Abstract
The aim of the present study was to investigate the transduction pathways elicited by prostaglandin E2 (PGE2) to inhibit hormone-stimulated adenosine 3′:5′-cyclic monophosphate (cyclic AMP) accumulation in the outer medullary collecting duct (OMCD) and medullary thick ascending limb (MTAL) microdissected from the rat nephron.
In the OMCD, 0.3 μM PGE2 and low concentrations of Ca2+ ionophores (10 nM ionomycin or 50 nM A23187) inhibited by about 50% a same pool of arginine vasopressin (AVP)-stimulated cyclic AMP content through a same process insensitive to Bordetella pertussis toxin (PTX).
Sulprostone, an agonist of the EP1/EP3 subtypes of the PGE2 receptor, decreased AVP-dependent cyclic AMP accumulation in OMCD and MTAL samples. The concentration eliciting half-maximal inhibition was of about 50 nM in OMCD and 0.1 nM in MTAL.
In MTAL, 1 nM sulprostone and PGE2 inhibited by about 90% a same pool of AVP-dependent cyclic AMP content through a PTX-sensitive, Ca2+-independent pathway.
In the OMCD, PGE2 decreased by about 50% glucagon-dependent cyclic AMP synthesis by a process sensitive to PTX and Ca2+-independent. Sulprostone 1 nM induced the same level of inhibition.
These results demonstrate that PGE2 decrease hormone-dependent cyclic AMP accumulation through a Gαi-mediated inhibition of adenylyl cyclase activity in MTAL cells and glucagon-sensitive cells of the OMCD or through a PTX-insensitive increase of intracellular Ca2+ concentration in AVP-sensitive cells of the OMCD.
Keywords: Cyclic AMP accumulation, arginine vasopressin, glucagon, Ca2+ionophores, prostaglandin E2, sulprostone, microdissected segments, rat kidney
Introduction
The urinary concentration process is regulated by arginine vasopressin (AVP) which increases intracellular adenosine 3′:5′ cyclic monophosphate (cyclic AMP) in two well-defined segments of the rat renal tubule, the thick ascending limb and the collecting tubule (Morel & Doucet, 1986). Previous studies on rat microdissected segments have established that the AVP-dependent cyclic AMP accumulation can be decreased by prostaglandin E2 (Torikai & Kurokawa, 1983; Chabardès et al., 1990; Aarab et al., 1993; Firsov et al., 1995; De Jesus Ferreira et al., 1998). However, depending on the segment studied, the transduction pathway involved in prostaglandin E2 (PGE2)-mediated regulation of AVP-dependent cyclic AMP accumulation appears different. In the medullary portion of the thick ascending limb (MTAL), PGE2 inhibits cyclic AMP synthesis (Torikai & Kurokawa, 1983; Firsov et al., 1995) by a process sensitive to Bordetella pertussis toxin (PTX) and thus presumably through interactions with GTP-dependent Gαi proteins. In contrast, in the outer medullary portion of the rat collecting duct (OMCD), PGE2-induced inhibition of AVP-dependent cyclic AMP accumulation appears mediated mainly by an increase of the cyclic AMP breakdown (Chabardès et al., 1990) by a process insensitive to PTX (Aarab et al., 1993). This increase of cyclic AMP catabolism might be linked to a rise of intracellular free concentration of Ca2+ ([Ca2+]i) since PGE2 increases [Ca2+]i in the OMCD and that the inhibitory effect of PGE2 on AVP-dependent cyclic AMP accumulation is not observed in Ca2+-depleted tubule samples (Aarab et al., 1993). In agreement with this hypothesis, it has been observed that the phosphodiesterase activity measured in isolated rat OMCD is stimulated by the addition of Ca2+ (Kusano et al., 1985).
AVP-dependent cyclic AMP accumulation in rat OMCD and MTAL is also inhibited by Ca2+ ionophores. However, the mechanism of action elicited by Ca2+ ionophores depends on the segment studied and/or on the concentration tested. In MTAL, Ca2+ ionophores decrease cyclic AMP accumulation by an effect restricted to the catabolism of cyclic AMP (Kusano et al., 1985). In the OMCD, high concentrations of Ca2+ ionophores (in the micromolar range) inhibit cyclic AMP synthesis whereas low concentrations are effective only on cyclic AMP catabolism (Kusano et al., 1985; Chabardès et al., 1996). These observations and the results obtained with PGE2 recalled above suggest that low concentrations of Ca2+ ionophores and PGE2 might have a same mechanism of action to inhibit AVP-dependent cyclic AMP accumulation in the rat OMCD.
Morphological studies have established that the rat OMCD contains mainly two cell types, the principal cell and the type A intercalated cell (Madsen & Tisher, 1986). As previously discussed, different experimental results support the conclusion that adenylyl cyclase activity is stimulated by AVP and glucagon in principal cells and intercalated cells, respectively (Morel & Doucet, 1986; Chabardès et al., 1996). In rat MTAL, there is only one morphological cell type (Madsen & Tisher, 1986) and different hormones including AVP and glucagon stimulate the same catalytic units of adenylyl cyclase (Morel et al., 1987). Thus, the different effects elicited by PGE2 in MTAL and AVP-sensitive cells of the OMCD reflect regulations present in two different well-defined cell types. To our knowledge, a possible action of PGE2 in glucagon-sensitive cells of the rat OMCD has not yet been described.
The present studies were designed to define the cell types sensitive to PGE2 and the mechanism of action of PGE2 in the rat OMCD and MTAL. Different approaches were used. The first approach was to compare the properties of regulation elicited by PGE2 and low concentrations of Ca2+ ionophores in the AVP-sensitive cells of the OMCD in order to study if these compounds inhibit hormone-dependent cyclic AMP accumulation by a same mechanism of action. The second approach was to compare the transduction pathway elicited by PGE2 and sulprostone, a selective agonist of the EP1/EP3 subtypes of the PGE2 receptor (Coleman et al., 1994) in the different cell types studied. Finally, we investigated a possible action of PGE2 in the glucagon-sensitive cells of the rat OMCD. The results establish that PGE2-mediated inhibition of hormone-dependent cyclic AMP accumulation is the consequence of a cell-specific coupling of PGE2 to either a PTX-sensitive inhibition of adenylyl cyclase activity or through an increase of [Ca2+]i insensitive to PTX.
Methods
Measurement of AVP-dependent cyclic AMP accumulation
The experimental conditions used to measure hormone-dependent cyclic AMP accumulation on an intact single segment have been detailed previously (Chabardès et al., 1990; Aarab et al., 1993; Firsov et al., 1995) and will be recalled briefly here. Experiments were performed on anaesthetized (pentobarbital, 6 mg 100 g−1 body weight) male Wistar rats (140–180 g of body weight, Iffa-Credo, France). After perfusion and hydrolysis of the left kidney with microdissection medium containing collagenase (collagenase A from Clostridium histolyticum, 0.406 U mg−1), single pieces (0.3–1.8 mm length) of collecting duct or thick ascending limb were microdissected at 4°C from the outer medulla. The standard microdissection medium was composed of (mM): NaCl 137; KCl 5; MgSO4 0.8; Na2HPO4 0.33; KH2PO4 0.44; MgCl2 1; NaHCO3 4; CH3COONa 10; CaCl2 1.0; glucose 5; 1.2, N-2-hydroxyethylpiperazine N′-2-ethanesulphonic acid (HEPES) 20, pH 7.4 and 0.1% (w/v) bovine serum albumin (fraction V). The medium was supplemented with 5 μM indomethacin and 0.5 u ml−1 adenosine deaminase. The effect of extracellular Ca2+ on cyclic AMP accumulation was investigated in tubule samples microdissected and incubated in media without CaCl2 and containing 0.1 mM [ethylene bis (oxyethylenenitrilo)] N, N′-tetraacetic acid (EGTA). This long duration in a Ca2+-free medium induces a marked depletion of intracellular Ca2+ pools as indicated by the lack of agonist-induced [Ca2+]i variations in such tubule samples. For long preincubation periods, in experiments performed with Bordetella pertussis toxin (PTX) or bis-(o-aminophenoxy)-ethane-N,N,N,′N′-tetracetic acid, tetra(acetoxymethyl)-ester (BAPTA/AM), all media contained essential and nonessential amino acids as well as vitamins (minimum Eagle's medium).
Due to the small number of cells per tubule sample (from about 200–600 cells), cyclic AMP accumulation can be measured only in the presence of a phosphodiesterase (PDE) inhibitor. The incubation medium had the same composition as the microdissection medium but included either 1 mM 3-isobutyl-1-methylxanthine (IBMX), which blocks all PDE types in the rat kidney (Hoey & Houslay, 1990), or 50 μM 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (Ro 20-1724), a specific inhibitor of the low Km cyclic AMP specific PDE family IV. PDE family IV is not regulated by Ca2+ (Beavo, 1995). Ro 20-1724 therefore allows to measure cyclic AMP accumulation resulting from effects on both cyclic AMP synthesis and partial cyclic AMP breakdown and was used in all experiments performed to study regulations of the response to AVP. Unless otherwise specified, each sample was preincubated 10 min at 30°C and then, after addition of incubation medium containing the agonists to be tested, incubated 4 min at 35°C. The amounts of cyclic AMP were measured on acetylated samples by radioimmunoassay. In our conditions, the basal level of cyclic AMP present in one single piece of tubule is close to, or below, the sensitivity threshold of the assay (2 fmol cyclic AMP per reaction tube) and thus only hormone-dependent cyclic AMP accumulation can be measured. AVP was used at concentrations which induce maximal stimulation of adenylyl cyclase activity, i.e., 1 and 10 nM in collecting duct and thick ascending limb tubule samples, respectively. PGE2, Ca2+ ionophores and sulprostone had no intrinsic agonist activity in our experiments.
The results were calculated in femtomoles of cyclic AMP accumulated per millimetre of segment per 4 min incubation time (fmol mm−1 4 min−1). In each experiment, different experimental conditions were tested on replicate tubule samples microdissected from a same rat kidney (6–10 samples per condition). The mean of the cyclic AMP values obtained in each condition was taken as one single data point and the results were expressed in absolute value or in percentage of the response to AVP or in percentage of inhibition calculated from the corresponding AVP value. Results were calculated from n different experiments or from n tubule samples in individual experiments.
Measurement of intracellular Ca2+ concentration
Intracellular free concentration of Ca2+ ([Ca2+]i) was measured in single tubule samples microdissected from collagenase-treated kidneys by using the calcium-sensitive fluorescence probe acetoxymethyl ester of fura-2 (fura-2 AM) as previously described (Aarab et al., 1993; Champigneulle et al., 1993). Briefly, the samples were loaded for 60 min with 10 μM fura-2 AM. Each loaded tubule was then transferred to a superfusion chamber fixed on the stage of an inverted fluorescence microscope (model IM 35; Zeiss, Oberkochen, Germany). The tubule was superfused at 37°C at a rate of 10–12 ml min−1, corresponding to about ten exchanges per min. The composition of the superfusion medium was similar to that used in cyclic AMP experiments, except that serum albumin, indomethacin and adenosine deaminase were not added since the superfusion medium was flushed continuously. After a 5- to 10-min equilibration period, agonists were added to the medium and superfused over tubule. An area of 60 μm diameter was selected over the tubule image and the fluorescence intensity emitted (during brief excitation periods at 340 and 380 nm alternatively, at a maximal rate of 30 cycles min−1) was recorded each 2 s. Calculations of [Ca2+]i was performed as previously described (Champigneulle et al., 1993). The results were obtained from different tubules (n) microdissected from several rats.
Statistical analysis
Data are presented as the means±s.e.mean. The statistical evaluation of the data was assessed either by unpaired Student's t-test or, for multiple comparison, by one-way analysis of variance on weighted means followed by LSD Fisher's t-test.
Materials
Unless otherwise specified, the compounds were from Merck (Darmstadt, Germany), Sigma (St Louis, MO, U.S.A.) And from Calbiochem (San Diego, CA, U.S.A.). Collagenase and adenosine deaminase were obtained from Boehringer (Mannheim, Germany). Amino acids and vitamins were obtained from Eurobio (Les Ulis, France) and bovine serum albumin was obtained from Pentex Miles Inc. (Kankakee, IL, U.S.A.). Glucagon (human) was purchased from Neosystem Laboratoire (Strasbourg, France). Sulprostone (16-phenoxy-ω-17,18,19,20-tetranor-PGE2-methylsulfonylamide) was purchased from Cayman Chemical Company (Ann Arbor, MI, U.S.A.). Specific cyclic AMP antibody and 125I-labelled cyclic AMP succinyl-tyrosine-methyl ester were purchased from either Sanofi Diagnostics Pasteur (Marnes-La-Coquette, France) or NEN Life Sciences Products (Le Blanc Mesnil, France).
Results
Effect of Ca2+ ionophores on AVP-dependent cyclic AMP accumulation and on [Ca2+]i in the outer medullary collecting duct
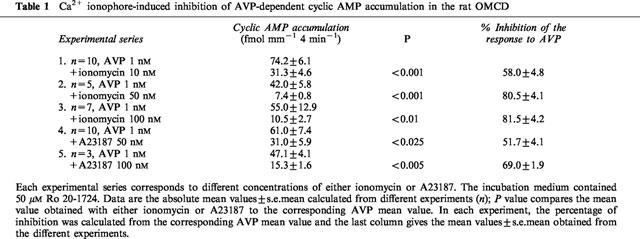
Ca2+ ionophores induced a dose-dependent inhibition of AVP-stimulated cyclic AMP accumulation (Table 1). For a same concentration of Ca2+ ionophore, the level of inhibition was more pronounced with ionomycin than with A23187. The response to 1 nM AVP was inhibited to a similar extent by 50 nM A23187 or 10 nM ionomycin (Table 1) and the percentage of inhibition observed in individual experiments ranged from about 40–70%. These values were similar to those previously observed with 0.3 μM PGE2 (Chabardès et al., 1990; Aarab et al., 1993). Thus, concentrations of 10 nM ionomycin or 50 nM A23187 were used in the further experiments and compared to the effect elicited by 0.3 μM PGE2.
Table 1.
Ca2+ ionophore-induced inhibition of AVP-dependent cyclic AMP accumulation in the rat OMCD
The [Ca2+]i variation induced by 10 nM ionomycin was characterized by a slow and progressive increase up to a maximal value which was maintained even after the end of ionomycin superfusion (Figure 1). The mean increase over basal value of [Ca2+]i calculated at the end of a 3 min perfusion period with 10 nM ionomycin was of 50.4±7.1 nM [Ca2+]i (n=11 single OMCD microdissected from five kidneys). As previously described (Aarab et al., 1993), a different pattern of [Ca2+]i was elicited by PGE2 which was characterized by a peak followed by a plateau (Figure 1). In a same OMCD sample, the response to ionomycin was not modified by a first superfusion with PGE2 (Figure 1 and data not shown).
Figure 1.
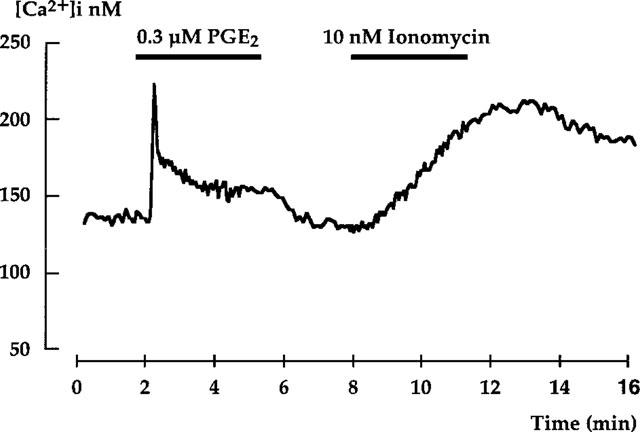
[Ca2+]i variations induced by PGE2 and ionomycin in the outer medullary collecting duct. Representative traces of [Ca2+]i variations elicited by adding 0.3 μM PGE2 and then 10 nM ionomycin in the superfusion medium. The superfusion period for each agent is indicated by the bar.
Increases in [Ca2+]i by ionomycin may induce activation of phospholipase A2 and thus the formation of metabolites which might account for the observed inhibition. However, indomethacin, which prevents endogenous synthesis of PGE2 by isolated segments, was present in all experiments (see Methods section). The further addition of quinacrine, a phospholipase A2 inhibitor, did not affect the inhibition induced by 10 nM ionomycin (57.6±8.9% of inhibition and 57.6±12.2% without and with the addition of 20 μM quinacrine, respectively, n=3). These results suggest that the inhibitory effect observed with 10 nM ionomycin was not due to an indirect regulation mediated by arachidonic acid metabolites.
Comparison of the inhibitory properties induced by Ca2+ ionophores and PGE2 on AVP-dependent cyclic AMP accumulation in the OMCD
The inhibition elicited by Ca2+ ionophores was no longer observed in OMCD studied in Ca2+-free medium. A23187 elicited 45.2±6.1% of the response to AVP in 1 mM Ca2+ medium (P<0.005 versus AVP) and 114.4±8.5% (NS) in Ca2+-free medium, n=6; with ionomycin, the corresponding values observed were of 55.7±9.4% (P<0.05) and 88.0±1.0 (NS), n=3. In the same experiments, PGE2-induced inhibition was also dependent from extracellular Ca2+ (31.0±2.2% of the response to AVP (P<0.001 and 98.3±6.2 (NS) in 1 mM Ca2+ and Ca2+-free medium, respectively, n=9).
A potential role of the GTP-dependent protein Gαi was investigated by using PTX. In each experiment, OMCD samples were microdissected from the same rat kidney, then pre-incubated (2 h at 30°C) in the presence or not (control) of PTX and incubated as usual. This long pre-incubation period decreased AVP-stimulated cyclic AMP accumulation in control OMCD but PTX improved this response (Figure 2A). The inhibition induced by 50 nM A23187 was of 49.2±6.8% and 58.2±10.8% in PTX-treated and control OMCD, respectively (n=3, Figure 2B). Studied in parallel, PGE2-induced inhibition was also slightly reduced but was still highly significant in each of the experiments performed (73.3±3.3% of inhibition and 63.0±4.9% in control and PTX-treated OMCD, respectively, Figure 2B). In another experimental series, 10 nM ionomycin led to a comparable observation with an inhibition of 61.5±11.0% and 66.7±15.9% in control and PTX-treated OMCD, respectively (n=3). In contrast, the inhibitory effect induced by the α2-adrenergic agonist clonidine was relieved by pertussis toxin (70.4±3.9% of inhibition and 14.8±8.9% in control and PTX-treated OMCD, respectively, n=3, Figure 2B).
Figure 2.
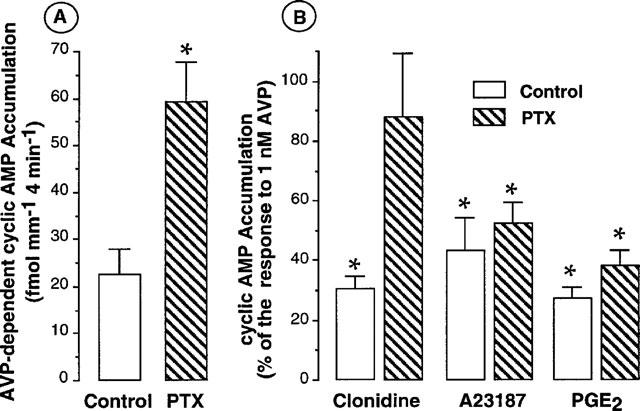
Effect of pertussis toxin on AVP-dependent cyclic AMP accumulation and on the inhibitory effect elicited by either clonidine, A23187 or PGE2 in the rat OMCD. (A) Absolute values of cyclic AMP accumulation induced by 1 nM AVP in the presence of 50 μM Ro 20-1724 in control and PTX-treated OMCD. Tubule samples were microdissected from the same kidney and preincubated 2 h at 30°C either in the control medium or in the presence of 500 ng ml−1 PTX. Data are the mean values±s.e.mean calculated from three experiments. *P<0.005 or 0.001 in individual experiments when compared to the corresponding response obtained with AVP in control tubule samples. (B) In experiments presented in (A), the effect elicited by 1 μM clonidine, 50 nM A23187 or 0.3 μM PGE2 on AVP-dependent cyclic AMP accumulation was tested in control and PTX-treated OMCD. Data are expressed in percentage of the corresponding response to 1 nM AVP and are given as the mean values±s.e.mean; *P<0.005 or 0.001 in individual experiments when compared to the corresponding response to AVP.
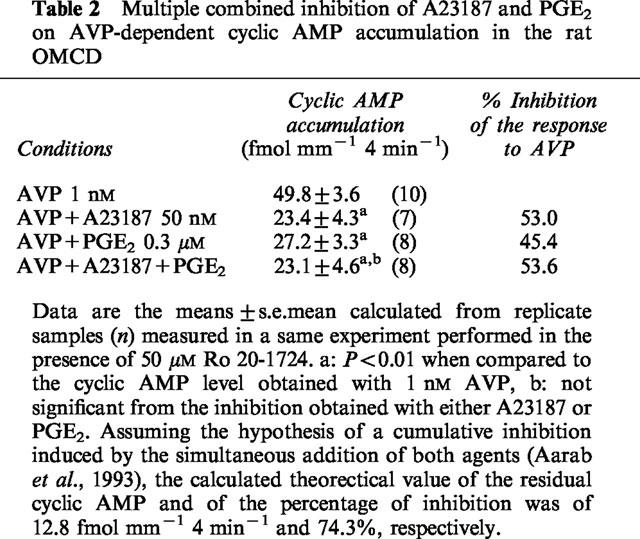
Experiments of multiple combined inhibition were performed to compare the inhibition induced by either PGE2 or a low concentration of A23187 to the inhibition elicited by the simultaneous addition of both agents. Although the concentration of Ca2+ ionophore used in these experiments did not induce a maximal inhibition (see Table 1), the validity of the summation experiments is fulfilled by the use of 0.3 μM PGE2, a concentration which elicits a maximal PGE2-induced inhibition of AVP-dependent cyclic AMP accumulation in the rat OMCD (Chabardès et al., 1990; Aarab et al., 1993). Table 2 gives the detailed results obtained in a typical experiment with A23187 and PGE2. The inhibition of AVP-dependent cyclic AMP accumulation induced by A23187 and PGE2 alone was not different from the inhibition observed with the simultaneous addition of both agents. In five different experiments, both agents inhibited by 61.1±2.6% the response to glucagon, an inhibition similar to the effect of A23187 alone (49.8±5.5%) or PGE2 alone (60.0±5.4%). The theoretical value calculated assuming a cumulative inhibition hypothesis was different from the mean value obtained with the addition of both A23187 and PGE2 (12.6±2.13 fmol mm−1 4 min−1 and 24.2±1.7, respectively, n=5, P<0.01). A23187 and PGE2 therefore were effective by a same mechanism to decrease AVP-dependent cyclic AMP accumulation.
Table 2.
Multiple combined inhibition of A23187 and PGE2 on AVP-dependent cyclic AMP accumulation in the rat OMCD
The role of [Ca2+]i increases to account for PGE2-induced inhibition was further studied in OMCD preincubated 30 min in the presence or not (control) of BAPTA (cell permeant form), a Ca2+ chelator which prevents agonists-stimulated increases in [Ca2+]i. PGE2 induced 49.5±7.8% of the response to 1 nM AVP (P<0.05) and 104.2±6.4 (NS) in control and 30 μM BAPTA-AM-treated OMCD, respectively, n=5.
Regulation of AVP-dependent cyclic AMP accumulation by the EP1/EP3 agonist of the PGE2 receptor, sulprostone
Tested at different concentrations, sulprostone had a higher efficiency to inhibit AVP-dependent cyclic AMP accumulation in MTAL than in OMCD samples. Indeed, 1 nM sulprostone induced a maximal inhibition in the MTAL but had no effect in the OMCD (Figure 3). As estimated from these results, the concentration eliciting half-maximal inhibition of AVP-dependent cyclic AMP accumulation was of about 0.1 nM in the MTAL and 50 nM in the OMCD (Figure 3). In the same experiments, the addition of 0.3 μM PGE2 decreased cyclic AMP values to 8.4±1.2% of the respone to AVP (n=3) and to 58.1±3.7% (n=4) in MTAL and OMCD, respectively. These values are similar to those induced by 0.1 μM sulprostone (10.7±3.3% of the response to AVP and 60.9±6.7% in MTAL and OMCD, respectively, Figure 3).
Figure 3.
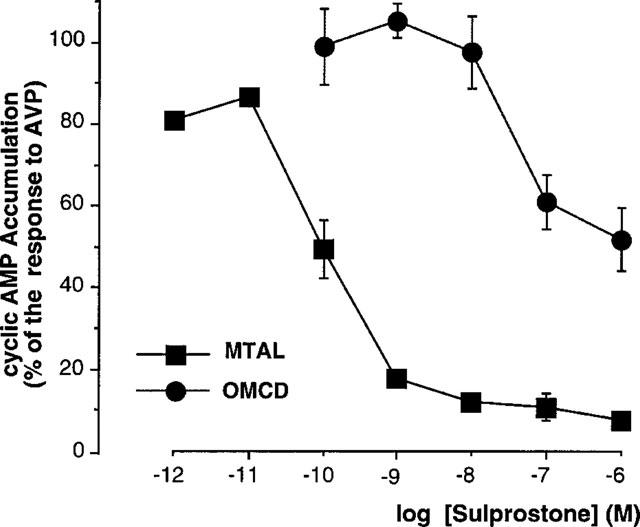
Dose-response curves of sulprostone-induced inhibition of AVP-dependent cyclic AMP accumulation in the rat MTAL and OMCD. Different concentrations of sulprostone were tested on cyclic AMP accumulation induced by 1 nM (OMCD) or 10 nM (MTAL) AVP in the presence of 50 μM Ro 20-1724. The results are expressed in percentage of the corresponding response obtained with AVP. The data are the mean values±s.e.mean calculated from three experiments in the MTAL and from four experiments in the OMCD. In each experiment, a statistical significant inhibition was observed from a concentration of either 0.1 nM in the MTAL (P<0.005–0.001 versus the corresponding AVP value) or 0.1 μM in the OMCD (P<0.025–0.005).
The pattern of [Ca2+]i increase induced by 0.1 μM Sulprostone in the OMCD was comparable to that observed with PGE2. The mean increase over basal value was of 42.6±5.3 nM [Ca2+]i and 21.2±1.7 nM at the peak and plateau phase, respectively (n=five single tubules microdissected from two kidneys). The superfusion of 1 nM sulprostone induced a barely detectable peak of [Ca2+]i (stimulated minus basal value=14.3±3.7, n=5) in the OMCD and had no effect on [Ca2+]i variations in the MTAL.
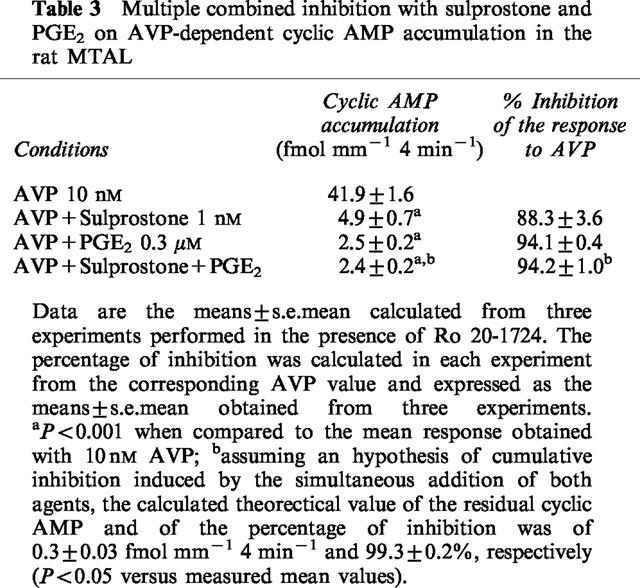
Different experimental protocols were performed in the MTAL in order to define the properties of inhibition elicited by sulprostone and PGE2: (i) the presence or not of Ca2+ in the incubation medium did not impair PGE2-induced inhibition of the response to 10 nM AVP (84.3±2.0% of inhibition and 82.1±6.1%, in 1 mM Ca2+ and Ca2+-free medium, respectively, n=5). A similar result was obtained with 1 nM sulprostone tested in some of these experiments (83.4±2.3% of inhibition and 77.8±1.8 in 1 mM Ca2+ and Ca2+-free medium, respectively, n=3); (ii) a preincubation of MTAL samples during 4 h at 35°C with 500 ng ml−1 PTX relieved the inhibition elicited by 1 nM sulprostone (Figure 4); (iii) in experiments of multiple combined inhibition, the simultaneous addition of 0.3 μM PGE2 and 1 nM sulprostone induced an inhibition similar to that elicited by PGE2 alone (Table 3).
Figure 4.
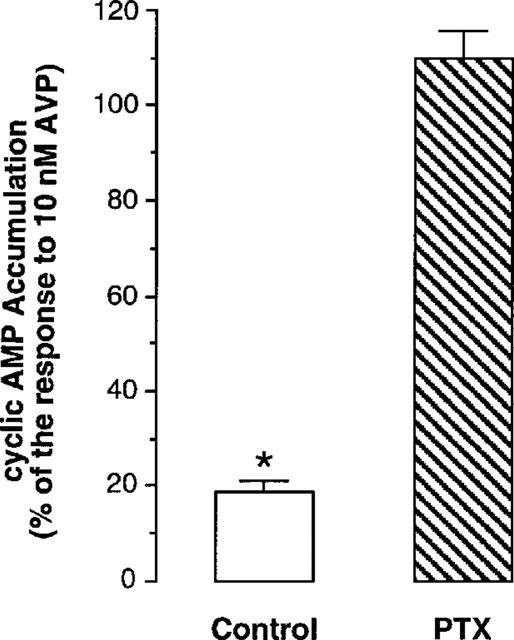
Effect of pertussis toxin on sulprostone-induced inhibition of AVP-dependent cyclic AMP accumulation in the MTAL. MTAL samples microdissected from the same kidney were preincubated 6 h at 35°C in the control medium or in the presence of 500 ng ml−1 PTX. The effect elicited by 1 μM sulprostone is expressed in percentage of the corresponding response to 10 nM AVP. Data are the mean values±s.e.mean calculated from four experiments performed in the presence of Ro 20-1724. *P<0.01–0.001 in individual experiments when compared to the corresponding response to AVP.
Table 3.
Multiple combined inhibition with sulprostone and PGE2 on AVP-dependent cyclic AMP accumulation in the rat MTAL
Effect of PGE2 on glucagon dependent cyclic AMP synthesis in the outer medullary collecting duct
These experiments were performed in the presence of 1 mM IBMX so that the variations of intracellular cyclic AMP content reflected regulations prevailing only on cyclic AMP synthesis.
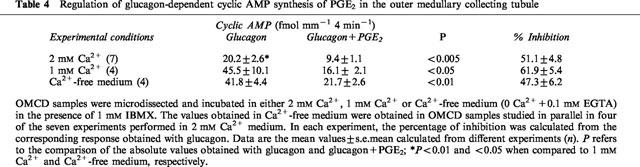
Glucagon-dependent cyclic AMP synthesis was measured in OMCD samples incubated in the presence of different extracellular Ca2+ concentrations (Table 4). The adenylyl cyclase sensitive to glucagon was lower in 2 mM Ca2+ medium as compared to 1 mM Ca2+ or Ca2+-free medium. In contrast to that observed in AVP-sensitive cells of the OMCD, glucagon-dependent cyclic AMP synthesis was inhibited with a similar efficiency by PGE2 whatever the Ca2+ concentration present in microdissection and incubation media (Table 4). Tested in 1 mM Ca2+ medium, 1 nM sulprostone inhibited the response to glucagon to the same extent than 0.3 μM PGE2 (57.7±2.6% of inhibition versus 1 μM glucagon, P<0.01, n=3).
Table 4.
Regulation of glucagon-dependent cyclic AMP synthesis of PGE2 in the outer medullary collecting tubule
The transduction pathway mediated by PGE2 was further studied on OMCD samples preincubated or not (control) with 500 ng ml−1 PTX during 2 h at 30°C. The inhibition elicited by PGE2 was markedly decreased in PTX-treated tubule samples (61.9±5.4% of inhibition and 19.4±6.0 in control and PTX-treated OMCD, respectively, Figure 5).
Figure 5.
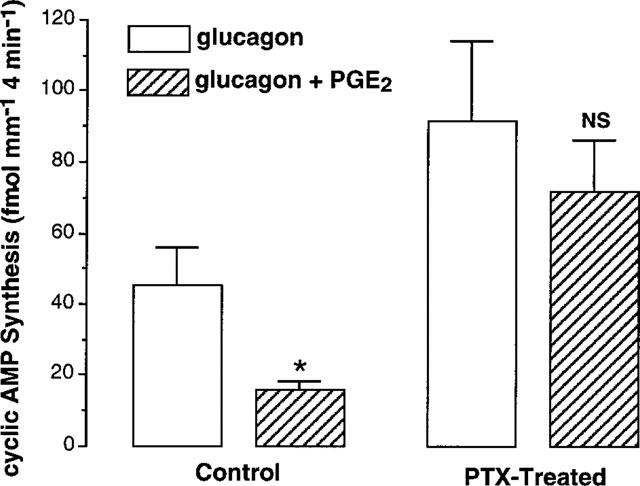
Effect of pertussis toxin on PGE2-mediated inhibition of glucagon-dependent cyclic AMP synthesis. In each experiment, OMCD samples were microdissected from the same rat kidney, preincubated (2 h at 30°C) and incubated (4 min at 35°C) in 1 mM Ca2+ medium without (control) or with 500 ng ml−1 PTX. Data are the mean values calculated from four experiments peformed in the presence of 1 mM IBMX. *Refers to the effect of 0.3 μM PGE2 on the cyclic AMP synthesis induced by 1 μM glucagon, P<0.001 in each experiment; NS indicates that the effect of PGE2 was not significantly different from the response to glucagon.
Discussion
The different results presented in this study allow the further characterization of two different transduction pathways elicited by PGE2 to inhibit hormone-dependent cyclic AMP accumulation along the rat renal tubule. The different properties of inhibition observed in this study and those previously obtained with PGE2 in the rat OMCD and MTAL are summarized in Table 5.
Table 5.
Properties of inhibition of PGE2 and/or sulprostone on hormone-dependent cyclic AMP content in AVP- and glucagon-sensitive segments
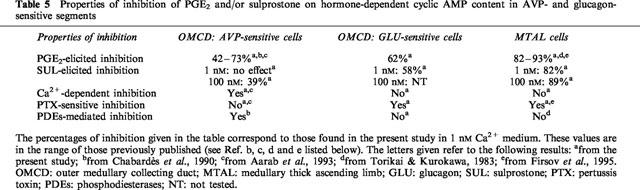
In the AVP-sensitive cells of OMCD, PGE2 and a low concentration of Ca2+ ionophore exhibited similar properties to inhibit a same pool of intracellular cyclic AMP content. Previous studies in the OMCD have shown that the response to forskolin is also inhibited by PGE2 and that PGE2-induced [Ca2+]i increase consists of two phases, a release of intracellular Ca2+ and an entry of extracellular Ca2+ (Chabardès et al., 1990; Aarab et al., 1993). The different observations made support the conclusion that PGE2-induced inhibition is linked to its capability to increase [Ca2+]i in the AVP-sensitive cells of OMCD and is due mainly to cyclic AMP hydrolysis. In the glucagon-sensitive cells of OMCD and in the MTAL cells, PGE2 decreased cyclic AMP synthesis through a process sensitive to PTX and independent from extracellular Ca2+ variations. The same properties were observed with sulprostone in MTAL and, in this segment, sulprostone and PGE2 decreased a same pool of cyclic AMP content. These data therefore support an interaction of PGE2 receptor with a GTP-dependent Gαi protein in the MTAL and the glucagon-sensitive cells of the OMCD.
The sensitivity of sulprostone to inhibit the response to AVP is much higher in MTAL then in OMCD. The concentration of sulprostone eliciting half-maximal inhibition is similar to that previously observed with PGE2 in the MTAL (Torikai & Kurokawa, 1983; Firsov et al., 1995) and the OMCD (Chabardès et al., 1990). These observations suggest that, in the rat nephron, sulprostone is as effective as PGE2 to decrease AVP-dependent cyclic AMP accumulation whatever the transduction pathway accounted for this regulation. An equipotent action of PGE2 and sulprostone to inhibit AVP-induced cyclic AMP synthesis through a pertussis toxin-sensitive process has already been described in rabbit kidney cells (Sonnenburg & Smith, 1988). However, the higher sensitivity of inhibition induced by sulprostone and PGE2 in MTAL than in OMCD suggests that these compounds have a higher potency to inhibit adenylyl cyclase activity than to elicit [Ca2+]i increases in renal segments. Although different concentrations of sulprostone were not tested in our experiments, the potency of sulprostone to inhibit glucagon-stimulated cyclic AMP synthesis in the OMCD appeared comparable to that observed in the MTAL cells.
The different transduction pathways elicited by PGE2 in MTAL and OMCD may result from the interaction of PGE2 to different subtypes of PGE2 receptor (Coleman et al., 1994). In different species, both EP1 and EP3 subtype mRNAs have been detected in the collecting duct and only EP3 subtype mRNA in the thick ascending limb (Breyer et al., 1993; 1996; Sugimoto et al., 1994; Takeuchi et al., 1994; Taniguchi et al., 1994). The expression of EP1 subtype mRNA in transfected cells or Xenopus oocytes results in [Ca2+]i increases but not stimulation or inhibition of adenylyl cyclase activity (Funk et al., 1993; Watabe et al., 1993). Multiple isoforms of the PGE2 receptor EP3 subtype mRNA have been cloned and one given isoform can be coupled to multiple second messenger systems (Coleman et al., 1994; An et al., 1994; Kotani et al., 1995; Schmid et al., 1995; Boie et al., 1997). Most EP3 isoforms decrease cyclic AMP content through a pertussis toxin-sensitive inhibition of adenylyl cyclase activity (Coleman et al., 1994; An et al., 1994; Kotani et al., 1995; Schmid et al., 1995). PGE2 and Sulprostone have a higher affinity to bind to EP3 than to EP1 subtype (Coleman et al., 1994; Boie et al., 1997; Kiriyama et al., 1997). In addition, tested on transduction pathways, a higher potency of sulprostone and PGE2 is observed in cells transfected with the rat EP3 subtype as compared to the EP1 subtype transfected cells (Boie et al., 1997). Thus, the localization of EP subtypes in the kidney, the signalling properties of PGE2 observed in our experiments and their comparison with the functional expression of EP1 or EP3 subtype in transfected cells suggest a different coupling of PGE2 along the rat renal tubule, i.e. to an EP1 subtype in the AVP-sensitive cells of the OMCD, and to an EP3 subtype in the glucagon-sensitive cells of the OMCD and the MTAL cells. The observations made with sulprostone and PGE2 in MTAL suggest that these agents interact with an EP3 isoform not associated to [Ca2+]i increases. In agreement with this hypothesis, PGE2 inhibits adenylyl cyclase activity and does not increase [Ca2+]i in the cortical portion of the rat thick ascending limb (De Jesus Ferreira et al., 1998).
The regulation elicited by PGE2 on the response to AVP in the rat collecting duct differs from the observations made in the rabbit collecting duct. Indeed, in the cortical portion (CCD) of the rabbit collecting duct, PGE2 stimulates a pertussis toxin-sensitive pathway which inhibits AVP-stimulated cyclic AMP production (Griffiths et al., 1993) and AVP-dependent permeability to water (Hébert et al., 1993). In the same segment, PGE2 decreases sodium reabsorption through a second pathway, Ca2+-dependent and pertussis toxin-insensitive (Hébert et al., 1993) but the precise cell type involved in this regulation remains to be defined. In the rat CCD, PGE2 does not regulate either AVP-dependent cyclic AMP content (Chabardès et al., 1990) or AVP-stimulated transepithelial water permeability or sodium transport (Chen et al., 1991). In the rat therefore, PGE2 inhibits the response to AVP in more terminal portions of collecting duct and this action is not mediated through a Gαi-dependent inhibition of adenylyl cyclase activity. In contrast to the species differences observed in the AVP-sensitive cells of the collecting duct, PGE2-mediated pertussis toxin-sensitive inhibition of adenylyl cyclase activity is observed in MTAL isolated from all species studied up to now, i.e. the rat, the mouse (Takaichi & Kurokawa, 1988) and the rabbit (Griffiths et al., 1993).
PGE2 and a low concentration of sulprostone also inhibited adenylyl cyclase activity in glucagon-sensitive cells of rat OMCD. A similar effect of PGE2 has been previously observed in the isoproterenol-sensitive cells of rat CCD (Chabardès et al., 1990). Glucagon- and isoproterenol-stimulated cyclic AMP synthesis are located in intercalated cells which are involved in proton secretion and/or bicarbonate reabsorption (Madsen & Tisher, 1986; Morel & Doucet, 1986). These observations therefore support a regulatory role of PGE2 in the control of acid-base balance, in addition to its well known inhibitory effect on the urinary concentration processes (Stokes, 1981).
In summary, this study demonstrates a differential cell-specific localization along the rat nephron of the transduction pathways elicited by PGE2 to inhibit hormone-dependent cyclic AMP accumulation. An interaction of PGE2 with Gαi-coupled inhibition of adenylyl cyclase is observed in MTAL cells and the glucagon-sensitive cells of the OMCD. In AVP-sensitive cells of the OMCD, the decrease of cyclic AMP content results from PGE2-mediated [Ca2+]i increases through a process insensitive to PTX. Compared to the low sensitivity of PGE2 in the OMCD (Chabardès et al., 1990), the high sensitivity of PGE2 (Torikai & Kurokawa, 1983; Firsov et al., 1995) and sulprostone (this study) to inhibit hormone-dependent cyclic AMP synthesis in the MTAL suggest that low concentrations of PGE2 can be very effective to regulate physiological functions of the rat thick ascending limb.
Acknowledgments
We are grateful to N. Griffiths and A. Doucet for critical advice and careful review of this manuscript and to M. Imbert-Teboul for her valuable advice in intracellular Ca2+ measurement. This work was supported by grants from the Centre Nationale de la Recherche Scientifique (URA 1859) and from the CEA (DBCM, SBCe). L. Aarab was supported in part by a grant from the Commissariat à l'Energie Atomique.
Abbreviations
- AVP
arginine vasopressin
- [Ca2+]i
intracellular free concentration of Ca2+
- CCD
cortical collecting duct
- cyclic AMP
adenosine 3′:5′-cyclic monophosphate
- MTAL
medullary thick ascending limb
- OMCD
outer medullary collecting duct
- PDE
phosphodiesterase
- PGE2
prostaglandin E2
- PTX
Bordetella pertussis toxin
References
- AARAB L., MONTÉGUT M., SIAUME-PEREZ S., IMBERT-TEBOUL M., CHABARDÈS D. PGE2-induced inhibition of AVP-dependent cAMP accumulation in the OMCD of the rat kidney is cumulative with respect to the effects of α2-adrenergic and A1-adenosine agonists, insensitive to pertussis toxin and dependent on extracellular calcium. Pflügers Arch. 1993;423:397–405. doi: 10.1007/BF00374933. [DOI] [PubMed] [Google Scholar]
- AN S., YANG J., SO S.W., ZENG L., GOETZL E.J. Isoforms of the EP3 subtype of human prostaglandin E2 receptor transduce both intracellular Calcium and cAMP signals. Biochemistry. 1994;33:14496–14502. doi: 10.1021/bi00252a016. [DOI] [PubMed] [Google Scholar]
- BEAVO J.A. Cyclic nucleotide phosphodiesterase: Functional implications of multiple isoforms. Physiol. Rev. 1995;75:725–748. doi: 10.1152/physrev.1995.75.4.725. [DOI] [PubMed] [Google Scholar]
- BOIE Y., STOCCO R., SAWYER N., SLIPETZ D.M., UNGRIN M.D., NEUSCHÄFER-RUBE F., PÜSCHEL G.P., METTERS K.M., ABRAMOVITZ M. Molecular cloning and characterization of the four rat prostaglandin E2 prostanoid receptor subtypes. Eur. J. Pharmacol. 1997;340:227–241. doi: 10.1016/s0014-2999(97)01383-6. [DOI] [PubMed] [Google Scholar]
- BREYER M.D., DAVIS L., JACOBSON H.R., BREYER R.M. Differential localization of prostaglandin E receptor subtypes in human kidney. Am. J. Physiol. 1996;270:F912–F918. doi: 10.1152/ajprenal.1996.270.5.F912. [DOI] [PubMed] [Google Scholar]
- BREYER M.D., JACOBSON H.R., DAVIS L.S., BREYER R.M. In Situ hybridization and localization of mRNA for the rabbit prostaglandin EP3 receptor. Kidney Inter. 1993;43:1372–1378. doi: 10.1038/ki.1993.391. [DOI] [PubMed] [Google Scholar]
- CHABARDÈS D., FIRSOV D., AARAB L., CLABECQ A., BELLANGER A.C., SIAUME-PEREZ S., ELALOUF J.M. Localization of mRNAs encoding Ca2+-inhibitable adenylyl cyclases along the renal tubule. Functional consequences for regulation of the cAMP content. J. Biol. Chem. 1996;271:19264–19271. doi: 10.1074/jbc.271.32.19264. [DOI] [PubMed] [Google Scholar]
- CHABARDÈS D., MONTÉGUT M., ZHOU Y., SIAUME-PEREZ S. Two mechanisms of inhibition by prostaglandin E2 of hormone-dependent cell cAMP in the rat collecting tubule. Mol. Cell. Endocrinol. 1990;73:111–121. doi: 10.1016/0303-7207(90)90124-q. [DOI] [PubMed] [Google Scholar]
- CHAMPGINEULLE A., SIGA E., VASSENT G., IMBERT-TEBOUL M. V2-like vasopressin receptor mobilizes intracellular Ca2+ in rat medullary collecting tubules. Am. J. Physiol. 1993;265:F35–F45. doi: 10.1152/ajprenal.1993.265.1.F35. [DOI] [PubMed] [Google Scholar]
- CHEN L., REIF M.C., SCHAFER J.A. Clonidine and PGE2 have different effects on Na+ and water transport in rat and rabbit CCD. Am. J. Physiol. 1991;261:F126–F136. doi: 10.1152/ajprenal.1991.261.1.F126. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. Classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- DE JESUS FERREIRA M.C., HÉLIÈS-TOUSSAINT C., IMBERT-TEBOUL M., BAILLY C., VERBAVATZ J.M., BELLANGER A.C., CHABARDÈS D. Co-expression of a Ca2+-inhibitable adenylyl cyclase and of a Ca2+-sensing receptor in the cortical thick ascending limb cell of the rat kidney. Inhibition of hormone-dependent cAMP accumulation by extracellular Ca2+ J. Biol. Chem. 1998;273:15192–15202. doi: 10.1074/jbc.273.24.15192. [DOI] [PubMed] [Google Scholar]
- FIRSOV D., AARAB L., MANDON B., SIAUME-PEREZ S., de ROUFFIGNAC C., CHABARDÈS D. Arachidonic acid inhibits hormone-stimulated cAMP accumulation in the medullary thick ascending limb of the rat kidney by a mechanism sensitive to pertussis toxin. Pflügers Archiv. 1995;429:636–646. doi: 10.1007/BF00373984. [DOI] [PubMed] [Google Scholar]
- FUNK C.D., FURCI L., FITZGERALD G.A., GRYGORCZYK R., ROCHETTE C., BAYNE M.A., ABRAMOVITZ M., METTERS K.M. Cloning and expression of a cDNA for the human prostaglandin E receptor EP1 subtype. J. Biol. Chem. 1993;268:26767–26772. [PubMed] [Google Scholar]
- GRIFFITHS N.M., BRICK-GHANNAM C., SIAUME-PEREZ S., CHABARDÈS D. Effect of prostaglandin E2 on agonist-stimulated cAMP accumulation in the distal convoluted tubule isolated from the rabbit kidney. Pflügers Archiv. 1993;422:577–584. doi: 10.1007/BF00374005. [DOI] [PubMed] [Google Scholar]
- HÉBERT R.L., JACOBSON H.R., FREDIN D., BREYER M.D. Evidence that separate PGE2 receptors modulate water and sodium transport in rabbit cortical collecting duct. Am. J. Physiol. 1993;265:F643–F650. doi: 10.1152/ajprenal.1993.265.5.F643. [DOI] [PubMed] [Google Scholar]
- HOEY M., HOUSLAY M.D. Identification and selective inhibition of four distinct soluble forms of cyclic nucleotide phosphodiesterase activity from kidney. Biochem. Pharmacol. 1990;40:193–202. doi: 10.1016/0006-2952(90)90678-e. [DOI] [PubMed] [Google Scholar]
- KIRIYAMA M., USHIKUBI F., KOBAYASHI T., HIRATA M., SUGIMOTO Y., NARUMIYA S. Ligand binding specificities of the eight types and subtypes of the mouse prostanoid receptors expressed in Chinese hamster ovary cells. Br. J. Pharmacol. 1997;122:217–244. doi: 10.1038/sj.bjp.0701367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOTANI M., TANAKA I., OGAWA Y., USUI T., MORI K., ICHIKAWA A., NARUMIYA S., YOSHIMI T., NAKAO K. Molecular cloning and expression of multiple isoforms of human prostaglandin E receptor EP3 subtype generated by alternative messenger RNA splicing: Multiple second messenger systems and tissue-specific distributions. Mol. Pharmacol. 1995;48:869–879. [PubMed] [Google Scholar]
- KUSANO E., MURAYAMA N., WERNESS J.L., CHRISTENSEN S., HOMMA S., YUSUFI A.N.K., DOUSA T.P. Effects of calcium on the vasopressin-sensitive cAMP metabolism in medullary tubules. Am. J. Physiol. 1985;249:F956–F966. doi: 10.1152/ajprenal.1985.249.6.F956. [DOI] [PubMed] [Google Scholar]
- MADSEN K.M., TISHER C.C. Structural-functional relationships along the distal nephron. Am. J. Physiol. 1986;250:F1–F15. doi: 10.1152/ajprenal.1986.250.1.F1. [DOI] [PubMed] [Google Scholar]
- MOREL F., DOUCET A. Hormonal control of kidney functions at the cell level. Physiol. Rev. 1986;66:377–468. doi: 10.1152/physrev.1986.66.2.377. [DOI] [PubMed] [Google Scholar]
- MOREL F., IMBERT-TEBOUL M., CHABARDÈS D. Receptors to vasopressin and other hormones in the mammalian kidney. Kidney Inter. 1987;31:512–520. doi: 10.1038/ki.1987.30. [DOI] [PubMed] [Google Scholar]
- SCHMID A., THIERAUCH K.-H., SCHLEUNING W.-D., DINTER H. Splice variants of the human EP3 receptor for prostaglandin E2. Eur. J. Biochem. 1995;228:23–30. doi: 10.1111/j.1432-1033.1995.tb20223.x. [DOI] [PubMed] [Google Scholar]
- SONNENBURG W.K., SMITH W.L. Regulation of cyclic AMP metabolism in rabbit cortical collecting tubule cells by prostaglandins. J. Biol. Chem. 1988;263:6155–6160. [PubMed] [Google Scholar]
- STOKES J.B. Integrated actions of renal medullary prostaglandins in the control of water excretion. Am. J. Physiol. 1981;240:F471–F480. doi: 10.1152/ajprenal.1981.240.6.F471. [DOI] [PubMed] [Google Scholar]
- SUGIMOTO Y., NAMBA T., SHIGEMOTO R., NEGISHI M., ICHIKAWA A., NARUMIYA S. Distinct cellular localization of mRNAs for three subtypes of prostaglandin E receptor in kidney. Am. J. Physiol. 1994;266:F823–F828. doi: 10.1152/ajprenal.1994.266.5.F823. [DOI] [PubMed] [Google Scholar]
- TAKAICHI K., KUROKAWA K. Inhibitory guanosine triphosphate-binding protein-mediated regulation of vasopressin action in isolated single medullary tubules of mouse kidney. J. Clin. Invest. 1988;82:1437–1444. doi: 10.1172/JCI113749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEUCHI K., TAKAHASHI N., ABE T., ABE K. Two isoforms of the rat kidney EP3 receptor derived by alternative RNA splicing: intrarenal expression co-localization. Biochem. Biophys. Res. Commun. 1994;199:834–840. doi: 10.1006/bbrc.1994.1304. [DOI] [PubMed] [Google Scholar]
- TANIGUCHI S., WATANABE T., NAKAO A., SEKI G., UWATOKO S., KUROKAWA K. Detection and quantitation of EP3 prostaglandin E2 receptor mRNA along mouse nephron segments by RT – PCR. Am. J. Physiol. 1994;266:C1453–C1458. doi: 10.1152/ajpcell.1994.266.5.C1453. [DOI] [PubMed] [Google Scholar]
- TORIKAI S, KUROKAWA K. Effect of PGE2 on vasopressin-dependent cell cAMP in isolated single nephron segments. Am. J. Physiol. 1983;245:F58–F66. doi: 10.1152/ajprenal.1983.245.1.F58. [DOI] [PubMed] [Google Scholar]
- WATABE A., SUGIMOTO Y., HONDA A., IRIE A., NAMBA T., NEGISHI M., ITO S., NARUMIYA S., ICHIKAWA A. Cloning and expression of cDNA for a mouse EP1 subtype of prostaglandin E receptor. J. Biol. Chem. 1993;268:20175–20178. [PubMed] [Google Scholar]


