Abstract
Adult primates have at least five known phenotypes of vomeronasal organ (VNO), ranging from the typical morphology seen in most other mammals to complete absence. With such morphological disparity, the phylogenetic value and any inferences on ancestral VNO morphology of the primate VNO are left uncertain. The present study investigated the VNO of embryonic and fetal Tarsius bancanus borneanus (n = 4) in comparison with prenatal specimens from four other species of primates in an effort to clarify adult morphological variations. In all except one of the fetal primates, the VNO communicated to the nasopalatine duct. One exception occurred in the largest fetal Tarsius (25 mm crown—rump length), in which the VNO communicated with the nasal cavity alone. The vomeronasal neuroepithelium was well differentiated from a thinner, non-sensory epithelium in all Tarsius and New World monkeys studied, as well as late embryonic and fetal Microcebus myoxinus. In anterior sections, this neuroepithelium was found in a more superior location in Tarsius and New World monkeys compared with Microcebus myoxinus. In all primates, masses of cell bodies were found superior to the VNO, intermingled with nerve fibres. These morphologically resembled luteinizing hormone-releasing hormone neurons described in other mammals, including humans, suggesting that a primitive association of these neurons with the VNO may exist in all primate taxa. The present study revealed that prenatal similarities exist in Tarsius and New World primates in VNO epithelial morphology. However, these are transient stages of morphology. If tarsiers and anthropoids do represent a clade (Haplorhini), then the atypical morphology seen in adult tarsiers and New World monkeys probably represents the adult VNO morphology of a haplorhine common ancestor.
Keywords: Haplorhini, Jacobson's organ, Prosimii, vomeronasal neuroepithelium
Introduction
Adult primates have at least five known phenotypes of vomeronasal organ (VNO), ranging from the typical morphology seen in most other mammals to complete absence (see Smith et al. 2001, 2003). Lemuriformes (lemurs and lorises) have VNOs that are rather typical of mammals, with an inferomedial, thick neuroepithelium, and a thinner, lateral non-sensory epithelium. The degree of disparity in VNO epithelial morphology among all other primates has led to confusion regarding homology and function when making interspecific comparisons (for further discussion see Smith & Bhatnagar, 2000; Smith et al. 2001), and leaves the systematic value of the VNO in doubt. This report concerns characteristics of the VNO in tarsiers, extant primates of debated phylogenetic affinity to other primate taxa (Hofer, 1979; Ankel-Simons, 2000).
In two alternative classifications of primates, the infraorder Tarsiiformes is variously classified within the Suborder Prosimii (with Lemuriformes) or the suborder Haplorhini (with Anthropoidea: monkeys, apes and humans) (Kay et al. 1997; Fleagle, 1999; Ankel-Simons, 2000; Ross, 2000). Because tarsiers have been phylogenetically separated from other primates for at least 45 million years (Kay et al. 1997), shared features with other primates may reflect the VNO morphology of a common ancestor. Moreover, because soft tissue structures such as the VNO and associated cartilages are not preserved in the fossil record, comparisons of extant taxa represent the only means for inferring the morphology of ancestral primate VNO epithelium. The vomeronasal complex of tarsiers has cartilaginous similarities to lemuriforms and epithelial similarities to anthropoids (Starck, 1975; Wöhrmann-Repenning & Bergmann, 2001). In adult tarsiers, the VNO itself has been described as vestigial (Hill, 1955) or well developed (Starck, 1975). Specifically, the VNO resembles that found in some New World monkeys, for which neither of these descriptors may be entirely suitable (Smith et al. 2001). As adult anthropoids exhibit most of the phenotypes described for the primate VNO, the ability to infer synapomorphic characteristics is particularly challenging. Recent studies show that an incomplete picture of VNO ontogeny in primates exacerbates this dilemma (Smith et al. 2001, 2003).
Previously, prenatal samples have been instrumental in demonstrating homology of variant forms of VNOs in adult primates (Smith & Bhatnagar, 2000), but cross-age samples of prenatal primates are rare. Herein, late embryonic and fetal tarsiers (Tarsius bancanus) from the Hubrecht Laboratory were described regarding epithelial organization of the VNO with a comparison to prenatal anthropoids and lemuriforms available from the Bluntschli collection (Department of Mammalogy, American Museum of Natural History).
Materials and methods
Specimens of Tarsisus bancanus ranged from 13 to 25 mm crown—rump length (CRL), including a specimen from a late embryonic stage (VNO fully formed, with unfused secondary palate) and three fetal specimens (Table 1). Three of these previously had been sectioned at 10–15 µm and were on loan to T.D.S. from the Hubrecht Laboratory. Also on loan from this collection was an unsectioned fetal head blocked in paraffin. This head was serially sectioned at 10 µm in the coronal plane and was stained with one-step Gomori trichrome and haematoxylin—eosin procedures. The comparative sample included 13 embryos and fetuses of Microcebus myoxinus, ranging from 8 to 32 mm CRL, one Aotus vociferans fetus (CRL not recorded), one Saimiri sciureus fetus (25 mm CRL) and one Alouatta seniculus fetus (29 mm CRL) (Table 1). (On the original hand-written specimen information card, the latter specimen was recorded as ‘Alouatta (Mycetes) auratus’, which is a synonym for A. seniculus – Elliot, 1913.) Only coronally sectioned heads were selected from the Bluntschli collection. The selected specimens were cut at 15–30 µm and stained with haematoxylin—eosin, Azan or other procedures (see Shimp et al. 2003, for further details on the Bluntschli collection).
Table 1.
Somatic and vomeronasal organ measurements in prenatal primates
| Sp. no. | Species | CRL (mm) | VNNE length (µm) | Source |
|---|---|---|---|---|
| M42 | Microcebus myoxinus (Pygmy mouse lemur) | 7.5 | 140 | B |
| M7 | Microcebus myoxinus | 8.0 | 405 | B |
| M23 | Microcebus myoxinus | 9.0 | 255 | B |
| M19 | M. myoxinus | 9.0 | 195 | B |
| M9 | M. myoxinus | 13.0 | 820 | B |
| M1 | M. myoxinus | 13.0 | 840 | B |
| M14 | M. myoxinus | 15.0 | 860 | B |
| M4 | M. myoxinus | 18.0 | 1050 | B |
| M26 | M. myoxinus | 18.0 | 915 | B |
| M15 | M. myoxinus | 21.0 | 860 | B |
| M31 | M. myoxinus | 24.0 | 870 | B |
| M24 | M. myoxinus | 31.0 | 1395 | B |
| M45 | M. myoxinus | 32.0 | NA | B |
| P21 | Aotus vociferans (Owl monkey) | ? | NA | B |
| P22 | Saimiri sciureus (Squirrel monkey) | 25.0 | 460 | B |
| P23 | Alouatta seniculus (Red howler monkey) | 29.0 | NA | B |
| 633 | Tarsius bancanus (Western tarsier) | 13.0 | NA | H |
| 72 | Tarsius bancanus | 20.0 | 370 | H |
| 808 | Tarsius bancanus | 20.0 | 435 | H |
| 981 | Tarsius bancanus | 25.0 | 495 | H |
Sp. no. = specimen number; CRL = crown—rump length; VNNE length = length of neuroepithelium of VNO; NA = VNNE length unknown because of deviation from coronal plane or section thickness unknown; Source: B = Bluntschli Collection; H = Hubrecht Collection.
All sections were examined at the Neurohistology Laboratory, School of Physical Therapy, Slippery Rock University, and at the Department of Mammalogy, American Museum of Natural History. The location of structures was described using terminology for adult rather than embryonic structures (e.g. ventral = anterior), in order to facilitate comparison with adult morphology described in the literature. Measurements from museum records on CRL along with VNO length (calculated as the number of sections exhibiting vomeronasal neuroepithelium multiplied by section thickness) were tabulated (Table 1).
Results
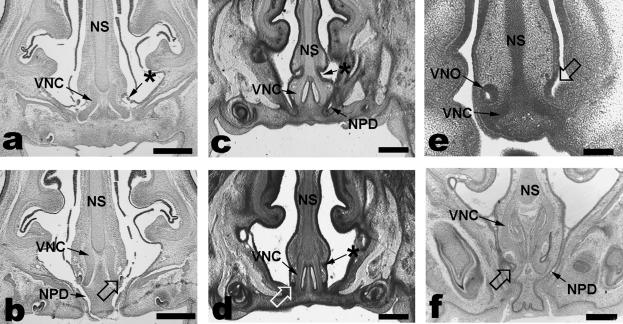
The smallest (13 mm CRL) tarsier embryo had VNOs opening bilaterally into the nasal and oral cavities (continuous at this stage via the primitive choana) through a short duct. The two 20-mm tarsier fetuses each had VNOs that communicated with the nasopalatine duct in the fully formed palate. In one of these fetuses, a groove was observed anterior to the vomeronasal duct communication to the nasopalatine duct (Fig. 1a,b), whereas the plane of section may have obscured any similar morphology in the other 20-mm fetus. The largest fetal tarsier (25 mm CRL) had a fused vomeronasal duct that did not communicate to the nasopalatine duct, which was found more anteriorly (Fig. 1c,d). In addition, this specimen had a more superiorly positioned epithelial tube, located just above the vomeronasal cartilages and anterior to the vomeronasal duct (Fig. 1c,d). This tube ended as a small epithelial mass after a length of about 105 µm, and communicated with the nasal cavities via a minute lateral aperture.
Fig. 1.
In one of the 20-mm CRL Tarsius, a groove (*) was seen that connected posteriorly with the intersection of the vomeronasal duct (open arrows) and nasopalatine duct (NPD, a,b). The largest fetal tarsier (25 mm CRL) had a fused vomeronasal duct that did not communicate with the nasopalatine duct (d), which was found more anteriorly (c). In addition, this specimen had a more superiorly positioned epithelial tube (*), located just above the vomeronasal cartilages (VNC, c,d). Microcebus had an embryonic VNO opening (open arrow) into the nasal cavity alone (e, 9-mm CRL embryo), but in fetuses (f, 33-mm CRL) the VNO opened directly into the nasopalatine duct. NS = nasal septum. Scale bars: a–d = 400 µm; e = 200 µm; f = 100 µm.
Among other primates, only the prenatal Microcebus myoxinus could be matched to tarsiers at all approximate stages of VNO maturation. The vomeronasal duct communication developed in embryos similarly to that observed for Tarsius, i.e. the VNO opened into the nasal cavity in the smallest embryos examined (Fig. 1e), but in specimens where secondary palate formation had occurred the VNO opened into the nasopalatine duct via a short duct (Fig. 1f). In contrast to the largest tarsier fetus, this communication was maintained in all older Microcebus fetuses, with a more prolonged vomeronasal duct. The secondary palate had already formed in all three New World primates examined, and in each case the VNO opened into the nasopalatine duct.
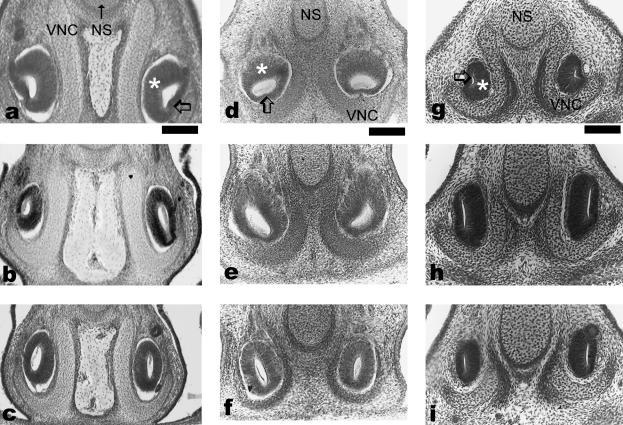
The VNO of the smallest tarsier could not be measured because section thickness was not known, but the three fetal tarsiers had VNO ranging from 370 to 495 µm in length (Table 1). The VNOs of the 13-mm Tarsius had a thickened (presumptive sensory) epithelium that was superior anteriorly (Figs 2a and 3e), and superomedial posteriorly. A thinner, presumptive non-sensory epithelium was found inferiorly in its ventral half and more medially near the midpoint. The two epithelial walls of the VNO were of nearly similar thickness toward the posterior end of the VNO. The VNOs of the 20-mm CRL fetuses had a similar organization of the VNO epithelium to the 13-mm embryo described above. The VNO of the 25-mm Tarsius resembled that in the embryo and fetuses except that the VNO epithelium was of nearly uniform thickness toward the posterior, blind end (Fig. 2a–c).
Fig. 2.
Organization of the vomeronasal organ epithelium at the 25th (top row), 50th (middle row) and 75th (bottom row) percentiles of VNO length. The left column (a–c) shows a 25-mm CRL Tarsius bancanus, the middle column (d–f) shows a 25-mm CRL Saimiri sciureus, and the right column (g–i) shows a 15-mm CRL Microcebus myoxinus. Scale bar = 100 µm.
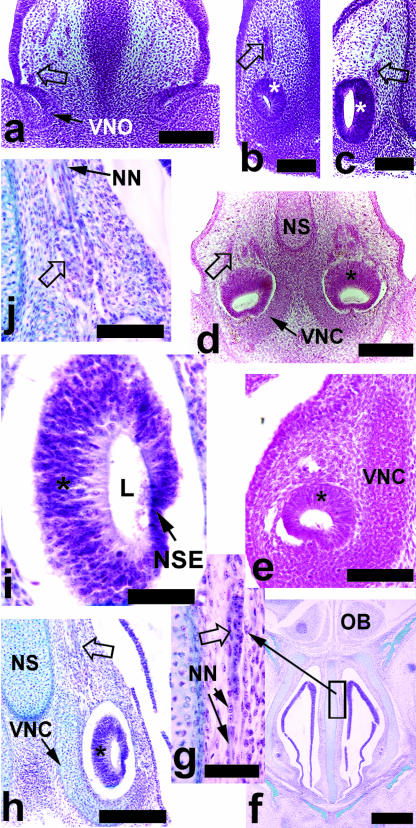
Fig. 3.
(a) An 8-mm CRL Microcebus myoxinus, showing the openings of the vomeronasal organ (VNO) with nerves and masses of paravomeronasal ganglia (open arrows); the similar cells were visible in association with the VNO itself in 9-mm embryos (b) and larger embryos (c, 13-mm CRL) of Microcebus. Note the mediolateral differentiation in the 13-mm CRL embryo, with a pronounced neuroepithelium medially (*, c). Anteriorly, the Saimiri sciureus embryo (d) had a more superiorly positioned neuroepithelium (*) and masses of paravomeronasal ganglia. (e) A 13-mm CRL embryonic tarsier had a similarly positioned neuroepithelium. A 20-mm CRL tarsier (f–j) had numerous neuronal cell bodies and ganglia visible in association with nerves (NN) anterior to the VNO (f and inset, g). (h) The posterior half of the VNO in the 20-mm fetal tarsier revealed a pronounced neuroepithelium (*) medially, with a thin lateral non-sensory epithelium (NSE, i). Ganglia and cells of neuroblastic appearance were visible superior to the VNO (open arrow, h, and enlarged in j). NS = nasal septum, VNC = vomeronasal cartilage. Scale bars: a,d = 150 µm; b,c,e,j = 100 µm; f = 500 µm; g,i = 50 µm; h = 200 µm.
Prenatal Microcebus had VNOs ranging from 140 to 1395 µm in length. In the smallest Microcebus embryos (7.5–9 mm CRL), the thickness of the vomeronasal epithelium was nearly equal on all sides (Fig. 3a,b). In all larger embryos and fetuses, a well-developed neuroepithelium was seen medially (Fig. 3c). In late embryos and early fetuses, the posterior-most part of the VNO epithelium had nearly equal thicknesses medially and laterally (Fig. 2g–i), whereas in the largest fetuses, the entire anteroposterior extent of the VNO was well divided into medial sensory and lateral non-sensory epithelia. All New World primates showed sensory and non-sensory regions. Anteriorly, the sensory epithelium was more superomedial compared with that in Microcebus. The fetal Saimiri sciureus showed a medial rotation of the sensory epithelium from anterior toward posterior parts of the VNO, with no medial–lateral differences in thickness posteriorly (Fig. 2d–f). Only the fetal Saimiri had a recorded sectional thickness, and the VNO was 460 µm long.
In all species examined, masses of cell bodies were observed superior to the VNO within the septal mucosa (Fig. 3a–d,h,j). These cell bodies varied in size from small, possibly neuroblastic cells to ganglionic bodies, and were found in close association with nerve fibres connecting to the VNO (Fig. 3j). In Microcebus, these cells were easiest to detect in embryos and early fetuses (Fig. 3a–c). The cells were not visible in the embryonic Tarsius, but dense eosinophilia of the tissue may have obscured them if present. All fetal tarsiers had ganglionic and/or other cell bodies present superior to the VNO (Fig. 3h,j). In addition, cells bodies were seen intermingled with nerve fibres in more anterior positions in one of the 20-mm Tarsius fetuses (Fig. 3f,g). Columns of cell bodies, without clearly associated nerve fibres, were seen in association with the superior blind-ending epithelial tube of the 25-mm Tarsius.
Discussion
Primitively, the mammalian VNO is composed of two distinct epithelial compartments, including a ventromedial neuroepithelium and a dorsolateral, non-sensory epithelium (or receptor-free epithelium, Breipohl et al. 1979; Sánchez-Villagra, 2001). Among mammals, primates and chiropterans exhibit the most extreme variations in the VNO epithelium (Bhatnagar & Meisami, 1998; Smith et al. 2001). Most, if not all, adult prosimians exhibit the primitive sensory/non-sensory distribution (called ‘well-developed’ by Smith et al. 2001). Anthropoid primates have four additional character states of the VNO: sensory epithelium only (neuroepithelium predominates, medially and laterally), interrupted sensory epithelium (similar, except interrupted by patches of non-sensory epithelium), displaced VNO (superiorly displaced, non-chemosensory VNO) or VNO absence (Smith et al. 2001, 2003). The communication point of the mammalian VNO varies, and the primitive condition is in debate. It occurs either as an isolated connection to the nasal cavity or at various points along the nasopalatine duct (Sánchez-Villagra, 2001; Wöhrmann-Repenning & Bergmann, 2001). Among primates, most lemuriforms appear to have a VNO communication with the nasopalatine duct, whereas anthropoids have much more variation (Smith et al. 2001; Wöhrmann-Repenning & Bergmann, 2001).
In adult tarsiers, characteristics of the vomeronasal complex are ambiguous in terms of phylogeny. Adult Tarsius has been reported to have a VNO opening into the most posterosuperior end of the nasopalatine duct, near the nasal cavity itself, and cartilaginous similarities to lemuriforms (Starck, 1975; Wöhrmann-Repenning & Bergmann, 2001). Existing descriptions of adult tarsiers imply a VNO epithelial morphology similar to that of some New World monkeys, specifically a uniform neuroepithelium (Woolard, 1925; Starck, 1975; Wöhrmann-Repenning & Bergmann, 2001).
The most notable characteristic seen in the prenatal tarsiers was the variable relationship of the VNO opening and nasopalatine duct. The lack of VNO communication to the nasopalatine duct found in the largest fetus cannot yet be adequately explained in terms of its relationship to adult morphology. The descriptions of adults show a more intimate relationship of the two ducts, although very near to the nasal cavity (Starck, 1975; Wöhrmann-Repenning & Bergmann, 2001). A larger fetal sample of tarsiers is needed to clarify this sequence of events. It is nonetheless strikingly different than the fetal mouse lemurs, which exhibited a consistent relationship of VNO duct and nasopalatine duct. The blind-ended tube observed in the largest fetal tarsier may be of interest for further scrutiny, especially if the associated columns of cells in the surrounding lamina propria indicated neurogenesis. It is also possible that the structure has an ontogenetic relationship to the groove found anterior to the VNO opening in the 20-mm fetus, each located at the superior margin of the vomeronasal cartilage.
The present study confirms that tarsiers are similar to mouse lemurs and New World monkeys in forming sensory and non-sensory divisions during fetal development. However, a more superior orientation of the neuroepithelium was evident in both Tarsius and the New World monkeys compared with Microcebus. Microcebus had a more medial or even inferomedial position to the sensory epithelium. A superior position of the neuroepithelium occurs initially in development in most mammals, but differential growth results in a more inferior (ventromedial) position soon after the VNO is formed (Garrosa et al. 1998).
A universal characteristic seen in this sample, at least in embryos and early fetuses, was the presence of cell bodies intermingled with nerves superior to the vomeronasal nerve. Ganglia and other cells were not as clearly identifiable in association with nerves of larger fetal Microcebus, but the stains used on these specimens were not ideal for recognizing them. The nerves, based on their location and connection to the VNO itself, corresponded to vomeronasal and terminal nerve branches, which are intimately associated with one another in fetal humans and other mammals (‘vomeronasal-terminal nerve complex’ of Brown, 1987). Morphologically, the masses of larger cell bodies resembled paravomeronasal ganglia previously described in bats (Bhatnagar & Kallen, 1974). These ganglia also had a strong resemblance to descriptions of luteinizing hormone-releasing hormone (LHRH) ganglia as seen in other mammals, including humans, which derive from olfactory placode and vomeronasal epithelia (Boehm et al. 1994). Scattered smaller-bodied cells superior to the VNO, and fusiform cells found more anteriorly in association with nerve fibres (possibly olfactory or terminal nerve branches; Fig. 3g), also resembled existing descriptions of LHRH cells (Boehm et al. 1994; Schwanzel-Fukuda, 1999).
Taken in the context of existing data on the primate VNO, there are possible implications regarding the ancestral vomeronasal system of primates. First, it is increasingly apparent that olfactory placode and its derivatives are a source of LHRH neurons in most, if not all, mammals (Schwanzel-Fukuda, 1999). In rodents, the majority of LHRH migration occurs before the VNO has fully formed (Schwanzel-Fukuda, 1999). Although immunohistochemical data are not available on these archival samples, the morphological observations herein suggest that humans are not unique in exhibiting a more prolonged peripheral presence of LHRH neurons in association with vomeronasal epithelia (Boehm et al. 1994; Kjær & Fischer Hansen, 1996).
A second point may have more systematic value. An unexpected finding was that the prenatal VNO morphology in Tarsius bancanus and the New World monkeys examined were contradictory to all existing descriptions of adults (Table 2). This is especially notable in light of existing, detailed descriptions of adult VNO morphology in Tarsius and Saimiri, each of which was previously described to have a uniform sensory epithelium comprising the VNO (Woolard, 1925; Starck, 1975; Mendoza et al. 1994; Wöhrmann-Repenning & Bergmann, 2001). Together, these findings support the recent suggestion that New World primates may pass transiently through a stage in which the VNO is (primitively) divided into sensory and non-sensory epithelia (Smith et al. 2003). Another transient feature of New World primates and Tarsius may be the communication point of the VNO opening. In adult New World primates and Tarsius, the VNO opens into a more posterosuperior part of the nasopalatine duct than in lemuriforms (Table 2). An explanation for the contradictory findings herein on Tarsius bancanus may lie in the results of Smith et al. (2003), who described a shifting association of VNO and nasopalatine duct in perinatal Saguinus geoffroyi, in which the VNO opened into the nasal cavity alone in neonates, but joined the nasopalatine duct by 1 month of age. Therefore, the apparently contradictory findings on fetuses, as well as inconsistencies among previous reports, are artefacts of ontogeny. Overall, both tarsiers and New World monkeys have transient phases of VNO development that end with a similar adult morphology (Table 2). These soft tissue transitions, along with shifting associations of the VNO and nasopalatine duct, are probably common developmental pathways of the VNO of tarsiers and New World monkeys. If tarsiers and anthropoids do represent a clade (Haplorhini), then the atypical morphology seen in adult tarsiers and New World monkeys probably represents the adult VNO morphology of a haplorhine common ancestor. This study underlines the utility of prenatal information for a complete understanding of the phylogenetic importance of adult morphology. Indeed, once VNO development in tarsiers and New World monkeys is taken into account, the VNO itself may provide evidence for the haplorhine clade.
Table 2. VNO morphology compared and contrasted in primates.
| Character states | Rodents1 prenatal and postnatal | Lemuriformes (lemurs, lorises) | Tarsiiformes (tarsiers) | Anthropoids (NWM, OWM, apes, humans) | |||
|---|---|---|---|---|---|---|---|
| prenatal2,3 | postnatal4 | prenatal3 | postnatal5,6,7,8 | prenatal2,3 | postnatal4,9 | ||
| VNO communicates with the nasopalatine duct | no* | yes | yes | transiently, yes | yes | variable?; not in humans | variable: adult NWM, yes; OWM and apes, no; infant tamarins, variable |
| VNO epithelium (see Smith et al. 2001) | well-developed | well-developed | well-developed | well-developed | uniformsensory | variable: NWM uniform sensory in adults, well-developed in neonates? | variable; uniform or interrupted sensory in NWM; displaced or absent in OWM, apes,and humans; well-developed in infant lion tamarins |
| Paravomeronasal ganglia | yes† | yes | ? | yes | ? | yes | ? |
VNO = vomeronasal organ; NWM = New World monkeys; OWM = Old World monkeys;
this study;
There is disagreement regarding the primitive VNO communication point for mammals; rodents have a more rostral, nasal VNO opening compared with lemuriforms and some other primates.
LHRH ganglia of rodents (Schwanzel-Fukuda, 1999) may represent the homologous structure.
Acknowledgments
We are greatly indebted to J. Narraway, Curator of the Embryological Collection of the Hubrecht Laboratory, for arranging a loan of tarsier specimens and numerous courtesies in providing information on this rare sample (including a translation of original notes in Dutch). S. B. McLaren and J. R. Wible of the Sections of Mammals, Carnegie Museum of Natural History, received the loan for T.D.S., and helped with numerous logistical difficulties in shipping. We also thank R. Randall and R. D. E. MacPhee of the Department of Mammalogy, AMNH, for arranging access to the Bluntschli collection. We are very grateful to P. M. Mikkelsen for allowing the use of her microscopic imaging system for examination of the Bluntschli series. Finally, we thank two anonymous reviewers and A. M. Burrows for valuable comments on the manuscript. This work was supported, in part, by a grant from the College of Health and Human Services, Slippery Rock University.
References
- Ankel-Simons F. Primate Anatomy: an Introduction. New York: Academic Press; 2000. [Google Scholar]
- Bhatnagar KP, Kallen FC. Morphology of the nasal cavities and associated structures in. Artibeus jamaicensis and Myotis lucifugus. Am. J. Anat. 1974;139:167–190. doi: 10.1002/aja.1001390203. [DOI] [PubMed] [Google Scholar]
- Bhatnagar KP, Meisami E. Vomeronasal organ in bats and primates: extremes of structural variability and its phylogenetic implications. Microsc. Res. Techn. 1998;43:465–475. doi: 10.1002/(SICI)1097-0029(19981215)43:6<465::AID-JEMT1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Boehm N, Roos J, Gasser B. Luteinizing hormone-releasing hormone (LHRH)-expressing cells in the nasal septum of human fetuses. Dev. Brain Res. 1994;82:175–180. doi: 10.1016/0165-3806(94)90160-0. [DOI] [PubMed] [Google Scholar]
- Breipohl W, Bhatnagar KP, Mendoza A. Fine structure of the receptor-free epithelium in the vomeronasal organ of the rat. Cell Tissue Res. 1979;200:383–395. doi: 10.1007/BF00234850. [DOI] [PubMed] [Google Scholar]
- Brown JW. The nervus terminalis in insectivorous bat embryos and notes on its presence during human ontogeny. Ann. New York Acad. Sci. 1987;287:184–200. doi: 10.1111/j.1749-6632.1987.tb36297.x. [DOI] [PubMed] [Google Scholar]
- Elliot DG. A Review of the Primates. Vol. 1. New York: American Museum of Natural History; 1913. Monograph 1. [Google Scholar]
- Fleagle JG. Primate Adaptation and Evolution. 2nd edn. San Diego: Academic Press; 1999. [Google Scholar]
- Garrosa M, Gayoso MJ, Esteban FJ. Prenatal development of the mammalian vomeronasal organ. Microsc. Res. Techn. 1998;41:456–470. doi: 10.1002/(SICI)1097-0029(19980615)41:6<456::AID-JEMT2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Hill WOC. Primates, Comparative Anatomy and Taxonomy. II. Haplorhini: Tarsioidea. Edinburgh: University Press; 1955. [Google Scholar]
- Hofer HO. The external nose of Tarsius bancanus borneanus Horsfield, 1821 (Primates, Tarsiiformes) Folia Primatol (Basel) 1979;32:180–192. doi: 10.1159/000155911. [DOI] [PubMed] [Google Scholar]
- Kay RF, Ross C, Williams BA. Anthropoid origins. Science. 1997;275:797–804. doi: 10.1126/science.275.5301.797. [DOI] [PubMed] [Google Scholar]
- Kjær I, Fischer Hansen B. The human vomeronasal organ: prenatal developmental stages and distribution of luteinizing hormone-releasing hormone. Eur. J. Oral Sci. 1996;104:34–40. doi: 10.1111/j.1600-0722.1996.tb00043.x. [DOI] [PubMed] [Google Scholar]
- Maier W. Nasal structures in Old and New World primates. In: Ciochon RL, Chiarelli AB, editors. Evolutionary Biology of the New World Monkeys and Continental Drift. New York: Plenum Press; 1980. pp. 219–241. [Google Scholar]
- Mendoza AS, Küderling I, Kuhn HJ, Kühnel W. The vomeronasal organ of the New World monkey Saguinus fuscicollis (Callitrichidae). A light and transmission electron microscope study. Ann. Anat. 1994;176:217–222. doi: 10.1016/s0940-9602(11)80481-4. [DOI] [PubMed] [Google Scholar]
- Ross CF. Into the light: the origin of Anthropoidea. Annu. Rev. Anthropol. 2000;29:147–194. [Google Scholar]
- Sánchez-Villagra MR. Ontogenetic and phylogenetic transformations of the vomeronasal complex and nasal floor elements in marsupial mammals. Zool. J. Linn. Soc. 2001;131:459–479. [Google Scholar]
- Schwanzel-Fukuda M. Origin and migration of luteinizing hormone-releasing hormone neurons in mammals. Microsc. Res. Techn. 1999;44:2–10. doi: 10.1002/(SICI)1097-0029(19990101)44:1<2::AID-JEMT2>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Shimp KL, Bhatnagar KP, Bonar CJ, Smith TD. Ontogeny of the nasopalatine duct in primates. Anat. Rec. 2003;274A:862–869. doi: 10.1002/ar.a.10101. [DOI] [PubMed] [Google Scholar]
- Smith TD, Bhatnagar KP. The human vomeronasal organ. Part II: prenatal development. J. Anat. 2000;197:421–436. doi: 10.1046/j.1469-7580.2000.19730421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TD, Bhatnagar KP, Bonar CJ, Shimp KL, Mooney MP, Siegel MI. Ontogenetic characteristics of the vomeronasal organ in Saguinus geoffroyi and Leontopithecus rosalia with comparisons to other primates. Am. J. Phys. Anthropol. 2003;121:342–353. doi: 10.1002/ajpa.10165. [DOI] [PubMed] [Google Scholar]
- Smith TD, Siegel MI, Bhatnagar KP. Reappraisal of the vomeronasal system of catarrhine primates: ontogeny, morphology, functionality, and persisting questions. Anat. Rec. (New Anat.) 2001;265:176–192. doi: 10.1002/ar.1152. [DOI] [PubMed] [Google Scholar]
- Starck D. Phylogeny of the primate higher taxa. The basicranial evidence. In: Luckett WP, Szalay FS, editors. Phyogeny of the Primates. New York: Plenum Press; 1975. pp. 91–125. [Google Scholar]
- Wöhrmann-Repenning A, Bergmann M. The vomeronasal complex in strepsirhine primates and Tarsius. Mamm. Biol. 2001;66:257–258. [Google Scholar]
- Woolard HH. The anatomy of Tarsius spectrum. Proc. Zool. Soc. Lond. Part 3. 1925;70:1071–1184. [Google Scholar]