Abstract
The return of Atlantic salmon (Salmon salar) to their home river for spawning coincides with drastic skeletal alterations in both sexes. Most prominent is the development of a kype (hook) at the tip of the lower jaw in males. Salmon that survive spawning have to cope with the kype throughout their life, unless it disappears after spawning, as was suggested in the early literature. To understand the fate of the kype skeleton, we compared morphological and histological features of kypes from pre-spawned mature anadromous males (grilse) with post-spawned males (kelts). The kype of male grilse is supported by fast-growing skeletal needles that differ from regular dentary bone. In kelts, growth of the kype skeleton has stopped and skeletal needles are resorbed apically by osteoclasts. Simultaneously, and despite the critical physiological condition of the animals, proximal parts of the kype skeleton are remodelled and converted into regular dentary bone. Apical resorption of the skeleton explains reports of a decrease of the kype in kelts. The conversion of basal kype skeleton into regular dentary bone contributes to the elongation of the dentary and probably also to the development of a larger kype in repetitive spawning males.
Keywords: bone growth, bone remodelling, hook, kype, secondary sexual character, spawning migration
Introduction
During upstream spawning migration of Atlantic salmon (Salmo salar L.), the skeleton undergoes considerable changes in both sexes, involving parts of the dermal skeleton (scale resorption and tooth replacement), the vertebral column (vertebrae resorption) and the skull (resorption and growth) (Tchernavin, 1937a,b, 1938a, 1944; Jones, 1959; Kacem et al. 1998; Witten & Hall, 2001). Resorption of scales and vertebrae are usually discussed in relation to one or more of: the animals high energy demands in a life history phase when salmon have stopped feeding and utilize their reserves for the metabolically expensive upstream migration; competition for mates; defending territories at the spawning grounds (Fleming, 1996). Also, having left the calcium-rich marine environment, the starving fish might have difficulties acquiring sufficient calcium from the mineral-poor freshwater and thus use the skeleton as a calcium source (Persson et al. 1998; Kacem et al. 2000).
More difficult to understand are processes that involve skeletal growth and development in starving anadromous salmon. The complete replacement of feeding teeth by a new set of breeding teeth (Tchernavin, 1938a) is astounding and not well understood. The skull also undergoes changes. As with the vertebral column and scales, some skull bones (branchiostegals, the postorbitalia and the elements of the gill cover) are partly resorbed and demineralized, whereas other elements of the snout (premaxilla, maxilla, dentary, palatine, supraethmoid, vomer) grow considerably (Tchernavin, 1938a,b; Kacem et al. 1998).
The most prominent alteration of the head skeleton is the development of a kype (hook) at the distal tip of the lower jaw of maturing males. During the sea-life stage, males and females are morphologically similar (Jones, 1959). Development of the kype during migration establishes, in part, the sexual dimorphism between males and females; female salmon do not develop a kype (Fig. 1a,b). The development of the kype is accompanied by formation of a large depression in the upper jaw into which the kype fits when the mouth is closed (Fig. 1c) (Witten & Hall, 2002). Early literature either refers to the kype in Atlantic salmon as entirely made of connective tissue (Day, 1887) or emphasizes its bony nature (Tchernavin, 1938c).Witten & Hall (2002) showed that the kype is supported at its base by fast-growing skeletal needles that differ considerably from the compact bone of the dentary by being composed of chondroid bone and Sharpey-fibre bone. Both types of tissue have been described in teleosts (Moss, 1961; Huysseune & Verraes, 1990; Hughes et al. 1994). Chondroid bone – cartilage-like cells surrounded by a collagen-rich mineralized matrix (Beresford, 1981) – is widespread among teleosts (Huysseune, 2000). The same applies to Sharpey's fibres. According to Moss (1961), bundles of uncalcified and calcified fibres that continue from the periosteum into the bone in teleosts are correctly considered to be Sharpey's fibres (for further discussions of definitions of chondroid bone and Sharpey's fibres see Knese, 1979; Beresford, 1981; Taylor, 1992). The unusual feature of the skeleton of the kype is the combination, in one tissue, of characteristics of chondroid bone and Sharpey-fibre bone (Witten & Hall, 2002).
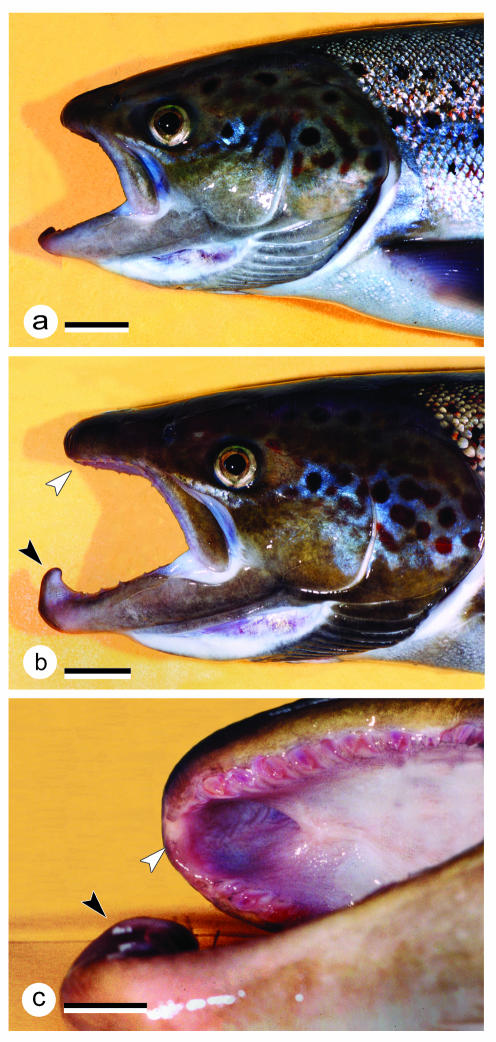
Fig. 1.
Heads of sexually mature female (a) and male (b) salmon grilse, ×0.6; scale bar = 20 mm. Comparison of the female with the male shows the prolongation of the jaws in males that takes place in the course of the animals’ return to their home rivers for spawning. In addition, males develop a kype (hook) at the tip of the lower jaw. The kype fits into a hole in the upper jaw, located between the two halves of the premaxilla (c). The black arrowhead indicates the kype, the white arrowhead points to the hole in the upper jaw.
The question remains, why do male salmon develop a kype? A possible purpose for the salmon kype was suggested by Darwin (1910), who considered that the kype is the product of sexual selection. Darwin viewed the kype as a tool for fighting among males, functioning either like a shield or a sword. By contrast, and because the kype seems to prevent the use of the breeding teeth, Tchernavin (1938a) regarded the kype as having no function. Mottley (1936) held a similar view, claiming that the kype is neither adaptive nor purposive from the evolutionary standpoint, being merely the result of a surplus of chemicals, not utilized for the production of sex products. Nowadays, and largely in agreement with Darwin (1910), the kype is regarded as a secondary sexual character displayed by males at the spawning grounds. Its function is assumed to be to establish a hierarchy among males, those with larger kypes being dominant over animals with smaller kypes (Morton, 1965; Hutchings & Myers, 1987; Järvi, 1990; Fleming, 1996; Taborsky, 2001).
Indeed, considering the extent of the alterations of the jaws (Tchernavin, 1938a; Stearly, 1992), the development of a kype must have an impact on the fitness of individual males, e.g. being advantageous in the mating process, but possibly disadvantageous for capturing prey for those male salmon that survive spawning (Witten & Hall, 2001). Repetitive spawners would have to cope with the lifelong presence of a kype, unless it disappeared during the subsequent sea-life phase. According to Tchernavin (1938b), changes in the salmon skull last only for the breeding season, after which the skull returns slowly to its earlier marine form. Roule (1933) also regarded the salmon kype as a temporary hyperplasia.
It is, however, questionable whether the kype is completely lost after spawning. Day (1887) commented that the kype in old fish does not disappear. This is also acknowledged by Tchernavin (1938a), who reports that the hook (kype) increases with the size of the fish to an extent that the hook appears disproportionate, even paradoxical. However, older literature (Roule, 1933; Tchernavin, 1938b), and personal observations, suggest the kype is reduced after spawning, although we do not know whether its regression is complete or how it is reduced. Insights into these processes should increase our understanding of the salmon life history, especially the consequences of kype retention for repetitive spawning males. Thus we addressed questions about the resorption and remodelling of the kype in post-spawning males (kelts) migrating downstream and about the mechanisms that contribute to its regression by comparing the morphology, microstructure and histology of the kype in males migrating upstream (fish from the fall run) with the kypes of males migrating downstream (surviving spawners from the following spring). The comparison reveals which parts of the kype skeleton are being resorbed, which parts grow and which parts are being remodelled. As a result of our studies, we put forward a hypothesis that could explain apparently contradictory reports about the decrease of the kype in kelts (Roule, 1933; Tchernavin, 1938b) and the increase of the kype in repetitive spawning males (Day, 1887; Tchernavin, 1938a).
Materials and methods
Sampling
This study includes material from ten male grilse migrating upstream (grilse are salmon returning from the sea for spawning the first time) and ten male kelts (males that survived the first spawning and are moving downstream). Grilse fish were collected during the autumn run in October 1999, shortly before spawning (Moore et al. 1995). Kelts were collected in April 2000. All animals were sampled at the south-west Miramichi River above Red Bank (New Brunswick, Canada) at 46°56.8′ N, 65°48.3′ W. Sampling was carried out under licence from the Canadian Federal Department of Fisheries and Oceans (DFO).
To demonstrate morphological alterations of the kype, the heads of all animals were photographed. Subsequently, the two halves of the lower jaws were separated in the midline. The left half was preserved for X-raying, whereas the right half was immediately processed and fixed for various histological and histochemical analyses.
Measurements
Weight and fork length (length from the tip of the snout to the fork in the caudal fin) were determined to calculate the condition factor (K = [weight (g)/fork length (cm3)] × 100) according to Arndt et al. (1996). Scales samples were taken to determine the animal's life history as part of an ongoing DFO monitoring programme. As revealed by scale reading (Shearer, 1992), all animals had spent one winter in the ocean (one sea winter salmon = 1 SW) before returning to their home rivers for the first spawning event. To monitor changes in the kype, morphometric data from the lower jaw were taken from the X-rays, using measures based on those of Tchernavin (1938a) along with additional measures (see Fig. 2a). As for other metric data and calculations (e.g. condition factor), we here give maximum, minimum and average values. In view of the small sample size, a non-parametric statistical test (Kruskal–Wallis anova analysis) was used to compare the measures given in Fig. 2(a) and to obtain information concerning the significance of the differences observed between grilse and kelts. P values lower than 0.05 indicate significant differences.
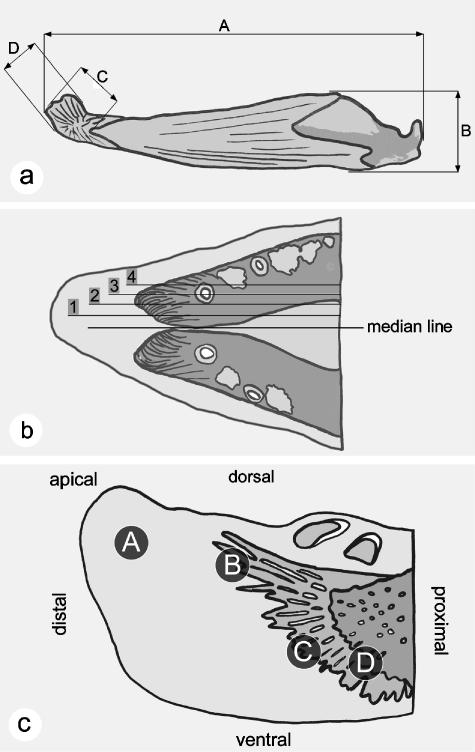
Fig. 2.
Measurements, levels of sagittal sections and areas of the kype that have been analysed. (a) Measurements taken from the lower jaw and the kype of grilse and kelts; values are given in Table 1. A, total length of the lower jaw (dentary, angular and articular). B, maximal height of the lower jaw. C, length of the kype skeleton. D, height of the kype skeleton. (b) Levels of sagittal sections at the tip of the lower jaw in grilse and kelts. Level 1 corresponds to Fig. 5(a,e), level 2 to Fig. 5(b,f), level 3 to Fig. 5(c,g), and level 4 to Fig. 5(d,h). (c) Areas of the salmon kype in grilse and kelts compared via the histological analysis. A, the apical connective tissue of the kype, corresponding to Fig. 6(a,e). B, the growth zone of the apically directed skeletal needles, corresponding to Fig. 6(b,f). C, the growth zone of the ventral kype skeleton, corresponding to Fig. 6(c,g). D, the transitional zone between compact bone of the dentary and the chondroid bone of the kype skeleton, corresponding to Fig. 6(d,h).
Radiology
High-resolution X-rays of the left lower jaws of all fish heads were taken using a portable ‘Mini X-ray HF80+’ machine (Mini X-ray Inc., Northbrock, USA) and ‘Kodak Industrex M Film Ready Pack II’ (Kodak Industry, France). No screens for increasing the X-ray beam were used. The settings of the X-ray unit were 70 kV, 15 mA, 2 s exposure time, and a distance of 40 cm between the beam source and the X-ray film. Radiographs were developed with Kodak chemicals according to the protocol of the manufacture.
Histological and histochemical procedures
Immediately after capture, right jaw halves were fixed in 10% neutral buffered parformaldehyde for 24 h, rinsed in tap water for 24 h and decalcified for 72 h in a 10% EDTA solution buffered with 0.1 m TRIS base, pH 7.0. After decalcification, samples were dehydrated with graded ethanols and embedded in Paraplast.
Starting from the mid sagittal plane, 10-µm serial sections (Fig. 2b) were prepared from the jaws of three grilse and three kelts. The sections were stained with Masson's Trichrome as the basic analytical procedure (Flint & Lyons, 1975; Benjamin et al. 2000). For a comparative analysis of structural details in grilse and kelts, four regions of the kype were chosen (Fig. 2c): A, the apical connective tissue part; B, the growth zone of the apically directed skeletal needles; C, the growth zone of the ventrally directed part of the kype skeleton; D, the transitional zone between the compact bone of the dentary and the chondroid bone of the kype, as defined by Witten & Hall (2002). Additional information about the cellular composition of the kype bone and its microstructure were obtained from Hall-Brunt Quadruple (HBQ) staining, from Toluidine blue staining and from demonstration of tartrate-resistant acid phosphatase (see below).
Masson's trichrome staining was carried out according to the following protocol: deparaffinized sections were stained for 10 min with Mayer's acid haematoxylin (Sigma MSH-32), exposed to running tap water for 10 min and rinsed in distilled water (distilled water was used for all following rinsing steps). Then, sections were stained with xylidene ponceau for 2 min (equal volumes of 0.5% xylidine ponceau 2R C.I. no. 16150, in 1% acetic acid and 0.5% acid fuchsin C.I. no. 42685, in 1% acetic), rinsed, treated for 4 min with 1% phosphomolybdic acid, rinsed, and stained with light green for 90 s (2% light green C.I. 42095 in 2% citric acid, diluted 1 : 10 with distilled water prior to use). Sections were then transferred into histoclear through a series of graded ethanol solutions and finally mounted with DPX (Fluka).
HBQ stain (Hall, 1986) was used to differentiate bone from cartilage and chondroid bone. Deparaffinized sections were stained for 2 min with an aqueous celestine blue solution (0.5% celestian blue C.I. no. 51050, 5% ammonium sulphate, 14% glycerin), rinsed, stained with Mayer's haematoxylin, exposed to running tap water for 10 min and rinsed again. Sections were then stained with alcian blue for 5 min (1% alcian blue 8 GX C.I. no. 74240, dissolved in 1% acetic acid, pH 2.6), rinsed, treated with 1% phosphomolybdic acid for 1 min, rinsed, stained with an aqueous direct red solution for 10 min (0.5% direct red 81 C.I. no. 28160), subsequently dehydrated and mounted (see above).
To demonstrate reversal lines (indicating the border between old and new bone) and bone remodelling, decalcified sections were stained with toluidine blue: Deparaffinized sections were brought into water through descending series of ethanol solutions, and stained with 1% aqueous toluidine blue solution (C.I. 52040) at pH 4.5 for 45 min.
To identify osteoclasts and bone surfaces under resorption, tartrate-resistant acid phosphatase (TRAP) was demonstrated following the protocol of Witten et al. (2001). Samples were (a) fixed in 10% TRIS-buffered formaldehyde for a maximum of 60 min, (b) rinsed in tap water for 1 h, (c) decalcified with EDTA (see above), (d) dehydrated in graded acetone solutions and (e) embedded in glycol-methacrylate. Sections of 5 µm were mounted on uncoated slides. For demonstration of TRAP activity, specimens were pre-incubated for 30 min at 20 °C in 100 mmol acetate buffer + 50 mmol L(+) di-sodium tartrate dehydrate at pH 4.5. Staining was performed in the presence of tartrate using naphthol AS-TR phosphate as substrate (final concentration 170 mmol L−1) and hexazotized pararosaniline (final concentration 1.58 mmol L−1) as an azo-coupling dye. Specimens were counterstained with Mayer's haematoxylin. Control procedures confirmed the specificity of the TRAP-staining and consisted of (a) heating of sections at 90 °C for 10 min prior to staining, (b) incubation without substrate and (c) adding the acid phosphatase inhibitor NaF to the staining solution at 10 mmol L−1 (Witten, 1997).
Results
Comparison of the fork lengths in grilse and kelts indicated an absence of somatic growth between spawning in October and downstream movement in the following April. In comparison to grilse, kelts lost about 40% of their body weight, while the average condition factor (K) dropped from 0.99 in grilse to 0.64 in kelts (Table 1).
Table 1.
Mean values obtained from the measurements of ten male grilse and ten male kelts. A = length of the lower jaw; B = height of the lower jaw; C = length of the kype skeleton; D = height of the kype skeleton; locations of measurements of the lower jaw are shown in Fig. 2(a). In addition, fork length (FL), weight (W) and the animals condition factor (K) are shown. For each trait the table gives the mean value, the standard deviation (SD), minimum (min.) and maximum (max.) and P values resulting from running a Kruskal–Wallis anova analysis (at P > 0.05 measured differences are regarded as being significant). P values suggest significant differences between the two groups except for the height of the lower jaw (B) and the fork length (FL)
| A (mm) | B (mm) | C (mm) | D (mm) | FL (mm) | W (kg) | K | |
|---|---|---|---|---|---|---|---|
| Grilse | |||||||
| mean | 78.9 | 14.3 | 10.9 | 7.3 | 601 | 2.2 | 0.99 |
| SD | 3.1 | 1.2 | 1.7 | 0.9 | 21 | 0.2 | 0.10 |
| min. | 74.4 | 11.9 | 8.3 | 5.3 | 554 | 2.0 | 0.88 |
| max. | 84.3 | 16.5 | 13.2 | 8.4 | 620 | 2.5 | 1.18 |
| Kelts | |||||||
| mean | 75.6 | 14.4 | 8.1 | 5.7 | 589 | 1.3 | 0.64 |
| SD | 3.2 | 0.7 | 0.7 | 0.6 | 17 | 0.1 | 0.04 |
| min. | 72.2 | 13.3 | 6.9 | 4.6 | 574 | 1.1 | 0.56 |
| max. | 82.8 | 15.6 | 9.6 | 6.9 | 608 | 1.5 | 0.68 |
| P | 0.0413 | 0.8490 | 0.0005 | 0.0017 | 0.0956 | 0.0001 | 0.0002 |
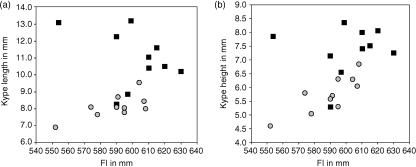
Measurements of the kype length and the height of skeleton in grilse and kelts (Fig. 2a) show variation among individuals (Table 1; Fig. 3). Nevertheless, comparison of the length and height of the kype skeleton from all individuals reveals a significant decrease in the size of the kype in kelts. The average length reduction of the kype skeleton (2.8 mm) is of roughly the same magnitude as the measured reduction of the length of the lower jaw (3.3 mm).
Fig. 3.
The distribution (mm) of length (a) and height (b) of the kype skeleton in grilse (black squares) and kelts (grey circles) from all 20 animals, plotted against fork length (mm) to show variation in the dimensions of the kype among individuals and the overall reduction of the size of the kype skeleton in kelts when compared with grilse.
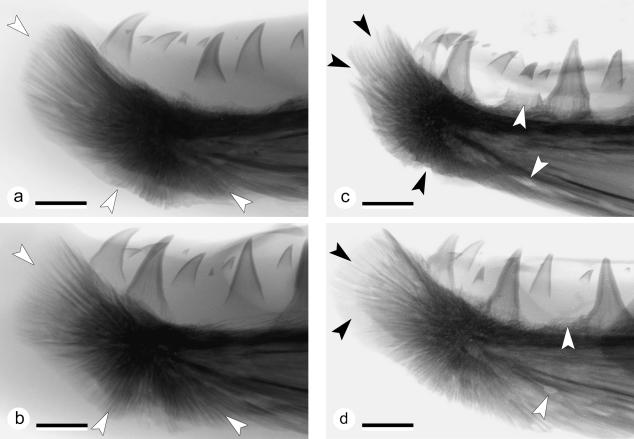
Figure 4 shows typical radiographs of the lower jaw tips from two grilse and two kelts and shows that the skeleton supporting the kype is composed of skeletal needles. Apart from supporting the kype apically, skeletal needles also extend ventrally and ventro-caudally. In agreement with the biometric data, radiographs show morphological variability of the kype skeleton among individuals: the kype skeleton in grilse can be long and narrow with a rounded tip (Fig. 4a), or relatively short and high with a flattened tip (Fig. 4b). X-rays taken from kelts show loss of skeletal needles, suggesting that bone has been resorbed (Fig. 4c). Less radiopaque skeletal needles suggest that the kype skeleton is also demineralized (Fig. 4d). Moreover, in kelts, the dentary bone itself appears less dense on X-rays and translucent, with areas indicating that the dentary is, or has been, partially resorbed. Kelts also show the loss of teeth, but similar to grilse the animals also develop new teeth (Fig. 4c,d).
Fig. 4.
Radiographs of the lower jaw tips from two grilse (a,b) and two kelts (c,d); colours inverted, ×3.5, scale bars = 4 mm. (a) X-ray of the tip of the lower jaw of a male grilse (FL = 58.7 cm, W = 2.2 kg, K = 1.04). The kype is supported by skeletal needles that extend apically, ventrally and ventro-caudally (white arrows). As shown by this animal the kype skeleton in grilse can be long and narrow with a rounded tip. Comparison with (b) reveals the variability of the kype skeleton among individuals. (b) X-ray of the tip of the lower jaw of a male grilse (FL = 61.5 cm, W = 2.5 kg, K = 1.08). The kype supporting skeletal needles extend apically, ventrally and ventro-caudally (white arrows). This kype skeleton is broad with a flattened tip (compare with a). (c) X-ray from the tip of the lower jaw of a male kelt (FL = 57.8 cm, W = 1.3 kg, K = 0.68), showing reduction of the kype skeleton by loss of apical, ventral and ventro-caudal skeletal needles (black arrows). Translucent zones in the dentary bone and the loss of teeth (white arrows) suggest that not only the kype skeleton is being resorbed. In comparison with the kype in grilse, the connective tissue is less radio-opaque. (d) X-ray of the reduced kype skeleton of a male kelt (FL = 57.4 cm, W = 1.2 kg, K = 0.63). An area of less radio-opaque skeletal needles at the tip of the kype skeleton (black arrows) indicates demineralization of skeletal needles rather than their removal. White arrows point to a site of tooth loss (upper arrow) and to a hole in the dentary bone that is not present in grilse.
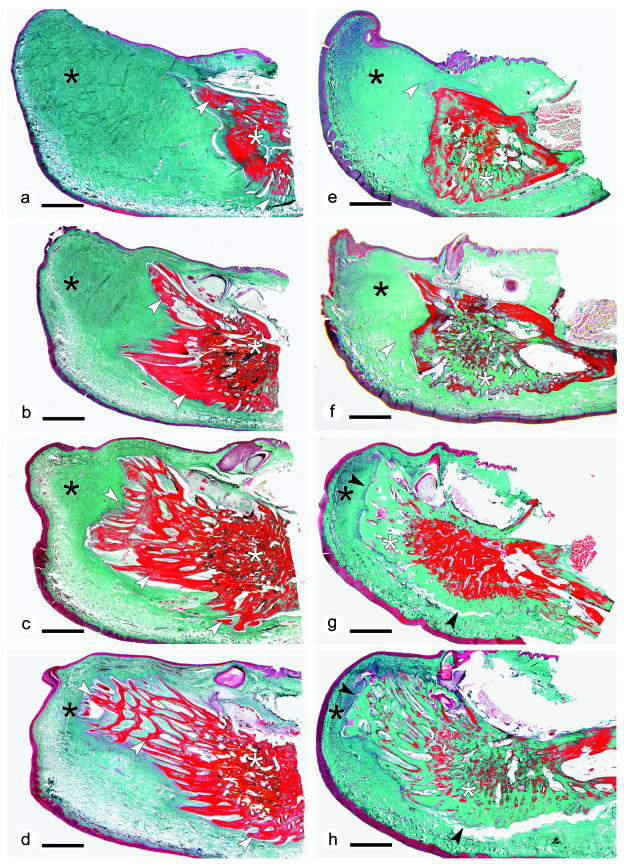
Comparison of serial sagittal sections through the kype of grilse (Fig. 5a–d) and kelts (Fig. 5e–h) reveals details of modification of the kype skeleton between the two seasons. Kelts show a loss of skeletal needles and a smooth surface of the remaining kype skeleton. The smooth apical bone surface indicates that, in addition to bone resorption, which results in loss of the skeletal needles (see below), new bone is also forming.
Fig. 5.
(a–d) Sagittal sections through the tip of the lower jaw of a male grilse (the same animal as shown in Fig. 4a), displaying connective tissue (black asterisks, green stain), the kype skeleton (made from skeletal needles, white arrows) and the compact bone of the dentary (white asterisks). Apical skeletal needles are free; proximal skeletal needles anastomose into a spongiosa-like meshwork. Apical skeletal needles are longer than ventral needles. The deep red-stained bone matrix suggests that the tissue is well mineralized. Masson's trichrome staining; ×6; scale bar = 2 mm. (e–h) Sagittal sections through the tip of the lower jaw of a male kelt (the same animal as shown in Fig. 4c), corresponding to the sections through the tip of the lower jaw in grilse shown in (a–d). The comparison with grilse reveals reduction of connective tissue (black asterisks, green stain) and the loss of apical skeletal needles (white arrows). In the absence of skeletal needles the surface of the kype skeleton is smooth (black arrows). Similar to the pattern seen in X-rays (Fig. 4c), the absence of red staining in large parts of the bone matrix (white asterisks) suggests that in comparison with grilse, the skeleton in kelts is less densely mineralized. Masson's trichrome staining; ×6; scale bar = 2 mm.
Proximal parts of the kype skeleton, consisting of a meshwork of anastomosed skeletal needles, display no obvious structural changes (but see the occurrence of resorption and remodelling described below). The general homogeneous red staining of the bone matrix of the kype in grilse (Fig. 5c,d), and the non-red staining in parts of the bony matrix in kelts (Fig. 5g,h) suggests loss of minerals in the bone of kelts (osteoid, non-mineralized newly synthesized bone matrix, stains green with Masson's trichome staining similar to the bone matrix of the kype skeleton on kelts).
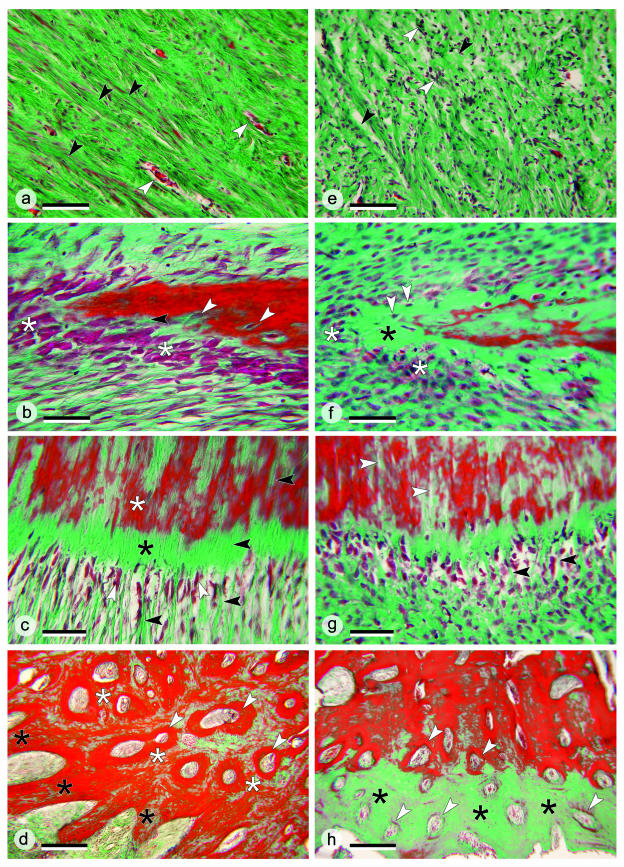
Comparison of histological details in the kype of grilse (Fig. 6a–d) and kelts (Fig. 6e–h) focuses on four areas (A–D) indicated in Fig. 2(c)
Fig. 6.
(a–d) Histological details in the kype of grilse; sections stained with Masson's trichrome. (a) Connective tissue supporting the kype of a grilse from area A in Fig. 2(c). Dense fibre bundles (black arrows) are arranged in parallel with blood vessels between (white arrows). ×160; scale bar = 100 µm. (b) Apical skeletal needle of the kype of a grilse from area B in Fig. 2(c). Numerous large osteoblasts (white asterisk) are located at the tip of a skeletal needle. The enclosure of osteoblasts (white arrows) into newly synthesized bone matrix and a small osteoid seam (black arrow, green stain) both indicate rapid bone formation and mineralization. ×400; scale bar = 40 µm. (c) Ventral part of the kype skeleton of a grilse from area C of Fig. 2(c). Osteoblasts (white arrows) are located between collagen fibre bundles (black arrows), adjoined by osteoid (black asterisk) and mineralized skeletal matrix (white asterisk). Continuation of collagen fibre bundles inside the bone matrix defines this part of the kype skeleton as Sharpey-fibre bone. ×250; scale bar = 60 µm. (d) The border between the kype skeleton and the compact bone of the dentary in a grilse (area D, Fig. 2c). Kype skeletal needles are composed of chondroid bone (black asterisks) adjoined by compact bone of the dentary (white asterisks) composed from osteons (white arrows). ×63; scale bar = 250 µm. (e–h) Histological details in the kype of kelts; sections stained with Masson's trichrome. (e) Apically located kype supporting connective tissue of a kelt (Fig. 2c, area A). Compared with grilse (a), collagen fibre bundles are much less organized and less dense (black arrows). Large numbers of unmasked cell nuclei are visible (white arrows). ×160; scale bar = 100 µm. (f) Apical skeletal needles of a kelt (Fig. 2c, area B). Compared with grilse (b) small and diminished numbers of osteoblasts (white asterisks) indicate that bone formation has largely stopped. The apparently ongoing transformation of osteoblasts into osteocytes (white arrows) suggests that kelts might still maintain slow bone formation, even if the matrix (black asterisk) is not mineralized. ×400; scale bar = 40 µm. (g) The ventral part of the kype skeleton of a kelt (Fig. 2c, area C). Compared with grilse (Fig. 6c) the shape of the osteoblasts has changed, similar to the changes shown in (f). Sharpey's fibres (black arrows) are less regularly arranged and appear less abundant. Gaps inside the bone matrix (white arrows) could be linked to the reduction of Sharpey's fibres. ×250; scale bar = 60 µm. (h) The border between the kype skeleton and compact bone of the dentary in a kelt (Fig. 2c, area D). Through the loss of skeletal needles and the ongoing development of osteons (white arrows), compact bone now reaches almost to the surface of the former kype skeleton. The absence of red staining in large parts of the bone matrix (black asterisks) suggests poor mineralization. ×63; scale bar = 250 µm.
Area A
(Fig. 6a,e). The connective tissue in grilse is rich in collagen fibres. Dense fibre bundles are arranged in parallel, with blood vessels in between (Fig. 6a). In kelts, collagen fibre bundles are much less regularly arranged and fibres appear less densely packed. A high number of cell nuclei are visible in kelts (Fig. 6e), probably not reflecting fibroblast proliferation. A reasonable interpretation is that the reduced collagen fibre density has unmasked cell nuclei that are hidden in grilse by the densely packed collagen fibres.
Area B
(Fig. 6b,f). The apical edge of the kype skeleton in grilse (Fig. 6b) displays large and numerous osteoblasts and the enclosure of osteoblasts in newly synthesized bone matrix, both of which indicate rapid bone deposition (Fig. 6b). The small osteoid seam shown in Fig. 6(b) is also indicative of rapid mineralization.
In kelts (Fig. 6f), osteoblasts are less prominent and much smaller when compared with those in grilse, indicating that bone formation has largely stopped. In contrast with the bone of the kype in grilse, a broad zone of presumably purely mineralized (green staining) bone matrix is visible. Osteoblasts that are apparently being embedded in the bone matrix (future osteocytes) could, however, indicate that kelts still maintain slow bone slow formation, even if the bone matrix is not mineralized.
Area C
(Fig. 6c,g). At the ventral edge of the kype skeleton, structural differences between grilse (Fig. 6c) and kelts (Fig. 6g) are less obvious. Nevertheless, in kelts, the shape and size alteration of osteoblasts and the less regular organization of collagen fibre bundles (Fig. 6g) resembles changes seen in the apical growth zone and in the connective tissue (Fig. 6e,f). In grilse, collagen fibre bundles extend from the connective tissue into the bone, defining this part of the kype skeleton as Sharpey-fibre bone (Fig. 6c) (Witten & Hall, 2002). In kelts, the number of Sharpey's fibres appears to be smaller and gaps that could result from the loss of Sharpey's fibres are visible inside the bone matrix (Fig. 6g).
Area D
(Fig. 6d,h). In grilse, the kype skeletal tissue is distinguished from the compact bone of the dentary by its structure – primary cancellous vs. compact bone – and its cellular composition – dual presence of osteocytes and chondrocytes in the kype skeleton vs. exclusively osteocytes in the compact bone of the dentary (see also Fig. 7b,d). At the border between the two types of skeletal tissue (Fig. 6d) new osteons arise in the process of converting kype skeletal tissue into compact bone (see also Witten & Hall, 2002).
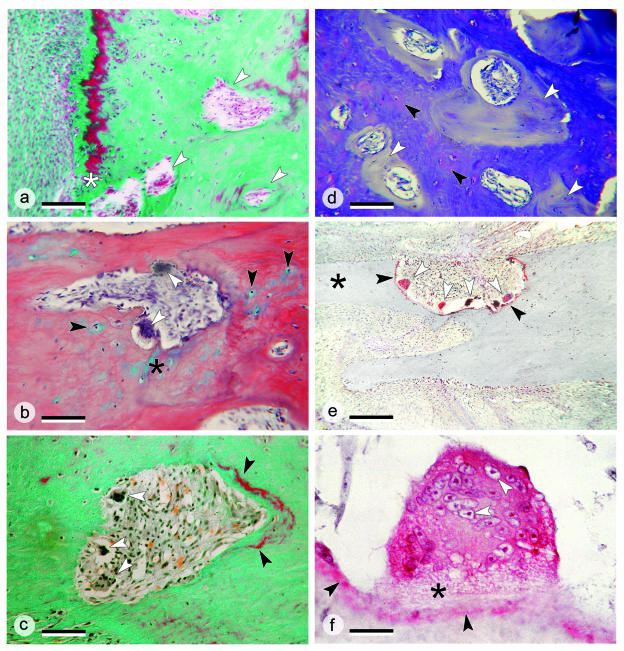
Fig. 7.
Details of the remodelling of the kype skeleton. (a) Higher magnification of the border between the kype skeleton and compact bone of the dentary in the kelt shown in Fig. 6(h). The ventral bone surface is to the left. Osteons arise in the rudiments of the kype skeleton (white arrows), a process that starts directly beneath the bone surface (white asterisk). Masson's Trichrome staining; ×100; scale bar = 150 µm. (b) Removal of apical kype skeletal tissue in a kelt by osteoclasts (white arrows). The tissue that is removed is a chondroid bone, i.e. a bone that contains chondrocytes (black arrows) and cartilage matrix (black asterisk). HBQ staining; ×250; scale bar = 60 µm. (c) Removal of kype skeletal tissue in a kelt by osteoclasts (white arrows) and deposition of newly mineralized bone (black arrows) as part of the process of converting kype skeletal tissue into compact bone. The ventral bone surface is located at the bottom left. Masson's Trichrome staining; ×250; scale bar = 60 µm. (d) Toluidine blue staining visualizes the transformation of kype skeletal tissue (seen as metachromatic chondroid bone, black arrows) into non-metachromatic compact bone (white arrows). Toluidine blue staining; ×100; scale bar = 150 µm. (e) The base of a skeletal needle (black asterisk) from the kype of a kelt is removed by bone resorption carried out by numerous large multinucleated osteoclasts (white arrows), which express the osteoclast marker enzyme tartrate-resistant acid phosphatase (TRAP, shown in red). The enzyme is released by the osteoclasts and thus also appears on the bone surface (black arrow). TRAP staining; ×100; scale bar = 150 µm. (f) Details of a bone-resorbing giant cell showing high intracellular TRAP activity in red, numerous cell nuclei (white arrows), and the osteoclasts’ ruffled border (black asterisk). Also, TRAP appears at the bone surface (black arrows). TRAP staining; ×1000; scale bar = 15 µm.
In parallel to regression of the skeletal needles in kelts, the border between the kype skeleton (made from chondroid bone) and the compact bone (made from osteons) shifts to the bone surface. Developing osteons, which in grilse are located far from the bone surface (Fig. 6d), in kelts are located immediately adjacent to the bone surface (Fig. 6h). A border defined by green vs. red staining of the bone matrix (Fig. 6h) suggests the presence of poorly mineralized (green) and of well-mineralized (red) parts of the kype bone but does not indicate structural differences. Developing osteons in the kelt kype skeleton are shown in Fig. 7(a,b) at higher magnification. The generation of an osteon, which can start immediately beneath the bone surface (Fig. 7a), involves resorption of the chondroid bone of the kype skeleton by multinucleated osteoclasts (Fig. 7b) and the deposition of new bone (Fig. 7c). The process is already underway in grilse (Witten & Hall, 2002) and is ongoing in kelts (Figs 6h and 7a). Toluidine blue-stained sections visualize the transformation of the metachromatic staining kype skeletal tissue into compact bone, which does not show any metachromatic staining (Fig. 7d).
Apart from remodelling of the kype skeleton, skeletal needles supporting the kype in kelts are directly removed by resorption. Figure 7(e) shows several large multinucleated osteoclasts, in the process of cutting off the base of an apical skeletal needle. These cells express the osteoclast marker enzyme TRAP, an enzyme that they secrete onto the bone surface. Figure 7(f) gives structural details of a bone-resorbing giant cell, showing the high intracellular TRAP activity, the ruffled border, and numerous cell nuclei. Figure 7(f) also shows the appearance of TRAP at the bone surface.
Discussion
Radiological and histological analysis reveals that the kype in grilse and in kelts is supported by both connective tissue and anatomizing skeletal needles, the latter consisting of chondroid bone and Sharpey-fibre bone. Skeletal needles supporting the kype can be distinguished from the dentary bone on radiographs and in histological sections. In grilse, the kype skeleton displays features of fast growth. In kelts, the kype skeleton (and its connective tissue) is significantly but not completely reduced, apparently by two processes: apical parts of the kype skeleton are resorbed by osteoclasts, whereas basal parts are remodelled into regular dentary bone. In addition, there are indications that large areas of the kype skeleton might be demineralized. Taking into account that grilse have stopped feeding, the fast growth of the kype skeleton in grilse is astounding. Considering the severe starvation undergone by kelts, ongoing remodelling of kype skeletal tissue into dentary bone in kelts is even more remarkable. In the following we discuss (a) how the cellular processes in the kype skeleton could relate to processes in other parts of the salmon skeleton; (b) the factors that could regulate growth and resorption of the kype skeleton; and (c) how reduction and remodelling of the kype could fit into the life history strategy of repetitive spawning males.
Because grilse are starving and utilize their energy reserves for gonad development, upstream migration and competition at the spawning grounds (Fleming, 1996; Persson et al. 1998; Booth et al. 1999), rapid development of the kype skeleton in male grilse is remarkable. Indeed, somatic growth in Atlantic salmon has stopped, the level of growth hormone is declining (Crim et al. 1992), and parts of the endoskeleton (vertebral column) and the dermal skeleton (scales) are being resorbed (Kacem et al. 1998; Persson et al. 1998). In other teleosts and in mammals, similar circumstances result in cessation of bone growth and loss of skeletal material (Goldstone et al. 2002; Yamada et al. 2002).
It has been proposed that resorption of postcranial skeletal elements in Atlantic salmon provide the calcium required for the growth of cranial bones and the kype (Kacem et al. 2000). There is, however, also the possibility that starving salmon resorb bone because of a lack of phosphorus. Recent studies by Vielma & Lall (1998) and by Yamada et al. (2002) present evidence that salmon (and other teleosts) utilize their skeleton as a phosphorus rather than a calcium depot.Tarlo (1964) suggested that the bone of early vertebrates was a phosphorus reservoir, arguing that phosphorus is a limiting factor in many ecosystems and is especially required during periods of starvation. Whereas salmon and other teleosts have unlimited access to calcium from the water, food is the only source for phosphorus (Steffens, 1985; Taylor, 1985). Because salmon mainly utilize lipids as an energy reserve (Jonsson et al. 1997; Booth et al. 1999) phosphorus and proteins for the kype skeleton might have to be mobilized from post-cranial bones. Regardless, that the kype is formed under difficult physiological conditions is reflected in its structure and development; the skeleton of the kype is laid down rapidly using as little material as possible (Witten & Hall, 2002).
Unlike its growth, reduction of the kype skeleton in kelts seems to be more in agreement with the general metabolic and physiological condition of the animals. Teleost cellular and acellular bone are metabolically active tissues (Takagi & Yamada, 1992; Witten et al. 1999, 2000, 2001; Huysseune, 2000) and resorption and demineralization can occur under a variety of deficiency conditions (Moss, 1962; Takagi et al. 1989; Carragher & Sumpter, 1991; Kacem et al. 1998; Yamada et al. 2002). Considering the low condition factor of kelts (average K = 0.64), resorption of the kype, a tissue that may no longer have a functional role, is understandable. Fat reserves of post-spawned starving Atlantic salmon decline by 80%, but tissue protein content is also reduced by 20% (Jonsson et al. 1997). Keeping in mind that skeletal resorption not only releases bone minerals but also matrix proteins (Holtrop, 1991), collagen from the matrix of the kype skeleton, together with that from the connective tissue portion of the kype, could provide an energy source for heavily starving salmon.
11-Keto-testosterone is thought to be the most important factor for the expression of male secondary sex characters (Taborsky, 2001). In male salmon, the level of 11-keto-testosterone rises in conjunction with development of the testes to levels at which it can overrule declining growth hormone levels, placing skeletal growth under androgen control (Crim et al. 1992). Bone formation in salmon and other teleosts is also enhanced by vitamin D, which increases intestinal uptake of calcium and phosphorus (Taylor, 1985; Vielma & Lall, 1998; Sasayama, 1999). Recently, the hormone leptin, which is released by adipose cells and present in all vertebrates, has been shown to stimulate bone formation (Zhang et al. 1994; Baker et al. 2000). In starving mice, leptin maintains the levels of osteocalcin, which is a marker of bone formation (Goldstone et al. 2002). Therefore, the drastic post-spawning decline of lipid resources in salmon (Booth et al. 1999) could decrease the leptin level and thus contribute to cessation of bone growth. Further studies are needed, however, to determine whether leptin can influence bone formation in salmon (Baker et al. 2000).
Oestradiol-17β (E2) is another systemic factor that could contribute to the reduction of the kype. In salmon, E2 increases calcium uptake to the plasma, both from the water and from the skeleton (Persson et al. 1998). However, the coexistence of opposing processes – kype skeletal growth and post-cranial skeletal resorption (Witten & Hall, 2001) – suggests that factors other than systemic ones alone regulate remodelling of the skeleton in grilse and kelts. Regulation of jaw development in vertebrates requires paracrine factors that act locally (MacDonald & Hall, 2001). Growth factors such as members of the bone morphogenetic proteins and fibroblast growth factor families, and transcription factors such as members of the muscle specific homeobox (Msx) gene family, regulate facial bone development in mice and are involved in dermal bone development in zebrafish (Poss et al. 2000; MacDonald & Hall, 2001; Quint et al. 2002). An interplay of locally produced growth factors probably regulates local bone formation in salmon. Because the skeletogenic cells that generate the jaws are neural crest-derived, and considering that skeletogenic cells of the kype develop into osteoblasts and chondroblasts (Hall, 2000; Witten & Hall, 2002), additional factors such as N-CAM (neural cell adhesion molecule) are probably involved in differentiation of the kype skeleton. N-CAM regulates the development of membrane bone scleroblasts into osteoblast and down-regulation of N-CAM is required for chondrogenic differentiation (Fang & Hall, 1997, 1999).
Chondroid bone that develops at the upper pharyngeal jaws of cichlids has been interpreted as an adaptation to fast skeletal development (Huysseune & Verraes, 1986; Huysseune, 1986). This interpretation could apply to the fast growing skeletal tissue of the salmon kype, which shares characteristics with locally fast growing bone tissue in mammals (observed under experimental and pathological conditions). X-rays images of the salmon kype display similarities with radiographs from periosteal osteosarcoma, which also show thin skeletal needles that protrude from the periosteum into the connective tissue (Breit et al. 2000). This situation of extremely rapid growth is known as ‘spiculated’, ‘hair on end’ or ‘sun burst’ condition (Fechner & Milles, 1993). Moreover, and as in the kype skeleton, cross-sections of periosteal osteosarcomas display a meshwork of skeletal needles that encircle loose vacularizing connective tissue (Fechner & Milles, 1992). However, differing from tumour tissue, and despite morphological variability (Fig. 4, Table 1), formation of kype tissue and its subsequent remodelling into compact bone appears to be a well-regulated process.
Induction of local (heterotopic) formation of chondroid bone has been achieved in mammals following application of bone morphogenetic protein (BMP) (Kawakami et al. 2001; Selvig et al. 2002). Also, mechanical stimuli can induce chondroid bone formation in mammals; chondroid bone occurs temporarily during fracture healing, especially during healing of mechanically unstable fractures (Ashhurst, 1992). In agreement with reports about BMP-mediated heterotopic bone formation, BMP-2 and BMP-4 mRNAs are expressed by immature prechondrogenic cells in mechanically unstable fractures. This expression pattern supports the idea that endogenous BMP-2 and BMP-4 are involved in local induction of bone and cartilage in mammals (Sato et al. 1999), possibly also in salmon. Whereas the mechanical stimuli in unstable fractures mainly consist of stress, mechanical load in general is a well-known stimulus of skeletal growth; absence of load leads to impaired growth and loss of bone structures (Bertram et al. 1997; Kostenuik et al. 1999). There should be little mechanical force on the kype if the structure is mainly used for display (Järvi, 1990) but Hutchings & Myers (1987) and Fleming (1996) assume that the kype is used in attacking sneaker males (males that mature as small juveniles and participate in the spawning of adult anadromous males. Sharpey's fibres that link the kype skeleton to the surrounding connective tissue also suggest that the kype is subjected to mechanical stress (Witten & Hall, 2002). Load could indeed assist in stimulating local growth of the kype skeleton in grilse, whereas absence of or reduced load in kelts could trigger resorption of the kype skeleton and loss of Sharpey's fibres. As in mammals (Burger et al. 1995; Heino et al. 2002), detection of skeletal load and regulation of bone remodelling in salmon is probably mediated by osteocytes because unlike in most teleosts, salmon bone contains osteocytes (Huysseune, 2000; Witten & Hall, 2002).
Interestingly, even after fish have starved for an entire winter, the kype is not fully resorbed, bone remodelling is ongoing, and new teeth even develop. If the kype is not required or is even disadvantageous for the sea life phase of salmon, as suggested by Mottley (1936), why are parts of the kype skeleton still present in kelts instead of having been completely resorbed to serve as a protein and mineral source facilitating the animals’ survival? An answer could come from the demonstration of ongoing conversion of kype skeletal tissue into solid dentary bone (Witten & Hall, 2002). At the tip of the lower jaw, the kype skeleton replaces the regular periosteum. Consequently, apical growth of the dentary is only possible via remodelling of the kype skeleton. That the skeletal tissue at the base of the kype is prospective dentary bone might explain why the new teeth insert into the basal skeleton of the kype (Witten & Hall, 2002). These teeth are not located on the temporary kype skeleton but on the future tip of the dentary. Utilizing the kype skeletal tissue to enlarge the dentary may also provide the basis for the development of a second, larger kype that grows out from the converted base of the previous kype in animals that return to spawn in the next season. The size of the kype is believed to determine male breeding success (Järvi, 1990; Fleming, 1996). By converting kype skeleton into elongated permanent dentary bone, kelts can increase this trait. This idea also provides an explanation for Tchernavin's observation that the kype increases with the size of the animal until the size of the kype appears paradoxically large (Tchernavin, 1938a). Our present observations neither support suggestions of periodic return of the lower jaw to its previous shape (Roule, 1933; Tchernavin, 1938b), nor the assumption that the bony part of the kype cannot be reabsorbed (Day, 1887).
Conclusions
Considering the animals’ severe starvation, it is striking that kelts do not completely resorb the bony skeleton of the kype. Instead, basal parts of the kype skeleton are remodelled into regular dentary bone. Reviewing known processes that direct bone formation and bone resorption in salmon, this remodelling appears to be uncoupled from the metabolism of the post-cranial skeleton and is probably controlled by local rather than systemic factors. Ongoing remodelling of the kype skeleton into regular dentary bone in kelts could prepare the animals for the next spawning season, providing the male salmon on its return with a larger kype and longer jaws, traits that increase the animals’ status in the hierarchy of spawning males. Future studies have to clarify whether conversion of kype tissue into dentary bone occurs in all male Atlantic salmon or if this trait mainly relates only to males from populations (like the salmon of the Miramichie River system) with a high percentage of repetitive spawners (Moore et al. 1995; Chaput et al. 2000).
Acknowledgments
We would especially like to thank Harald Rosenthal (Institute of Marine Research, Kiel, Germany) for his continuous promotion and support. We also thank our Canadian colleagues Gerald Chaput, Michael Chadwick, Mark Hambrook, David Moore, Joe Sheasgreen, John Hayward and Lawrence Taylor, and our German colleagues Wolfgang Villwock, Volker Hilge, Lothar Renwrantz, Wiebke Schmidt and Gudrun Schulze. Technical support was provided by the Zoological Institute and Zoological Museum, University of Hamburg (Germany), by the DFO-Moncton (Canada) and by the Miramichie Salmon Hatchery (Canada). Funding was granted from the Deutsche Forschungsgemeinschaft (RO 380/9-1), from the German–Canadian Science and Technology Cooperation (CAN 01/014), and from the Natural Sciences and Engineering Research Council of Canada (Grant A5056).
References
- Arndt SKA, Benfey TJ, Cunjak RA. Effect of temporary reductions in feeding on protein synthesis and energy storage of juvenile Atlantic salmon. J. Fish. Biol. 1996;49:257–276. [Google Scholar]
- Ashhurst DE. Macromolecular synthesis and mechanical stability during fracture repair. In: Hall BK, editor. Bone, Vol. 5: Fracture Repair and Regeneration. Boca Raton: CRC Press; 1992. pp. 61–121. [Google Scholar]
- Baker DM, Larsen DA, Swanson P, Dickhoff WW. Long-term peripheral treatment of immature coho salmon (Oncorhynchus kisutch) with human leptin has no clear physiologic effect. Gen. Comp. Endocrinol. 2000;118:134–138. doi: 10.1006/gcen.1999.7450. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Rufai A, Ralphs JR. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum. 2000;43:576–583. doi: 10.1002/1529-0131(200003)43:3<576::AID-ANR14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Beresford WA. Chondroid Bone, Secondary Cartilage and Metaplasia. Baltimore: Urban & Schwarzenberg; 1981. [Google Scholar]
- Bertram JEA, Greenberg LS, Miyake T, Hall BK. Paralysis and long bone growth in the chick: growth shape trajectories of the pelvic limb. Growth Dev. Aging. 1997;61:51–60. [PubMed] [Google Scholar]
- Booth RK, McKinley RS, Ballantyne JS. Plasma non-esterified fatty acid profiles in wild Atlantic salmon during their freshwater migration and spawning. J. Fish. Biol. 1999;55:260–273. [Google Scholar]
- Breit R, Van Der Wall H, Emmett L, Storey G, Allman K. Sunburst periosteal reaction in a bony metastasis. Clin. Nucl. Med. 2000;25:392–393. doi: 10.1097/00003072-200005000-00025. [DOI] [PubMed] [Google Scholar]
- Burger EH, Klein-Mullend J, Van Der Plas A. Function of osteocytes in bone – their role in mechanotransduction. J. Nutr. 1995;125(Suppl.):202S–2023S. doi: 10.1093/jn/125.suppl_7.2020S. [DOI] [PubMed] [Google Scholar]
- Carragher JF, Sumpter J. The mobilization of calcium from calcified tissues of rainbow trout (Oncorhynchus mykiss) induced to synthesize vitellogenin. Comp Biochem. Physiol. 1991;99A:169–172. [Google Scholar]
- Chaput G, Moore D, Hayward J, Shaesgreen J, Dubee B. Stock Status of Atlantic Salmon (Salmo Salar) in the Miramichi River, 1999. Ottawa: Canadian Stock Assessment Secretariat; 2000. , Research Document 2000/004. [Google Scholar]
- Crim LW, Wilson CE, So YP, Idler DR, Johnston CE. Feeding, reconditioning, and rematuration responses of captive Atlantic salmon (Salmo salar) kelt. Can. J. Fish. Aquat. Sci. 1992;49:1835–1842. [Google Scholar]
- Darwin CD. The Origin of Species by Means of Natural Selection. London: John Murray; 1910. Popular impression of the corrected copyright edition. [Google Scholar]
- Day F. British and Irish Salmonidae. London: Williams and Norgate; 1887. [Google Scholar]
- Fang J, Hall BK. Chondrogenic cell differentiation from membrane bone periostea. Anat. Embryol. 1997;196:349–363. doi: 10.1007/s004290050104. [DOI] [PubMed] [Google Scholar]
- Fang J, Hall BK. N-CAM is not required for initiation of secondary chondrogenesis: the role of N-CAM in skeletal condensation and differentiation. Int. J. Dev. Biol. 1999;43:335–342. [PubMed] [Google Scholar]
- Fechner RE, Mills SE. Tumors of the Bones and Joints. Part 8. 3rd Series. Washington, DC: Armed Forces Institute of Pathology; 1993. [Google Scholar]
- Fleming IA. Reproductive strategies of Atlantic salmon: ecology and evolution. Rev. Fish. Biol. Fish. 1996;6:379–416. [Google Scholar]
- Flint MH, Lyons MF. The effect of heating and denaturation on the staining of collagen by Masson's Trichrome procedure. Histochem. J. 1975;7:547–555. [Google Scholar]
- Goldstone AP, Howard JK, Lord GM, Ghatei MA, Gardiner JV, Wang ZL, et al. Leptin prevents the fall in plasma osteocalcin during starvation in male mice. Biochem. Biophys. Res. Commun. 2002;295:475–481. doi: 10.1016/s0006-291x(02)00697-6. [DOI] [PubMed] [Google Scholar]
- Hall BK. The role of movement and tissue interactions in the development and growth of bone and secondary cartilage in the clavicle of the embryonic chick. J. Embryol. Exp. Morph. 1986;93:l33–l52. [PubMed] [Google Scholar]
- Hall BK. The neural crest as a fourth germ layer and vertebrates as quadroblastic not tribloblastic. Evol. Dev. 2000;2:3–5. doi: 10.1046/j.1525-142x.2000.00032.x. [DOI] [PubMed] [Google Scholar]
- Heino TJ, Hentunen TA, Vaananen HK. Osteocytes inhibit osteoclastic bone resorption through transforming growth factor-beta: enhancement by estrogen. J. Cell. Biochem. 2002;85:185–197. doi: 10.1002/jcb.10109. [DOI] [PubMed] [Google Scholar]
- Holtrop ME. Light and electron microscopic structures of osteoclasts. In: Hall BK, editor. Bone, Vol. 2: the Osteoclast. Boca Raton: CRS Press; 1991. pp. 1–29. [Google Scholar]
- Hughes DR, Bassett JR, Moffat LA. Histological identification of osteocytes in the allegedly acellular bone of the sea breams Acanthopagrus australis, Pagrus auratus and Rhabdosargus sarba(Sparidage, Perciformes, Teleostei). Anat. Embryol. Berl. 1994;190:163–179. doi: 10.1007/BF00193413. [DOI] [PubMed] [Google Scholar]
- Hutchings JA, Myers RA. Escalation of an asymmetric contest: mortality resumption from mate competition in Atlantic salmon, Salmo salar. Can. J. Zool. 1987;65:766–768. [Google Scholar]
- Huysseune A. Late skeletal development at the articulation between upper pharyngeal jaws and neurocranial base in the fish, Astatotilapia elegans, with the participation of a chondroid form of bone. Am. J. Anat. 1986;177:119–137. doi: 10.1002/aja.1001770113. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Verraes W. Chondroid bone on the upper pharyngeal jaws and neurocranial base in the adult fish Astatotilapia elegans. Am. J. Anat. 1986;177:527–535. doi: 10.1002/aja.1001770411. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Verraes W. Carbohydrate histochemistry of mature chondroid bone in Astatotilapia elegans (Teleostei: Cichlidae) with a comparison to acellular bone and cartilage. Ann. Sci. Nat. Zool. 1990;13:29–43. [Google Scholar]
- Huysseune A. Skeletal system. In: Ostrander GK, editor. The Laboratory Fish Part 4 Microscopic Functional Anatomy. San Diego: Academic Press; 2000. pp. 307–317. [Google Scholar]
- Järvi T. The effects of male dominance, secondary sexual characteristics and female choice on mating success of male Atlantic salmon Salmo salar. Ethology. 1990;84:123–132. [Google Scholar]
- Jones JW. The Salmon. New York: Harper & Brothers; 1959. [Google Scholar]
- Jonsson N, Jonsson B, Hansen LP. Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon Salmo salar. J. Anim. Ecol. 1997;66:425–436. [Google Scholar]
- Kacem A, Meunier FJ, Bagliniere JL. A quantitative study of morphological and histological changes in the skeleton of Salmo salar during its anadromous migration. J. Fish Biol. 1998;53:1096–1109. [Google Scholar]
- Kacem A, Bagliniere JL, Meunier FJ. Salmon osteocytic osteolysis at the vertebral column. Comp. Biochem. Physiol. A. 2000;125:497–484. [Google Scholar]
- Kawakami T, Kawai T, Kimura A, Hasegawa H, Tsujigiwa H, Gunduz M, et al. Characteristics of bone morphogenetic protein-induced chondroid bone: Histochemical, immunohistochemical and in situ hybridization examinations. J. Int. Med. Res. 2001;29:480–487. doi: 10.1177/147323000102900603. [DOI] [PubMed] [Google Scholar]
- Knese KH. Stützgewebe und Skelettsystem. Handbuch der mikroskopischen Anatomie des Menschen. Berlin: Springer Verlag; 1979. Bd. 2 die Gewebe, Teil 5. [Google Scholar]
- Kostenuik PJ, Harris J, Halloran BP, Turner RT, Morey-Holton ER, Bikle DD. Skeletal unloading causes resistance of osteoprogenitor cells to parathyroid hormone and insulin-like growth factor-I. J. Bone Mineral. Res. 1999;14:21–31. doi: 10.1359/jbmr.1999.14.1.21. [DOI] [PubMed] [Google Scholar]
- MacDonald ME, Hall BK. Altered timing of the extracellular-matrix-mediated epithelial–mesenchymal interaction that initiates mandibular skeletogenesis in three inbred strains of mice: development, heterochrony, and evolutionary change in morphology. J. Exp. Zool. 2001;291:258–273. doi: 10.1002/jez.1102.abs. [DOI] [PubMed] [Google Scholar]
- Moore DS, Chaput GJ, Pickard PR. The effect of fisheries on the biological characteristics and survival of mature Atlantic salmon (Salmo salar) from the Miramichie river. Can. Spec. Publ. Fish. Aquat. Sci. 1995;123:229–247. [Google Scholar]
- Morton WM. The taxonomic significance of the kype in American salmonids. Copeia. 1965;1965:14–19. [Google Scholar]
- Moss ML. Studies of the acellular bone of teleost fish. 1. Morphological and systematic variations. Acta Anat. 1961;46:343–462. [PubMed] [Google Scholar]
- Moss ML. Studies of the acellular bone of teleost fish. 2. Response to fracture under normal and acalcemic variations. Acta Anat. 1962;48:46–60. doi: 10.1159/000141826. [DOI] [PubMed] [Google Scholar]
- Mottley CMcC. The hooked snout in the salmonidae. Fish. Res. Bd. Can., Pac. Biol. Station Prog. Rept. 1936;30:9–10. [Google Scholar]
- Persson P, Sundell K, Bjšrnsson BT, Lundqvist H. Calcium metabolism and osmoregulation during sexual maturation of river running Atlantic salmon. J. Fish Biol. 1998;52:334–349. [Google Scholar]
- Poss KD, Shen J, Nechiporuk A, McMahon G, Thisse B, Thisse C, et al. Roles for Fgf signaling during Zebrafish fin regeneration. Dev.Biol. 2000;222:347–358. doi: 10.1006/dbio.2000.9722. [DOI] [PubMed] [Google Scholar]
- Quint E, Smith A, Avaron F, Laforest L, Miles J, Gaffield W, et al. Bone patterning is altered in the regenerating zebrafish caudal fin after ectopic expression of sonic hedgehog and bmp2b or exposure to cyclopamine. Proc. Natl. Acad. Sci. USA. 2002;99:8713–8718. doi: 10.1073/pnas.122571799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roule L. Fishes. Their Journeys and Migrations. New York: W.W. Norton; 1933. [Google Scholar]
- Sasayama Y. Hormonal control of Ca homeostasis in lower vertebrates: considering the evolution. Zool. Sci. 1999;16:857–869. [Google Scholar]
- Sato M, Ochi T, Nakase T, Hirota S, Kitamura Y, Nomura S, et al. Mechanical tension-stress induces expression of bone morphogenetic protein (BMP)-2 and BMP-4, but not BMP-6, BMP-7, and GDF-5 mRNA, during distraction osteogenesis. J. Bone Mineral. Res. 1999;14:1084–1095. doi: 10.1359/jbmr.1999.14.7.1084. [DOI] [PubMed] [Google Scholar]
- Selvig KA, Sorensen RG, Wozney JM, Wikesjo UME. Bone repair following recombinant morphogenetic proteine-2 stimulated periodontal regeneration. J. Periodontol. 2002;73:1020–1029. doi: 10.1902/jop.2002.73.9.1020. [DOI] [PubMed] [Google Scholar]
- Shearer WM. Atlantic Salmon Scale Reading Guidelines. Copenhagen: ICES; 1992. ICES cooperative research report 188. [Google Scholar]
- Stearly RF. Historical ecology of salmoninae, with special references to Oncorhynchus. In: Mayden RL, editor. Systematics, Historical Ecology, and North American Freshwater Fishes. Stanford: Stanford University Press; 1992. pp. 622–658. [Google Scholar]
- Steffens W. Grundlagen der Fischernaehrung. Jena: Gustav Fischer Verlag; 1985. [Google Scholar]
- Taborsky M. The evolution of bourgeois, parasitic, and cooperative reproductive behaviours in fishes. J. Hered. 2001;92:100–110. doi: 10.1093/jhered/92.2.100. [DOI] [PubMed] [Google Scholar]
- Takagi Y, Hirano T, Yamada J. Scale regeneration of tilapia (Oreochromis niloticus) under various ambient and dietary calcium concentrations. Comp. Biochem. Physiol. 1989;92A:605–608. [Google Scholar]
- Takagi Y, Yamada J. Effects of calcium deprivation on the metabolism of acellular bone in tilapia, Oreochromis niloticus. Comp. Biochem. Physiol. 1992;102A:481–485. [Google Scholar]
- Tarlo LBH. The origin of bone. In: Blackwood HJJ, editor. Bone and Tooth. Proceedings of the First European Symposium, Oxford, April 1963. New York: Macmillan; 1964. pp. 3–15. [Google Scholar]
- Taylor CW. Calcium regulation in vertebrates: An overview. Comp. Biochem. Physiol. 1985;82A:249–255. [Google Scholar]
- Taylor JF. The periosteum and bone growth. In: Hall BK, editor. Bone, Vol. 6: Bone Growth – A. Boca Raton: CRC Press; 1992. pp. 21–52. [Google Scholar]
- Tchernavin V. Preliminary account of the breeding changes in the skulls of Salmo and Oncorhynchus. Proc. Linn. Soc. Lond. 1937a;149:11–19. [Google Scholar]
- Tchernavin V. Skulls of salmon and trout. A brief study of their differences and breeding changes. Salmon Trout Magazine. 1937b;88:235–242. [Google Scholar]
- Tchernavin V. The mystery of a salmon kype. Does it serve any purpose? Salmon Trout Magazine. 1938a;90:37–44. [Google Scholar]
- Tchernavin V. Changes in the salmon skull. Trans. Zool. Soc. Lond. 1938b;24:103–185. [Google Scholar]
- Tchernavin V. The absorption of bones in the skull of salmon during their migration to rivers. Fishery Board for Scotland, Salmon Fish. 1938c;6:1–5. [Google Scholar]
- Tchernavin V. The breeding characters of salmon in relation to their size. Proc. Zool. Soc. Lond. 1944;113:206–232. [Google Scholar]
- Vielma J, Lall SP. Control of phosphorus homeostasis of Atlantic salmon (Salmo salar) in fresh water. Fish Physiol. Biochem. 1998;19:83–93. [Google Scholar]
- Witten PE. Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae) Cell Tissue Res. 1997;287:591–599. doi: 10.1007/s004410050782. [DOI] [PubMed] [Google Scholar]
- Witten PE, Holliday LS, Delling G, Hall BK. Immunohistochemical identification of a vacuolar proton pump (V-ATPase) in bone-resorbing cells of an advanced teleost species (Oreochromis niloticus, Teleostei, Cichlidae) J. Fish Biol. 1999;55:1258–1272. [Google Scholar]
- Witten PE, Villwock W, Peters N, Hall BK. Bone resorption and bone remodeling in juvenile carp (Cyprinus carpio) J. Appl. Ichthyol. 2000;16:254–261. [Google Scholar]
- Witten PE, Hall BK. The salmon kype. How does it grow? What's its purpose? Does it disappear after spawning? Atl. Salm. J. 2001;50:36–39. [Google Scholar]
- Witten PE, Hansen A, Hall BK. Features of mono and multinucleated bone resorbing cells of the zebrafish Danio rerio and their contribution to skeletal development, remodeling and growth. J. Morph. 2001;250:197–207. doi: 10.1002/jmor.1065. [DOI] [PubMed] [Google Scholar]
- Witten PE, Hall BK. Differentiation and growth of kype skeletal tissues in anadromous male Atlantic Salmon (Salmo salar) Int. J. Dev. Biol. 2002;46:719–730. [PubMed] [Google Scholar]
- Yamada Y, Okamura A, Tanaka S, Utoh T, Horie N, Mikawa N, et al. The roles of bone and muscle as phosphorus reservoirs during the sexual maturation of female Japanese eels, Anguilla japonica Temminck and Schlegel (Anguilliformes) Fish Physiol. Biochem. 2002;24:327–334. [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]