Abstract
The stable prostacyclin analogue, iloprost relaxes a variety of blood vessels and increases cyclic AMP, although the relationship between adenosine 3′ : 5′-cyclic monophosphate (cyclic AMP) and vasorelaxation remains unclear. We therefore investigated the effect of the adenylyl cyclase inhibitor, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ22536) on iloprost-mediated relaxation and cyclic AMP elevation in endothelium-denuded aortic strips. Iloprost (1–1000 nM) caused a concentration-dependent inhibition of phenylephrine (1–6 μM) contractions, the responses being unaffected by pre-incubation with SQ22536 (100 μM) for 30 min. In other experiments 60 nM iloprost caused a 64% inhibition of phenylephrine contractions concomitant with a 3 fold rise in cyclic AMP. SQ22536 completely abolished the iloprost-induced elevation in cyclic AMP while having no significant effect on relaxation. Our results therefore strongly suggest that cyclic AMP-independent pathways are responsible for the vasorelaxant effects of iloprost in guinea-pig aorta.
Keywords: Vascular smooth muscle, iloprost, guinea-pig aorta, cyclic AMP measurement, adenylyl cyclase inhibitor, SQ22536, vasorelaxation
Introduction
Iloprost is a synthetic carbacyclin derivative of prostacyclin (PGI2), mediating its effects through activation of cell surface prostanoid (IP) receptors which are known to be coupled to adenylyl cyclase through the G-protein, GS (Coleman et al., 1994). Like PGI2, iloprost is a potent inhibitor of platelet aggregation and produces profound vasodilation in vivo as evidenced by its ability to relax a variety of isolated arterial preparations in vitro (Grant & Goa, 1992). However, the cellular mechanism(s) underlying the vascular action of iloprost remains to be elucidated. Because IP agonists increase cyclic AMP in many cell types, including smooth muscle (Coleman et al., 1994), and responses to these agents can be potentiated by phosphodiesterase inhibitors (Holzmann et al., 1980; Riva et al., 1990), it has been proposed that cyclic AMP is the mediator of iloprost-induced vasorelaxation. This is consistent with the observation that iloprost causes concentration-dependent increases in cyclic AMP in arteries denuded of endothelium (Schroder et al., 1987; Ozaki et al., 1996) and that vasodilation in rat tail artery can be reduced by inhibitors of cyclic AMP-dependent protein kinase A (Schubert et al., 1997). In contrast, low concentrations of PGI2 have been reported to induce relaxation that is either associated with no detectable change or even a fall in cyclic AMP (see Frolich, 1990). Furthermore, in rabbit aorta PGI2 increases cyclic AMP causing contraction, not relaxation (Vegesna & Diamond, 1986). Thus the link between cyclic AMP and tissue relaxation remains controversial (Grant & Goa, 1992; Wise & Jones, 1996). Surprisingly, there have been no studies in vascular muscle using IP agonists where tension and cyclic AMP levels have been measured either in the same tissue or with adenylyl cyclase inhibitors. Therefore, the aim of the present study was to investigate the effect of a direct inhibitor of adenylyl cyclase, 9-(tetrahydro-2-furanyl)-9H-purin-6-amine (SQ22356; Haslam et al., 1978) on iloprost-induced relaxation and cyclic AMP levels in isolated guinea-pig aorta.
Methods
Organ bath studies
Male guinea-pigs (Duncan Harver, 250–300 g) were killed by cervical dislocation and the upper thoracic aorta removed and placed in physiological saline solution (PSS) containing in mM; NaCl 112, KCl 5, CaCl2 1.8, MgCl2 1, NaHCO3 25, KH2PO4 0.5, NaH2PO4 0.5, glucose 10 and 0.03 phenol red (pH 7.4 with 95%O2/5% CO2). The tissue was cut into rings (∼2 mm wide, and cut longitudinally) or helical strips (∼2 mm wide, 10 mm long). The endothelium was denuded by gently rubbing the lumen of the muscle with filter paper. Tissues were mounted in an organ bath (0.3 ml) and tension measured isometrically. Muscle strips were subjected to a basal tension of 1.25 g and continuously perfused with PSS (1.0 ml min−1 at 37°C) for 30 min before being precontracted with phenylephrine (1 or 6 μM). Removal of the endothelium was confirmed by lack of relaxation to acethylcholine (10 μM for 2 min). Concentration-response curves were obtained by cumulatively applying iloprost (1–1000 nM) in the absence or presence of the adenylyl cyclase inhibitor, SQ22536 (100 μM). SQ22536 was given 30 min before the addition of iloprost and remained throughout. A 1 h wash-out period was allowed between concentration-response curves.
Determination of cyclic AMP
In other experiments, helical strips were precontracted with phenylephrine (1 μM) for 40 min before being exposed to iloprost (60 nM) for 5 min in the presence or absence of SQ22536 (100 μM). SQ22536 was applied for 30 min beforehand. At the end of the protocol, tissues were snap frozen in liquid N2 and stored at −80°C for up to 2 weeks. To extract cyclic AMP, tissues were placed in acidified ethanol (∼pH 3) containing 0.5 mM 3-isobutyl-1-methylxanthine (IBMX), homogenized and sonicated for 15 min. Homogenates were stored at 4°C for 18 h, and then centrifuged (12,000 r.p.m.) for 3 min. The supernatant was collected and the pellet resuspended in 66% ethanol followed by centrifugation. The total supernatant was evaporated to dryness at 55°C under a flow of N2 gas and the residue resuspended in Tris buffer containing 0.4 mM ethylene-diaminetetracetic acid (EDTA). The cyclic AMP content was measured using a radioreceptor assay kit supplied by Amersham (Buckinghamshire, U.K.). Tissue pellets were dissolved in 0.5 mM NaOH and protein content determined using the Lowry method.
Drugs and statistics
Phenylephrine and IBMX were purchased from Sigma Chemical Co. (Poole, Dorset, U.K.), SQ22536 from Alexis Corporation (Bingham, Nottingham, U.K.) and iloprost was kindly donated by Schering AG (Berlin, Germany). All data are expressed as mean±s.e.mean of n observations and significance determined using Student's t-test or ANOVA with correction for pairwise comparisons of groups (Student-Newman-Keuls Method). P values <0.05 were considered significant. The concentration of iloprost causing 50% relaxation of phenylephrine contractions is expressed as the log EC50 value.
Results
Aortic rings precontracted with 6 μM phenylephrine were relaxed by iloprost (1–1000 nM) in a concentration- dependent manner with an log EC50 value for inhibition of phenylephrine-induced tone of −7.54±0.03 (n=17; Figure 1). Under these conditions, the maximal relaxation produced by iloprost was 70.9±5.3% (n=17). Pre-incubation for 30 min with the adenylyl cyclase inhibitor, SQ22536 (100 μM) produced no significant changes in tone, nor did it affect the log EC50 (−7.45±0.1; n=11) or the maximal relaxation (71.4±5.4%; n=11) elicited by iloprost (P>0.30, two tailed t-test; Figure 1). In order to ascertain that SQ22536 was effectively inhibiting any rise in cyclic AMP induced by iloprost, cyclic AMP levels were measured in tissues that had been contracted with phenylephrine and exposed to a single concentration of iloprost (60 nM) for 5 min (Figure 2a). Under these conditions, iloprost relaxed phenyl-ephrine (1 μM) contractions on average by 63.9±5.0% (n=9) and produced a 3 fold rise in cyclic AMP, increasing it from 3.64±0.80–10.48±1.46 pmol mg−1 protein (Figure 2c). This rise in cyclic AMP could be completely inhibited by a 30 min pre-incubation with 100 μM SQ22536 (Figure 2c), although it had no significant effect on the magnitude of relaxation (61.2±4.7%; n=9) induced by iloprost (Figure 2b).
Figure 1.
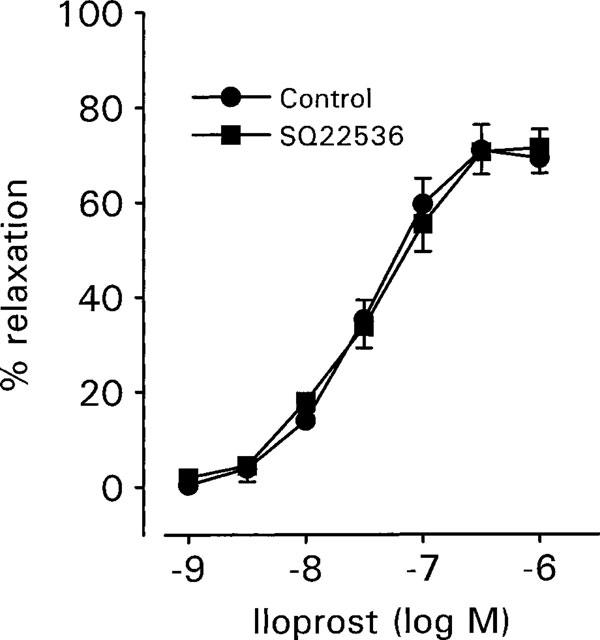
Cumulative concentration-response curves to iloprost (1–1000 nM) measured in the absence (control) and presence of the adenylyl cyclase inhibitor, SQ22536. Aortic rings, denuded of endothelium, were precontracted with 6 μM phenylephrine and each concentration of iloprost applied for 5 min. SQ22536 (100 μM) was applied 30 min before the first application of iloprost. Data are expressed as the mean±s.e.mean of 11–17 experiments from nine animals.
Figure 2.
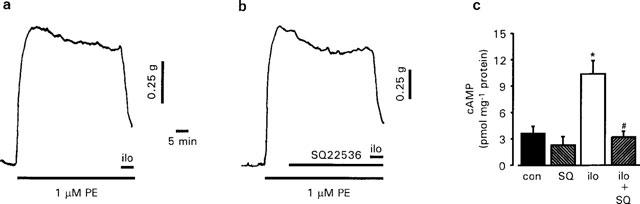
The effect of iloprost (ilo; 60 nM) on endothelium-denuded helical strips precontracted with 1 μM phenylephrine under control conditions (a) or in the presence of 100 μM SQ22536 (b). Determination of cyclic AMP was carried out at the end of the experiment and found to be 7.71 and 1.91 pmol mg−1 protein in a and b, respectively. (c) Influence of SQ22536 (SQ) on cyclic AMP levels measured in the absence and presence of iloprost (ilo). Data are expressed as mean±s.e.mean of nine experiments from nine animals. *P<0.05 when compared to control value, #P<0.05 when compared to iloprost (one way ANOVA).
Discussion
The data presented in this paper provides strong evidence that cyclic AMP does not play a major role in vasorelaxation induced by the IP agonist, iloprost in guinea-pig aortic smooth muscle. This conclusion is based on the findings that the adenylyl cyclase inhibitor SQ22536 failed to affect relaxation induced by iloprost at any concentration tested, and yet it was able to completely inhibit the rise in cyclic AMP induced by a near maximal concentration of iloprost. To our knowledge this is the first study where the effects of IP agonists on tension and cyclic AMP levels have been measured in the same tissue. Moreover, unlike most studies to date, cyclic AMP was measured in tissues not exposed to phosphodiesterase inhibitors, thus allowing a direct comparison between tension and cyclic AMP under conditions where breakdown is not artificially prevented. This is important because not only do phosphodiesterase inhibitors elevate cyclic AMP to extremely high (and probably unphysiological) levels, they often cause substantial relaxation by themselves, and in many cases will also elevate cyclic GMP, thus complicating the interpretation of such data.
In this study we used SQ22536 in preference to other known adenylyl cyclase inhibitors (e.g. 2′, 5′-dideoxyadenosine) since this was the only inhibitor out of several tested that we found could completely block the rise in cyclic AMP evoked by activators of adenylyl cyclase; all others reduced cyclic AMP levels by ∼50% (Turcato & Clapp, unpublished observations). Whilst we have shown that SQ22536 was unable to block the functional effects of iloprost in this study, results from other studies demonstrate that this inhibitor at similar concentrations, does oppose the action of IP agonists in other cell types. For example, SQ22536 was reported to completely reverse the protective effects of iloprost against neutrophil-induced lung injury (Riva et al., 1990) and also reduce the inhibitory action of IP agonists on platelet aggregation (Armstrong et al., 1986). Thus, in some situations, cyclic AMP does appear to play an important role in mediating the biological effects of IP agonists.
Our previous observations in guinea-pig aorta showing that relaxations to iloprost were markedly inhibited by blockers of Ca2+-activated K+ channels, whereas those to forskolin, a direct activator of adenylyl cyclase, were minimally affected (Clapp et al., 1998), would also support the idea that cyclic AMP-independent pathways are involved in mediating relaxation to iloprost in this tissue. Consistent with this, we have observed that the cyclic AMP-dependent protein kinase inhibitor, H-89 did not inhibit the effects of iloprost (Turcato & Clapp, 1998). Likewise, vasodilator responses to iloprost in the rabbit coronary artery were found to be sensitive to the KATP channel blocker glibenclamide but not those to forskolin or dibutyryl cyclic AMP (Jackson et al., 1993). Although the above investigations are in direct contrast to studies implicating a role for cyclic AMP (Holzmann et al., 1980; Schroder et al., 1987; Schubert et al., 1997), cellular mechanisms may alter depending on the presence of the endothelium, which is known to enhance the response to IP agonists (Schroder et al., 1987). One possibility is that cyclic AMP plays an important role in controlling the release of endothelium-derived relaxing factors. Indeed iloprost-stimulated cyclic AMP levels have been observed to be significantly higher in endothelium intact vessels (Schroder et al., 1987). Regardless of the underlying biochemical mechanisms associated with IP receptor activation, recent evidence suggests these receptors can couple to other G-protein signalling pathways, including Gi, Gp and possibly Go (Wise & Jones, 1996). However, which, if any of these pathways mediates the vascular effects of iloprost clearly needs to be investigated, although we cannot at this stage rule out the possibility that Gs is involved as an intracellular mediator.
In conclusion, we suggest that cyclic AMP does not play a major role in mediating the vascular effects of iloprost in guinea-pig aorta. Moreover, in the light of recent evidence that IP receptors can couple to multiple signalling pathways, there needs to be a closer examination of the classical notion that cyclic AMP mediates the cardiovascular effects of IP receptor agonists.
Acknowledgments
S.T. is funded by a PhD studentship from the British Heart Foundation and L.H.C. is an MRC Senior Research Fellow. This work was supported by a grant from the British Heart Foundation (FS/96058). We thank Schering AG (Berlin, Germany) for the gift of iloprost.
Abbreviations
- IBMX
3-isobutyl-1-methylxanthine
- PSS
physiological saline solution
- PGI2
prostacyclin
- SQ22536
9-(tetrahydro-2-furanyl)-9H-purin-6-amine
References
- ARMSTRONG R.A., JONES R.L., MACDERMOT J., WILSON N.H. Prostaglandin endoperoxide analogues which are both thromboxane receptor antagonists and prostacyclin mimetics. Br. J. Pharmacol. 1986;87:543–551. doi: 10.1111/j.1476-5381.1986.tb10196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLAPP L.H., TURCATO S., HALL S.J., BALOCH M. Evidence that Ca2+-activated K+ channels play a major role in mediating the vascular effects of iloprost and cicaprost. Eur. J. Pharmacol. 1998;356:215–224. doi: 10.1016/s0014-2999(98)00549-4. [DOI] [PubMed] [Google Scholar]
- COLEMAN R.A., SMITH W.L., NARUMIYA S. International union of pharmacology classification of prostanoid receptors: properties, distribution, and structure of the receptors and their subtypes. Pharmacol. Rev. 1994;46:205–229. [PubMed] [Google Scholar]
- FROLICH J.C. Prostacyclin in hypertension. J. Hyperten. 1990;8:S73–S78. [PubMed] [Google Scholar]
- GRANT S.M., GOA K.L. Iloprost. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in peripheral vascular disease, myocardial ischaemia and extracorporeal circulation procedures. Drugs. 1992;43:889–924. doi: 10.2165/00003495-199243060-00008. [DOI] [PubMed] [Google Scholar]
- HASLAM R.J., DAVIDSON M.M., DESJARDINS J.V. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the undirectional control of platelet function by cyclic AMP. Biochem. J. 1978;176:83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLZMANN S., KUKOVETZ W.R., SCHMIDT K. Mode of action of coronary arterial relaxation by prostacyclin. J. Cyclic. Nucleoltide. Res. 1980;6:451–460. [PubMed] [Google Scholar]
- JACKSON W.F., KONIG A., DAMBACHER T., BUSSE R. Prostascyclin-induced vasodilation in rabbit heart is mediated by ATP-sensitive potassium channels. Am. J. Physiol. 1993;264:H238–H243. doi: 10.1152/ajpheart.1993.264.1.H238. [DOI] [PubMed] [Google Scholar]
- OZAKI H., ABE A., UEHIGASHI Y., KINOSHITA M., HORI M., MITSUISALTO M., KARAKI H. Effects of a prostaglandin I2 analog iloprost on cytoplasmic Ca2+ levels and muscle contraction in isolated guinea pig aorta. Jpn. J. Pharmacol. 1996;71:231–237. doi: 10.1254/jjp.71.231. [DOI] [PubMed] [Google Scholar]
- RIVA C.M., MORGANROTH M.L., LJUNGMAN A.G., SCHOENEICH S.O., MARKS R.M., TODD R.F., WARD P.A., BOXER L.A. Iloprost inhibits neutrophil-induced lung injury and neutrophil adherence to endothelial monolayers. Am. J. Respir. Cell. Molec. Biol. 1990;3:301–309. doi: 10.1165/ajrcmb/3.4.301. [DOI] [PubMed] [Google Scholar]
- SCHRODER G., BECKMANN R., SCHILLINGER E.Studies on the vasorelaxant effects of and mechanisms of iloprost in isolated preparations Prostacyclin and its stable analogue iloprost 1987Berlin: Springer-Verlag; 129–137.ed. Gryglewski, R.J. & Stock, G. pp [Google Scholar]
- SCHUBERT R., SEREBRYAKOV V.N., MEWES H., HOPP H.H. Iloprost dilates rat small arteries: Role of KATP- and KCa- channel activation by cAMP-dependent protein kinase. Am. J. Physiol. 1997;272:H1147–H1156. doi: 10.1152/ajpheart.1997.272.3.H1147. [DOI] [PubMed] [Google Scholar]
- TURCATO S., CLAPP L.H. Evidence that vasorelaxation induced by Gs coupled receptors is largely independent of cAMP in guinea-pig aorta. J. Mol. Cell. Cardiol. 1998;30:A190. [Google Scholar]
- VEGESNA R.V., DIAMOND J. Elevation of cyclic AMP by prostacyclin is accompanied by relaxation of bovine coronary arteries and contraction of rabbit aortic rings. Eur. J. Pharmacol. 1986;128:25–31. doi: 10.1016/0014-2999(86)90553-4. [DOI] [PubMed] [Google Scholar]
- WISE H., JONES R.L. Focus on prostacyclin and its novel mimetics. Trends. Pharmacol. Sci. 1996;17:17–21. doi: 10.1016/0165-6147(96)81565-3. [DOI] [PubMed] [Google Scholar]


