportions (Frey & Riede, 2003). The size and structure of the female larynx corresponds to that of other bovids (Kleinschmidt, 1961; Frey & Riede, 2003).
Fig. 1.
Head and neck of a male Mongolian gazelle (Procapra gutturosa) in its summer coat. The larynx bulges the ventral neck region considerably. Length of mandibula and contour of the transverse process of the atlas are indicated to elucidate the relative position of the larynx. (Photo: C. Tittmann 2002.)
Mongolian gazelles, like some other ruminant species (e.g. the red deer and fallow deer), are polygynous. Onset of rut is in late November, and it continues through December into early January. Adult dominant males establish individual territories in which they retain several females, thus forming a harem. Territories are occupied for 2–3 weeks and, together with the respective harems, are defended against male conspecifics. Mating takes place in December, more or less synchronously across the whole population. Mating is preceded by a fairly prolonged courtship during which the harem males pursue and chase females and utter their loud, guttural, low-frequency bellows as a kind of acoustic display. Matings occur predominantly at night (Heptner et al. 1989; Sokolov & Luschekina, 1997).
Kleinschmidt (1961, 1962) described the anatomy of the pharynx and larynx of the Mongolian gazelle after investigation of one adult male, one juvenile male and one adult female. In addition, he described the extrinsic laryngeal musculature using the conventional macroscopic dissection method in air, which yielded rather unsatisfactory original photographs of the dissection. He gave no full account of the hyoid apparatus.
Kleinschmidt (1961, pp. 9, 22) erroneously stated that, in contrast to other bovids, a thyrohyoid muscle (‘Schildknorpel-Zungenbeinmuskel’) was found in P. gutturosa. However, a thyrohyoid muscle does normally occur in domestic ruminants (Nickel et al. 1987, p. 42) and also in other bovids, e.g. in the takin, Budorcas taxicolor, both sexes of which have an enlarged larynx (Frey & Hofmann, 2000). Considering the evolutionarily enlarged larynx of the male P. gutturosa, and thus its greater weight, one would expect a higher demand for muscular laryngeal support and a corresponding enlargement of this muscle, which is essential for the suspension of the larynx from the hyoid.
Frey & Riede (2003) gave an account of the sexual dimorphism of the larynx of P. gutturosa on the basis of two specimens (one adult male, one adult female), focusing on the laryngeal cartilages, the intrinsic laryngeal muscles, the lateral laryngeal ventricle in the male and the male vocal fold with its peculiar fibroelastic support. The epiglottis of both larynges could not be investigated as it had been severely damaged by local hunters during sample collection.
The aims of the present study were: (1) to base the description of the anatomy of the head and ventral neck region including laryngeal and vocal tract specializations of P. gutturosa on a larger sample size (three additional adult specimens); (2) to re-investigate the thyrohyoid muscle in P. gutturosa with reference to Kleinschmidt's original account; (3) to provide high-quality original photographs of the extrinsic laryngeal muscles and the suspension apparatus of the larynx including the hyoid bones by applying a special dissection method with the specimen submersed in water; (4) to describe the epiglottis of P. gutturosa including its in situ position and its relationships with surrounding structures; (5) to confirm the occurrence of a lateral laryngeal ventricle in additional specimens; (6) to confirm the peculiar structure of the vocal fold; (7) to compare the anatomical results obtained by conventional macroscopic dissection methods with computer-tomographic images of the entire head and neck region of an adult specimen; (8) to provide measurements of the vocal tract length; and (9) to present a preliminary spectrogram of the Mongolian gazelle's vocalization.
Materials and methods
One entire head and neck of a wild adult male Mongolian gazelle (Procapra gutturosa Pallas 1777) was imported from the Republic of Mongolia. The specimen was collected during the official hunting season in the winter of 1999/2000 and transported deep-frozen to the IZW by veterinarian H. Mix (Nature Conservation International). It was dissected with special reference to (1) the extrinsic laryngeal musculature in the neck and throat region, (2) the topographic position of the larynx and the palatinal pharyngeal pouch, (3) the hyoid apparatus, (4) the epiglottis and the laryngeal cartilages, (5) the thyroarytenoid muscle and the paired lateral laryngeal ventricle, (6) the laryngeal cavity and the vocal fold including its supporting fibroelastic pad, and (7) measurements of the vocal tract.
The specimen was dissected macroscopically while being submerged in water and kept overnight at a temperature of ∼0 °C. Consecutive dissection steps were documented by a series of analogue photographic slides and also by digital images using a Nikon digital camera (Coolpix 950), compact flash-cards and the appropriate software (Nikonview). Major stages were recorded in detailed drawings. Slide projection via a mirror (kept at an angle of 45°) directly onto the drawing board guaranteed that correct proportions were maintained. The undissected right halves of two isolated larynges (one adult male, one adult female, both without epiglottis) were used as a control for certain aspects of the dissection. The results obtained by dissection of the left halves have been published elsewhere (Frey & Riede, 2003).
Samples of the left and right fibroelastic pad (FEP) were processed histologically according to routine procedures (Van Gieson as well as Orcein staining) in order to determine qualitatively the ratio of collagen and elastic fibres.
In August 2002 one of the authors (R.F.) participated in the German–Mongolian expedition ‘Semi-desert Mammalogy’ to the Republic of Mongolia led by Dr I. W. Stuermer, University of Göttingen, Germany. In the course of this expedition four additional specimens (the entire head and neck of three adult males and one adult female) were collected with the assistance of local hunters. For this purpose, an exceptional hunting permit was obtained from the Mongolian Ministry of Nature and Environment, from the State University of Ulaanbaatar (Zoological faculty), and from the local authorities of Manlay (province of Dornodgobi). The specimens were deep frozen immediately after death and transported to the IZW.
In addition, the first preliminary acoustic recordings of the vocalizations of P. gutturosa were made by means of a DAT (digital audio tape) recorder (Sony TCD – D 100) and a directional microphone with wind cover (Sennheiser ME 80 including a pre-amplifier K3U). Recordings were made at a sample frequency of 48 kHz and with 16-bit resolution. To our knowledge, acoustic recordings of P. gutturosa are not yet available in the greater acoustic archives worldwide. Auditory sensitivity curves, likewise, have not been reported for the Mongolian gazelle.
The acoustic recordings had to be taken at night because (1) the gazelles vocalize more frequently in the dark, and (2) it was impossible to approach the gazelles closely enough during daylight. Recordings were taken while following a small herd on foot for 5–6 h. The distance of the animals to the microphone during nocturnal recordings was estimated on the basis of a dawn observation of vocalizing gazelles as 150–200 m. The recordings were made in Dornodgobi province in mid August 2002. The geographical position, ascertained by GPS (global positioning system) was 45°22.4′–23.4′N, 111°35.8′E at an elevation of approximately 880–930 m. About 150 calls of around 50 adult and young animals were recorded, of which only a few could be used for acoustic analysis.
The spectrograms of representative calls were generated by means of the software program CoolEdit 2000 (Syntrillium). The considerable distance between the sound source and the microphone prevented us from making high-intensity recordings. In addition, the quality of the recordings was affected by wind, resulting in a small signal-to-noise ratio. Thus a 150-Hz high-pass filter had to be applied. The spectrograms were generated with the Praat program (v.4.0.38, www.praat.org) using the following settings: (1) an analysis width of 0.08 s, (2) time steps of 0.001 s, (3) frequency steps of 10 Hz along a range of 5000 Hz and (4) by applying a Hamming window function.
Computer tomographic scanning of the entire deep-frozen heads and necks of two adult male Mongolian gazelles was done by means of a GE Lightspeed 4-Slice Spiral CT. Slice thickness of the scans was 0.6 mm. Processing of the obtained data was carried out at a working station applying the General Electrics volume rendering software (VolumeViewer). The device-specific raw data were transformed into bitmap files and then labelled by means of Adobe Photoshop 5.5.
Our results of the features investigated by Kleinschmidt (1961) confirm his findings. Thus, we are confident that, despite the small sample size, our data in general are representative of laryngeal morphology in P. gutturosa and not due to individual aberrations or pathologically altered conditions.
Anatomical terms follow Schaller (1992) and are in accordance with the 4th edition of the Nomina Anatomica Veterinaria (NAV, 1994) except for certain terms introduced by us to describe specific vocal tract specializations of the male Mongolian gazelle. In the following the paired structures of the larynx are generally referred to in the singular. Anatomical abbreviations are given in Table 1
Table 1.
Anatomical abbreviations
| App. hyoid. | hyoid apparatus |
| Arc. cart. cric. | arc of cricoid cartilage |
| Art. cricthyr. | cricothyroid articulation |
| Art. thyrhyo. | thyrohyoid articulation |
| Basihyo. | Basihyoideum |
| Burs. pal. phar. | palatinal pouch of pharynx |
| Burs. tymp. phar. | tympanic pouch of pharynx |
| C1, C2, C3, C4, C5 | cervical vertebrae 1–5 |
| Cart. aryt. (sin.) | (left) arytenoid cartilage |
| Cart. cric. | cricoid cartilage |
| Cart. thyr. | thyroid cartilage |
| Cart. thyrhyo. | thyrohyoid cartilage (rod-like cartilaginous elongation of thyrohyoideum) |
| Cav. infrglott. | infraglottic cavity |
| Cav. nasi | nasal cavity |
| Cav. oris | oral cavity |
| Cerathyo. | Ceratohyoideum |
| Choan. | choanae |
| Conch. nas. ventr. | ventral nasal concha |
| conn. tiss. | connective tissue |
| Cont. gl. par. | contour of parotid gland |
| Cont. ling. | contour of the tongue |
| Cont. Mm. constr. phar. | contour of the pharyngeal constrictor muscles |
| Corn. caud. | caudal horn of thyroid cartilage |
| Corn. rostr. | rostral horn of thyroid cartilage |
| Epigl. | epiglottis |
| Epihyo. | Epihyoideum |
| ethm. gap | ethmoidal gap |
| Fasc. cerv., lam. supf. | superficial layer of neck fascia |
| fibrelast. pad | fibroelastic pad of vocal fold |
| FEP | fibroelastic pad of vocal fold |
| Front. | Frontal |
| Gll. bucc. | buccal salivary glands |
| Gl. mand. | mandibular gland |
| Gl. par. | parotid gland |
| Gl. thyr. | thyroid gland |
| Gl. zyg. | zygomatic gland |
| I:M. omoh. | insertion of omohyoid muscle |
| I:M. sternoh. | insertion of sternohyoid muscle |
| I:M. ventric. | insertion of ventricularis muscle |
| Incis. | Incisive (Premaxillary) |
| Lacr. | Lacrimal |
| Lam. cart. cric. | lamina of cricoid cartilage |
| Lam. cart. thyr. | lamina of thyroid cartilage |
| Lig. arycorn. | arycorniculate ligament |
| Lig. nuch. | nuchal ligament |
| Ling. | tongue |
| LLV (dex.) | (right) lateral laryngeal ventricle |
| Mand. | Mandibula (Dentary) |
| Max. | Maxilla (Maxillary) |
| M. cleidceph., p. mast. | mastoid portion of cleidocephalic muscle |
| M. cleidceph., p. occ. | occipital portion of cleidocephalic muscle |
| Mm. constr. phar. caud. | caudal constrictor muscles of the pharynx |
| M. cricaryt. dors. | dorsal cricoarytenoid muscle |
| M. cricaryt. lat. | lateral cricoarytenoid muscle |
| M. cutan. fac. | cutaneous muscle of the head |
| M. depr. lab. inf. | depressor muscle of the lower lip |
| M. digastr. | digastric muscle |
| M. lev. nasol. | nasolabial levator muscle |
| M. mal., p. caud. | caudal portion of malaris muscle |
| M. mal., p. lacr. | lacrimal portion of malaris muscle |
| M. mass. | masseter muscle |
| M. myloh. | mylohyoid muscle |
| M. omoh. | omohyoid muscle |
| M. parotaur. | parotidoauricularis muscle |
| M. retr. ang. oc. lat. | retractor muscle of lateral eye angle |
| M. sternman. | sternomandibular muscle (M. sterno cephalicus, pars mandibularis) |
| M. sternman., p. dors. | dorsal portion of double-bellied sternomandibular muscle |
| M. sternman., p. ventr. | ventral portion of double-bellied sternomandibular muscle |
| M. sternoh. | sternohyoid muscle |
| M. sternthyr. | sternothyroid muscle |
| M. temp. | temporalis muscle |
| M. thyrhyo. | thyrohyoid muscle |
| M. ventric. | ventricularis muscle |
| M. voc. | vocalis muscle |
| M. zyg. | zygomatic muscle |
| M. zygaur. | zygomaticoauricularis muscle |
| Nar. dex. | right nostril |
| Nas. | Nasal |
| Nl. mand. | mandibular lymph node |
| O: M. cricaryt. dors. | origin of dorsal cricoarytenoid muscle |
| O: M. cricthyr. | origin of cricothyroid muscle |
| Occip. | Occipital |
| Oesoph. | oesophagus |
| Pal. | Palatine |
| Palat. dur. | hard palate |
| Pariet. | Parietal |
| Plic. aryepigl. | aryepiglottic fold |
| Plic. voc. (dex.) | (right) vocal fold |
| poorb. fat pad | postorbital fat pad |
| Por. ac. ext. | opening of osseous part of external acoustic meatus |
| PPP | palatinal pharyngeal pouch |
| Proc. corn. | corniculate process of arytenoid cartilage |
| Proc. cuneif. | cuneiform process of epiglottis |
| Proc. musc. | muscular process of arytenoid cartilage |
| Proc. parac. | paracondylar process |
| Proc. voc. | vocal process of arytenoid cartilage |
| Prom. lar. | ventral prominence of thyroid cartilage |
| Rad. ling. | root of the tongue |
| Rec. lar. med. caud. | caudal median laryngeal recess |
| Rec. lar. med. rostr. | rostral median laryngeal recess |
| res. cush. | resilient cushion of connective tissue |
| rostr. bulge | rostral bulge of thyroid cartilage |
| Stylhyo. | stylohyoideum |
| Temp. | Temporal |
| Thyrhyo. | Thyrohyoideum |
| tip proc. corn. | dorsal tip of corniculate process of arytenoid cartilage |
| TPP (dex.) | (right) tympanic pharyngeal pouch |
| Trach. | trachea |
| Tymphyo. | Tympanohyoideum |
| V. jug. ext. | external jugular vein |
| ventr. bulge | rostroventral bulge of thyroid keel |
| ventr. keel | ventral keel of thyroid cartilage |
| Ventr. lar. lat. | lateral laryngeal ventricle |
| Vest. lar. | laryngeal vestibule |
| VTL | vocal tract length |
| Zyg. | Zygomatic |
Results
Extrinsic laryngeal and ventral hyoid muscles and weight of the larynx
These muscles were dissected in the head and throat region only. Thus, the origins of the longer muscles arising from the sternum are not given. The M. hyoepiglotticus is treated together with the epiglottis (see below).
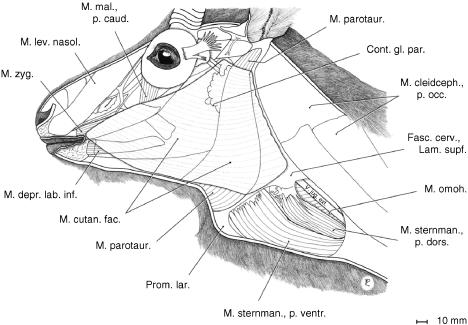
In addition to the ventral hyoid and extrinsic laryngeal muscles (omohyoid, sternohyoid, sternothyroid and thyrohyoid), the larynx is covered laterally and ventrally (1) by the mandibular portion of the sternocephalic muscle (M. sternomandibularis) and (2) by the thin cutaneous muscle of the head and by the parotidoauricularis muscles (Fig. 2).
Fig. 2.
Cutaneous muscle of the head and parotidoauricularis muscle of an adult male Mongolian gazelle. The ventrally enlarged parotidoauricularis muscle, a depressor of the auricular concha, supports the epiglottal region in a hammock-like sling.
The M. parotidoauricularis takes its origin from the parotidomasseteric fascia and, ventromedially, from the fibres of its contralateral counterpart. Its fibres converge continuously in the dorsal direction towards its insertion to the auricular concha. As in the takin (Frey & Hofmann, 1998, 2000), the broad ventral portions of both parotidoauriculares muscles support the epiglottal region of the larynx in a hammock-like sling (Figs 2 and 3).
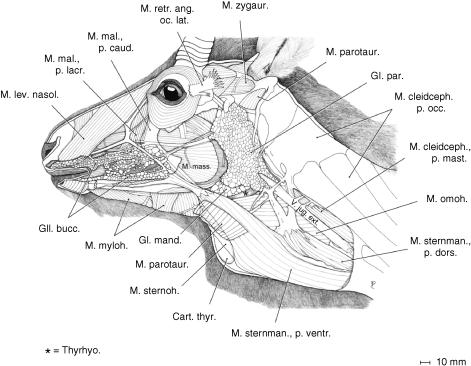
Fig. 3.
Superficial musculature of the head and upper neck region of an adult male Mongolian gazelle after removal of the cutaneous muscle and most of the parotidoauricularis muscle. The strong double-bellied mandibular portion of the sternocephalic muscle (M. sternomandibularis) and the strong omohyoid muscle lend effective lateral support to the large and heavy larynx.
In contrast to what is seen in other bovids, the powerful M. sternomandibularis is double-bellied. The stronger ventral portion inserts with a strong tendon to the superficial aponeurosis of M. masseter at the level of the vascular notch of the mandibula and reaches up to the caudally directed muscle fibres of the caudal portion of the M. malaris (Fig. 3). The weaker dorsal portion inserts via an aponeurosis, which is traversed by the external jugular vein to the parotid fascia and, superficially, to the ventral edge of M. cleidocephalicus at the level of the transverse process of the atlas (Ala atlantis).
The broad and strong M. omohyoideus (basically an M. cervicohyoideus) arises from fasciae lateral to the transverse processes of cervicals C2–C4. It passes deep to the external jugular vein, covering the junction of pharynx and larynx. Together with M. sternohyoideus, it inserts to the basihyoideum (Figs 3 and 4).
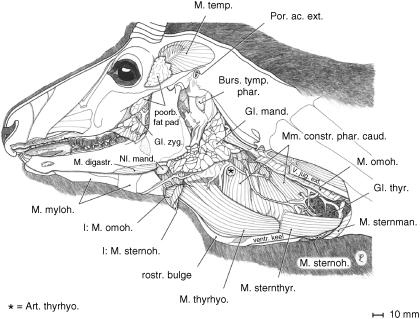
Fig. 4.
Deep musculature of the laryngeal region of an adult male Mongolian gazelle. Parotid gland, left auricula, masseter, sternomandibularis, sternohyoid and omohyoid muscles removed. Corresponding to the evolutionarily enlarged larynx, the constrictor muscles of the pharynx are also enlarged. Broad thyrohyoid muscle and sternothyroid muscle exposed.
The slender M. sternohyoideus is the weakest of the extrinsic laryngeal muscles. Medial to M. sternomandibularis, it passes as a long, parallel-fibred narrow band along the trachea and the keel of the thyroid cartilage (see below) and, ventral to M. omohyoideus, inserts to the basihyoideum (Fig. 4).
The M. sternothyroideus, also a long, parallel-fibred band, passes laterally along the trachea touching the ventral circumference of the thyroid gland. It inserts onto the caudal edge and the caudolateral surface of the thyroid cartilage separated from the origin of M. thyrohyoideus merely by a tendinous strip (Fig. 4).
The M. thyrohyoideus arises immediately rostral to the insertion of M. sternothyroideus, broadens and covers the rostrolateral surface of the thyroid cartilage. In contrast to other bovids, the M. thyrohyoideus is broad and strong. It inserts not only onto the thyrohyoideum but also onto the basihyoideum (Fig. 4).
The weight of the excised larynx, including the epiglottis, the caudal portion of the pharynx, the ventral portions of the hyoid apparatus and the anterior seven tracheal rings but excluding the extrinsic laryngeal muscles, amounted to 650 g. Thus a reasonable estimate of the average weight of the isolated larynx in the adult males of P. gutturosa is 500–600 g.
Topographic position of larynx, palatinal and tympanic pharyngeal pouch
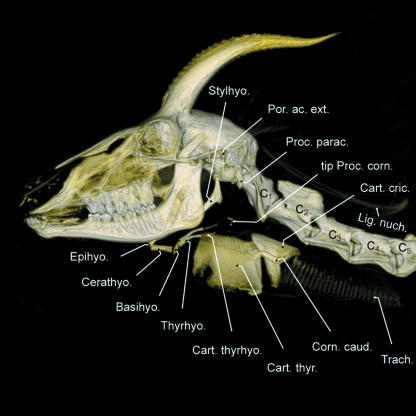
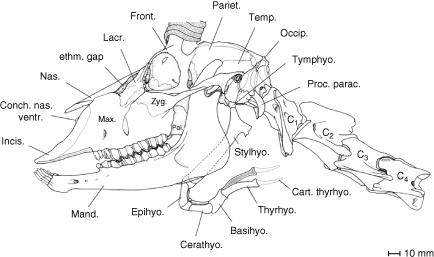
The larynx of the male Mongolian gazelle causes the ventral neck region to bulge conspicuously. The contours of the larynx can easily be detected from outside, especially in males wearing their summer coat (Fig. 1). The rostroventral prominence of the thyroid cartilage in this dead individual corresponds approximately with the level of the tip of the transverse process of the atlas (Ala atlantis), the prominence of which can also be seen from outside below the ear. The caudal contour of the larynx is marked by the step-like transition of the cricoid cartilage into the trachea. The contours of the larynx, as seen from outside, roughly circumscribe a rectangle of about 125 mm in craniocaudal length and 75 mm in dorsoventral height. The larynx lies ventral to the anterior 2–3 cervical vertebrae (Fig. 5). The position of the larynx as determined from anatomical dissection was about one vertebral length (50–70 mm) more caudal than that obtained by computer tomographic imaging (Figs 1, 5 and 6). The rostral horn of the thyroid cartilage articulates with the rod-like cartilaginous elongation of the thyrohyoideum (Fig. 5). The dorsal contour of the pharyngeal constrictor muscles touches the ventral surfaces of the adjacent neck musculature (from medial to lateral: M. longus colli, M. longus capitis, M. cleidocephalicus).
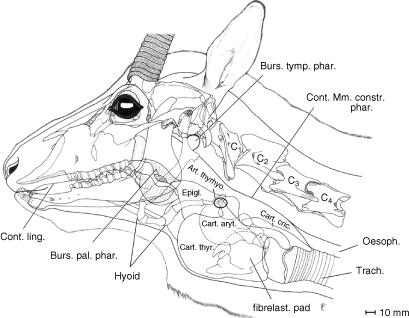
Fig. 5.
Overlay comprising head contour, skull, anterior cervical vertebrae, hyoid apparatus, palatinal and tympanic pharyngeal pouches, and larynx of an adult male Mongolian gazelle illustrating relative topographic positions. The unpaired palatinal pharyngeal pouch, a ventromedian sac of the soft palate, is intercalated between the root of the tongue and the epiglottis.
Fig. 6.
Computer tomographic image of the head and neck of an adult male Mongolian gazelle showing relative positions of skull, anterior cervical vertebrae, hyoid apparatus and larynx. Note the rod-like cartilaginous elongation (thyrohyoid cartilage) of the dorsally convex thyrohyoideum. Surprisingly, the rostral horn of the thyroid cartilage is not visible (cf. Fig. 5). Owing to a certain amount of calcification, the dorsal tip of the corniculate process can be identified.
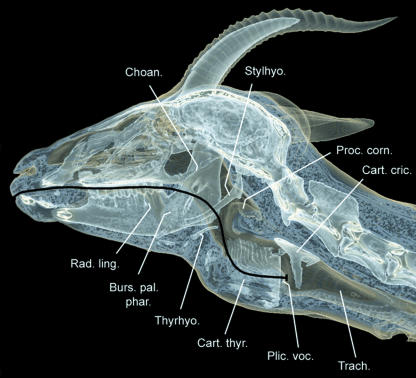
The peculiar palatinal pharyngeal pouch (PPP) of the male Mongolian gazelle consists of an unpaired ventromedian sac of the soft palate. Rostrally, it is fastened to the palatinal and pterygoidal bones, forming the capacious choanae. Its caudal edge, ending with the palatoglossal arcs, is dorsally overlapped by the tip of the epiglottis. Laterally, the PPP is bordered on both sides by the stylohyoidea and, further laterally, by the angles of the mandibulae. Additional lateral support is provided by mucous membrane folds (cf. Kleinschmidt, 1961). Ventrally, the PPP lies in the depression between the torus of the tongue and the ventral prominence of the epiglottis (Fig. 5).
The position of the PPP differs from that described by Kleinschmidt (1961). He found the PPP (‘Bursa faucium’ in his nomenclature) protruding rostrally into the oral cavity between the hard palate and the tongue (see figures 6–12 in Kleinschmidt, 1961). However, in our specimen the PPP hung down from the soft palate between the root of the tongue and the epiglottis (see Figs 5, 10 and 14).
Fig. 12.
Measurement of vocal tract length (VTL = lips to glottis) in a computer tomographic image. Computed mediosagittal section of the head and neck of an adult male Mongolian gazelle (same specimen as in Fig. 6); medial view of the right half. The vocal fold between the caudal edge of the thyroid cartilage and the rostral edge of the cricoid arc can be clearly identified.
Fig. 10.
Palatinal pharyngeal pouch, laryngeal cavity and vocal fold of an adult male Mongolian gazelle. Left halves of mandibula and larynx removed; rostral and caudal tube-like spaces between thyroid cartilage and resilient cushion damaged by the section; cricoid lamina broken by bullet. Large laryngeal entrance and vestibule. The caudally directed bow-like vocal fold is thick and tough and lacks any rostrally directed flexible structures. It is supported by a cymbal-like fibroelastic pad. Scale bar = 50 mm.
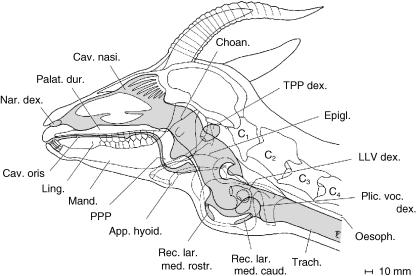
Fig. 14.
Sagittal section of the head and neck summarizing vocal tract specializations of the adult male Mongolian gazelle, based upon anatomical dissection and computer tomographic images. Nasal conchae schematic. Shaded areas: air-filled spaces.
The PPP is approximately 42 mm in craniocaudal length and 35 mm in dorsoventral height. The mucous membrane of the PPP is deeply wrinkled outwards and inwards. In our specimen it was not of the dark bluish grey colour reported by Kleinschmidt, but of a light flesh-coloured appearance as were the surrounding structures of the pharynx. As Kleinschmidt's specimens originated from a commercial meat import from China in winter 1958/59, we infer that they were also immediately deep-frozen after death. However, the colour of the pharyngeal mucous membranes may be influenced by physiological status (rut), locally increased circulation in the course of calling, etc.
Compared with domestic ruminants, the nasopharynx of the male Mongolian gazelle is elongated by the formation of a PPP (cf. Nickel et al. 1987, pp. 48–49). Consequently, the rostral edge of the thyroid cartilage is not immediately adjacent to the root of the tongue as in domestic ruminants. Instead, the larynx has been displaced caudally by 40–50 mm as already stated by Kleinschmidt (1961, p. 10). The perpendicular osseous walls surrounding the choanae along the base of the skull, i.e. the pterygoidal, palatinal and sphenoidal bones, are correspondingly elongated. Thus, the opening by which the nasal and the laryngeal portion of the pharynx communicate (Ostium intrapharyngeum) has also been caudally displaced (Kleinschmidt, 1961, p. 17).
An additional specialized structure of the pharynx is the thin-walled inflatable tympanic pharyngeal pouch (TPP), a paired sac-like protrusion of the dorsal pharyngeal wall between the base of the stylohyoideum and the tympanic bulla. Rostrally, it is in contact with the tympanohyoideum and the angle of the stylohyoideum and caudally it touches the paracondylar process and the occipitohyoid muscle (Fig. 5). This peculiar TPP was described by Kleinschmidt (1961, p. 10) as ‘tympanic sinus’. Approximate measurements of the TPP, when being artificially inflated to an extent that it tightly fits the space provided by the surrounding structures, are 25 mm in dorsoventral height and 25 mm in rostrocaudal width.
Hyoid apparatus
The basic structure of the hyoid apparatus of the male Mongolian gazelle, despite profound specializations of the larynx, largely conforms to that of other bovids (Figs 6 and 7). The short and cartilaginous tympanohyoideum connects the hyoid apparatus to the styloid process of the petrosal portion of the temporal bone. The stylohyoideum, as mentioned above, embraces the PPP on both sides. The connection between the rostral edge of the thyroid cartilage and the basi- and thyrohyoideum is established by a relatively long (20–30 mm) thyrohyoid membrane. In contrast to what is seen in other bovids, the thyrohyoideum is elongated caudally by a rod-like cartilaginous element. The caudal tip of this thin thyrohyoid cartilage connects to the rostral horn of the thyroid cartilage to form the synovial thyrohyoid articulation (Figs 5–7). It comprises a capsule containing synovial fluid. In addition, this synovial junction is strikingly loose, and thus a high mobility of this thyrohyoid articulation can be assumed (Fig. 5).
Fig. 7.
Skull, hyoid apparatus and anterior cervical vertebrae of an adult male Mongolian gazelle. Supposedly, the elevated basihyoideum can turn down caudoventrally and, thus, contributes to the rostrocaudal mobility of the larynx. The thyrohyoid cartilage forms an easily mobile synovial thyrohyoid articulation with the rostral horn of the thyroid cartilage (cf. Fig. 5).
A strong triangular-shaped ceratohyoideus muscle (not shown) arises from the caudal edge of the ceratohyoideum and inserts onto the dorsal edge of the thyrohyoideum. It reduces the angle between these two osseus elements and may thereby pull the larynx rostrodorsally.
Thyroarytenoid muscle and lateral laryngeal ventricle
The intrinsic laryngeal muscles including the thyroarytenoid muscle have already been described in context elsewhere (Frey & Riede, 2003).
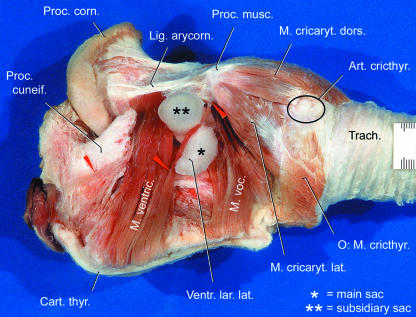
The large thyroarytenoid muscle of the male Mongolian gazelle is exposed after removal of the left half of the thyroid cartilage and a rostroventral fat cover. In contrast to what is seen in other bovids but in accordance with those mammalian taxa possessing a lateral laryngeal ventricle (see below), this muscle is clearly divided into two portions: a rostral ventricularis and a caudal vocalis muscle (Fig. 8). Surprisingly, the in situ aspect of the thyroarytenoid muscle reveals a saccular structure projecting laterally between the ventricularis and vocalis muscles (Figs 8 and 9).
Fig. 8.
Bipartite thyroarytenoid muscle and lateral laryngeal ventricle of an adult male Mongolian gazelle. Left lateral view. Main portion of epiglottis, left half of thyroid cartilage and cricothyroid muscle removed. In contrast to that in other bovids, the thyroarytenoid muscle is clearly divided into a rostral ventricularis and a caudal vocalis muscle. Between them, the two-chambered inflatable lateral laryngeal ventricle projects laterally. Scale bar is in millimetres. After Frey & Riede (2003).
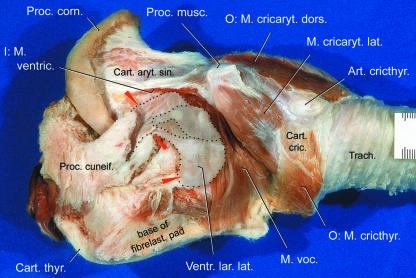
Fig. 9.
Isolated larynx of adult male Mongolian gazelle. Left lateral view. Main portion of epiglottis, left half of thyroid cartilage and ventricularis muscle removed. The V-shaped cuneiform process embraces the neck of the two-chambered lateral laryngeal ventricle (between upper two arrows). The rostrally directed opening of the LLV (not shown) lies between the corniculate process of the arytenoid cartilage and the cuneiform process. Scale bar is in millimetres. After Frey & Riede (2003).
This inflatable saccular structure constitutes the paired, thin-walled lateral laryngeal ventricle (LLV) of the male Mongolian gazelle. Such an LLV is present in some mammalian taxa, e.g. insectivores, primates, rodents, carnivores, perissodactyls, artiodactyls (suidae), but is lacking in other bovid and ruminant species (Göppert, 1937, p. 858; Nickel et al. 1987, p. 246). The two-chambered LLV of the male Mongolian gazelle consists of a roughly half moon-shaped main sac (length, ∼70 mm; diameter, 20–25 mm) and a smaller subsidiary sac (length, ∼25 mm; diameter, ∼15 mm) projecting laterally from the main sac (Figs 8 and 9). The subsidiary sac projects laterally either through the cleft between the ventricularis and vocalis muscles or through a cleft within the dorsal portion of the vocalis muscle.
In contrast to that seen in other mammalian species with an LLV, the slit-like rostral opening of the LLV in the male Mongolian gazelle (diameter, ∼15 mm) is not situated medial but lateral to the corniculate process of the arytenoid cartilage. The ‘neck’ of the main sac, between rostral opening and subsidiary sac, is kept in place by the two branches of the V-shaped cuneiform process of the epiglottis (see below), the rostral opening being located between the corniculate and cuneiform processes. The LLV covers the slightly convex lateral surface of the fibroelastic pad (Figs 8, 9 and 11).
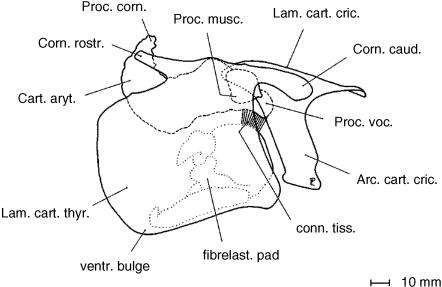
Fig. 11.
Laryngeal cartilages and fibroelastic pad of a male Mongolian gazelle assembled in near natural position. The vocal process of the arytenoid cartilage (broken line) is remarkably short. The vocal fold is supported by a cymbal-like fibroelastic pad. The dorsal portion of this resilient fibroelastic pad connects to the vocal process and its ventral portion to the keel of the thyroid cartilage. After Frey & Riede (2003).
Epiglottis and laryngeal cartilages
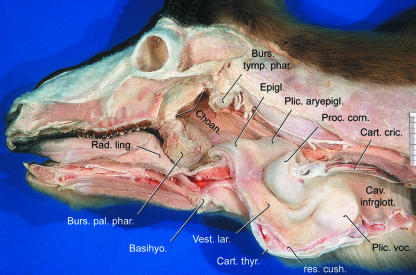
The epiglottis of the male Mongolian gazelle is very large. Its greatest craniocaudal length including the large and thick aryepiglottic fold is almost the same as that of the lamina of the thyroid cartilage. Correspondingly, the M. hyoepiglotticus is also large. It arises from the cerato- and basihyoideum and inserts onto the rostroventral surface of the epiglottis. Together with the hyoepiglottic and thyroepiglottic ligaments and a cover of fat and other connective tissue, it forms the pronounced rostroventral prominence of the epiglottis (Figs 5 and 10).
As a consequence of the remarkable size of the epiglottis and the aryepiglottic fold (Fig. 10), a long and deep piriform recess is formed. This is the mucous membrane groove through which soft, finely ground food particles and liquid are guided laterally around the epiglottis and the laryngeal entrance into the oesophagus. In accordance with Kleinschmidt (1961, p. 20), its rostrocaudal length is approximately 90 mm and dorsoventral depth 20 mm. Accordingly, the male Mongolian gazelle has an exceptionally large entrance to the larynx. Laterally, the piriform recess is supported by the thyrohyoideum, the thyrohyoid membrane and the thyroid cartilage and, medially, by the epiglottis, the aryepiglottic fold and the arytenoid cartilage.
Owing to the intercalation of the PPP, the epiglottis does not reach the root of the tongue as in domestic ruminants (cf. Nickel et al. 1987, pp. 48, 49). Instead, it is caudally displaced, its rostral tip covering the caudal edge of the PPP (Figs 5 and 10). Of particular interest is the cuneiform process of the epiglottis of the male Mongolian gazelle, which is lacking in domestic ruminants but is present in the dog and in the domestic horse. As with the epiglottic cartilage, the cuneiform process consists of elastic cartilaginous tissue (Nickel et al. 1987). The cuneiform process of the male Mongolian gazelle is bifurcated (V-shaped). It has no direct cartilaginous connection to the epiglottic cartilage but is attached to the laterocaudal base of the epiglottis via connective tissue. The two caudodorsally orientated branches of the cuneiform process embrace the neck of the lateral laryngeal ventricle (see below and Fig. 9).
The laryngeal cartilages of the male and female Mongolian gazelle have been described in detail elsewhere (Frey & Riede, 2003; Fig. 11).
Laryngeal cavity, vocal fold and fibroelastic pad
Owing to the large epiglottis, the laryngeal vestibule, i.e. the space from the laryngeal entrance up to the glottis, and the laryngeal entrance itself, between the inner aspects of epiglottis, aryepiglottic folds and the corniculate tubercula, are correspondingly large. The space between the ventral wall of the laryngeal vestibule and the dorsal surface of the keel of the thyroid cartilage is filled by a resilient cushion of connective tissue. This cushion is subducted by tube-like hollow spaces from rostrally and from caudally. The rostral tube, about 20 mm in length, resembles the median laryngeal recess of equids (Göppert, 1937, p. 859; Nickel et al. 1987, pp. 248, 251). Access to it is by a circular opening about 5 mm in diameter from the floor of the laryngeal vestibule. The caudal tube has been described by Kleinschmidt (1961 figure 12, a, p. 20ff). In our specimen it measures about 30 mm in length and its 4- to 5-mm-wide opening lies immediately rostroventral to the vocal cleft.
Subsequent to the laryngeal entrance, rostral to the corniculate processes of the arytenoid cartilages, is a narrow passage along which the mucous membrane is folded longitudinally (cf. Kleinschmidt, 1961, p. 21). Then, ventral to the corniculate processes, the laryngeal vestibule widens again to form a large central space. The caudal portion of the laryngeal vestibule narrows again along the large vocal folds up to the glottis. Subsequent to the glottis, the cricoid cartilage surrounds a large infraglottic cavity, which, at the caudal edge of the cricoid arch, narrows step-like to the diameter of the trachea (Fig. 10).
The dorsoventral diameter of the caudally directed bow-like vocal fold of the male Mongolian gazelle is large owing to an evolutionary increase in dorsoventral height of the thyroid cartilage (Frey & Riede, 2003). The vocal fold is approximately 40 mm in dorsoventral height and 17 mm in rostrocaudal length. Its transverse diameter is also large owing to the thick and tough fibroelastic pad (FEP, cf. Frey & Riede, 2003) and, as in other bovid species (cf.Albrecht, 1896; Nickel et al. 1987, pp. 248, 287), lacks any rostrally directed flexible structures. Its transverse diameter decreases from ∼10 mm at its base to 5–6 mm at its caudal edge.
The vocal fold projects from the wall of the laryngeal vestibulum obliquely in caudomedial direction, thereby forming the vocal cleft, approximately at the level of the gap between thyroid and cricoid cartilages bridged by the cricothyroid ligament (Fig. 10).
Surprisingly, the vocal fold of the male Mongolian gazelle is supported by a peculiar, roughly pan- or cymbal-like, FEP. The dorsal portion of this resilient FEP connects to the short vocal process of the arytenoid cartilage and its ventral portion to the dorsal surface of the keel of the thyroid cartilage (Fig. 11). The FEP's slightly convex lateral surface forms the lateral edge of the vocal fold adjacent to the infraglottic cavity and its slightly concave medial surface forms the medial edge of the vocal fold adjacent to the laryngeal vestibule up to the vocal cleft.
Histologically, the FEP was found to consist mainly of collagen fibres (roughly 90%) and few elastic fibres (about 10%).
The free portion of the vocal fold projecting caudomedially into the laryngeal cavity is much smaller (about one-third the size) than the whole, almost spherical, FEP. Thus, the vocal fold is deeply rooted within the wall of the laryngeal vestibule by the FEP (Figs 10 and 11).
Measurements of the vocal tract
Vocal-tract length (VTL) in the dissected male Mongolian gazelle (string measure) was 370 mm (cf. Fig. 10). In the deep-frozen specimens used for computer tomographic imaging the larynx was situated further rostrally than the position determined in the course of anatomical dissection. Consequently, VTL measured in the computer tomographic images was only 310 mm (Fig. 12).
Spectrograms and call parameters
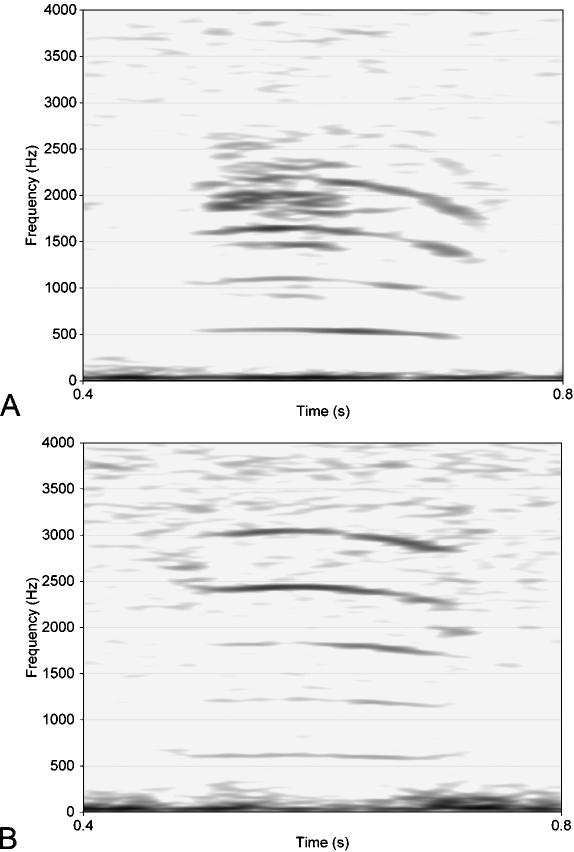
Two preliminary spectrograms of representative vocalizations of one adult male and a juvenile for comparison are shown in Fig. 13. Assigning calls with the lowest pitch to adult males and calls with the highest pitch to juveniles is based upon visual observation prior to nocturnal recording of the calls. Duration of a call is 250 ms on average (n = 128). (Here and in the following n is the number of analysed calls. It may contain several calls from the same individual.) The sound energy of the adult male's call is concentrated in three or four bands around centre frequencies of a harmonic ratio. In addition, noisy portions and, in some calls, a certain amount of subharmonics are found (Fig. 13A). The centre frequency of the lowest band is about 500 Hz (n = 41). The mean distance between harmonic bands of a single call is approximately 410 Hz. Highest intensities are achieved at 0.4–2.4 kHz. The position of the band with the lowest frequency and (more pronounced) that of the bands with the higher frequencies may vary along a single call by up to 200 Hz. Usually, frequencies increase at the beginning of a call and decrease towards the end.
Fig. 13.
Spectrograms of representative calls of an adult male (A) and of a juvenile (B) Mongolian gazelle. Call duration is about 0.25 s in both cases. In the calls of adult males (n = 41) the lowest-frequency band averages 500 Hz. Possibly the noisy parts in A are indicative of non-linear phenomena. In the calls of juveniles (n = 21) the lowest-frequency band is ∼790 Hz.
The average frequency of the lowest band of the juvenile's call is approximately 790 Hz (n = 21), i.e. about 300 Hz higher than that of an adult male. Compared with the calls of adult males, frequency bands in the calls of juveniles are also of harmonic ratio but vary less along a single call (Fig. 13B). The average distance between harmonic bands is about 640 Hz. Frequencies decrease slightly towards the end of the call.
The remaining intermediate calls could not be assigned to either male or female individuals. Their lowest frequency band is at approximately 600 Hz. The average distance between harmonic bands is ∼530 Hz (n = 35).
Discussion
Extrinsic laryngeal muscles and laryngeal weight
In the male Mongolian gazelle, with an average body weight of 31.5 kg (Sokolov & Lushchekina, 1997, cf.Frey & Riede, 2003), evolution has led to a larynx of more than 0.5 kg weight suspended from the anterior neck region. It was assumed that this evolutionary transformation had been favoured by sexual selection via male–male competition and female choice (Frey & Riede, 2003). This increase in laryngeal mass must have been accompanied by a strengthening of the laryngeal suspension and stabilization.
Together with the parotidoauricularis muscle, the extrinsic laryngeal muscles support the larynx laterally and ventrally. The ventrally and caudally situated sternohyoid and sternothyroid muscles of the male P. gutturosa seem to have retained the shape they have in other bovids: as long and slender muscular bands, they connect the cranial portion of the sternum to the hyoid and to the thyroid cartilage, respectively. Contraction of these band-like muscles may therefore pull the hyoid and/or the larynx caudally. By contrast, the laterally situated thyrohyoid and especially the omohyoid and sternomandibularis muscles are markedly enlarged in width and also in strength (thickness) as is the vostral M. hyoepiglotticus. The sternomandibularis muscle even has evolved an additional dorsal portion with added insertions onto the laterodorsal neck musculature and onto the parotid fascia.
Compared with that seen in other bovids of comparable body size, the larynx of the male P. gutturosa has been evolutionarily enlarged to about double the original size (Frey & Riede, 2003) and, thus, its mass, owing to geometrical relationships, has increased three-fold. This substantial gain in laryngeal mass seems to demand strong lateral support to diminish lateral swinging movements of the larynx during fast locomotion as in amble or gallop. This improved lateral support is provided by three lateral muscle layers (sternothyroid plus thyrohyoid, sternohyoid plus omohyoid, double-bellied mandibular portion of sternocephalic muscles).
Supportive action of the extrinsic laryngeal, ventral hyoid and sternomandibularis muscles is supplemented by the interconnected broad ventral portions of the parotidoauriculares muscles, which, hammock-like, surround the epiglottal region.
Epiglottis and laryngeal descent
The epiglottis of the adult male P. gutturosa is exceptionally large, almost equalling the craniocaudal linear dimensions of the thyroid cartilage. Epiglottal enlargement, of course, is related to the evolutionary enlargement of the larynx itself. However, a further disproportional enlargement may have been caused by the caudoventral displacement of the larynx by 40–50 mm. This may have occurred prior to or concomitant with the evolution of a PPP between the root of the tongue and the base of the epiglottis, resulting in a limited and permanent evolutionary descent of the male larynx (cf. Fitch & Reby, 2001). In consequence, the topographic relations of the likewise descended epiglottis in the male P. gutturosa have changed in so far as the nasopharynx became elongated caudally (see above) and the epiglottis became enlarged and elongated rostrally. Compared with the typical situation in bovids, the anatomical ‘decoupling’ of the larynx from the choanae (despite their caudal elongation), the loose synovial thyrohyoid articulation via a thin and flexible cartilaginous rod and the strong ceratohyoid muscle suggest an increased mobility of the larynx in rostrocaudal directions. Contrary to the assumption in Frey & Riede (2003), a comparison of the in situ dissection with the computer tomographic images points to a laryngeal mobility of about one vertebral length, i.e. 50–70 mm. This may be called a physiological momentary descent, which presumably occurs during vocalization (cf.Fitch, 2000b; Fitch & Reby, 2001).
Retention of the rostral tip of the epiglottis within the caudal opening of the nasopharynx (i.e. at the caudal edge of the PPP) and the necessity for an adequate closure of the laryngeal entrance in the course of swallowing also probably demanded for a disproportional enlargement of the epiglottis. In soricoid and erinaceid insectivores, in canid and ursid carnivores, in murid and arvicolid rodents (Göppert, 1937, p. 844ff), in primates (Starck, 1995, p. 508) and in the domestic horse (Nickel et al. 1987, p. 245) the lateral base of the epiglottis is equipped with a wedge-shaped elastic cartilaginous process. This dorsocaudally directed cuneiform process is missing in domestic ruminants (Nickel et al. 1987, p. 245) and in cervids (Köhler, 1982). However, the epiglottis of the adult male P. gutturosa has a V-shaped elastic cuneiform process attached to the lateral base of the epiglottis by connective tissue. This V-shape is probably achieved by resorption of the central and caudal portions of the primary ‘wedge’. This bifurcated cuneiform process may serve two functions. First, it may stabilize the ventrocaudal portion of the long aryepiglottic fold, and correspondingly the long and deep piriform recess, and thereby the ventrocaudal portion of the laryngeal entrance itself. It is reasonable to assume that an enlarged larynx with an exceptionally large laryngeal entrance is particularly susceptible to choking owing to the crossing of respiratory and digestive tracts within the pharynx. Stabilization of the long aryepiglottic fold of the male P. gutturosa might therefore be essential to prevent choking and to guarantee safe passage of food particles through the exceptionally deep piriform recess around the large laryngeal aditus into the oesophagus. In addition, the enlarged corniculate process of the arytenoid cartilage (cf. Frey & Riede, 2003) and a pronounced rostrocaudal elevation and lowering movement of the larynx (see above) may contribute to an adequate closure of the laryngeal entrance in the course of swallowing. The second function may be to allow topographic fixation of the opening and neck of the LLV between its dorsal and ventral branches. Lack of a direct cartilaginous connection between epiglottis and cuneiform process, i.e. their separation, is assumed to be a derived character state. In the primitive situation (see above), the median and the two lateral portions of the epiglottis are united, i.e. the cuneiform process is a continuation of the lateral base of the epiglottis (Göppert, 1937, p. 844ff).
The above considerations may serve to give a functional explanation as to why the male P. gutturosa has a well-developed specialized cuneiform process. Phylogenetically, this appears to be a newly developed (secondarily derived) character state because a cuneiform process is missing in other bovids.
The evolutionary descent of the larynx and the caudal elongation of the nasopharynx in the male P. gutturosa, although less pronounced, is reminiscent of the pharyngo-laryngeal topography in the roaring felids of the subfamily Pantherinae (Weissengruber et al. 2002). Interestingly, one precondition for roaring in the five Panthera species is the presence of a proportionally large undivided vocal fold (i.e. lacking a sharp rostral edge) being rostrally supported by a large pad of fibroelastic tissue (Hast, 1986, 1989; Peters & Hast, 1994; Weissengruber et al. 2002). The structure of the vocal fold in the male P. gutturosa is quite similar.
Vocal fold, fibroelastic pad and implications for sound production
The lowest producible fundamental frequency of phonation is determined by the length and mass of the vocal folds: the larger the folds the lower the fundamental frequency (Fitch, 1997). The vocal fold of P. gutturosa is large and of peculiar shape and consistency. As in the roaring Pantherine species within the Felidae (Hast, 1986; 1989; Peters & Hast, 1994; Weissengruber et al. 2002), it is undivided, i.e. not clearly divided into a cartilaginous portion (supported by the vocal process of the arytenoid cartilage) and a membraneous portion (extending from the vocal process to the dorsal surface of the thyroid cartilage – cf. Titze, 1994, p. 173). Instead, the robust and bulky vocal fold of the male P. gutturosa bulges out uniformly in a bow-like manner towards the infraglottic cavity lacking any flexible membraneous structures (cf. Frey & Riede, 2003). Thus, the distinction between an ‘effective vocal fold length’ as that portion of the vocal fold actually vibrating during phonation from a non-vibrating portion anchored within the laryngeal wall (Titze, 1994, p. 173) is not applicable in the male P. gutturosa. The situation becomes even more complex by comparison with the three-layered structure of a standard vocal fold (medial to lateral: mucosa, ligament, muscle – Titze, 1994, p. 18). The intermediate layer of the vocal fold in the male P. gutturosa, i.e. the homologue of the vocal ligament, has evolutionarily hypertrophied into a large cymbal-like FEP two-thirds of which are embedded in the lateral wall of the laryngeal vestibulum and only one-third of which extends freely towards the infraglottic cavity. Thereby, a substantial mass increase of the vocal fold is induced. In consequence, we may infer that the massive vocal fold of the male P. gutturosa is evolutionarily designed to produce a low fundamental frequency. This is further enhanced by an evolutionary shortening of the vocal process. The absolute lengths of the vocal processes in female and male adult individuals of this species, despite a size relation of the entire larynges of 1 (female) to 2 (male) are almost identical (Frey & Riede, 2003). Thus, the length of the male vocal process, relative to the size of the larynx, is only half that of the female's approximately. If the vocal fold, as in a standard larynx, were extending along a straight line between the vocal process and the thyroid cartilage, a selective shortening of the vocal process alone would result in a corresponding increase in vocal fold length. However, vocal fold length in the male P. gutturosa is further increased by the convex bow-like curvature of the caudal edge of the vocal fold. Thus, in addition to the evolutionary mass increase of the vocal fold, the vocal fold length has also been increased. Functionally, both features tend to produce a low fundamental frequency that is perceived as a low pitch (see below).
Vocal tract and potential resonance spaces
The evolutionary intercalation of a PPP between the root of the tongue and the epiglottis in the male P. gutturosa probably caused an evolutionary descent of the larynx by ∼40–50 mm supplemented by a physiological momentary descent by ∼50–70 mm during vocalization. Although less pronounced than in the Pantherine felid species (Weissengruber et al. 2002) or in certain cervids (Fitch & Reby, 2001), both the evolutionary and the momentary laryngeal descent in the male Mongolian gazelle, more precisely, the correlated elongation of the vocal tract, can also be expected to lead to a lowering of its call's formant frequencies (Fitch, 1997; 2000a,b; Fitch & Reby, 2001; Weissengruber et al. 2002). In contrast to the pitch-correlated fundamental frequency, formants affect the timbre of a call. Apart from the length of the vocal tract, its specific three-dimensional shape also influences the filtering of the fundamental frequency plus its harmonics provided by the vocal folds (Fant, 1960; Titze, 1994, p. 137ff – see above). A formant is a resonance frequency of the vocal tract (Titze, 1994, p. 143). According to varying diameters along its rostrocaudal extension, the vocal tract is a composite filter of several (band-pass) filters, one for each formant (Titze, 1994, p. 161). The vocal tract of P. gutturosa is very peculiar in this respect. It has several subsidiary closed-end tubes of different diameters connected to the main acoustic tube (i.e. the vocal tract): an unpaired PPP, paired TPPs and LLVs and unpaired rostral and caudal median laryngeal recesses subjacent to the laryngeal vestibulum (Fig. 14). In addition, the diameters and volumina of PPP, TPP and LLV are probably variable owing to extensible walls. These subsidiary closed-end tubes might act as additional resonator devices within the vocal tract of P. gutturosa, ultimately increasing the amplitude of certain frequencies relative to others (Sundberg, 1991, p. 33). However, at present there is no further evidence for this assumption.
Comparative aspects – dulaa of the dromedary and air sacs of primates
Surprisingly, the isolated occurrence of a pouch of the soft palate (dulaa, Qulla, vesica palatina, palatine diverticulum) comparable to the PPP of the Mongolian gazelle has been documented in a phylogenetically distant taxon: the dromedary (Camelus ferus f. dromedarius, Camelidae, Tylopoda). Both sexes of the dromedary have a dulaa, although it is better developed in the male (Arnautovic & Abdel Magid, 1974; Smuts & Bezuidenhout, 1987). By contrast, a pouch of the soft palate is lacking in the two-humped camel and in the llama (Lesbre, 1903; Hegazi, 1949). The dulaa of the dromedary conforms with the corresponding structure of the Mongolian gazelle in the following points: (1) the palatinal pouch is located within a spacious depression between the larynx and the root of the tongue immediately caudal to the isthmus faucium, and (2) the mucosal wall of the palatinal pouch is strongly folded in the non-inflated state.
The dulaa is produced by the male during the rut or when being severely irritated. Projection of the dulaa outside the mouth cavity is accompanied by extensive salivation and by the production of odd gurgling sounds. The dulaa is considered to be functionless in the female dromedary (Hegazi, 1949).
Although the wall of the PPP in the male Mongolian gazelle is strongly folded, its dorsally directed opening seems to impede an inflation mechanism similar to that of the dulaa in the dromedary by raising the soft palate for closure of the choanae (Arnautovic & Abdel Magid, 1974).
This apart, no one has observed the PPP protruding from the mouth of a male Mongolian gazelle during the rut. However, matings occur at night (Heptner et al. 1989; Sokolov & Lushchekina, 1997) and therefore protrusion of the PPP in the course of immediate circumcopulatory vocal behaviour might has escaped attention. Thus, at present, the following points remain unclear: (1) can the PPP be inflated in the Mongolian gazelle, and (2), if yes, by what mechanism is it inflated, (3) is the PPP projecting outside the mouth cavity in the inflated state as in the dromedary, and (4) does the PPP play a role in the vocalization of the Mongolian gazelle?
In apes, in the larger species of gibbons and in species of the Colobinae, paired lateral ventricular air sacs occur in the ventral neck region and upper thorax (Hewitt et al. 2002). They arise as appendices of the lateral laryngeal ventricles and, thus, are homologues of the LLV in the Mongolian gazelle. The large ventricular air sacs in primates are assumed to play a role in vocalization, possibly as resonance chambers. Intensity of the booming calls in De Brazza's monkey (Cercopithecus neglectus) was reduced after puncturing the air sacs experimentally. Additionally, males producing louder calls than females have larger air sacs in cercopithecines (Starck & Schneider, 1960; Hewitt et al. 2002). Radiological analyses confirm that air enters the air sacs during exhalation and leaves during inhalation. Lateral ventricular air sacs in the Orang Utan (Pongo pygmaeus) can extend to 6000 cm3 in volume when being inflated (Starck & Schneider, 1960). Hewitt et al. (2002) argue that rebreathing air during vocalizations from air sacs could help to prevent hyperventilation.
The volume of each LLV in the Mongolian gazelle amounts to about 30 cm3 in the non-inflated state, roughly calculated according to the measurements given in Frey & Riede (2003). In addition, the inflation of the LLVs in the Mongolian gazelle is considerably restricted by the cartilaginous framework of the larynx. In consequence, the LLV volumes seem too small to allow for rebreathing and prevention of hyperventilation during call series. In view of the rostrally directed openings of the LLVs, their filling mechanism has yet to be elucidated. At present, the role of the LLVs in the vocalization of the Mongolian gazelle can only be a matter of conjecture.
Call structure and habitat
Vocalizations comprise two components: the primary sound production by a source characterized by its fundamental frequency, and the modification (filtering) of the primary sound signal within resonance cavities rostral to the sound source characterized by formant frequencies (source-filter theory, Fant, 1960; Titze, 1994). This implies a distinct correlation between the specific anatomy of a certain vocal tract and its corresponding vocalizations.
Owing to unfavourable recording conditions, the quality of our acoustic raw data is not optimal. Therefore, we need to be cautious in drawing conclusions from the acoustic analysis. The social context of the calls could not be ascertained, but a relationship of the calls to rutting behaviour can be excluded as the recordings were taken in August. If assuming that laryngeal specializations of males only come into play in the context of rut, non-rut calls may be inappropriate acoustic signals for study. Nocturnal recording of the calls impeded the identification of calling individuals.
It is possible that, owing to the small signal-to-noise ratio of the recordings, the beginning and the end of a call are not completely detectable. Consequently, the duration of a single call might be somewhat longer than assumed by us (see above). The occurrence of call parts below a certain amplitude, e.g. noisy regions, subharmonics, etc., might be obscured by the small signal-to-noise ratio. In addition, the frequency range of the calls could be larger than shown in the spectrograms (Fig. 13).
The observed call structures (Fig. 13) may be explained in two ways.
The lowest frequency band is the fundamental frequency (f0), i.e. the oscillation frequency of the sound-producing vocal folds. Consequently, the other bands represent higher harmonics as integer multiples of f0.
All 3–4 main frequency bands in the spectrograms are formants, i.e. resonance frequencies of the vocal tract (cf.Ungeheuer, 1958, 1962; Fant, 1960; Fellbaum, 1984).
We assume that the first case applies: the frequency bands are f0 and its higher harmonics. The intensities of the two bands between 1.5 and 2.5 kHz are higher than that of the band around 1 kHz (Fig. 13B). Possibly, this points to a stronger attenuation of the first harmonic frequency of f0 compared with the second and third harmonic frequencies by the filter function of the vocal tract. Additional evidence for the frequency bands being f0 and its higher harmonics are their relative constant distances. In contrast to the integer ratio of the fundamental frequency f0 and its harmonics, distances between formants may vary according to the diverse resonance spaces of the vocal tract of P. gutturosa (see above). Unfortunately, the fundamental frequency could be determined only in a few calls. In most calls the glottal beats could not be resolved from stochastic fluctuations.
The calls of adult males of P. gutturosa feature a clearly lower fundamental frequency than those of juveniles and remaining individuals. Probably the main causes for this are the overproportional evolutionary mass increase and elongation of the vocal folds in the male (see above), leading to an increase in the largest producible wavelength, i.e. to a decrease in the lowest producible frequency of the glottal source.
The evolution of the pronounced sexual dimorphism of the larynx of P. gutturosa has been related to its mating system and, ultimately, to sexual selection (Frey & Riede, 2003).
Habitat has an influence on acoustic communication. Higher vegetation (trees, shrubs, thickets, etc.), in particular, affects sound propagation by reflecting, scattering and attenuating acoustical waves (Martens & Marler, 1977; Waser & Waser, 1977; Richards & Wiley, 1980; Bullen & Fricke, 1982; Dabelsteen et al. 1993; – cf.Frey & Hofmann, 2000). However, the habitat of P. gutturosa, i.e. the flat Mongolian steppe, is devoid of higher vegetation. In such an environment an almost undisturbed sound propagation around the head of a vocalizing animal can be assumed. The lower the emitted frequency, the more homogeneous is the directivity of the call (Gebler, 2001). Therefore, in the habitat of P. gutturosa a call with a low pitch may be best suited for multidirectional advertisement calls during the rut (Bradbury & Vehrencamp, 1998).
The acoustical properties of the ground also have an influence on the potential range of an acoustic signal. Firm ground devoid of higher vegetation and with only a sparse cover of low vegetation, as found in the habitat of P. gutturosa, is capable of reflecting emitted sound waves. The distance that ground-reflected waves have to travel from the head of a sending animal to the head of a potential receiver (heads ∼1 m above the ground) is longer than that for non-reflected waves. Interference between the direct and the reflected sound field may destroy or at least reduce the signal depending on its wavelength (Embleton, 1996).
Transmission functions of sound in air over different ground types have been investigated by several authors (see Embleton, 1996). The relative range of a call can be estimated by defining cut-off frequencies, i.e. frequencies at which the sound pressure level has decreased by 3 dB (source height: 1.2 m; Embleton, 1996). According to such estimates, the signal range of a call of an adult male P. gutturosa in its natural habitat can be expected to be approximately up to three-fold larger than that of a juvenile (cf.Embleton, 1996, fig. 5). However, actual acoustic conditions near the ground are difficult to determine and therefore relative signal ranges may deviate from this value.
Sound propagation is also affected by atmospheric conditions. The amount of absorption depends mainly on temperature and air humidity. In general, higher frequencies are more intensely absorbed than lower frequencies. Considering the meteorological characteristics of the Mongolian gazelle's habitat, i.e. 30–60% relative air humidity and about 13 °C of daily temperature fluctuation (www.wetter.com: Dalanzadgad, Mongolia), the absorptive effects caused by humidity are almost equal for frequencies within the range from about 400 Hz to 800 Hz and can therefore be neglected (cf.Embleton, 1996, figure 3). The velocity of sound propagation is influenced by vertical temperature gradients ultimately leading to a curved propagation path. At night the sparsely covered ground cools faster than the air above owing to heat radiation. As a result of such temperature inversion part of the sound energy, which normally would travel upwards, turns down, thereby increasing the sound level at the receiver above expected values. The frequencies of the Mongolian gazelle's calls are likely to be affected by this phenomenon. Within the frequency range of calls of P. gutturosa this effect depends heavily on the extent of the temperature gradient (Embleton, 1996, figure 13). However, a reliable estimate cannot be given yet as actual temperature gradients were not measured.
Acknowledgments
We wish to thank the Mongolian Academy of Sciences, in particular its general secretary, Dr T. Galbaatar, and the National University of Ulaanbaatar, in particular Professor Dr R. Samjaa, Zoological Faculty, for support in providing the specimens. One specimen was collected and transported to the IZW by veterinarian H. M. Mix (Nature Conservation International) in December 2000, the others by the German–Mongolian expedition ‘semidesert mammalogy’ to the Republic of Mongolia in August 2002 led by Dr I. W. Stuermer, University of Göttingen. Special thanks to R. Tungalag (Mongolian botanist), to B. Sodnomdarj (Mongolian driver) and to Mr Gantumur and his son (Mongolian herdsmen) who assisted in obtaining acoustical records of the Mongolian gazelle. Dr K.-H. Frommolt (archive for animal acoustics, Museum of Natural History, Berlin) lent technical support for making the records and for acoustic analysis. Dr S. Badtke (Radiological Department, Ruppiner Kliniken, Neuruppin, Germany) provided technical assistance in computer tomographic scanning. Scanning and processing of the data was done by V. Vasuthevan and B. Landgraf (General Electrics Medical Systems, Solingen, Germany). The original photograph of the Mongolian gazelle was kindly provided by C. Tittmann, University of Göttingen. B. Paschmionka, dissector at the IZW, assisted in processing of the specimens. Histological sections and staining were done by D. Viertel, technical assistant at the IZW. Three referees critically reviewed the manuscript. Special thanks to our librarian, Mrs B. Peters, who provided unvaluable help in procuring the literature.
References
- Albrecht H. Beitrag zur vergleichenden Anatomie des Säugethier-Kehlkopfes. SB Kais. Akad. Wiss. Math.-Naturwiss. Cla. 1896;105:227–322. [Google Scholar]
- Arnautovic I, Abdel Magid AM. Anatomy and mechanism of distension of the dulaa of the one-humped camel. Acta Anat. 1974;88:115–124. doi: 10.1159/000144229. [DOI] [PubMed] [Google Scholar]
- Bradbury JW, Vehrencamp SL. Principles of Animal Communication. Sunderland, MA: Sinauer Associates; 1998. [Google Scholar]
- Bullen R, Fricke F. Sound propagation through vegetation. J. Sound Vibration. 1982;80:11–23. [Google Scholar]
- Dabelsteen T, Larsen ON, Pedersen SB. Habitat-induced degradation of sound signals: quantifying the effects of communication sounds and bird location on blur ratio, excess attenuation, and signal-to-noise ratio in blackbird song. J. Acoust. Soc. Am. 1993;93:2206–2220. [Google Scholar]
- Embleton TFW. Tutorial on sound propagation outdoors. J. Acoust. Soc. Am. 1996;100:31–48. [Google Scholar]
- Fant G. Acoustic Theory of Speech Production. S-Gravenhage: Mouton; 1960. [Google Scholar]
- Fellbaum K. Sprachverarbeitung und Sprachübertragung. Berlin: Springer Verlag; 1984. [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J. Acoust. Soc. Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Skull dimensions in relation to body size in nonhuman mammals: the causal basis for acoustic allometry. Zoology. 2000a;103:40–58. [Google Scholar]
- Fitch WT. The phonetic potential of non-human vocal tracts: comparative cineradiographic observations on vocalizing animals. Phonetica. 2000b;57:205–218. doi: 10.1159/000028474. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Reby D. The descended larynx is not uniquely human. Proc. R. Soc. London B. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Hofmann RR. Salivary glands of the takin (Budorcas taxicolor, Mammalia, Bovidae) with special consideration of the glandula zygomatica. Zool. Anz. 1998;237:139–153. [Google Scholar]
- Frey R, Hofmann RR. Larynx and vocalization of the takin (Budorcas taxicolor Hodgson 1850 – Mammalia Bovidae) Zool. Anz. 2000;239:197–214. [Google Scholar]
- Frey R, Riede T. Sexual dimorphism of the larynx of the Mongolian gazelle (Procapra gutturosa Pallas, 1777) Zool. Anz. 2003;242:33–62. [Google Scholar]
- Gebler A. Diploma Thesis. Berlin: Humboldt Universität; 2001. Richtcharakteristik der Lautgebung beim Haushund (Canis Lupus f. familiaris) [Google Scholar]
- Göppert E. Atmungssystem (Organe der Luftatmung) I. Kehlkopf und Trachea. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere. Berlin und Wien: Urban & Schwarzenberg; 1937. pp. 797–866. [Google Scholar]
- Hast MH. The larynx of roaring and non-roaring cats. J. Anat. 1986;149:221–222. [PMC free article] [PubMed] [Google Scholar]
- Hast MH. The larynx of roaring and nonroaring cats. J. Anat. 1989;163:117–121. [PMC free article] [PubMed] [Google Scholar]
- Hegazi AH. The soft palate of the camel. Br. Vet. J. 1949;105:325–328. [Google Scholar]
- Heptner VG, Nasimovich AA, Bannikov AG. In: Mammals of the Soviet Union. Heptner VG, Naumov NP, editors. Vol. 1. Leiden: EJ Brill; 1989. Ungulates. XXVII + 1147, pp. [Google Scholar]
- Hewitt G, MacLarnon A, Jones KE. The functions of laryngeal air sacs in primates: a new hypothesis. Folia Primatol. 2002;73:70–94. doi: 10.1159/000064786. [DOI] [PubMed] [Google Scholar]
- Kleinschmidt A. Über die große mongolische Kropfgazelle (Procapra gutturosa Pallas 1777) Stuttgarter Beitr. Naturk. 1961;79:1–24. [Google Scholar]
- Kleinschmidt A. Die Schlund- und Maulblase (Bursa faucium) bei Kamel und Großer Kropfgazelle. Die Naturwissenschaften. 1962;49:140–141. [Google Scholar]
- Köhler H. Schriften des Arbeitskreises für Wildbiologie und Jagdwissenschaft an der Justus-Liebig-Universität Gießen, Heft 8. Stuttgart: Ferdinand Enke; 1982. Vergleichend-anatomische Untersuchungen am Kehlkopf von Cerviden: Rotwild (Cervus elaphus Linné, 1758), Damwild (Cervus dama Linné 1758), Rehwild (Capreolus capreolus Linné, 1758) und Elchwild (Alces alces Linné 1758) [Google Scholar]
- Lesbre FX. Recherches anatomiques sur les camélidés. Arch. Mus. Hist. Nat. Lyon. 1903;8:1–195. [Google Scholar]
- Lhagvasuren B, Dulamtseren S, Amgalan L. Antelopes. Part 4: North Africa, the Middle East, and Asia. Global Survey and Regional Action Plans. SSC Antelope Specialist Group (compilers Mallon DP, Kingswood SC) Switzerland; Cambridge, UK: IUCN; Gland; 2001. Chapter 32. Mongolia; pp. 159–167. [Google Scholar]
- Lhagvasuren B, Milner-Gulland EJ. The status and management of the Mongolian gazelle Procapra gutturosa population. Oryx. 1997;31:127–134. [Google Scholar]
- Lushchekina AA. Abstract of PhD Thesis. Moscow: Russian Academy of Sciences; 1990. Eco-geographical fundamentals for the conservation and rational use of the Mongolian gazelle (Procapra gutturosa) in the People's Republic of Mongolia. (in Russian) [Google Scholar]
- Martens K, Marler P. Sound transmission and its significance for animal vocalization. I. Temperate Habitats. Behav. Ecol. Sociobiol. 1977;2:271–290. [Google Scholar]
- NAV. Nomina Anatomica Veterinaria. 4th edn. Zürich: World Ass. Vet. Anat; 1994. [Google Scholar]
- Nickel R, Schummer A, Seiferle E. Lehrbuch der Anatomie der Haustiere. Berlin: Paul Parey; 1987. 6. Aufl. Bd. 2. [Google Scholar]
- Peters G, Hast MH. Hyoid structure, laryngeal anatomy, and vocalization in felids (Mammalia: Carnivora: Felidae) Z. Säugetierk. 1994;59:87–104. [Google Scholar]
- Richards DG, Wiley RH. Reverberations and amplitude fluctuations in the propagation of sound in a forest: implications for animal communication. Am. Nat. 1980;115:381–399. [Google Scholar]
- Schaller O, editor. Illustrated Veterinary Anatomical Nomenclature. Stuttgart: Ferdinand Enke; 1992. [Google Scholar]
- Smuts MMS, Bezuidenhout AJ. Anatomy of the Dromedary. Oxford: Clarendon Press; 1987. [Google Scholar]
- Sokolov VE, Dash Y, Lushchekina AA, Neronov VM. Current Distribution and Numbers of Mongolian Gazelles in Mongolia. Zoological Research in Mongolia. Moscow: Nauka; 1982. (in Russian) [Google Scholar]
- Sokolov VE, Lushchekina AA. Mammalian Species no 571. Lawrence,KA: Allan Press Inc; 1997. Procapra gutturosa. American Society of Mammalogists. [Google Scholar]
- Starck D. 5. Teil 1/2: Säugetiere. In: Starck D, editor. Lehrbuch der Speziellen Zoologie, Bd. II: Wirbeltiere. Jena: Gustav. Fischer Verlag; 1995. [Google Scholar]
- Starck D, Schneider R. Respirationsorgane A. Larynx. Primatologia. 1960;3:423–587. [Google Scholar]
- Sundberg J. The Science of Musical Sounds. San Diego: Academic Press; 1991. [Google Scholar]
- Titze IR. Principles of Voice Production. Englewood Cliffs, NJ: Prentice Hall; 1994. [Google Scholar]
- Ungeheuer G. Die Eigenwerttheorie der Formanten und das System der Vokale. Z. Phonetik u. Allgem. Sprachwiss. 1958;11:36–48. [Google Scholar]
- Ungeheuer G. Elemente einer akustischen Theorie der Vokalartikulation. Berlin: Springer Verlag; 1962. [Google Scholar]
- Waser PM, Waser MS. Experimental studies of primate vocalization: specializations for long-distance propagation. Z. Tierpsychol. 1977;43:239–263. [Google Scholar]
- Weissengruber GE, Forstenpointner G, Peters G, Kübber-Heiss A, Fitch WT. Hyoid apparatus and pharynx in the lion (Panthera leo), jaguar (Panthera onca), tiger (Panthera tigris), cheetah (Acinonyx jubatus) and domestic cat (Felis sylvestris f. catus) J. Anat. 2002;201:195–209. doi: 10.1046/j.1469-7580.2002.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]