Abstract
When injected intraperitoneally into mice in doses larger than those used clinically, all the amino derivatives of bisphosphonates (aminoBPs) tested induce a variety of inflammatory reactions such as induction of histidine decarboxylase (HDC, the histamine-forming enzyme), hypertrophy of the spleen, atrophy of the thymus, hypoglycaemia, ascites and accumulation of exudate in the thorax, and an increase in the number of macrophages and/or granulocytes in the peritoneal cavity of blood. On the other hand, dichloromethylene bisphosphonate (Cl2MBP) a typical non-aminoBP, has no such inflammatory actions. In the present study, we found that this agent can suppress the inflammatory actions of aminoBPs.
Cl2MBP, when injected into mice before or after injection of 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid (AHBuBP; a typical aminoBP), inhibited the induction of HDC activity by AHBuBP in a dose- and time-dependent manner. The increase in HDC activity induced by AHBuBP was largely suppressed by the injection of an equimolar dose of Cl2MBP. Cl2MBP also inhibited other AHBuBP-induced inflammatory reactions, as well as the inflammatory actions of two other aminoBPs. However, Cl2MBP did not inhibit the increase in HDC activity induced by lipopolysaccharide (LPS).
We have previously reported that AHBuBP augments the elevation of HDC activity and the production of interleukin-1β (IL-1β) that are induced by LPS. These actions of AHBuBP were also inhibited by Cl2MBP.
Based on these results and reported actions of bisphosphonates, the mechanisms underlying the contrasting effects of aminoBPs and Cl2MBP, a non-aminoBP are discussed. The results suggest that combined administration of Cl2MBP and an aminoBP in patients might be a useful way of suppressing the inflammatory side effects of aminoBPs.
Keywords: Bisphosphonates, aminobisphosphonates, histidine decarboxylase, histamine, lipopolysaccharide (LPS), interleukin-1 (IL-1)
Introduction
Many derivatives of bisphosphonates (BPs), inhibitors of bone resorption, have been developed as promising agents for the treatment of conditions in which there is enhanced bone resorption, such as Paget's disease, tumoral osteolysis, tumoral hypercalcaemia, osteoporosis and rheumatoid arthritis (Green et al., 1994; Bonjour et al., 1994; Geddes et al., 1994). Among these derivatives, the aminobisphosphonates (aminoBPs) are particularly powerful (Geddes et al., 1994). However, undesirable acute phase responses, including fever, occur in humans after administration of aminoBPs (Adami et al., 1987). Recently, Sauty et al. (1996) suggested that an increase in the plasma levels of IL-6 and TNF may be responsible for the acute phase reactions induced by aminoPBs.
We found some time ago that injection of aminoBPs into mice, in doses larger than those used clinically, enhances the activity of histidine decarboxylase (HDC), the enzyme that forms histamine, in the bone marrow, spleen, lung and liver, resulting in an increase in histamine levels in these tissues (Endo et al., 1993). In addition, we have also found that aminoBPs induce hypertrophy of the spleen, atrophy of the thymus, ascites and accumulation of exudate in the thorax, and an increase in the number of granulocytes, macrophages and even osteoclasts (Endo et al., 1993), although the activity of osteoclasts is impaired. The inflammatory actions of aminoBPs described above are assumed to affect immune responses. Indeed, we have demonstrated that aminoBPs exacerbate the arthritis induced in mice by co-injection of a type II collagen and adjuvant, and we proposed the idea that this effect might be related to the ability of aminoBPs to increase HDC activity and/or to increase macrophages and granulocytes (Nakamura et al., 1996). Recently, we have shown that administration of an aminoBP augments both the production of interleukin-1 (IL-1) and the induction of HDC by a lipopolysaccharide (LPS) (Sugawara et al., 1998).
On the other hand, dichloromethylene bisphosphonate (Cl2MBP), a typical non-aminoBP with a weak bone-resorbing action, has none of the effects described above and has an anti-inflammatory action on collagen-induced arthritis (Nakamura et al., 1996 and references cited therein). In the present study, we found that Cl2MBP can inhibit or suppress the inflammatory actions of all three of the aminoBPs we tested.
Methods
Mice and reagents
Male BALB/c mice (6–7-weeks-old) were obtained from the facility for experimental animals in our university. Dichloromethylene bisphosphonate (Cl2MBP) and 4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid (AHBuBP) were synthesized by ourselves. Cycloheptylaminomethylene bisphosphonate (CHAMBP) and 3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid (MP-AHPrBP) were provided by Yamanouchi Pharmaceutical Co. (Tokyo, Japan) and Boehringer Mannheim (Mannheim, Germany), respectively. Bisphosphonates (BPs) were dissolved in sterile saline and adjusted to pH 7 with NaOH or HCl. Lipopolysaccharide (LPS) from Escherichia coli O55 : B5 prepared by Boivin's method was obtained from Difco Laboratories (Detroit, MI, U.S.A.). These solutions were injected intraperitoneally (i.p.) (0.1 ml per 10 g body weight) as indicated in the text. All experiments complied with the guidelines for care and use of laboratory animals in Tohoku University.
Assay of HDC activity
HDC activity was assayed by a previously described method (Endo et al., 1992a). HDC activity in the liver is expressed as nmol of histamine formed in 1 h by the enzyme contained in 1 g of each tissue (nmol h−1 g−1). HDC activity in the bone marrow is expressed as the activity in 1 g of tibia plus femur, because these bone tissues were themselves assayed for HDC activity (Endo et al., 1992a).
Determination of cytokines in the serum
IL-1β and TNFα were assayed using ELISA kits (Endogen, Cambridge, MA, U.S.A.), the assay procedures being performed exactly as described by the manufacturer. The blood was collected directly into test tubes following decapitation of the mice. Serum was recovered by centrifugation at 2000×g at 4°C, then stored at −80°C until used. The amount of each cytokine is expressed as pg per ml serum.
Determination of exudate in thorax
After the thorax had been opened with scissors, the exudate present in the thorax was absorbed using small pre-weighed pieces of filter paper and the amount of exudate was measured as the increase in the weight of the filter paper.
Determination of cell number of peritoneal cavity
Peritoneal exudate cells were obtained as follows. Sterile saline (10 ml) was injected into the peritoneal cavity of ether-anaesthetized mice, and the cavity was massaged. Then, the suspension of cells in the saline (5 ml) was recovered using a syringe, and the number of cells in the suspension was counted after appropriate dilution.
Determination of serum glucose
Serum was prepared as described above, and serum glucose levels were measured by the glucose oxidase method using a glucometer (Accutrend; Boehringer-Mannheim, Mannheim, Germany).
Data analysis
Experimental values are given as mean±standard deviation. The statistical significance of differences was analysed by Dunnett's multiple comparison test after ANOVA, P values less than 0.05 being considered to indicate significance.
Results
Effects of Cl2MBP and AHBuBP on HDC activity in mouse tissues
The effects of Cl2MBP and AHBuBP (separately or in combination) on HDC activity in mouse tissues are shown in Figure 1. Three days after a single intraperitoneal injection of AHBuBP (40 μmol kg−1) there was a marked elevation of HDC activity in the lung, liver, spleen and bone marrow. On the other hand, Cl2MBP had no such effects, even at 800 μmol kg−1. The combined injection of these agents (injection of Cl2MBP immediately after injection of AHBuBP) completely abolished the AHBuBP-induced elevation of HDC activity in all the tissues examined. Bone marrow is the tissue with the highest HDC activity in normal mice (Endo et al., 1992a). It should be noted that Cl2MBP did not decrease the normal level of HDC activity in the bone marrow or in the other tissues examined.
Figure 1.
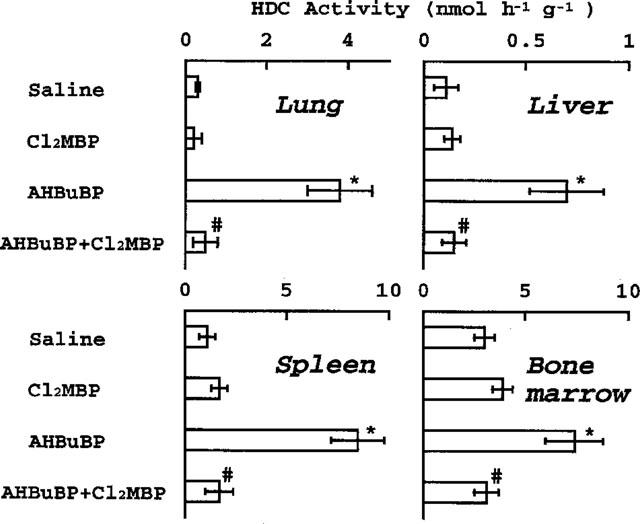
Effects of Cl2MBP and AHBuBP on HDC activity in mouse tissues. Cl2MBP (800 μmol kg−1) was injected (i.p.) immediately after injection of AHBuBP (40 μmol kg−1, i.p.), and the mice were sacrificed 3 days later. Each value is the mean±s.d. from four mice. *P<0.001 vs saline. #P<0.001 vs AHBuBP.
Dose-dependent inhibitory effect of Cl2MBP on the AHBuBP-induced elevation of HDC activity
Injection of Cl2MBP immediately after the injection of AHBuBP produced a dose-dependent inhibition of the AHBuBP-induced elevation of HDC activity in the lung, liver, spleen and bone marrow (Figure 2). Cl2MBP at 32 μmol kg−1 strongly inhibited the HDC elevation induced by AHBuBP (40 μmol kg−1). The inhibitory effect of Cl2MBP in the spleen was significant even at 6.4 μmol kg−1.
Figure 2.
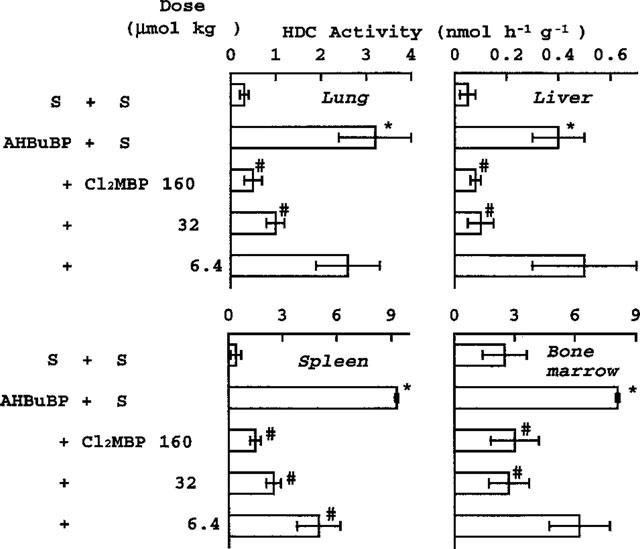
Dose-dependent inhibitory effect of Cl2MBP on the AHBuBP-induced elevation of HDC activity. Saline (S) or Cl2MBP (6.4–160 μmol kg−1) was injected (i.p.) immediately after injection of saline or AHBuBP (40 μmol kg−1, i.p.). The mice were sacrificed 3 days later. Each value is the mean±s.d. from four mice. *P<0.001 vs S+S. #P<0.001 vs AHBuBP+S.
Time-dependent inhibitory effect of Cl2MBP on the AHBuBP-induced elevation of HDC activity
The magnitude of the inhibition of the AHBuBP (40 μmol kg−1)-induced elevation of HDC activity was dependent on the time at which Cl2MBP (160 μmol kg−1) was injected (Figure 3). The inhibitory effect of Cl2MBP was greatest when it was injected simultaneously with AHBuBP, and it declined as the interval between the injections was increased. In the liver and spleen, there was still a significant inhibition when Cl2MBP was injected as much as 2 h after the injection of AHBuBP.
Figure 3.
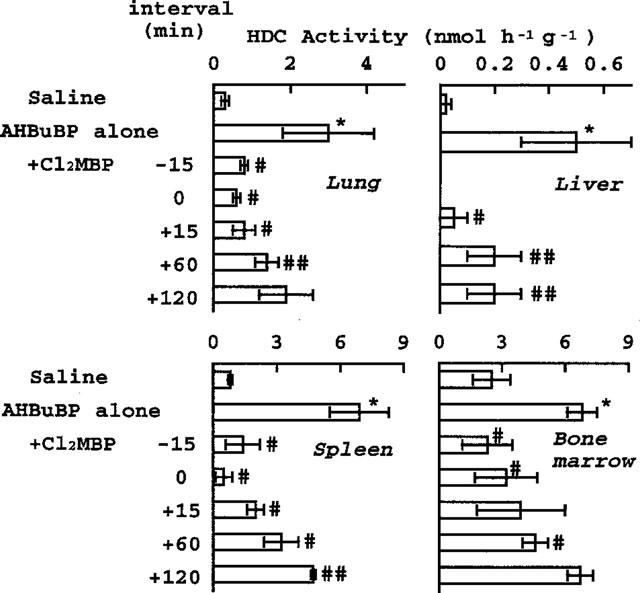
Time-dependent inhibitory effect of Cl2MBP on the AHBuBP-induced elevation of HDC activity. Cl2MBP (160 μmol kg−1) was injected (i.p.) into mice 15 min before (−), simultaneously with, or 15–120 min after (+) injection of AHBuBP (40 μmol kg−1, i.p.). The mice were sacrificed 3 days later. Each value is the mean±s.d. from four mice. *P<0.001 vs normal control. #P<0.01, ##P<0.05 vs AHBuBP alone.
Effects of Cl2MBP on other inflammatory reactions induced by AHBuBP
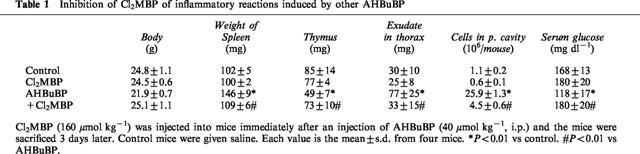
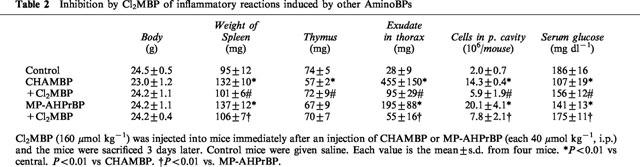
AHBuBP induces in mice a variety of inflammatory reactions, such as hypertrophy of the spleen, atrophy of the thymus, accumulation of exudate in the thorax and peritoneal cavity, and accumulation of macrophages and granulocytes in the peritoneal cavity. The AHBuBP-induced hypertrophy of the spleen was also inhibited (both dose-dependently and time-dependently) by Cl2MBP (Figure 4). The atrophy of the thymus, the accumulation of exudate in the thorax, and the accumulation of cells in the peritoneal cavity were also suppressed by Cl2MBP (Table 1). In the present study, it was found that AHBuBP induced hypoglycaemia, and this effect was also prevented by Cl2MBP (Table 1).
Figure 4.
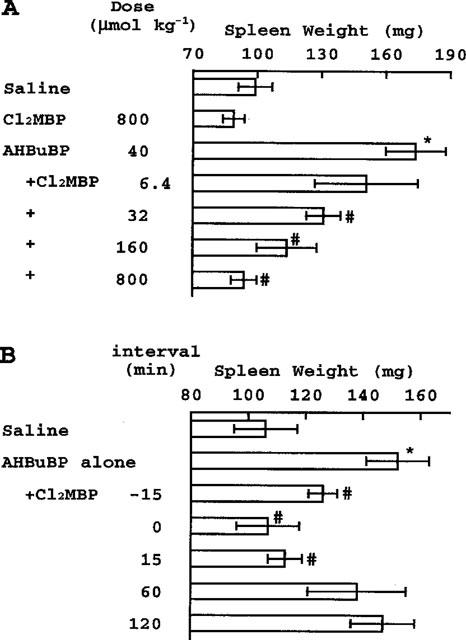
(A) Dose-dependent inhibitory effect of Cl2MBP on the AHBuBP-induced hypertrophy of spleen. Spleen weights in the experiments shown in Figures 1 and 2 are shown. *P<0.001 vs Saline. #P<0.001 vs AHBuBP. (B) Time-dependent inhibitory effect of Cl2MBP on the AHBuBP-induced hypertrophy of spleen. Spleen weights in the experiment shown in Figure 3 are shown. *P<0.001 vs Saline. #P<0.01 vs AHBuBP alone.
Table 1.
Inhibition of Cl2MBP of inflammatory reactions induced by other AHBuBP
Effects of Cl2MBP on inflammatory reactions induced by other aminoBPs
Like AHBuBP, two other aminoBPs (CHAMBP and MP-AHPrBP) induced elevations of tissue HDC activity and other inflammatory reactions. Cl2MBP (160 μmol kg−1) also inhibited these reactions. (Figure 5 and Table 2).
Figure 5.
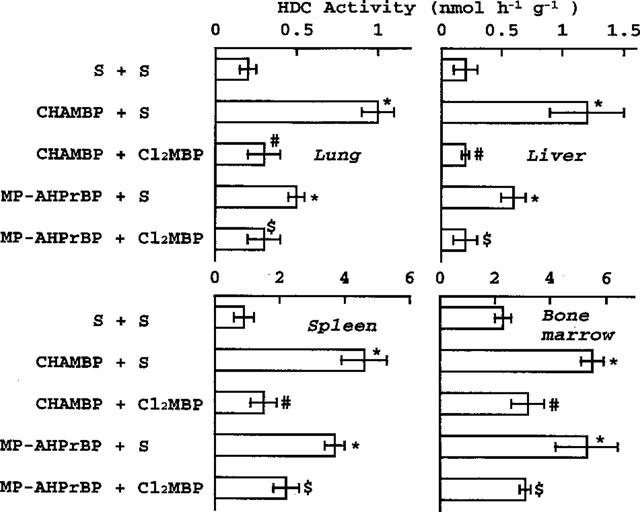
Effects of Cl2MBP on the elevation of HDC activity induced by other aminoBPs. Saline (S) or Cl2MBP (160 μmol kg−1) was injected (i.p.) into mice immediately after injection of saline or of CHAMBP or MP-AHPrBP (each 40 μmol kg−1, i.p.). The mice were sacrificed 3 days later. *P<0.001 vs saline+saline. #P<0.01 vs CHAMBP+S. $P<0.01 vs MP-AHPrBP+S.
Table 2.
Inhibition by Cl2MBP of inflammatory reactions induced by other AminoBPs
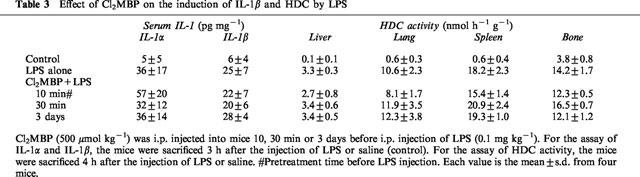
Effect of Cl2MBP on the induction of HDC by LPS
LPS is a potent inducer of HDC in various tissues, as well as in haematopoietic organs (Endo, 1982; 1983). Some cytokines have been implicated in the induction of HDC by LPS (see Discussion). In contrast to the HDC induction induced by AHBuBP, the induction by LPS is transient: HDC activity induced by an injection of LPS in mice peaks within 3–5 h of the injection and returns to the normal level within 20 h (Endo, 1982). Cl2MBP (500 μmol kg−1), when injected (i.p.) 10, 30 min or 3 days before LPS injection (0.1 mg kg−1, i.p.), did not inhibit the LPS-induced production of IL-1β and elevation of HDC activity in the liver, spleen, lung and bone (Table 3).
Table 3.
Effect of Cl2MBP on the induction of IL-1β and HDC by LPS
Effects of Cl2MBP on the augmentation by AHBuBP of the elevation of HDC activity and production of IL-1 induced by LPS
Recently, we have shown that AHBuBP pretreatment augments the LPS-induced elevation of HDC activity and production of IL-1 (both α and β), but suppresses the LPS-induced production of TNF (Sugawara et al., 1998). As shown in Figure 6, there were elevations of HDC activity in the lung, liver and spleen at 2 h after an i.v. injection of LPS. In mice treated with AHBuBP 3 days before the injection of LPS, there was a very considerable augmentation of the effect of LPS on HDC activity (note that the scale for HDC activity in Figure 6 is different from those in Figures 1, 2, 3 and 5). This augmentation was largely inhibited by Cl2MBP. In the present experiment, the contrasting effects of AHBuBP on the production of IL-1 and TNF were confirmed (Figure 7). The AHBuBP-induced augmentation of IL-1 production was completely prevented by Cl2MBP. Interestingly, Cl2MBP showed a tendency to reverse the inhibition of TNFα production induced by AHBuBP, although this effect of Cl2MBP was not statistically significant.
Figure 6.
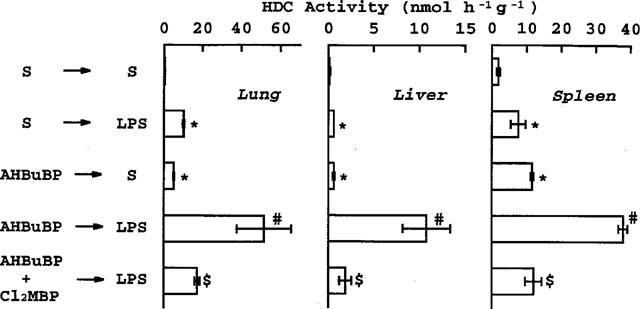
Effect of Cl2MBP on the augmentation by AHBuBP of the LPS-induced elevation of HDC activity. Saline (S), AHBuBP (40 μmol mg kg−1) or AHBuBP+Cl2MBP (160 μmol kg−1, immediately after AHBuBP) was injected intraperitoneally. Three days later, LPS (100 μg kg−1) or S was injected intravenously into the mice and tissues were removed 2 h later. Each value is the mean±s.d. from four mice. *P<0.01 vs S+S. #P<0.01 vs S+LPS and AHBuBP+S. $P<0.01 vs AHBuBP+LPS.
Figure 7.
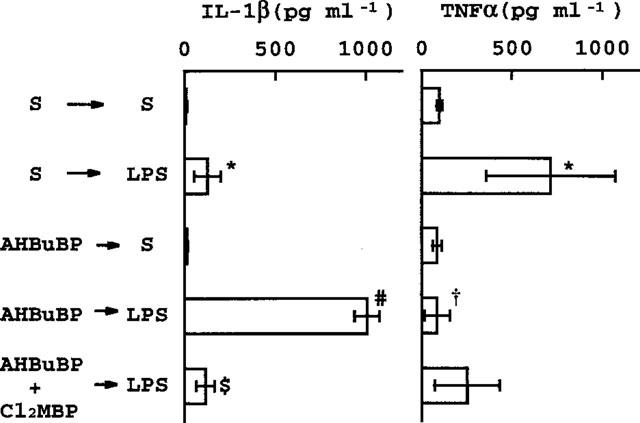
Effect of Cl2MBP on the influence of AHBuBP on the LPS-induced production of IL-1β and TNFα. The blood of mice in the experiment shown in Figure 6 was assayed for the cytokines. *P<0.01 vs S+S. #P<0.01 vs S+LPS and AHBuBP+S. $P<0.01 vs AHBuBP+LPS. †P<0.01 vs S+LPS.
Discussion
Intraperitoneal injection of any of the aminoBPs we have tested so far induces inflammatory reactions. The doses used in the present study are higher than those used clinically. Therefore, our experimental system is indeed an animal model to induce inflammation by aminoBPs in mice. However, it is the fact that even at clinical doses, aminoBPs have inflammatory side effects. By contrast, the non-aminoBPs tested did not produce such inflammatory reactions. In the present study, we found that the development of the inflammatory reactions induced by these aminoBPs was largely prevented by Cl2MBP, a typical non-aminoBP. Our findings highlight several interesting and important areas, all of which, however, will require more work before they can be clarified. We discuss these points in the following paragraphs.
Inhibition by Cl2MBP of inflammatory actions of aminoBPs
AminoBPs and Cl2MBP are structurally related analogues of pyrophosphate with a non-hydrolyzable P-C-P structure (Figure 8). It has been shown that Cl2MBP injected i.v. into mice (100 μmol kg−1) is cleared rapidly from the plasma, spleen and liver and disappears almost completely within 2 h of its injection (Mönkkönen et al., 1989). In contrast, a high concentration of aminoBPs (AHPrBP and AHBuBP) have been shown to be retained for a much longer time in the liver, spleen and lung of mice and rats (Mönkkönen et al., 1989; Lin et al., 1992). Therefore, the suppressive effect of Cl2MBP may depend on its concentration immediately after its injection. In addition, the effect of Cl2MBP was greatest when it was injected simultaneously with AHBuBP and was markedly lowered when it was injected 2 h after injection of AHBuBP (Figures 3 and 4). Therefore, our findings are likely to suggest the following two possibilities. First, aminoBPs and Cl2MBP may bind to common receptors involved in the induction of inflammatory reactions, and they may act on these receptors as an agonist or as an antagonist, respectively. Second, both aminoBPs and Cl2MBP may positively or negatively affect a step in a biochemical pathway(s) in which a certain kind of phosphate may be involved. For example, Schmidt et al. (1996) reported that AHBuBP inhibits protein-tyrosine phosphatase, and they suggested that the activity of this enzyme may be important in the function and formation of osteoclasts. However, this pathway seems unlikely to be the common step on which aminoBPs act to induce inflammation, because 1-hydroxyethylidene-1,1-bisphosphonate (HEBP, a non-aminoBP) inhibits the activity of this enzyme with an IC50 value similar to that of AHBuBP (Schmidt et al., 1996), and yet HEBP does not induce inflammatory reactions (Endo et al., 1993). However, the testing of these hypotheses will require further experiments.
Figure 8.
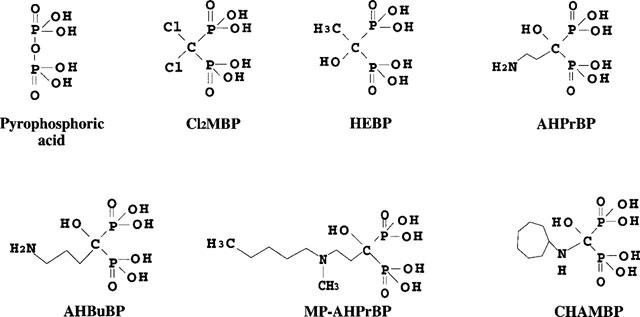
Structure of the bisphosphonates described in this paper.
On the other hand, Mönkkönen and his co-workers found that non-aminoBPs, clodronate (Cl2MBP) and tiludronate, inhibit LPS-induced production of IL-1β, IL-6 and TNFα (Pennanen et al., 1995; Mönkkönen et al., 1998) by a macrophage cell line (RAW 264) in vitro. They explained that this effect may be attributed to toxic metabolites formed after non-aminoBPs are ingested within cells (Frith et al., 1997; Auriola et al., 1997). We found that administration of Cl2MBP-liposomes, which selectively deplete phagocytic macrophages in vivo (Van Rooijen & Sanders, 1994), largely abolishes the augmentation of LPS-induced production of IL-1 in AHBuBP-treated mice (Sugawara et al., 1998). Therefore, the formation of the cytotoxic metabolite of Cl2MBP may underlie the depletion of macrophages. However, it is not clear whether the toxic metabolite is formed when Cl2MBP itself is given to mice as a free drug. As described above, Cl2MBP is cleared rapidly from the plasma, spleen and liver (Mönkkönen et al., 1989). Moreover, when free Cl2MBP exerts its inhibitory effect, 10–20 times larger doses of Cl2MBP are required than when it is encapsulated in liposomes (Pennanen et al., 1995). Therefore, to clarify whether the toxic metabolite of Cl2MBP is involved in its suppressive effect on the inflammatory actions of aminoBPs, its formation in vivo following the injection of Cl2MBP itself, requires investigation.
Cl2MBP does not inhibit the inflammatory actions of LPS
Cl2MBP itself, when injected 10, 30 min or 3 days before LPS-injection, was entirely ineffective in suppressing both the LPS-induced production of IL-1β and elevation of HDC activity. Although Cl2MBP accumulates almost irreversibly in bone, it does not accumulate in soft tissues including liver and spleen of mice (Mönkkönen et al., 1989). Therefore, the reasons why Cl2MBP does not inhibit the actions of LPS in mice not treated with an aminoBP are likely that (i) Cl2MBP may be not ingested in macrophages in a sufficient amount to induce its toxic effect and/or (ii) the major cells in the mice responsible for both the LPS-induced production of IL-1β and elevation of HDC activity may be not phagocytic macrophages as suggested by previous studies (Salkowski et al., 1995; Endo et al., 1995).
Mechanisms underlying the inflammatory actions of aminoBPs
Among the cytokines capable of inducing HDC activity (including IL-1, TNF, granulocyte-macrophage colony-stimulating factor (GM-CSF) and granulocyte colony-stimulating factor (G-CSF)), IL-1 is the most potent (Endo, 1989; Endo et al., 1992b). In the present study, it was found that aminoBPs induce hypoglycaemia, and it is known that injection of a low dose of IL-1 is also capable of inducing hypoglycaemia (Endo et al., 1985; Rey & Besedovsky, 1989; Endo, 1991). We found that AHBuBP greatly augments in vivo both the LPS-induced production of IL-1 (both α and β) and the LPS-induced elevation of HDC activity (Sugawara et al., 1998). In contrast, the LPS-induced production of TNFα was completely inhibited in AHBuBP-treated mice (Sugawara et al., 1998 and Figure 7 in the present study). Although IL-1 is barely detectable by current methods in the serum of mice injected with AHBuBP alone, we found that the cells of the peritoneal cavity, spleen and bone marrow in AHBuBP-injected mice spontaneously produced a higher level of IL-1 in vitro than the cells of normal mice (Sugawara et al., 1998). In addition, Northern blot analysis showed that there is a large increase in IL-1 mRNA, but not TNF- and GM-CSF-mRNAs, in the spleen of AHBuBP-injected mice (unpublished data). Moreover, our recent study using IL-1 (α/β)-deficient mice prepared by gene targeting indicated that in these mice, the elevation of HDC activity and most of other inflammatory reactions induced by aminoBPs are very small (unpublished data). These results suggest that a very slight but prolonged production of IL-1 by macrophages and/or other cells capable of producing IL-1 (such as endothelial cells) might be an important cause of the inflammatory action of aminoBPs, although the underlying mechanisms remain to be clarified.
Mönkkönen's group reported that aminoBPs are not converted to the toxic metabolite (Frith et al., 1997). However, they reported that pamidronate (AHPrBP) at higher concentrations inhibited the LPS-induced production of IL-1β in vitro by a macrophage cell line, RAW264, although this agent showed no significant effect at lower concentrations (Pennanen et al., 1995). In their experiments, they showed that the production of IL-6 was enhanced by the agent at its lower concentrations. By contrast, using the same experimental system, they recently reported that ibandronate (MP-AHPrBP) did not inhibit but did markedly enhance the LPS-induced secretion of IL-1β (Mönkkönen et al., 1998). Therefore, whether the effects of aminoBPs observed in these in vitro experiments can explain the in vivo inflammatory actions of aminoBPs is unclear. However, their finding that AHBuBP, but not non-aminoBPs, deposits in a large amount in the liver and spleen of mice (Mönkkönen et al., 1989) seems to be an important cause to induce its inflammatory actions.
Possible involvement of histamine in the inflammatory and haematopoietic actions of aminoPBs
In addition to the actions on the microcirculation, histamine has been shown to stimulate the proliferation of haematopoietic precursor cells in vitro (Byron, 1977; 1980; Gross & Worthington-White, 1984; Shounan & You-Heng, 1988; Nakaya & Tasaka, 1988; Schneider et al., 1990; Dy et al., 1993). Indeed, the HDC activity in the bone marrow is the highest found in the tissues of normal mice (Endo et al., 1992a). Moreover, the haematopoietic cytokines, interleukin-3 (IL-3), GM-CSF and G-CSF, induce HDC activity in vivo only in the haematopoietic organs of the mouse (spleen and bone marrow) (Lebel et al., 1990; Endo et al., 1992b). It is known that haematopoiesis in the spleen is inactive in adult rats and, although LPS strongly induces HDC activity in the spleen of adult mice, it does not have this effect in the spleen of adult rats (Endo et al., 1995). Moreover, histamine has been shown to exert various effects on the immune system (Beer et al., 1983; Falus & Merétey, 1992). Collectively, these data suggest that histamine may be involved in the inflammatory and haematopoietic actions (increase in macrophages and granulocytes) of aminoBPs. However, further work is required to investigate this.
Relationship between inflammatory or anti-inflammatory actions of BPs and their inhibitory actions on bone-resorption
The potency with which Cl2MBP inhibits bone resorption in vivo has been shown to be much less than those of aminoBPs (Mühlbauer et al., 1991; Geddes et al., 1994). Reitsma et al. (1982) have reported that the in vitro action on bone resorption of 3-amino-1-hydroxypropylidene-1,1-bisphosphonate (AHPrBP), an aminoBP which also induces inflammatory reactions (Endo et al., 1993), is different from that of Cl2MBP. Various mechanisms have been proposed to explain the inhibitory action of BPs on bone resorption. These include a physico-chemical stabilization of the bone resulting from the binding of BPs to hydroxyapatite, a cytotoxic action on osteoclasts, an inhibition of osteoclast formation or recruitment, a metabolic inhibition that is specific to osteoclasts (Geddes et al., 1994; Rodan & Fleisch, 1996), an osteoblast-mediated inhibition (Sahni et al., 1993), apoptosis (Hughes et al., 1995) and inhibition of a protein-tyrosine phosphatase (Schmidt et al., 1996). In our present study, the inflammatory reactions induced by 40 μmol kg−1 of aminoBPs were all significantly inhibited by co-injection of an equimolar dose of Cl2MBP (Figures 2 and 4). It could be of considerable interest to determine whether the mechanism by which aminoBPs inhibit bone resorption is independent of their inflammatory actions, and also whether the mechanism by which Cl2MBP inhibits bone resorption is independent of its anti-inflammatory actions. It is also of interest to examine whether the suppressed production of TNF might be involved in the action of aminoBPs to inhibit bone resorption.
Possibility that other non-aminoBPs have anti-inflammatory actions
We have pointed out previously (Nakamura et al., 1996) that while aminoBPs are not effective at suppressing experimental arthritis in rats and mice (in fact, they exacerbate the arthritis), non-aminoPBs are effective. Dunn et al. (1993a and 1993b) and Nugent et al. (1993) have reported that pyrazoline BPs (non-aminoBPs) resemble Cl2MBP in having anti-inflammatory and anti-arthritic activities. On this basis, it seems likely that other non-aminoBPs, including pyrazoline BPs, will prove, like Cl2MBP, to suppress the inflammatory actions of aminoBPs.
Proposal of a combined clinical use of an aminoBP and non-aminoBP
Finally, it has been shown that administration of aminoBPs produces inflammatory responses, including fever, in 10–50% of treated patients (Adami et al., 1987; Sauty et al., 1996). In addition, inflammatory side effects have also been reported in the field of ophthalmology (Siris, 1993; Macarol & Frauenfelder, 1994). Our findings suggest that the combined administration of an aminoBP (a potent inhibitor of bone resorption) with Cl2MBP (a less potent inhibitor of bone resorption) may be a useful regimen in that it might produce much weaker inflammatory side effects than use of an aminoBP alone, while possibly producing a stronger inhibitory effect on bone resorption than that obtained by the separate use of either agent.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education of Japan (No. 09470409).
Abbreviations
- aminoBP
aminobisphosphonate
- AHBuBP
4-amino-1-hydroxybutylidene-1,1-bisphosphonic acid
- BP
bisphos-phonate
- CHAMBP
cycloheptylaminomethylene bisphosphonate
- Cl2MBP
dichloromethylene bisphosphonate
- HDC
histidine decarboxylase
- IL
interleukin
- LPS
lipopolysaccharide
- HEBP
1-hydroxyethylidene-1,1-bisphosphonate
- MP-AHPrBP
3-(N-methyl-N-pentyl)amino-1-hydroxypropane-1,1-diphosphonic acid
- TNF
tumour necrosis factor
References
- ADAMI S., BHALLA A.K., DORIZZI R., MONTESANI F., ROSINI S., SALVAGNO G., LO CASCIO V. The acute phase response after bisphosphonate administration. Calcif. Tissue Int. 1987;41:326–331. doi: 10.1007/BF02556671. [DOI] [PubMed] [Google Scholar]
- AURIOLA S., FRITH J.C., ROGERS M.J., KOIVUNIEMI A., MÖNKKÖNEN J. Identification of adenine nucleotide-containing metabolites of bisphosphonate drugs using ion-pair liquid chromatography-electrospray mass spectrometry. J. Chromatogr. B. 1997;704:187–195. doi: 10.1016/s0378-4347(97)00490-8. [DOI] [PubMed] [Google Scholar]
- BEER J.D., MALTOFF S.M., ROCKLIN R.E. The influence of histamine on immune and inflammatory responses. Adv. Immunol. 1983;35:209–268. doi: 10.1016/s0065-2776(08)60577-5. [DOI] [PubMed] [Google Scholar]
- BONJOUR J.P., RIZZOLI R., AMMANN P., CHEVALLEY T.Bisphosphonates in clinical medicine Bone and Mineral Research 19948Amsterdam: Elsevier Science BV; 205–264.ed. Heershe, N.M. & Kanis, J.K. pp [Google Scholar]
- BYRON J.W. Mechanism for histamine H2-receptor induced cell-cycle changes in the bone marrow stem cell. Agents Actions. 1977;7:209–213. doi: 10.1007/BF01969974. [DOI] [PubMed] [Google Scholar]
- BYRON J.W. Pharmacodynamic basis for the interaction of cimetidine with the bone marrow stem cells (CFUs) Exp. Hematol. 1980;8:256–263. [PubMed] [Google Scholar]
- DUNN C.J., DOYLE D.V., WILLOUGHBY D.A. Investigation of the acute and chronic anti-inflammatory properties of diphosphonates using a broad spectrum of immune and non-immune inflammatory reactions. Drug Develop. Res. 1993b;28:47–55. [Google Scholar]
- DUNN C.J., GALINET L.A., WU H., NUGENT R.A., SCHLACHTER S.T., STAITE N.D., ASPAR D.G., ELIOTT G.A., ESSANI N.A., ROHLOFF N.A., SMITH R.J. Demonstration of novel anti-arthritic and anti-inflammatory effects of diphosphonates. J. Pharmacol. Exp. Ther. 1993a;266:1691–1698. [PubMed] [Google Scholar]
- DY M., MACHAVOINE F., LEBEL B., ICHIKAWA A., GASTINEL L.N., SCHNEIDER E. Interleukin 3 promotes histamine synthesis in hematopietic progenitors by increasing histidine decarboxylase mRNA expression. Biochem. Biophys. Res. Commun. 1993;192:167–173. doi: 10.1006/bbrc.1993.1396. [DOI] [PubMed] [Google Scholar]
- ENDO Y. Simultaneous induction of histidine and ornithine decarboxylases and changes in their product amines following the injection of Escherichia coli lipopolysaccharide into mice. Biochem. Pharmacol. 1982;31:1643–1647. doi: 10.1016/0006-2952(82)90394-x. [DOI] [PubMed] [Google Scholar]
- ENDO Y. Induction of histidine decarboxylase in mouse tissues by mitogens in vivo. Biochem. Pharmacol. 1983;32:3835–3838. doi: 10.1016/0006-2952(83)90157-0. [DOI] [PubMed] [Google Scholar]
- ENDO Y. Induction of histidine and ornithine decarboxylase activities in mouse tissues by recombinant interleukin-1 and tumor necrosis factor. Biochem. Pharmacol. 1989;38:1287–1292. doi: 10.1016/0006-2952(89)90335-3. [DOI] [PubMed] [Google Scholar]
- ENDO Y. Parallel relationship between the increase in serotonin in the liver and the hypoglycaemia induced in mice by interleukin-1 and tumor necrosis factor. Immunol. Lett. 1991;27:75–80. doi: 10.1016/0165-2478(91)90248-9. [DOI] [PubMed] [Google Scholar]
- ENDO Y., KIKUCHI T., NAKAMURA M., SHINODA H. Determination of histamine and polyamines in calcified tissues of mice: contribution of mast cells and histidine decarboxylase to the amount of histamine in the bone. Calcif. Tissue Int. 1992a;51:67–71. doi: 10.1007/BF00296220. [DOI] [PubMed] [Google Scholar]
- ENDO Y., KIKUCHI T., TAKEDA Y., NITTA Y., RIKIISHI H, KUMAGAI K. GM-CSF and G-CSF stimulate the synthesis of histamine and putrescine in the hematopoietic organs in vivo. Immunol. Lett. 1992b;33:9–14. doi: 10.1016/0165-2478(92)90086-4. [DOI] [PubMed] [Google Scholar]
- ENDO Y., NAKAMURA N., KIKUCHI T., SHINODA H., TAKEDA K., NITTA Y., KUMAGAI K. Aminoalkylbisphosphonates, potent inhibitors of bone resorption, induce a prolonged stimulation of histamine synthesis and increase macrophages, granulocytes and osteoclasts in vivo. Calcif. Tissue Int. 1993;52:248–254. doi: 10.1007/BF00298728. [DOI] [PubMed] [Google Scholar]
- ENDO Y., NAKAMURA M., NITTA Y., KUMAGAI K. Effect of macrophage depletion on the induction of histidine decarboxylase by lipopolysaccharide interleukin-1 and tumor necrosis factor. Br. J. Pharmacol. 1995;114:187–193. doi: 10.1111/j.1476-5381.1995.tb14924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDO Y., SUZUKI R., KUMAGAI K. Interleukin-1-like factor can accumulate 5-hydroxytrytamine in the liver of mice and can induce hypoglycaemia. Biochem. Biophys. Acta. 1985;840:37–42. doi: 10.1016/0304-4165(85)90159-x. [DOI] [PubMed] [Google Scholar]
- FALUS A., MERÉTEY K. Histamine: an early messenger in inflammatory and immune reactions. Immunol. Today. 1992;13:154–156. doi: 10.1016/0167-5699(92)90117-p. [DOI] [PubMed] [Google Scholar]
- FRITH J.C., MÖNKKÖNEN J., BLACKBURN G.M., RUSSEL R.G., ROGERS M.J. Clodronate and liposome-encapsulated clodronate are metabolized to a toxic ATP analog, adenosine 5′-(beta, gamma-dichloromethylene) triphosphate, by mammalian cells in vitro. J. Bone Miner. Res. 1997;12:1358–1367. doi: 10.1359/jbmr.1997.12.9.1358. [DOI] [PubMed] [Google Scholar]
- GEDDES A.D., D'SOUZA S.M., EBENTINO F.H., KENNETH J.I.Bisphosphonates: structure-activity relationships and therapeutic implications Bone and Mineral Research. 19948Amsterdam: Elsevier Science BV; 265–306.ed. Heershe, N.M. & Kanis, J.K. pp [Google Scholar]
- GREEN J.R., MÜLLER G.K., JAEGGI K.A. Preclinical pharmacology of CGP 42′446, a new, potent, heterocyclic bisphosphonate compound. J. Bone Mineral Res. 1994;9:745–751. doi: 10.1002/jbmr.5650090521. [DOI] [PubMed] [Google Scholar]
- GROSS S., WORTHINGTON-WHITE D.A. Cimetidine suppression of CFU-C in male. Am. J. Hematol. 1984;17:279–286. doi: 10.1002/ajh.2830170308. [DOI] [PubMed] [Google Scholar]
- HUGHES D.E., WRIGHT K.R., UY H.L., SASAKI A., YONEDA T., ROODMAN G.D., MUNDY G.R., BOYCE B.F. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Miner. Res. 1995;10:1478–1487. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- LEBEL B., SCHNEIDER E., PIQUET-PELLORCE C., MACHAVOINE F., KINDER V., LUFFAU G., DY M. Antigenic challenge of immunized mice induces endogenous production of IL-3 that increases histamine synthesis in hematopoietic organs. J. Immunol. 1990;145:1222–1226. [PubMed] [Google Scholar]
- LIN J.H., CHEN I.-W., DUGGAN D.E. Effects of dose, sex, and age on the disposition of alendronate, a potent antiosteolytic bisphosphonate, in rats. Drug Metab. Dispos. 1992;20:473–478. [PubMed] [Google Scholar]
- MACAROL V., FRAUENFELDER F.T. Pamidronate disodium and possible ocular adverse drug reactions. Am. J. Ophthamol. 1994;118:220–224. doi: 10.1016/s0002-9394(14)72902-2. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN J., KOPONEN H.-M., YLITALO P. Comparison of the distribution of three bisphosphonates in mice. Pharmacol. Toxicol. 1989;65:294–298. doi: 10.1111/j.1600-0773.1990.tb00750.x. [DOI] [PubMed] [Google Scholar]
- MÖNKKÖNEN J., SIMILÄ J., ROGERS M.J.Effects of tiludronate and ibandronate on the secretion of proinflammatory cytokines and nitric oxide from macrophages in vitro Life Science 19986295–102.PL [DOI] [PubMed] [Google Scholar]
- MÜHLBAUER R.C., BAUSS F., SCHENK R., JANNER M., BOSIES E., STREIN K., FLEISCH H. BM 21.0955, a new bisphosphonate to inhibit bone resorption. J. Bone Mineral Res. 1991;6:1003–1011. doi: 10.1002/jbmr.5650060915. [DOI] [PubMed] [Google Scholar]
- NAKAMURA M., ANDO T., MASATAKA A., KUMAGAI K., ENDO Y. Contrast between effects of aminobisphosphonates and non-aminobisphosphonates on collagen-induced arthritis in mice. Br. J. Pharmacol. 1996;119:205–212. doi: 10.1111/j.1476-5381.1996.tb15972.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAYA N., TASAKA K. The influence of histamine on precursors of granulocytic leukocytes in murine bone marrow. Life Sci. 1988;42:999–1010. doi: 10.1016/0024-3205(88)90430-4. [DOI] [PubMed] [Google Scholar]
- NUGENT R.A., MURPHY M., SCHLACHTER S.T., DUNN C.J., SMITH R.J., STAITE N.D., GALINET L.A., SHEILDS S.K., ASPAR D.G., RICHARD K.A., ROHLOFF N.A. Pyrazoline bisphosphonate esters as novel anti-inflammatory and anti-arthritic agents. J. Med. Chem. 1993;36:134–139. doi: 10.1021/jm00053a017. [DOI] [PubMed] [Google Scholar]
- PENNANEN N., LAPINJOKI S., URTTI A., MÖNKKÖNEN J. Effect of liposomal and free bisphosphonates on the IL-1β, IL-6 and TNFα secretion from RAW264 cells in vitro. Pharmaceutical Res. 1995;12:916–922. doi: 10.1023/a:1016281608773. [DOI] [PubMed] [Google Scholar]
- REITSMA P.H., TEITELBAUM S.L., BIJVOET O.L.M., KAHN A.J. Differential action of the bisphosphonates (3-amino-1-hydroxypropylidene)-1,1-bisphosphonate (APD) and disodium dichloromethylidene bisphosphonate (Cl2MDP) on rat macrophage-mediated bone resorption in vitro. J. Clin. Invest. 1982;70:927–933. doi: 10.1172/JCI110704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REY A., BESEDOVSKY H. Antidiabetic effects of interleukin 1. Proc. Natl. Acad. Sci. U.S.A. 1989;86:5943–5947. doi: 10.1073/pnas.86.15.5943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RODAN G.A., FLEISCH H.A. Bisphosphonates: Mechanism of Action. J. Clin. Invest. 1996;97:2692–2696. doi: 10.1172/JCI118722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAHNI M., GUENTHER H.L., FLEISCH H., COLLIN P., MARTIN T.J. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J. Clin. Invest. 1993;91:2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALKOWSKI C.A., NETA R., WHYNN T.A., STRASSMANN G., VAN ROOIJEN N., VOGEL S.N. Effect of liposome-mediated macrophage depletion on LPS-induced cytokine expression and radioprotection. J. Immunol. 1995;155:3168–3179. [PubMed] [Google Scholar]
- SAUTY A., PECHERSTORFER M., ZIMMER-ROTH I., FIORONI P., JUILLERAT L., MARKERT M., LUDWIG H., LEUENBERGER P., BURCKHARDT P., THIÉBAUD D. Interleukin-6 and tumor necrosis factor α leveles after bisphosponates treatment in vitro and in patients with malignancy. Bone. 1996;18:133–139. doi: 10.1016/8756-3282(95)00448-3. [DOI] [PubMed] [Google Scholar]
- SCHMIDT A., RUTLEDGE S.J., ENDO N., OPAS E.E., TANAKA H., WESOLOWSKI G., LEU C.T., HUANG Z., RAMACHANDARAN C. Protein-tyrosine phosphatase activity regulates osteoclast formation and function: inhibition by alendronate. Proc. Natl. Acad. Sci. U.S.A. 1996;93:3068–3073. doi: 10.1073/pnas.93.7.3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHNEIDER E., PIQUET-PELLORCE C., DY M. New role for histamine in interleukin-3-induced proliferation of hematopoietic stem cells. J. Cell. Physiol. 1990;143:337–343. doi: 10.1002/jcp.1041430218. [DOI] [PubMed] [Google Scholar]
- SHOUNAN Y., YOU-HENG X. The influence of histamine at various concentrations on the cell cycle state of hematopoietic stem cells (CFU-s) Int. J. Cell Cloning. 1988;6:290–295. doi: 10.1002/stem.5530060406. [DOI] [PubMed] [Google Scholar]
- SIRIS E. Bisphosphonateas and iritis. Lancet. 1993;ii:436–437. doi: 10.1016/0140-6736(93)93029-z. [DOI] [PubMed] [Google Scholar]
- SUGAWARA S., SHIBAZAKI M., TAKADA H., KOSUGI K., ENDO Y. Contrasting effects of an aminobisphosphonate, a potent inhibitor of bone resorption, on the lipopolysaccharide-induced production of interleukin-1 and tumour necrosis factor in mice. Br. J. Pharmacol. 1998;125:1735–1740. doi: 10.1038/sj.bjp.0702151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN ROOIJEN N., SANDERS A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J. Immunol. Meth. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]


