Abstract
The aim of this study was to determine the main stages of the lacrimal gland's developmental process in humans and to establish its precise morphogenetic timetable. Its onset is generally assumed to take place at O'Rahilly's stage 21, arising from an epithelial thickening of the superior extreme of the temporary conjunctival fornix. However, the present study points to a prior stage in the process: the presence of epithelial–mesenchymal changes in embryos at O'Rahilly's stage 19. The study was performed using light microscopy on serial sections of 37 human specimens: 23 embryos and 14 fetuses ranging from 15 to 137 mm crown–rump length (7–116 weeks of development). Three stages in lacrimal gland morphogenesis were identified: (1) the presumptive glandular stage, O'Rahilly's stages 19–20, characterized by a thickening of the superior fornix epithelium together with surrounding mesenchymal condensation; (2) the bud stage, generally assumed to be the first manifestation of glandular origin, characterized initially by the appearance of nodular formations in the region of the superior conjunctival fornix and concluding with the appearance of lumina within the epithelial buds; and (3) the glandular maturity stage, weeks 9–16, the period in which the gland begins to take on the morphology of adulthood.
Keywords: development, embryology, embryonic stage, epithelial gland, epithelial yolk
Introduction
The development of the human lacrimal gland has been studied by numerous authors (Keibel & Elze, 1908, 1911; Duke-Elder & Cook, 1963; Jakobiec & Iwamoto, 1982; Murube, 1982; Ozanics & Jakobiec, 1982; Tripathi & Tripathi, 1990). Most of them agreed that the gland arises from the ectoderm of the superior conjunctival fornix in human embryos of 22–24 mm crown–rump length (CR). However, Tripathi & Tripathi (1990) used immunohystochemical techniques to affirm that the lacrimal gland arose from the neural crest. More recently, various authors studying other mammals (Lovicu et al. 1999; Wahl & Noden, 2000) have confirmed that the origin of the lacrimal gland emerges from the surface ectoderm and not from cells deriving from the neural crest. As stated by Johnston et al. (1979) in avian species, it is the mesenchyme surrounding the glandular primordium which derives from the neural crest and not the gland itself.
During the embryogenesis of mammals, the epithelial–mesenchymal interactions play a decisive role in the design and development of tissue (Grobstein, 1953; Martin, 1998; Lovicu et al. 1999). This interaction is essential not only for the development of the main lacrimal gland (Kammandel et al. 1999) but also for the development in mammals of the lung (Hogan, 1999), limbs (Martin, 1998; Sekine et al. 1999), teeth (Sanders, 1988) and salivary glands (Denny et al. 1997). It was also pointed out that the mesenchyme determined the patterning of glandular arborization (Denny et al. 1997).
The human lacrimal gland is made up of two lobes: the palpebral and the orbital. The orbital lobe originates from the proliferation of conjunctival fornix epithelial cells in the form of five or six epithelial buds (Duke-Elder & Cook, 1963; Jakobiec & Iwamoto, 1982; Murube, 1982; Ozanics & Jakobiec, 1982). Its formation concludes towards the end of the second month and subsequently other epithelial buds initiate the onset of the palpebral lobe (Murube, 1982; Ozanics & Jakobiec, 1982). The lobes are separated by the levator muscle tendon, which appears in the third week of development (Duke-Elder & Cook, 1963; Murube, 1982).
The purpose of this work was to study the development of the human lacrimal gland during the fetal and embryonic periods and to establish the precise morphogenetic timetable.
Materials and methods
Thirty-seven human specimens were studied (23 embryos and 14 fetuses) ranging from the 7th to the 16th week of development.
All the specimens studied belong to the collection of the Institute of Embryology of La Universidad Complutense de Madrid. The specimens were obtained after miscarriages and ectopic pregnancies from the department of obstetrics. Absence of external and internal congenital malformations was verified. Specimens were fixed in 10% formaldehyde and transferred to the laboratory. The parameters used to determine age of gestation were CR, weight and cranial perimetry (O'Rahilly & Mü ller, 1996). Embryonic and fetal periods are shown in Table 1. Specimens were then dehydrated in graded ethanol and finally embedded in paraffin wax. The usual laboratory procedures were used to prepare 10–20-µ m-thick transverse, frontal and sagittal serial sections, which were stained with haematoxylin eosin and azan (McManus & Mowry, 1968) for light microscopy study.
Table 1.
Details of the embryonic and fetal periods
| O'Rahilly stage | Section plane | CR length (mm) | Embryo | Results |
|---|---|---|---|---|
| 18 | F | 15 | NO | Palpebral primordial |
| F | 15 | GV-8 | ||
| T | 15.5 | GO-2 | ||
| F | 16 | HL-10 | ||
| 19 | F | 17 | ESC-14 | Epithelial thickening by surrounfing mesenchymal condensation ‘Prospective glandular Area’ |
| T | 17 | PA | ||
| T | 18 | MAR | ||
| 20 | F | 18.5 | PT-5 | Well defined ovoid shaped ‘Prospective glandular Area’ |
| S | 19 | PR | ||
| F | 20 | CAS | ||
| S | 20 | BR-3 | ||
| T | 20 | Be-1 | ||
| S | 21 | BO | ||
| F | 21.5 | AR | ||
| 21 | F | 22 | GV6 | Epithelial buds condense in the superior conjunctival fornix |
| S | 22 | GV7 | ||
| T | 23 | MAR | ||
| 22 | F | 26 | PT-10 | Artery and lacrimal gland approach glandual primordium |
| F | 26.5 | GI-4 | ||
| 23 | F | 27 | J-2 | Formation of central lumen in the interior of the epithelial buds. Insertion of superior rectus muscle in the sclera |
| F | 28 | BR-4 | ||
| T | 28 | Ci-11 | ||
| T | 28.5 | BR-2 |
| WK of development | Section plane | CR length (mm) | Foetus | Results |
|---|---|---|---|---|
| 9 | F | 38 | OY-2 | Levator palpebrae superioris muscle |
| S | 38 | OY | ||
| 10 | F | 47 | FAUS-7 | Expansion of the levator palpebrae superioris muscle, which divides the gland into two lobes |
| F | 52 | Ca-6 | ||
| F | 55 | JR-1 | ||
| 11 | F | 62 | Be-403 | |
| F | 67 | VR | ||
| 12 | F | 74.5 | VR-2 | |
| 13 | T | 90 | Be-608 | Glandular acinis. Anastomosis of zigomatic and lacrimal nerves |
| T | 93 | Bu-18 | ||
| 14 | F | 107 | Bu-007 | Ramification of the glandular parenchyma and surrounding isolated |
| F | 113 | B062 | ||
| 15 | T | 116 | B29 | The stroma condenses around the acini |
| 16 | F | 137 | Cu-2 | The acino-stromal union forms glandular lobes |
Results
O'Rahilly's stage 18
During this stage, initiation of the palpebral primordium is observed. At the level of the superior conjunctival fornix there is no appreciable morphological change that might indicate the onset of the lacrimal gland.
O'Rahilly's stages 19 and 20
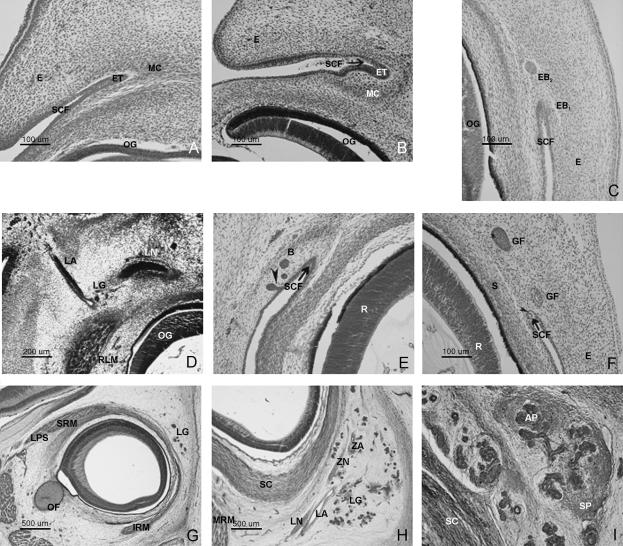
In embryos at O'Rahilly's stage 19, the superior conjunctival fornix is observed to thicken as the surrounding mesenchyme condenses (Fig. 1A). An intermediate area remains characterized by scarcity or lack of cells.
Fig. 1.
(A) Human embryo Esc-14 (17 mm CR; O'Rahilly's stage 18). Frontal section (×40 magnification). (B) Human embryo CAS 20 (20 mm CR; O'Rahilly's stage 20). Oblique fronto-transverse section (×40). (C) Human embryo GV-6 (22 mm CR; O'Rahilly's stage 21). Frontal section (×40) (D) Human embryo PT-10 (26 mm CR; O'Rahilly's stage 22). Frontal section (×10). (E) Human embryo J2 (27 mm CR; O'Rahilly's stage 23). Frontal section (×20). (F) Human embryo BR-2 (28.5 mm CR; O'Rahilly's stage 23). Transverse section (×20). (G) Human fetus Ca-6 (52 mm CR; week 10 of development). Transverse section (×10). Fibrous expansion of the levator palpebrae superioris appears. (H) Human fetus Bu-18 (93 mm CR; week 13 of development). Transverse section (×10). The lacrimal and zygomatic nerves are observed to be accompanied by their respective arteries at the dorsomedial portion of the lacrimal gland (LG). (I) Human fetus B29 (116 mm CR; week 15 of development). Transversal section (×20 magnification). The lacrimal gland, now with both a stromal portion (SP) and an acinose portion (AP), takes on an adult-like appearance.
In specimens at O'Rahilly's stage 20, the epithelial thickening takes on the form of a nodular-shaped cell grouping. The most notable feature at this stage is the organization of the mesenchyme surrounding the superior fornix epithelium, which adopts an ovoid form (Fig. 1B) This epithelial thickening together with the mesenchymal condensation constitute the presumptive glandular area.
During this stage the palpebral primordia are clearly defined; the upper eyelid appears before the lower one, although there is still considerable distance between them.
O'Rahilly's stage 21
During this stage, rounded epithelial buds condense into the superior conjunctival fornix (Fig. 1C) and subsequently invaginate into the surrounding mesenchyme. These buds appear in the superior and exterior area of epithelial condensation and, surrounded by the mesenchyme, constitute the glandular primordium.
O'Rahilly's stage 22
During this stage the epithelial glandular clusters receive the artery and the lacrimal nerve (Fig. 1D) The lacrimal nerve approaches the posterior and medial extreme of the gland primordium. The gland primordium, in which lumen has not yet appeared, corresponds to the orbital portion of the gland.
O'Rahilly's stage 23
At the end the embryonal period, lumina appear within the glandular epithelial buds. As shown in Fig. 1(E), some formations maintain a temporary epithelial connection by means of a pedicule and other formations isolated in the surrounding mesenchyme. The presence of lumen is evident in 28.5-mm CR embryos (Fig. 1F). The upper rectus muscle, encircled by mesenchymal condensation, approaches the ocular sclera. During this stage, eyelid closure is initiated.
9th–12th weeks
In the 9th week of development the levator palpebrae superioris muscle initiates its formation.
During the 10th week of development, the expansion of the levator palpebrae superioris is observed (Fig. 1G). The aponeurotic expansion divides the lacrimal gland into two portions: the orbital and palpebral lobes.
During this period, epithelial buds continue to invaginate from the fornix epithelium.
13th–14th weeks
Two outstanding features occur during these weeks of development: the arborization of the glandular parenchyma and the anastomosis in the interior of the gland between the lacrimal and the zygomatic nerves (Fig. 1H). Both occurrences coincide with an increase in glandular vascularization. The stroma appears isolated and scarcely condensed.
15th and 16th weeks
During these weeks of development, the stroma condenses and the gland organizes itself and forms glandular lobes (Fig. 1I). The formation of epithelial buds at the level of the fornix continues to be observed during the 15th week of development. By the 16th week each lobe receives its own vessels.
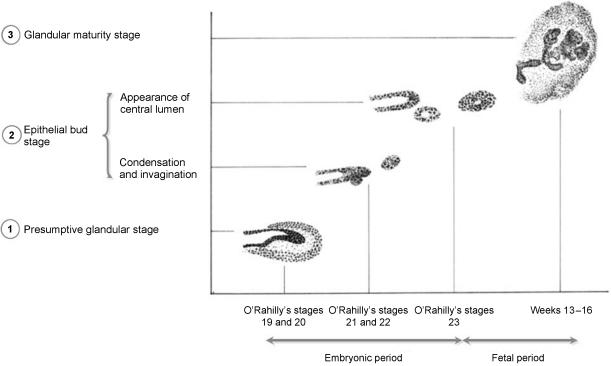
The results are summarized in Fig. 2
Fig. 2.
Results summary.
Discussion
Our observations enabled us to identify three distinct phases in human lacrimal gland morphogenesis:
presumptive glandular stage (O'Rahilly's stages 19–20);
epithelial bud stage (O'Rahilly's stages 21–23); and
glandular maturity stage from the 9th week of development onward.
We have observed that thickening of the superior conjunctival fornix epithelium accompanied by condensation of the surrounding mesenchyme occurs during the presumptive glandular stage.
Epithelial–mesenchymal interaction is considered by Grobstein (1953), Sanders (1988) and Martin (1998) as being responsible for the processes of morphogenesis, organogenesis, cell differentiation and growth. Indeed, the development of the lacrimal gland is an example of such epithelial–mesenchymal interaction (Kammandel et al. 1999). The majority of authors hold that these mesenchymal cells, which finally form the gland stroma, arise from the neural crest (e.g. Johnston et al. 1979).
The following phase, the bud stage (O'Rahilly's stages 21–23), is characterized by the formation of buds of epithelial origin at the level of the superior conjunctival fornix. It has generally been agreed that these epithelial formations constitute the onset of glandular development in embryos between 22 and 25 mm (Duke-Elder & Cook, 1963; Murube, 1982; Ozanics & Jakobiec, 1982); it is also generally assumed that these formations arise from the surface ectoderm.Tripathi & Tripathi (1990), however, situated their origin at the neural crest. The results of the present study coincide with mainstream opinion that the gland primordium arises from the surface epithelium.
The present study indicates that the epithelial bud stage concludes at the end of the embryonal period, i.e. 28.5 mm CR embryos with the appearance of lumina in the epithelial clusters. However, there is a disagreement regarding the emergence of lumina in these formations: 55 mm (Murube, 1982); between 40 and 70 mm (Ozanics & Jacobiec, 1982); 55–60 mm (Duke-Elder & Cook, 1963). In the present study, the arrival of vascular and nerve structures is observed to precede the emergence of lumen.
The presence of these nerve-vascular structures has not been reported by other authors in the period under study but rather as a later development.
The present study locates the occurrence of the glandular maturity stage from the ninth week of development onward because it is at this period that the gland prepares itself to attain adult morphology, disposition and relationships. This interval is characterized by (among other phenomena) the emergence of the levator palpebrae superioris expansion, which will subsequently divide the lacrimal gland into the palpebral and orbital portions, the formation of the acini and glandular stroma, together with the development of vascularization and glandular innervation. It is important to note that the formation of epithelial buds continues even in fetuses at this stage.
However, the most significant occurrence during this period is the emergence of the levator palpebrae superioris muscle expansion whose primordium many authors fix at 38 mm with total definition at 60 mm (e.g. Murube, 1982). Our observations place the emergence of the primordium in fetuses between 38 and 48 mm (weeks 9–10 of development). At 52 mm (week 10) this muscle, whose expansion divides the gland into the palpebral and orbital portions, is clearly defined.
During the 13th week, anastomosis between the lacrimal and zygomatic nerves may be observed. We founf no bibliographic reference on the subject of anastomosis during the embryonic and fetal periods; nor in fact has any reference been found on the onset of glandular vascularization during this period of development.
Analysis of our observations leads us to consider that the lacrimal gland has a double origin; the glandular parenchyma derives from the epithelium and the surrounding mesenchymal stroma.
Glandular innervation and vascularization begin during O'Rahilly's stage 22 (i.e. the bud stage), and at the onset of the fetal period the gland reveals relationships similar to those of adulthood.
Acknowledgments
We wish to express our gratitude to the following for their technical assistance in the elaboration of this paper: Mr Richard Smithson, Mrs Montserrat Juanilla and Mrs Cristina Navarro.
Glossary
Abbreviations
- AP
Acinose portion
- B
Bud
- E
Eyelid
- EB1 & EB2
Epithelial buds
- ET
Epithelial thickening
- GF
Glandular formations
- IRM
Inferior rectus muscle
- LA
Lacrimal artery
- LG
Lacrimal gland
- LN
Lacrimal nerve
- LPS
Levator palpebrae superioris muscle
- MC
Mesenchymal condensation
- MRM
Medialis rectus muscle
- OF
Optic fascicle
- OG
Ocular globe
- R
Retina
- RLM
Rectus lateralis muscle
- S
Sclera
- SC
Scleral condensation
- SCF
Superior conjunctival fornix
- SP
Stromal portion
- SRM
Superior rectus muscle
- ZA
Zygomatic artery
- ZN
Zygomatic nerve
References
- Denny PC, Ball WD, Redman RS. Salivary glands: a paradigm for diversity of gland development. Crit. Rev. Oral. Biol. Med. 1997;8:51–75. doi: 10.1177/10454411970080010301. [DOI] [PubMed] [Google Scholar]
- Duke-Elder S, Cook C. Normal and abnormal development. Part I. Embryology. In: Duke-Elder S, editor. System of Opthalmology. III. London: Kimpton; 1963. pp. 239–241. [Google Scholar]
- Grobstein C. Epithelio-mesenchymal specificity in the morphogenesis of mouse sub-mandibular rudiments in vitro. J. Exp. Zool. 1953;124:383–413. [Google Scholar]
- Hogan BLM. Morphogenesis. Cell. 1999;96:225–233. doi: 10.1016/s0092-8674(00)80562-0. [DOI] [PubMed] [Google Scholar]
- Jakobiec F, Iwamoto T. Ocular adnexae: introduction to lids, conjunctiva and orbit. In: Jakobiec F, editor. Ocular Anatomy, Embryology and Teratology. Philadelphia: Harper & Row; 1982. pp. 677–731. [Google Scholar]
- Johnston MC, McNoden DM, Hazelton RD, Coulombre JI, Coulombre AJ. Origins of avian ocular and periocular tissues. Exp. Eye Res. 1979;29:27–43. doi: 10.1016/0014-4835(79)90164-7. [DOI] [PubMed] [Google Scholar]
- Kammandel B, Chowdhury K, Stoykova A, Aparicio S, Brenner S, Gruss P. Distinc cis-essential modules direct the time space pattern of the pax6 gene activity. Dev. Biol. 1999;205:79–97. doi: 10.1006/dbio.1998.9128. [DOI] [PubMed] [Google Scholar]
- Keibel F, Elze C. Tabla De Desarrollo Cronológico Del Ser Humano. Jena: Verlag von Gustav Fischer; 1908. [Google Scholar]
- Keibel F, Elze C. Manual Del Desarrollo Del Ser Humano. Leipzig: Hirtzel; 1911. p. 243. [Google Scholar]
- Lovicu FJ, Kao WW, Overbeek PA. Ectopic gland induction by lens-specific expression of keratinocyte growth factor (FGF-7) in transgenic mice. Mech. Dev. 1999;88:43–53. doi: 10.1016/s0925-4773(99)00169-0. [DOI] [PubMed] [Google Scholar]
- Martin GR. The roles of FGFs in the early development of vertebrate limbs. Gene. Dev. 1998;12:1571–1586. doi: 10.1101/gad.12.11.1571. [DOI] [PubMed] [Google Scholar]
- McManus JFA, Mowry MR. Técnica Histológica. Madrid: Atika; 1968. [Google Scholar]
- Murube del Castillo J. Dacryología básica. Madrid: Murube del Castillo; 1982. pp. 57–59. [Google Scholar]
- O'Rahilly R, Müller F. Human embryology and Teratology. 2nd edn. New York: Wiley-Liss; 1996. pp. 81–104. [Google Scholar]
- Ozanics V, Jakobiec FA. Prenatal development of the eye and its adnexa. In: Jakobiec F, editor. Ocular Anatomy, Embryology and Teratology. Philadelphia: Harper & Row; 1982. pp. 11–96. [Google Scholar]
- Sanders EJ. The roles of ephithelial–mesenchymal cell interactions in developmental proceses. Biochem. Cell. Biol. 1988;66:530–540. doi: 10.1139/o88-063. [DOI] [PubMed] [Google Scholar]
- Sekine K, Ohuchi H, Fujiwara M, Yamasaki M, Yoshizawa T, Sato T, et al. Fgf 10 is essential for limb and lung formation. Nat. Genet. 1999;21:138–141. doi: 10.1038/5096. [DOI] [PubMed] [Google Scholar]
- Tripathi BJ, Tripathi R. Evidence for the neuroectodermal origin of the human lacrimal gland. Invest. Ophthl. Vis. Sci. 1990;31:393–395. [PubMed] [Google Scholar]
- Wahl C, Noden DM. Defining the environment around the eye. In: Tasman W, Jaeger E, editors. Duane's Clinical Opthalmologhy on CD-ROM. Philadelphia: Lippincott-Raven; 2000. [Google Scholar]