Abstract
The present study was designed to characterize the adenosine receptors involved in the relaxation of the pig intravesical ureter, and to investigate the action of adenosine on the non adrenergic non cholinergic (NANC) excitatory ureteral neurotransmission.
In U46619 (10−7 M)-contracted strips treated with the adenosine uptake inhibitor, nitrobenzylthioinosine (NBTI, 10−6 M), adenosine and related analogues induced relaxations with the following potency order: 5′-N-ethylcarboxamidoadenosine (NECA)=5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA)=2-chloroadenosine (2-CA)>adenosine>cyclopentyladenosine (CPA)=N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide (IB-MECA)=2-[p-(carboxyethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine (CGS21680).
Epithelium removal or incubation with indomethacin (3×10−6 M) and L-NG-nitroarginine (L-NOARG, 3×10−5 M), inhibitors of prostanoids and nitric oxide (NO) synthase, respectively, failed to modify the relaxations to adenosine.
1,3-dipropyl-8-cyclopentylxanthine (DPCPX, 10−8 M) and 4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385, 3×10−8 M and 10−7 M), A1 and A2A receptor selective antagonists, respectively, did not modify the relaxations to adenosine or NECA. 8-phenyltheophylline (8-PT, 10−5 M) and DPCPX (10−6 M), which block A1/A2-receptors, reduced such relaxations.
In strips treated with guanethidine (10−5 M), atropine (10−7 M), L-NOARG (3×10−5 M) and indomethacin (3×10−6 M), both electrical field stimulation (EFS, 5 Hz) and exogenous ATP (10−4 M) induced contractions of preparations. 8-PT (10−5 M) increased both contractions. DPCPX (10−8 M), NECA (10−4 M), CPCA, (10−4 M) and 2-CA (10−4 M) did not alter the contractions to EFS.
The present results suggest that adenosine relaxes the pig intravesical ureter, independently of prostanoids or NO, through activation of A2B-receptors located in the smooth muscle. This relaxation may modulate the ureteral NANC excitatory neurotransmission through a postsynaptic mechanism.
Keywords: Pig intravesical ureter, adenosine receptors, electrical field stimulation, 8-PT, DPCPX, ZM 241385, NANC neurotransmission
Introduction
ATP and related nucleotides and nucleosides have been described to be involved in non adrenergic non cholinergic (NANC) neurotransmission (Burnstock, 1972; Burnstock et al., 1978). ATP is converted to adenosine by the action of ecto-ATPases and nucleotidases, which are present in the surroundings of the nerve terminal (McDonald & White, 1984; Cusack & Hourani, 1984; Cusack et al., 1988). Once formed, adenosine acts on presynaptic receptors to depress further ATP release (Khakh & Kennedy, 1998).
Physiological and pharmacological effects of purines are mediated via two main classes of receptors, designated as P1 and P2, which primarily respond to adenosine and ATP, respectively (Burnstock, 1978). Smooth muscle P1-purinoceptors generally cause relaxation (Burnstock, 1978) although in a number of smooth muscle preparations adenosine cause contraction via these receptors (Bailey et al., 1992). P2-purinoceptors have recently been termed P2 receptors since they are receptors for a purine or pyrimidine nucleotide or dinucleotide. These receptors have been classified into the ionotropic P2X and the metabotropic P2Y. P2X operate via intrinsic ion channels mediating fast and transient responses while P2Y are G protein-coupled receptors which mediate slower responses (Abbracchio & Burnstock, 1994; Fredholm et al., 1994; 1997).
P1-receptors have been divided in four subtypes, designated as A1, A2A, A2B and A3 (Fredholm et al., 1994; Olah & Stiles, 1995). All four are G protein-coupled receptors; the A1 and A3 subtypes are coupled to inhibitory G proteins, while activation of the A2A- and A2B-receptors leads to adenylate cyclase stimulation (Collis & Hourani, 1993; Alexander et al., 1994; Fredholm et al., 1994). A1- and A2-receptor subtypes have been defined according to the relative potency on them of adenosine analogues, as well as considering the potency shown by A1-selective antagonists such as 1,3-dipropyl-8-cyclopentyl xanthine (DPCPX). Thus, on A1-receptors, N6 substituted analogues such as N6-cyclopentyladenosine (CPA) are more potent than 5′-substituted analogues such as 5′-N-ethylcarboxamidoadenosine (NECA). Xanthines such as 8-phenyltheophylline (8-PT) and 8-sulphophenyltheophylline (8-SPT) are antagonists at both A1- and A2-receptors, whereas DPCPX has nanomolar affinity at A1-receptors and micromolar affinity at A2 receptors, and thus effectively discriminates between the two types of receptors (Lohse et al., 1987; Bruns, 1990; Collis et al., 1989).
A2-receptors have been divided further into A2A and A2B subtypes, based on the potency shown by some two-substituted adenosine analogues, such as 2-[p-(carboxyethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine (CGS21680). Thus, CGS21680 is a potent agonist at A2A receptors but is virtually inactive at A2B sites (Jacobson, 1990). Recently, a non-xanthine antagonist, 4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol, (ZM 241385) had shown high selectivity for A2A receptors (Poucher et al., 1995).
A3-receptors, which are considered to be xanthine-insensitive, have been cloned from rat striatum. These receptors are coupled to an inhibitory G protein (Meyerhof et al., 1991; Zhou et al., 1992). N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide (IB-MECA) has been reported as a selective A3-receptor agonist (Jacobson et al., 1993; 1995; Gallo-Rodríguez et al., 1994). A3-receptors are resistant to blockade with methylxanthines, although it has been reported that compounds such as 1,3-dipropyl-8-(4-acrylate)phenylxanthine (BWA1433) block them (Jacobson et al., 1995).
The autonomic nervous system plays an essential role in the maintenance of ureteral peristalsis. Thus, a rich catecholaminergic innervation forming dense neuromuscular, perivascular and subepithelial plexuses has been found in the intravesical ureter (Schulman, 1985; Prieto et al., 1993; 1994). Both noradrenaline (Hernández et al., 1992) and acetylcholine (Hernández et al., 1993; 1995a; Prieto et al., 1994) stimulate the two contractile components of intravesical ureteral smooth muscle (phasic activity and tone). In addition to the adrenergic and cholinergic systems, several neuropeptides, such as neuropeptide Y (NPY), vasoactive intestinal peptide and somatostatin have been identified in ureteral nerves, coexisting with different neurotransmitters (Edyvane et al., 1994; Smet et al., 1994; Prieto et al., 1997). These peptides act by modulating the neurotransmitter-evoked activity. Thus, NPY has been reported to enhance the noradrenaline-induced contraction in the equine intravesical ureter through Y2-receptors (Prieto et al., 1997). Together with the above mentioned peptides, nitric oxide (NO) (Hernández et al., 1995b) and calcitonin gene related peptide (CGRP) (Maggi & Giuliani, 1991) have been involved in the ureteral non adrenergic non cholinergic (NANC) inhibitory neurotransmission, in processes operated through guanylate cyclase (Hernández et al., 1997) and adenylate cyclase (Maggi et al., 1994; 1995), respectively, involving in both cases the activation of glibenclamide-sensitive K+ channels.
Adenosine has been reported to modulate the NANC mouse urinary bladder neurotransmission (Acevedo et al., 1992). The possible role exerted by adenosine in the intravesical ureter has not been described. Therefore, the present study was designed to characterize the functionally active adenosine receptor subtypes involved in the relaxation of porcine ureter, and to investigate the possible modulatory action of adenosine in the NANC motor transmission of the pig intravesical ureter.
Methods
Adult pigs of either sex with no lesions in their urinary tract were selected from the local slaughterhouse. Urinary bladders with attached ureters were removed immediately after the animals were killed, and kept in chilled physiological saline solution (PSS) at 4°C. The adjacent connective and fatty tissues were removed with care and longitudinal preparations (4–6 mm long and 2–3 mm wide) of the intravesical ureter were isolated from the bladder by dissection, as previously described (Hernández et al., 1992). The ureteral strips were suspended horizontally and placed parallel between two platinum electrodes, with one end connected to an isometric transducer (Grass FT 03C) and the other one to a micrometer screw which regulates the tension applied to the preparations, in 5 ml organ baths. The signal was continuously recorded on a polygraph (Graphtec Multicorder MC 6621, Hugo Sachs Elektronik, Germany). Passive tension of 2 g was applied to the ureteral preparations and they were allowed to equilibrate for 60 min.
Experimental procedure
The contractile ability of the preparations was determined by exposing the ureteral strips to 124 mM potassium-rich physiological saline solution (KPSS). Relaxations to adenosine and structurally related analogues were performed on strips incubated for 30 min with the adenosine uptake inhibitor nitrobenzylthioinosine (NBTI, 10−6 M) and further contracted with the thromboxane analogue U46619 (10−7 M). Afterwards, the tissues were washed every 20 min during 1.5 h. To study the effect of antagonists on adenosine or NECA relaxations, the preparations were incubated with NBTI alone (control curve) or NBTI plus the antagonist (30 min, except for 8-PT, which was incubated for 45 min). At the end of each experiment with adenosine and its structural analogues, papaverine (10−4 M) was added to the organ bath with the aim of obtaining the maximal relaxation of preparations.
In electrical field stimulation (EFS) experiments, adrenergic neurotransmission, muscarinic receptors, NO synthase activity and prostaglandins were blocked by incubation with guanethidine (10−5 M), atropine (10−7 M), L-NG-nitroarginine (L-NOARG, 3×10−5 M) and indomethacin (3×10−6 M), respectively, during a period of 1 h, washing every 20 min, and these drugs were present throughout the experiment. EFS was performed with rectangular pulses (1 ms duration, 5 Hz, 20 s trains), at 3 min intervals, from a Cibertec CS20 stimulator (Barcelona, Spain) with constant current output adjusted to 75 mA. The strips were repeatedly washed and allowed to equilibrate for at least 1 h before they were incubated with blocking agents.
Drugs and solutions
The following drugs were used: acetylcholine (ACh), adenosine, adenosine 5′-triphosphate (ATP), atropine sulphate, guanethidine, 5-hydroxytryptamine (5-HT), indomethacin, L-NOARG, papaverine, 9, 11-dideoxy-11α, 9α-epoxymethano-prostaglandin F2α (U46619) and tetrodotoxin (Sigma, U.S.A.). N6-cyclopentyladenosine (CPA), 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA), 2-chloroadenosine (2-CA), 2-[p-(carboxyethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine (CGS21680), 1,3-dipropyl-8-cyclopentyl xanthine (DPCPX), N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide (IB-MECA), nitrobenzylthioinosine (NBTI), 5′-N-ethylcarboxamidoadenosine (NECA), 8-phenyl-theophylline (8-PT) (RBI, U.S.A), 4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385) (Tocris Cookson, U.S.A.). ACh, adenosine, ATP, guanethidine, 5-HT, L-NOARG and tetrodotoxin were dissolved in double distilled water. CGS21680, DPCPX, NBTI, NECA, IB-MECA and ZM 241385 were dissolved in dimethyl sulphoxide (DMSO). CPCA was dissolved in 0.1N HCl. 8-PT was dissolved in 25% ethanol plus 30 mM NaOH. U46619, indomethacin, 2-CA, CPA and papaverine were dissolved in 96% ethanol. These solvents, in the amount applied, had no effect on pig intravesical ureteral activity. The composition of PSS was (mM): NaCl 119, KCl 4.6, MgCl2 1.2, NaHCO3 24.9, glucose 11, CaCl2 1.5, KH2PO4 1.2, EDTA (ethylenediamine tetraacetic acid) 0.027. The solution was continuously gassed at 37°C with 95% O2 and 5% CO2, to maintain pH at 7.4. KPSS was PSS with KCl exchanged for NaCl on an equimolar basis. Stock solutions were prepared daily in double-distilled water.
Calculations and statistics
For each concentration-response relaxation curve to adenosine and related analogues, the drug concentration required to give 40% relaxation (EC40) of U46619 induced contraction was estimated by regression of the linear portion of the curve, by computerized linear regression analysis (Inplot, GraphPad, U.S.A.). The sensitivity of the drugs is expressed in terms of pEC40, where pEC40 is defined as the negative logarithm of EC40 (pEC40=−logEC40 (M). Each parameter was determined from ureters of at least 4–6 different animals. Statistical significance of differences was calculated by Student's t-test, for paired observations for individual concentrations or frequencies and variance analysis (ANOVA) for multiple comparisons, followed by an a posteriori Bonferroni test (Wallestein et al., 1980). Differences were considered significant with a probability level of P<0.05.
Results
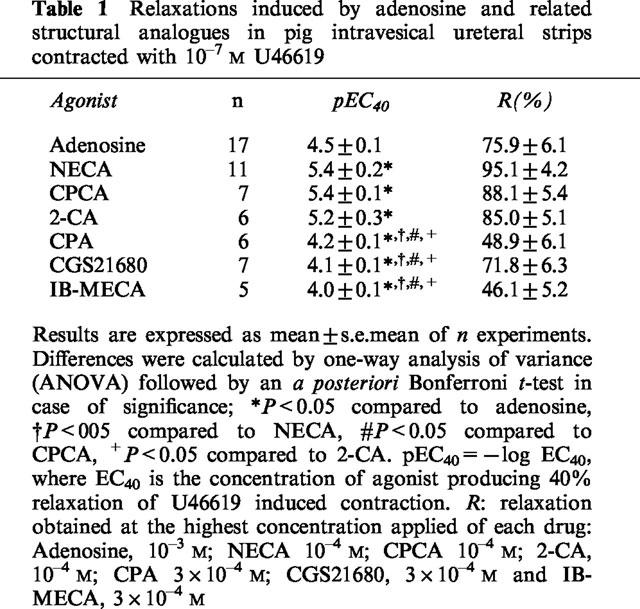
The pig intravesical ureteral preparations were equilibrated to a passive tension of 1.8±0.3 g (n=87). In NBTI (10−6 M)-pretreated tissues, the thromboxane analogue, U46619 (10−7 M) induced sustained contractions of 1.9±0.3 g (n=75). Adenosine, NECA, 2-CA, CPCA, CGS 21680, CPA and IB-MECA all concentration-dependently relaxed the U46619-contracted intravesical ureteral strips, the order of potency being: NECA=CPCA=2-CA>adenosine>CPA=IB-MECA=CGS21680 (Figure 1, Table 1). This order of potency suggests that the adenosine receptors that relax the pig intravesical ureteral smooth muscle belong to the A2B subtype.
Figure 1.
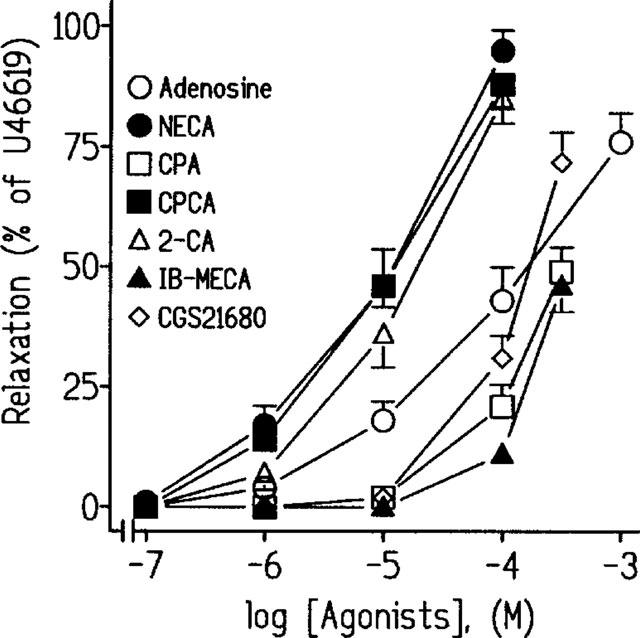
Log concentration-response relaxation curves to adenosine, 5′-N-ethylcarboxamidoadenosine (NECA), N6-cyclopentyladenosine (CPA), 5′-(N-cyclopropyl)-carboxamidoadenosine (CPCA), 2-chloroadenosine (2-CA), N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide (IB-MECA) and 2-[p-(carboxyethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine (CGS21680), in pig intravesical ureteral strips contracted to 10−7 M U46619. Relaxations are expressed as a percentage of the U46619-induced contraction. Results represent means and vertical line s.e. of the mean of 5–17 preparations.
Table 1.
Relaxations induced by adenosine and related structural analogues in pig intravesical ureteral strips contracted with 10−7 M U46619
The relaxations to adenosine (10−7–10−3 M) and NECA (10−7–10−4 M) were reproducible. Thus, pEC40 values and the relaxations to 10−3 M adenosine were 4.1±0.2 and 73.2±7.0%, and 4.0±0.2 and 70.2±6.8% (P>0.05, paired t-test, n=9), in a first and second concentration-response curve performed in the same preparation, respectively. pEC40 values and the relaxations to 10−4 M NECA were 5.2±0.2 and 92.1±5.2% and 5.1±0.3 and 88.3±4.8% (P>0.05, paired t-test, n=7), in two consecutive curves.
Removal of ureteral epithelium did not modify the adenosine concentration-relaxation curve, the pEC40 values being 4.0±0.2 and 4.1±0.2, (P>0.05, paired t-test, n=6) in the absence and in the presence of epithelium, respectively. Epithelium removal had also no effect on the relaxations to 10−3 M adenosine. These relaxations were 70.1±4.7% and 63.2±5.2% (P>0.05, paired t-test, n=6), in the absence and in the presence of epithelium, respectively (Figure 2a).
Figure 2.
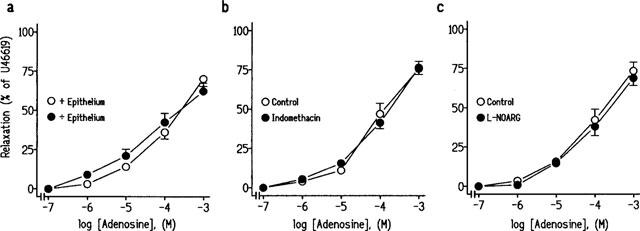
(a) Log concentration-response relaxation curves to adenosine in pig intravesical ureteral strips contracted to 10−7M U46619 in the presence and in the absence of ureteral epithelium. (b and c) Log concentration-response relaxation curves to adenosine in control conditions or in the presence of (b) 3×10−6 M indomethacin or (c) 3×10−5 M L-NG-nitroarginine (L-NOARG). The relaxations are expressed as a percentage of the U46619-induced contraction. Results represent means and vertical line s.e.mean of 6–7 preparations.
Indomethacin (3×10−6 M) and L-NOARG (3×10−5 M), inhibitors of prostanoid production and NO synthase activity, respectively, did not modify the relaxations to adenosine. The pEC40 values and the relaxations to 10−3 M adenosine were 4.1±0.1 and 75.7±4.8% and 4.1±0.2 and 76.4±4.3% (P>0.05, paired t-test, n=6), in control conditions and in the presence of indomethacin, respectively (Figure 2b). pEC40 values and the relaxations to 10−3 M adenosine in the experiments with L-NOARG (3×10−5 M) were 4.1±0.2 and 73.4±5.5% and 4.0±0.2 and 68.8±4.7% (P>0.05, paired t-test, n=7), in the absence and in the presence of L-NOARG, respectively (Figure 2c).
Effects of 8-PT, DPCPX and ZM 241385 on relaxations to adenosine and NECA
We examined whether 8-PT (10−5 M), an inhibitor of both A1- and A2-adenosine receptors, and DPCPX (10−8 M and 10−6 M), inhibited the relaxations to adenosine or NECA in U46619-precontracted strips obtained from pig intravesical ureter. Pretreatment of strips with 8-PT (10−5 M), did not modify the ureteral basal tone (1.6±0.4 g, in control conditions and in the presence of 8-PT), but reduced the relaxations to adenosine (Figure 4a, Table 2) and NECA (Figure 3 and 4b, Table 1). Incubation with 10−8 M DPCPX, a concentration selective for A1-receptors, did not modify the relaxations to adenosine or to NECA. However, the use of 10−6 M DPCPX, a concentration which blocks the A2-receptors, significantly reduced the relaxations to adenosine or NECA on ureteral strips (Figure 5a and b, Table 2). ZM 241385 (3×10−8 M and 10−7 M), a non-xanthine antagonist which shows high selectivity for A2A did not modify the relaxations to adenosine or to NECA (Figure 5c and d, Table 2).
Figure 4.
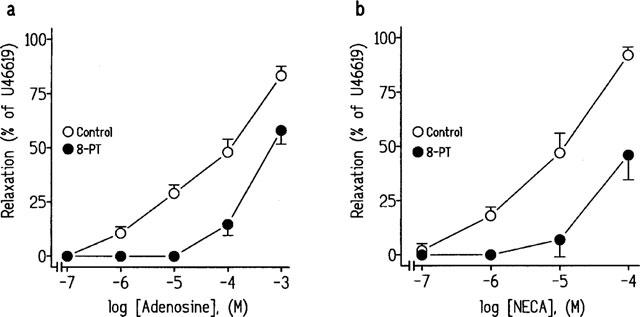
Log concentration-response relaxation curves to (a) adenosine and (b) 5′-N-ethylcarboxamidoadenosine (NECA) in pig intravesical ureteral strips contracted to 10−7 M U46619, in control conditions and in the presence of the A1 and A2 adenosine receptors inhibitor, 8-phenyltheophylline (8-PT, 10−5 M). The relaxations are expressed as a percentage of the U46619-induced contraction. Results represent means and vertical line s.e.mean of 6–7 preparations.
Table 2.
Effect of 8-PT (10−5 M), DPCPX (10−8 M), DPCPX (10−6 M), ZM 241385 (3×10−8 M) and ZM 241385 (10−7 M) on relaxations to adenosine and NECA of pig intravesical ureter contracted to U46619 (10−7 M)
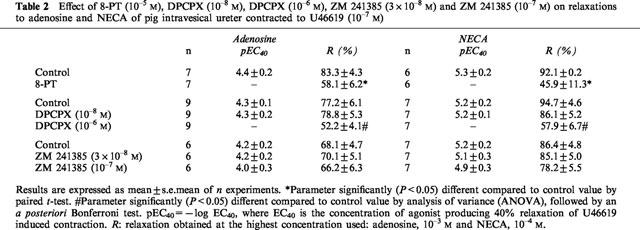
Figure 3.

Isometric force recordings showing the effect of 8-phenyltheophylline (8-PT, 10−5 M) on the relaxant response to 5′-N-ethylcarboxamidoadenosine (NECA, 10−7–10−4 M), in U46619 (10−7 M)-contracted pig intravesical ureteral strips. At the end of the experiment, papaverine (PAP, 10−4 M) was added to obtain the maximal relaxation of the ureteral strips. Vertical bar shows tension in g and horizontal bar time in min. Numbers indicate molar concentration in the bath. W: wash out.
Figure 5.
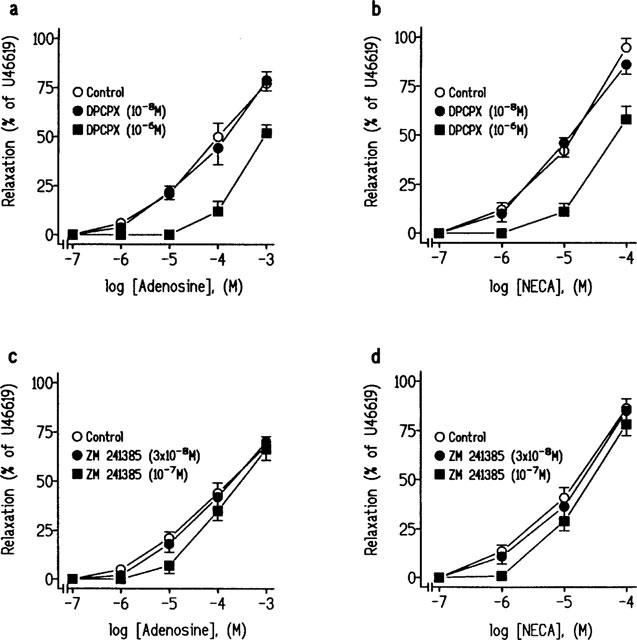
Log concentration-response relaxation curves to (a and c) adenosine and (b and d) 5′-N-ethylcarboxamidoadenosine (NECA) in pig intravesical ureteral strips contracted to 10−7 M U46619, in control conditions and in the presence of the A1-adenosine receptors inhibitor, 3-dipropyl-8-cyclopentylxanthine (DPCPX), (10−8 M) or (10−6 M) (a and b) and the A2A selective receptors antagonist, 4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol (ZM 241385), (3×10−8 M) or (10−7 M) (c and d). The relaxations are expressed as a percentage of the U46619-induced contraction. Results represent means and vertical line s.e.mean of 6–9 preparations.
Effects of 8-PT and DPCPX on contractions evoked by EFS and exogenous ATP
Pig intravesical ureteral strips were treated with guanethidine (10−5 M), atropine (10−7 M), L-NOARG (3×10−5 M) and indomethacin (3×10−6 M), with the aim to block adrenergic neurotransmission, muscarinic receptors, NO synthase activity and prostanoids, respectively. In such conditions, both EFS (5 Hz) and exogenous ATP (10−4 M) evoked reproducible contractions of 1.3±0.2 g and 1.2±0.2 g (P>0.05, paired t-test, n=7) and 0.4±0.1 g and 0.4±0.2 g (P>0.05, paired t-test, n=5), in a first and second addition, respectively, on the basal tone of preparations. Treatment of ureteral strips with 8-PT (10−5 M) significantly potentiated the contractions induced by both EFS (1.1±0.3 g and 1.7±0.4 g, in control conditions and in the presence of 10−5 M 8-PT, respectively, n=9, P<0.05, paired t-test) (Figure 6a and b) or exogenous ATP (0.5±0.2 g and 1.1±0.2 g in the absence and in the presence of 10−5 M 8-PT, respectively, n=11, P<0.05, paired t-test) (Figure 6a and c). The incubation with a selective A1-receptor antagonist, DPCPX (10−8 M), did not modify the contractions induced by EFS (0.9±0.2 g and 1.0±0.3 g, P>0.05, paired t-test, n=7, in control conditions and in the presence of 10−8 M DPCPX, respectively) or exogenous ATP (0.6±0.2 g and 0.5±0.2 g, P>0.05 paired t-test, n=8, in the absence and in the presence of 10−8 M DPCPX, respectively). The contractions elicited by EFS (5 Hz) were abolished by including 10−6 M tetrodotoxin in the bath, thus providing evidence for the neurogenic origin of these contractions.
Figure 6.
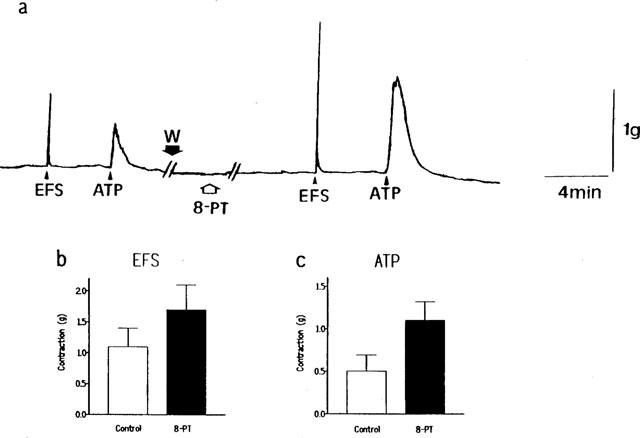
(a) Isometric force recordings showing the effect of 8-phenyltheophylline (8-PT, 10−5 M) on contractions induced by both EFS (5 Hz, 1 ms duration, 20 s trains, at 3 min intervals) and exogenous ATP (10−4 M), in pig intravesical ureteral strips pretreated with guanethidine (10−5 M), atropine (10−7 M), L-NG-nitroarginine (L-NOARG, 3×10−5 M) and indomethacin (3×10−6 M), to block adrenergic neurotransmission, muscarinic receptors, NO synthase activity and prostaglandins, respectively. Vertical bar shows tension in g and horizontal bar time in min. W: wash out. Contraction (g) induced by (b) EFS (5 Hz) or (c) ATP (10−4 M), in the absence or presence of 10−5 M 8-phenyltheophylline in the pig intravesical ureter. Results represent means and vertical line s.e.mean of 9–11 preparations.
Effects of NECA, CPCA and 2-CA on contractions evoked by EFS
In the conditions above mentioned, the incubation during 5 min of the preparations with a maximal (10−4 M) concentration of NECA, CPCA and 2-CA did not modify the contractions to EFS (5 Hz). Thus, EFS evoked contractions of 1.0±0.3 g and 1.1±0.3 g (P>0.05, paired t-test, n=7); 0.9±0.2 g and 0.8±0.2 g (P>0.05, paired t-test, n=5); 1.1±0.3 g and 1.2±0.4 g, (P>0.05, paired t-test, n=5) in control conditions and in the presence of NECA, CPCA and 2-CA, respectively.
Effects of NECA on contractions elicited by exogenous ATP
Pretreatment of ureteral strips for 5 min with NECA (10−4 M), reduced the contractions to exogenous ATP (10−4 M). Thus, ATP (10−4 M) induced contractions of 0.5±0.2 g and 0.2±0.2 g (P<0.05, paired t-test, n=5) in the absence or presence of NECA (10−4 M), respectively.
Effects of 8-PT on contractions induced by exogenous ACh and 5-HT
Incubation of preparations with 8-PT (10−5 M) increased the contractions to ACh (10−5 M) or 5-HT (10−5 M) in the pig intravesical ureter. ACh (10−5 M) induced contractions of 0.9±0.2 g and 1.3±0.3 g (P<0.05, paired t-test, n=7) in the absence or presence of 8-PT (10−5 M), respectively. 5-HT (10−5 M) evoked contractions of 1.3±0.2 g and 1.8±0.2 g (P<0.05, paired t-test, n=5) in control conditions and in the presence of 8-PT (10−5 M), respectively.
Discussion
In the present study adenosine and six of its related structural analogues, NECA, 2-CA, CPCA, CGS21680, CPA and IB-MECA concentration-dependently and epithelium-independently relaxed the pig intravesical ureteral strips precontracted with U46619. The relaxing potency order obtained (NECA= CPCA = 2-CA>adenosine>CPA = IB-MECA=CGS21680) together with the lack of effect of indomethacin and L-NOARG is consistent with a prostanoid- and NO-independent activation of A2B receptors by these agonists. The potentiation induced by 8-PT on the contraction evoked by EFS and exogenous ATP suggests that endogenous adenosine could be modulating the non adrenergic non cholinergic neurotransmission of the pig intravesical ureter.
The relaxation curves to adenosine and NECA were monophasic, suggesting that both agonists activate one receptor site to cause relaxation in the pig intravesical ureter. Removal of the ureteral epithelium did not change the relaxations to adenosine, indicating that these responses are mediated via a receptor located on the ureteral smooth muscle. Both prostanoids and NO have been reported to mediate the relaxation induced by adenosine in different tissues. Thus, part of the relaxing effect produced by adenosine in the rabbit isolated heart is mediated through release of prostaglandins (Ciabattoni & Wennmalm, 1985; Karwatowska-Prokopczuk et al., 1988). Adenosine, on the other hand, promotes the release of NO from vascular endothelium (Vials & Burnstock, 1993; Abebe et al., 1995; Li et al., 1995; Danialou et al., 1997) and smooth muscle (Ikeda et al., 1997). However, in the pig intravesical ureter, indomethacin and L-NOARG, which inhibit prostanoid production and NO-synthase activity, respectively, did not modify the relaxations to adenosine, suggesting that adenosine relaxes the ureteral smooth muscle via a prostaglandins- and NO-independent mechanism. These results are consistent with those found in the frog aorta, where adenosine induces vasodilatation without involving prostanoid or NO production (Knight & Burnstock, 1996).
The blocker of A1- and A2-receptors, 8-PT (10−5 M) potently reduced the relaxations induced by adenosine and NECA, suggesting that these relaxations are mediated through P1-purinoceptors. The fact that 10−6 M DPCPX, a concentration high enough to block A2-receptors, inhibits the relaxations, indicates that they are A2-receptor-mediated. This hypothesis is in accordance with the general notion that smooth muscle A2-receptors mediate relaxation, whereas A1-receptors, which are presynaptically located, operate as a negative feedback mechanism, regulating transmitter release and/or postsynaptic contractile responses (White, 1988; Huidobro-Toro & Parada, 1989; Acevedo et al., 1992). This notion, however, does not unequivocally mean that any adenosine mediated relaxation is exerted through an action at the A2-receptor, as there are references describing A1-receptor mediated relaxations. Thus, in the adenosine-mediated relaxation of the rat duodenum (Nicholls et al., 1992), in the pig coronary arteries (Merkel et al., 1992; Dart & Standen, 1993) and in rat diaphragmatic arterioles (Danialou et al., 1997), the involvement of both the A1- and A2-receptor subtypes has been suggested. In the present study, the contribution of the A1-receptor to pig intravesical ureter relaxation has been discarded because of the low potency shown by adenosine and CPA, as well as by the lack of effect of 10−8 M DPCPX, a concentration selective for blocking the A1-receptor, on relaxations to adenosine and NECA.
A2-receptors are divided into A2A- and A2B-subtypes on the basis of the potency of CGS21680 (15 nM on A2A and practically inactive on A2B) (Jacobson, 1990; Jacobson et al., 1995). Thus, the rabbit aorta, mesenteric and coeliac arteries show A2-receptor mediated vasodilatation, but in those preparations CGS21680 has a relaxing potency which is similar to that exhibited by NECA, so the A2A subtype has been the one implicated in the vasodilatation (Balwierczak et al., 1991). However, in vascular tissues such as the dog coronary artery and saphenous vein, guinea-pig aorta (Balwierczak et al., 1991), rat mesenteric bed (Rubino et al., 1995; Prentice et al., 1997) as well as in the rat duodenum and urinary bladder (Nicholls et al., 1992) CGS21680 is very weak whereas NECA is a potent agonist, thus suggesting the presence of the A2B subtype in those tissues. In the pig intravesical ureter, the low potency showed by CGS21680, as well as the lack of blocking effect produced by ZM 241385, a selective antagonist of A2A adenosine receptors, on relaxations to adenosine or NECA (Poucher et al., 1995), suggest that the adenosine-induced relaxation is mediated through activation of the A2B-subtype. The low potency exhibited by CGS21680 is in accordance with those found in the rat duodenum and urinary bladder (Nicholls et al., 1992).
A3-receptors are clearly different from the A1- and A2-receptors, as the A3-receptors show resistance to methylxanthine blockade. IB-MECA has been reported as a selective A3-receptors agonist (Jacobson et al., 1993; 1995; Gallo-Rodríguez et al., 1994). In the pig intravesical ureter, the A3-receptor subtype does not seem to be involved in the adenosine-mediated relaxation, as this relaxation was potently blocked by 8-PT, whereas the selective A3-receptor agonist, IB-MECA (Gallo-Rodríguez et al., 1994) produced relaxation of the ureteral smooth muscle only at high concentrations.
Adenosine is a regulator at a variety of synapses or neuroeffector junctions where ATP participates in neurotransmission, either as the transmitter itself or as a cotransmitter. Adenosine has also been reported as a paracrine neuromodulator (Acevedo et al., 1992). Adenosine exerts its actions through a multiplicity of mechanisms. Thus, A1-receptor activation may lead to modulation of adenylcyclase activity and to a negative regulation of Ca2+ fluxes (Ribeiro et al., 1979; Fredholm & Dunwiddie, 1988), protein kinase C stimulation, opening of K+ channels (Fredholm et al., 1990), as well as to tonic inhibition of the release of ATP via presynaptic receptors (Sebastiao & Ribeiro, 1986; Huidobro-Toro & Parada, 1989). A2-receptors may be concerned with blockade of Ca2+ currents or stimulation of adenylcyclase activity (Fredholm et al., 1990). The multiciplity of mechanisms operated by adenosine, as well as the pre- and postsynaptic localization of the A1- and A2-receptors in the bladder, could explain the lack of selectivity of the adenosine-induced ATP antagonism. Thus, the adenosine analogue, NECA reduced the contractions induced not only by ATP but also those evoked by 5-HT or ACh (Acevedo et al., 1992). These authors suggested that endogenous adenosine tone modulates the mouse urinary bladder neurotransmission. This conclusion has been reached on the basis of the potentiation evoked by 8-PT on the contraction induced by electrical stimulation and exogenous ATP (Acevedo et al., 1992). In the present study, EFS and exogenous ATP evoked contractions of the intravesical ureteral strips, which were potentiated as a consequence of the blockade of adenosine receptors with 8-PT. These results suggest that adenosine could exert a physiological tonic influence on the ureteral NANC transmission and would thus be consistent with those obtained in the mouse (Acevedo et al., 1992) and rat (Parija et al., 1991) urinary bladder. The fact that 8-PT increased the contractions induced by two non purinergic agonists (ACh and 5-HT) could indicate that adenosine formed from ATP, either released from nerves or exogenously added, would be diminishing the contractions of the tissue nonspecifically. These results are in accordance with those found in the mouse urinary bladder smooth muscle (Acevedo et al., 1992) where NECA reduced the contractions induced by ATP, ACh, 5-HT and prostaglandin F2α. The existence of a basal level of adenosine in the pig intravesical ureter does not seem likely as incubation of preparations with 8-PT failed to modify the ureteral basal tone. The lack of effect exhibited by 10−8 M DPCPX, on contractions evoked by EFS or exogenous ATP in the pig intravesical ureter, suggests the absence of an A1-presynaptic receptor that could be inhibiting the release of excitatory transmitters from ureteral nerves. Moreover, the fact that adenosine analogues, NECA, CPCA and 2-CA did not modify the contractions to EFS suggests the absence of inhibitory A2-receptors presynaptically located, which could be modulating the neurotransmitter release. NECA, however, reduced the contractions evoked by exogenous ATP. All these results suggest that adenosine could be acting via postsynaptic A2B-receptors in the porcine ureter.
In summary, the results of the present investigation suggest that the adenosine receptors that cause relaxation of the intravesical ureteral smooth muscle are of the A2B-subtype. Moreover, evidence is presented showing that adenosine could modulate the NANC excitatory neurotransmission of the pig intravesical ureter, possibly through a postsynaptic mechanism. The adenosine-induced modulation could be favouring the dilatation of ureteral lumen, thus contributing to urine boli transport through ureter and its discharge to urinary bladder.
Acknowledgments
The authors thank Mr Manuel Perales and Mr Francisco Puente for their technical assistance. They also wish to thank Municipal slaughterhouse (Villaviciosa de Odón, Madrid) for kindly donating the ureters.
Abbreviations
- 2-CA
2-chloroadenosine
- 8-PT
8-phenyltheophylline
- ACh
acetylcholine
- CGS21680
2-[p-(carboxyethyl)-phenylethylamino]-5′-N-ethylcarboxamidoadenosine
- CPA
cyclopentyladenosine
- CPCA
5′-(N-cyclopropyl)-carboxamidoadenosine
- DPCPX
1,3-dipropyl-8-cyclopentylxanthine
- IB-MECA
N6-(3-iodobenzyl)-adenosine-5′-N-methylcarboxamide
- NBTI
nitrobenzylthioinosine
- NECA
5′-N-ethylcarboxamidoadenosine
- ZM 241385
4-(2-[7-amino-2-(2-furyl) [1,2,4]-triazolo[2,3-a][1,3,5]triazin-5-ylamino]ethyl)phenol
References
- ABBRACCHIO M.P., BURNSTOCK G. Purinoceptors: are there families of P2X and P2Y purinoceptors. Pharmacol. Ther. 1994;64:445–475. doi: 10.1016/0163-7258(94)00048-4. [DOI] [PubMed] [Google Scholar]
- ABEBE W., HUSSAIN T., OLANREWAJU H., MUSTAFA J. Role of nitric oxide in adenosine receptor-mediated relaxation of porcine coronary artery. Am. J. Physiol. 1995;269:H1672–H1678. doi: 10.1152/ajpheart.1995.269.5.H1672. [DOI] [PubMed] [Google Scholar]
- ACEVEDO C.G., CONTRERAS E., ESCALONA J., LEWIN J., HUIDOBRO-TORO J.P. Pharmacological characterization of adenosine A1 and A2 receptors in the bladder: evidence for a modulatory adenosine tone regulating non adrenergic non cholinergic neurotransmission. Br. J. Pharmacol. 1992;107:120–126. doi: 10.1111/j.1476-5381.1992.tb14473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ALEXANDER S.P.H., LOSINSKI A., KENDALL D. A., HILL S.J. A comparison of A2 adenosine receptor stimulated cyclic AMP generation in cerebral cortex and relaxation of precontracted aorta. Br. J. Pharmacol. 1994;111:185–190. doi: 10.1111/j.1476-5381.1994.tb14042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAILEY S.J., HICKMAN D., HOURANI S.M. O. Characterization of the P1 -purinoceptors mediating contraction of the rat colon muscularis mucosae. Br. J. Pharmacol. 1992;105:400–404. doi: 10.1111/j.1476-5381.1992.tb14265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALWIERCZAK J.L., SHARIF R., KRULAN C.M., FIELD F.P., WEISS G.B., MILLER M.J.S. Comparative effects of a selective adenosine A2 receptor agonist, CGS21680, and nitroprusside in vascular smooth muscle. Eur. J. Pharmacol. 1991;196:117–123. doi: 10.1016/0014-2999(91)90416-n. [DOI] [PubMed] [Google Scholar]
- BRUNS R.F. Adenosine receptors: roles and pharmacology. Ann. N.Y. Acad. Sci. 1990;603:211–226. doi: 10.1111/j.1749-6632.1990.tb37674.x. [DOI] [PubMed] [Google Scholar]
- BURNSTOCK G. Purinergic nerves. Pharmacol. Rev. 1972;24:509–581. [PubMed] [Google Scholar]
- BURNSTOCK G.A basis for distinguishing two types of purinergic receptor Cell Membrane Receptors for Drugs and Hormones: a Multidisciplinary Approach 1978New York: Raven Press; 107–118.ed. Straub, R.W. & Bolis, L. pp [Google Scholar]
- BURNSTOCK G., COCKS T., KASAKOV L., WONG H.K. Direct evidence for ATP release from non-adrenergic non-cholinergic (‘purinergic') nerves in the guinea-pig taenia coli and bladder. Eur. J. Pharmacol. 1978;49:145–149. doi: 10.1016/0014-2999(78)90070-5. [DOI] [PubMed] [Google Scholar]
- CIABATTONI G., WENNMALM A. Adenosine-induced coronary release of prostacyclin at normal and low pH in isolated heart of rabbit. Br. J. Pharmacol. 1985;85:557–563. doi: 10.1111/j.1476-5381.1985.tb08893.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COLLIS M.G., HOURANI S.M.O. Adenosine receptor subtypes. Trends Pharmacol. Sci. 1993;14:360–366. doi: 10.1016/0165-6147(93)90094-z. [DOI] [PubMed] [Google Scholar]
- COLLIS M.G., STOGALL S.M., MARTIN F.M. Apparent affinity of 1,3-dipropyl-8-cyclopentylxanthine for adenosine A1 and A2 receptors in isolated tissues from guinea-pigs. Br. J. Pharmacol. 1989;97:1274–1278. doi: 10.1111/j.1476-5381.1989.tb12589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSACK N.J., HOURANI S.O.M. Some pharmacological and biochemicals interactions of the enantiomers of adenylyl 5′-(β, γ-methylene)-diphosphonate with the guinea-pig urinary bladder. Br. J. Pharmacol. 1984;82:155–159. doi: 10.1111/j.1476-5381.1984.tb16453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CUSACK N.J., HOURANI S.O.M., WELFORD L.A.The role of ectonucleotidases in pharmacological responses to nucleotide analogues Adenosine and Adenine Nucleotides 1988London: Taylor; 93–100.ed Paton, D. M. pp [Google Scholar]
- DANIALOU G., VICAUT E., SAMBE A., AUBIER M., BOCZKOWSKI J. Predominant role of A1 adenosine receptors in mediating adenosine induced vasodilatation of rat diaphragmatic arterioles: involvement of nitric oxide and the ATP-dependent K+ channels. Br. J. Pharmacol. 1997;121:1355–1363. doi: 10.1038/sj.bjp.0701247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DART C., STANDEN N. Adenosine-activated potassium current in smooth muscle isolated from the pig coronary artery. J. Physiol. 1993;471:767–786. doi: 10.1113/jphysiol.1993.sp019927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDYVANE K.A., SMET P.J., TRUSELL D.C., JONAVICIUS J., MARSHALL V.R. Patterns of neuronal colocalization of tyrosine hydroxylase neuropeptide Y, vasoactive intestinal polypeptide, calcitonin gene-related peptide and substance P in human ureter. J. Auton. Nerv. Syst. 1994;48:241–255. doi: 10.1016/0165-1838(94)90053-1. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DALY J.W., HARDEN T.K., JACOBSON K.A., LEFF P., WILLIAMS M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., ABBRACCHIO M.P., BURNSTOCK G., DUBYAK G.R., HARDEN T.K., JACOBSON K.A., SCHWABE U., WILLIAMS M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREDHOLM B.B., DUNER-ENGSTROM M., FASTBOM J., HU P.S., VAN DER PLOEG I. Role of G proteins, cyclic AMP, and ion channels in the inhibition of transmitter release by adenosine. Ann. N. Y. Acad. Sci. 1990;604:276–288. doi: 10.1111/j.1749-6632.1990.tb32000.x. [DOI] [PubMed] [Google Scholar]
- FREDHOLM B.B., DUNWIDDIE T.V. How does adenosine inhibit transmitter release. Trends Pharmacol. Sci. 1988;9:130–134. doi: 10.1016/0165-6147(88)90194-0. [DOI] [PubMed] [Google Scholar]
- GALLO-RODRIGUEZ C., JI X., MELMAN N., SIEGMAN B.D., SANDERS L.H., ORLINA J., FISHER B., PU Q., OLAH ME., VAN GALEN J.M., STILES G.L., JACOBSON K.A. Structure-activity relationships of N6-benzyladenosine-5′-uronamides as A3-selective adenosine agonists. J. Med. Chem. 1994;37:636–646. doi: 10.1021/jm00031a014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ M., GARCIA-SACRISTAN A., ORENSANZ L.M. Muscarinic binding sites of the pig intravesical ureter. J. Auton. Pharmacol. 1995a;15:351–359. doi: 10.1111/j.1474-8673.1995.tb00401.x. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ M., PRIETO D., ORENSANZ L. M., BARAHONA M.V., GARCIA-SACRISTAN A., SIMONSEN U. Nitric oxide is involved in the non-adrenergic non-cholinergic inhibitory neurotransmission of the pig intravesical ureter. Neurosci. Lett. 1995b;186:33–36. doi: 10.1016/0304-3940(94)11275-n. [DOI] [PubMed] [Google Scholar]
- HERNANDEZ M., PRIETO D., ORENSANZ L.M., BARAHONA M.V., JIMENEZ-CIDRE M., RIVERA L., GARCIA-SACRISTAN A., SIMONSEN U. Involvement of a glibenclamide-sensitive mechanism in the nitrergic neurotransmission of the pig intravesical ureter. Br. J. Pharmacol. 1997;120:609–616. doi: 10.1038/sj.bjp.0700952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ M., PRIETO D., SIMONSEN U., RIVERA L., BARAHONA M.V., GARCIA-SACRISTAN A. Noradrenaline modulates smooth muscle activity of the isolated intravesical ureter of the pig through different types of adrenoceptors. Br. J. Pharmacol. 1992;107:924–931. doi: 10.1111/j.1476-5381.1992.tb13387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERNANDEZ M., SIMONSEN U., PRIETO D., RIVERA L., GARCIA P., ORDAZ E., GARCIA-SACRISTAN A. Different muscarinic receptor subtypes mediating the phasic activity and basal tone of pig isolated intravesical ureter. Br. J. Pharmacol. 1993;110:1413–1420. doi: 10.1111/j.1476-5381.1993.tb13978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUIDOBRO-TORO J. P., PARADA S.Pharmacological characterization of A1 and A2-adenosine receptors in the rat vas deferens neuroeffector junction Adenosine Receptors in the Nervous System 1989London: Taylor & Francis; 199ed. Ribeiro, J. A. pp [Google Scholar]
- IKEDA U., KUROSAKI K., OHYA K., SHIMADA K. Adenosine stimulates nitric oxide synthesis in vascular smooth muscle cells. Cardiovasc. Res. 1997;35:168–174. doi: 10.1016/s0008-6363(97)00068-0. [DOI] [PubMed] [Google Scholar]
- JACOBSON K.A.Adenosine (P1) and ATP (P2) receptors Comprehensive Medicinal Chemistry. Membranes and Receptors 1990Oxford: Pergamon Press; ed. Emmett J.C [Google Scholar]
- JACOBSON K.A., KIM H.O., SIDDIQI S.M., OLAH M.E., STILES G.L., VON LUBITZ D.K.J.E. A3-adenosine receptors: design of selective ligands and therapeutic prospects. Drugs of the Future. 1995;20:689–699. doi: 10.1358/dof.1995.020.07.531583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JACOBSON K.A., NIKODIJEVIC O., SHI D., GALLO-RODRIGUEZ C., OLAH M.E., STILES G.L., DALY J.W. A role for central A3-adenosine receptors. Mediation of behavioural depressant effects. FEBS Lett. 1993;336:57–60. doi: 10.1016/0014-5793(93)81608-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KARWATOWSKA-PROKOPCZUK E., CIABATTONI G., WENNMALM A. Effect of adenosine on the formation of prostacyclin in the rabbit isolated heart. Br. J. Pharmacol. 1988;94:721–728. doi: 10.1111/j.1476-5381.1988.tb11581.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHAKH B.S., KENNEDY C. Adenosine and ATP: progress in their receptors' structures and functions. Trends Pharmacol. Sci. 1998;19:39–41. doi: 10.1016/s0165-6147(97)01158-9. [DOI] [PubMed] [Google Scholar]
- KNIGHT G.E., BURNSTOCK G. The effect of purine compounds on the isolated aorta of the frog Rana temporaria. Br. J. Pharmacol. 1996;117:873–878. doi: 10.1111/j.1476-5381.1996.tb15274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LI J.M., FENTON R., CUTLER B., DOBSON J., JR Adenosine enhances nitric oxide by vascular endothelial cells. Am. J. Physiol. 1995;269:C519–C523. doi: 10.1152/ajpcell.1995.269.2.C519. [DOI] [PubMed] [Google Scholar]
- LOHSE M.J. , KLOTZ K.-N., LINDENBORN-FOTINOS J., REDDINGTON M., SCHWABE U., OLSSON R.A. 8-Cyclopentyl-1,3-dipropylxanthine (DPCPX) a selective high affinity antagonist radioligand for A1 adenosine receptors. Naunyn-Schmiedeberg's Arch. Pharmacol. 1987;336:204–210. doi: 10.1007/BF00165806. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S. The neurotransmitter role of CGRP in the rat and guinea-pig ureter: effect of a CGRP antagonist and species-related differences in the action of omega conotoxin on CGRP release from primary afferents. Neuroscience. 1991;43:261–271. doi: 10.1016/0306-4522(91)90433-o. [DOI] [PubMed] [Google Scholar]
- MAGGI C.A., GIULIANI S., SANTICIOLI P. Effect of cromakalim and glibenclamide on spontaneous and evoked motility of the guinea-pig isolated renal pelvis and ureter. Br. J. Pharmacol. 1994;111:687–694. doi: 10.1111/j.1476-5381.1994.tb14792.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAGGI C.A., SANTICIOLI P., GIULIANI S. Role of cyclic AMP and protein kinase A in K+ channel activation by calcitonin gene-related peptide (CGRP) in the guinea-pig ureter. J. Auton. Pharmacol. 1995;15:403–419. doi: 10.1111/j.1474-8673.1995.tb00406.x. [DOI] [PubMed] [Google Scholar]
- McDONALD W.F., WHITE T.D. Adenosine released from synaptosomes is derived from the extracellular dephosphonylation of released ATP. Prog. Neuropsychopharmacol. Biol. Psychiatr. 1984;8:487–494. [Google Scholar]
- MERKEL L.A., LAPPE R.W., RIVERA L.M., COX B.F., PERRONE M.H. Demonstration of vasorelaxant activity with an A1-selective adenosine agonist in porcine coronary artery: involvement of potassium channels. J. Pharmacol. Exp. Ther. 1992;260:437–443. [PubMed] [Google Scholar]
- MEYERHOF W., MULLER-BRECHLIN R., RICHTER D. Molecular cloning of a novel putative G-protein coupled receptor expressed during rat spermiogenesis. FEBS Lett. 1991;284:155–160. doi: 10.1016/0014-5793(91)80674-r. [DOI] [PubMed] [Google Scholar]
- NICHOLLS J., HOURANI S.M.O., KITCHEN I. Characterization of P1-purinoceptors on rat duodenum and urinary bladder. Br. J. Pharmacol. 1992;105:639–642. doi: 10.1111/j.1476-5381.1992.tb09032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OLAH M.E., STILES G.L. Adenosine receptor subtypes: characterization and therapeutic regulation. Annu. Rev. Pharmacol. Toxicol. 1995;35:581–606. doi: 10.1146/annurev.pa.35.040195.003053. [DOI] [PubMed] [Google Scholar]
- PARIJA S.C., RAVIPRAKASH V., MISHRA S.K. Adenosine- and alpha, beta-methylene ATP-induced differential inhibition of cholinergic and non-cholinergic neurogenic responses in rat urinary bladder. Br. J. Pharmacol. 1991;102:396–400. doi: 10.1111/j.1476-5381.1991.tb12185.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POUCHER S.M., KEDDIE J.R., SINGH P., STOGGALL S.M., CAULKETT P.W.R., JONES G., COLLIS M.G. The in vitro pharmacology of ZM 241385, a potent, non-xanthine, A2a selective adenosine receptor antagonist. Br. J. Pharmacol. 1995;115:1096–1102. doi: 10.1111/j.1476-5381.1995.tb15923.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRENTICE D.J., PAYNE S.L., HOURANI S.M.O. Activation of two sites by adenosine receptor agonists to cause relaxation in rat isolated mesenteric artery. Br. J. Pharmacol. 1997;122:1509–1515. doi: 10.1038/sj.bjp.0701524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PRIETO D., HERNANDEZ M., RIVERA L., GARCIA-SACRISTAN A., SIMONSEN U. Distribution and functional effects of neuropeptide Y on equine ureteral smooth muscle and resistance arteries. Regul. Pept. 1997;69:155–165. doi: 10.1016/s0167-0115(97)00003-7. [DOI] [PubMed] [Google Scholar]
- PRIETO D., HERNANDEZ M., RIVERA L., ORDAZ E., GARCIA-SACRISTAN A. Catecholaminergic innervation of the equine ureter. Res. Vet. Sci. 1993;54:312–318. doi: 10.1016/0034-5288(93)90128-3. [DOI] [PubMed] [Google Scholar]
- PRIETO D., SIMONSEN U., MARTIN J., HERNANDEZ M., RIVERA L., LEMA L., GARCIA P., GARCIA-SACRISTAN A. Histochemical and functional evidence for a cholinergic innervation of the equine ureter. J. Auton. Nerv. Syst. 1994;47:159–170. doi: 10.1016/0165-1838(94)90177-5. [DOI] [PubMed] [Google Scholar]
- RIBEIRO J.A., SA-ALMEDIA A.M., NAMORDO J.M. Adenosine and adenosine triphosphate decrease calcium uptake by synaptosomes stimulated by potassium. Biochem. Pharmacol. 1979;28:1297–1300. doi: 10.1016/0006-2952(79)90428-3. [DOI] [PubMed] [Google Scholar]
- RUBINO A., RALEVIC V., BURNSTOCK G. Contribution of P1-(A2b subtype) and P2-purinoceptors to the control of vascular tone in the rat isolated mesenteric arterial bed. Br. J. Pharmacol. 1995;115:648–652. doi: 10.1111/j.1476-5381.1995.tb14981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHULMAN C.C.Innervation of the ureter: a histochemical and ultrastructural study Urodynamics. Upper and Lower Urinary Tract II 1985292–361.ed. Lutzeyer, W. & Hannapel, I. ppBerlin: Springer-Verlag
- SEBASTIAO A.M., RIBEIRO J.A. Enhancement of transmission at the frog neuromuscular junction by adenosine deaminase: evidence for an inhibitory role of endogenous adenosine on neuromuscular transmission. Neurosci. Lett. 1986;62:267–270. doi: 10.1016/0304-3940(85)90366-0. [DOI] [PubMed] [Google Scholar]
- SMET P.J., EDYVANE K.A., JONAVICIUS J., MARSHALL V.R. Colocalization of nitric oxide synthase with vasoactive intestinal peptide, neuropeptide Y, and tyrosine hydroxylase in nerves supplying the human ureter. J. Urol. 1994;152:1292–1296. doi: 10.1016/s0022-5347(17)32570-3. [DOI] [PubMed] [Google Scholar]
- VIALS A., BURNSTOCK G. A2-purinoceptor-mediated relaxation in the guinea-pig coronary vasculature: a role for nitric oxide. Br. J. Pharmacol. 1993;109:424–429. doi: 10.1111/j.1476-5381.1993.tb13586.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLESTEIN S., ZUCKER G.L., FLEISS J.L. Some statistical methods useful in circulation research. Circ. Res. 1980;47:1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- WHITE T.D. Role of adenine compounds in autonomic neurotransmission. Pharmacol. Ther. 1988;38:129–168. doi: 10.1016/0163-7258(88)90095-2. [DOI] [PubMed] [Google Scholar]
- ZHOU Q.Y., LI C., OLAH M.E., JOHNSON R.A., STILES G.L., CIVELLI O. Molecular cloning and characterization of an adenosine receptor: the A3 adenosine receptor. Proc. Natl. Acad. Sci. U.S.A. 1992;89:7432–7436. doi: 10.1073/pnas.89.16.7432. [DOI] [PMC free article] [PubMed] [Google Scholar]


