Abstract
Injury to the adult mammalian central nervous system (CNS) often results in permanent loss of sensory and motor function. This is due to the failure of injured axons to regenerate. The inhibitory nature of the CNS can be attributed to several factors, including formation of the glial scar, the presence of several molecules, associated with myelin, which inhibit axonal regrowth, and the intrinsic growth state of these neurons. Encouraging regeneration in the adult mammalian CNS therefore will require targeting one or all of these factors following injury. Here we illustrate recent work from our laboratory that identifies some of the signalling components involved in modulation of the intrinsic growth state of adult neurons. When activated, these signalling pathways can induce axonal regeneration in the presence of the myelin-associated inhibitors both in vitro and in vivo.
Keywords: cAMP, myelin-associated glycoprotein (MAG), polyamines, regeneration
Introduction
Axons of the adult mammalian central nervous system (CNS) fail to regenerate after injury. Equivalent embryonic or peripheral nervous system (PNS) neurons will, however, exhibit substantial regeneration and functional recovery. The main factor in the ability of neurons to regenerate spontaneously appears to be the local, extracellular environment. Indeed, this is evident from the fact that, if CNS neurons are provided with a permissive environment, such as a graft of PNS tissue, regeneration may be observed (David & Aguayo, 1981). Many factors account for the poor regenerative capacity of CNS neurons, including a deficiency in neurotrophic factors, the presence of myelin-associated inhibitory molecules, formation of the glial scar and, as we will discuss here, a change in the intracellular levels of cyclical nucleotides. Following injury, however, the two major obstacles to regeneration appear to be the myelin-associated inhibitors and the formation of the glial scar. The glial scar, which forms a physical regenerative barrier, also contains molecules, such as chondroitin sulphate proteoglycans (CSPGs), which act as inhibitors of regeneration (McKeon et al. 1991; Niederost et al. 1999; Bradbury et al. 2002). However, as the glial scar may often take several weeks to form fully, it appears likely that there is a ‘window of opportunity’, immediately following injury, during which the only impediments to regeneration are the myelin-associated inhibitors.
To date, three major myelin-associated inhibitors have been identified: myelin-associated glycoprotein (MAG) (McKerracher et al. 1994; Mukhopadhyay et al. 1994); Nogo-A, which consists of two separate inhibitory domains – a 66-amino extracellular loop termed nogo-66 (GrandPre et al. 2000) and the amino-terminus region (Chen et al. 2000; Prinjha et al. 2000); and oligodendrocyte-myelin glycoprotein (OMgp) (Kottis et al. 2002; Wang et al. 2002b). Although these three inhibitory proteins have very different physical structures, they all appear to be present at the periaxonal surface of the myelin membrane and thus may encounter regenerating axons. In addition, recent and surprising evidence points to the fact they all appear to bind to the same neuronal receptor (Domeniconi et al. 2002; Liu et al. 2002; Wang et al. 2002b). This glycosylphosphatidyl-inositol (GPI)-linked protein, termed the Nogo-66 receptor (NgR) since it was first identified as the receptor for the 66 amino acid loop of Nogo (Fournier et al. 2001), binds all three inhibitors with similar affinities and with a proposed overlap in binding domain, suggesting a redundancy in the activity of these molecules. Because NgR is a GPI-linked protein and therefore lacks a transmembrane domain, it requires a binding partner in order to transduce the inhibitory signal to the neuron. This co-receptor appears to be none other than the p75 neurotrophin receptor (p75NTR) (Wang et al. 2002a; Wong et al. 2002). Although little is known about the signalling cascade, it has been demonstrated that the small GTPase, RhoA, is one of the downstream components (Lehmann et al. 1999; Yamashita et al. 2002). Nevertheless, the NgR–p75NTR receptor complex and RhoA provide two potential targets for intervention after injury that may result in an increase in CNS regeneration by blocking the inhibitory signalling.
There is, however, another potential method for inducing CNS regeneration after injury: altering the intrinsic growth state of the adult neuron so that they no longer respond to these inhibitors. In this review, we illustrate some of our recent work that elucidates the signalling molecules involved in these processes and some methods that have been employed to facilitate axonal regeneration.
Neurotrophins and cyclical AMP
Despite the inhibitory nature of the mature CNS environment, several studies have shown that limited regeneration is indeed possible if the axons are provided with a more permissive environment (David & Aguayo, 1981; Berry et al. 1996; Bregman et al. 1998). In some cases, regenerating axons were also able to extend beyond the grafted tissue and into the white matter, suggesting a change in the ability of the neurons to respond to the inhibitory cues. Our investigations into this proposed change began when we noted that the regenerating axons were exposed to specific neurotrophic factors (BDNF or NT-3) for a period prior to encountering the inhibitory environment (Bregman et al. 1998). With this in mind, we examined the ability of postnatal CNS neurons to grow on an inhibitory substrate after exposure to neurotrophins. We found that although neurons grown on a substrate of MAG-expressing Chinese Hamster Ovary (CHO) cells or purified myelin fail to regenerate in the presence of neurotrophins, they can extend long neurites on the same substrates if they are first ‘primed’ overnight with these neurotrophic factors. This suggests that the completion of some downstream signalling mechanisms are required prior to the induction of the inhibitory signalling. What, however, are the components of this signalling pathway?
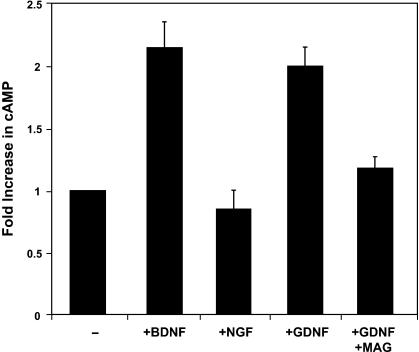
To determine this, we tested a number of known signalling agents. Of those tested, only the cAMP analogue, dibutyryl-cAMP (db-cAMP), was able to mimic the effect of neurotrophin priming. Interestingly, we also found that the regeneration-inducing effect of db-cAMP does not require a ‘priming’ period prior to inhibitor exposure. This suggests that cAMP may be an integral downstream component of the neurotrophin signalling pathway. And, indeed, competitive immunoassay measurements indicate that there is a two-fold increase in endogenous cAMP levels following neurotrophin treatment (Fig. 1). Furthermore, this regenerative effect is dependant on the activity of the cAMP-dependent protein kinase (PKA), because blocking the activity of this enzyme abrogates the improved axonal regeneration observed following both priming with neurotrophins or treatment with db-cAMP (Cai et al. 1999). Together, these data suggest that following treatment with neurotrophins, the endogenous levels of cAMP become elevated by an as yet unknown mechanism, as a result of which the neurons are no longer responsive to the inhibitory cues provided by myelin.
Fig. 1.
Cerebellar neurons that are treated with Brain-derived neurotrophic factor (BDNF) or Glial-derived neurotrophic factor (GDNF), but not nerve growth factor (NGF), exhibit a significant increase in endogenous cAMP levels that can be blocked by the addition of MAG. (Reproduced from Cai et al. 1999, with permission.)
Endogenous cAMP and development
It is well known that embryonic and, in some cases, perinatal, CNS neurons will spontaneously regenerate both in vitro and in vivo whereas their postnatal counterparts will not. Does this switch in regenerative ability reflect a change in the extracellular environment, a change in receptor expression or simply a change in responsiveness to the inhibitory cues? Current evidence suggests that the major myelin inhibitors are indeed present during embryonic development and although it is yet unclear if the NgR receptor is indeed present in embryonic neurons, recent findings from our laboratory suggest that such arguments may be irrelevant.
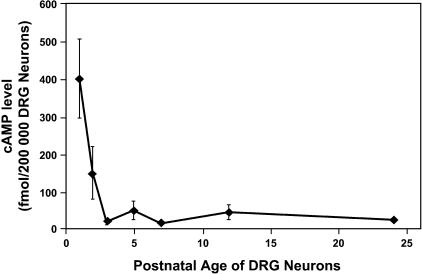
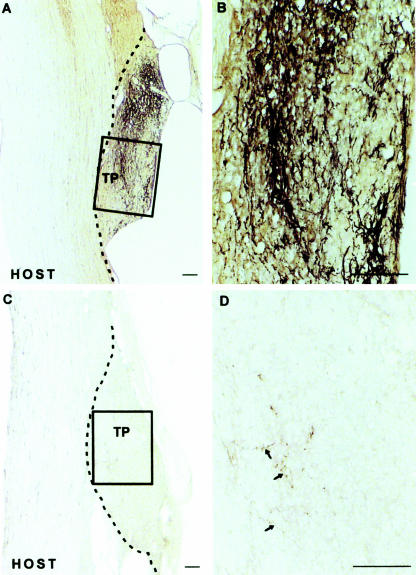
As it had been previously shown that elevation of cellular cAMP levels is necessary for attractive growth cone guidance cues (Ming et al. 1997; Song et al. 1997, 1998), and given our previous results suggesting that such elevations can induce axonal regeneration, we sought to determine if the endogenous levels of cAMP may, in fact, influence the embryonic regenerative capacity observed during development. Indeed, quantification of cAMP levels in prenatal and postnatal CNS neurons by both competitive immunoassay and by immunostaining of primary neurons indicates that endogenous cAMP levels are greatly elevated in the young neurons and these elevated levels decrease precipitously after birth. The time course of this change in endogenous cAMP temporally coincides with the lack of regeneration observed from older neurons (Fig. 2). In further support of this hypothesis, we find that we can abrogate the improved regenerative capacity of embryonic neurons in vitro by blocking the cAMP signalling pathway by either PKA inhibition or via application of a cAMP antagonist. Finally, we examined the role of cAMP in the spontaneous regeneration of the neonatal rat spinal cord via application of a dorsal column hemisection. In the young animal, these axotomized neurons normally regenerate, but, if an inhibitor of PKA is applied simultaneously to the lesion site, this regeneration is completely blocked (Fig. 3) (Cai et al. 2001). Thus is seems evident that a sudden decrease in cAMP levels at or near birth signals a change from the developmental stage and this coincides with the inability of these neurons to regenerate. This further suggests that elevated cAMP levels and the activation of the downstream signalling mechanisms are a major contributor to the induction of spontaneous regeneration both in vitro and in vivo.
Fig. 2.
The dramatic decrease of endogenous cAMP levels in DRG neurons coincides precisely with the end of the developmental stage at which they are no longer able to exhibit spontaneous regeneration. (Reproduced from Cai et al. 2001, with permission.)
Fig. 3.
Regeneration of neonatal corticospinal axons after overhemisection lesioning, which can be seen in the untreated animals (A and B), can be completely abrogated via the addition of an inhibitor of PKA (C and D). Axons are labelled anterogradely with BDA. (Reproduced from Cai et al. 2001, with permission.)
Endogenous cAMP and the conditioning-lesion effect
An interesting and useful tool for the study of regeneration of CNS vs. PNS neurons is the dorsal root ganglion (DRG) population. These sensory neurons differ from other neurons in that they have two branches, one which extends into the CNS and another which radiates peripherally. As expected, if the peripheral branch is lesioned, spontaneous regeneration is observed. The same is not true for the central branch. However, several studies have shown that if one lesions the peripheral branch of these DRG neurons and then, 1 week later, lesions the central branch, extensive regeneration into and beyond the lesion site can be observed (Richardson & Issa, 1984; Richardson & Verge, 1986; Neumann & Woolf, 1999). This phenomenon is called the ‘conditioning lesion’ effect. The mechanism by which this effect acts is, as yet, unknown. An intriguing possibility is that this conditioning lesion results in an elevation of endogenous cAMP, which, in turn, allows for the improved central-branch regeneration.
To test this hypothesis, we began by measuring the endogenous cAMP levels of DRG neurons 1 day and 1 week after application of a sciatic nerve lesion. Again, immunostaining and a competitive immunoassay showed a robust increase in the cAMP levels of the axotomized DRG neurons by 1 day post-lesion, but these elevated levels had returned to control levels after 1 week. Furthermore, if these conditioning-lesioned neurons are grown on a substrate of either MAG-expressing CHO cells or purified myelin, they will extend long neurites without the application of neurotrophins or db-cAMP in a PKA-dependent manner. These findings add further support for the necessity of cAMP in the induction of regeneration in these neurons, but is the elevation of endogenous cAMP in itself sufficient to induce this effect? Recent evidence suggests that it is. A single injection of the cAMP analogue db-cAMP into dorsal root ganglia in vivo is sufficient to effectively mimic the conditioning lesion effect in the absence of sciatic nerve section when these neurons are grown on either MAG or purified myelin. In addition, extensive regeneration is observed following a dorsal column lesion applied 1 week after a single injection of db-cAMP (Neumann et al. 2002; Qiu et al. 2002). Thus, it appears evident that elevation of neuronal cAMP levels is not only necessary but also sufficient to allow spontaneous regeneration following injury in the inhibitory CNS environment.
Downstream effectors
Although we have demonstrated that cAMP and PKA activation are integral components of the regenerative pathway, the downstream effectors of this system are as yet unknown. Because PKA is a known activator of cellular transcription factors, it is possible that the next step in this pathway involves transcription and new protein synthesis. This does indeed seem to be the case as we find that simultaneous treatment of primary neurons with an inhibitor of RNA polymerase can effectively block the axonal regeneration induced by either neurotrophins or db-cAMP. With this in mind, we sought to identify some of the key genes that are up-regulated in response to elevation of endogenous cAMP and play a role in the regenerative pathway.
One potential candidate that we investigated was a gene for an enzyme that plays a role in the synthesis of cellular polyamines, called Arginase I. This gene was selected for study because it has been shown to be up-regulated in other cells types in response to cAMP elevation (Nebes & Morris, 1988; Morris et al. 1998) and also because several studies indicate that polyamines may play a role in both nervous system developmental growth (Slotkin & Bartolome, 1986; Slotkin et al. 1982) and axonal regeneration after injury (Ingoglia et al. 1982; Gilad & Gilad, 1988; Gilad et al. 1996).
Our data show that both transcription of the ArgI gene and subsequent Arginase I expression is up-regulated by treatment of cerebellar neurons with either neurotrophins or db-cAMP. Furthermore, this increase in expression is functionally significant as we also observed an increase in the synthesis of the downstream polyamine product putrescine in neurons treated with neurotrophins or db-cAMP. Does this increase in polyamine synthesis actually induce neuronal regeneration?
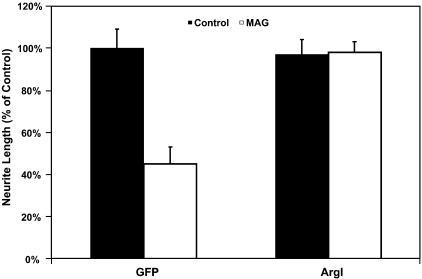
To answer this question, we infected primary cerebellar neurons with an adenoviral vector containing the ArgI gene. Infected neurons which over-express the ArgI construct extend significantly longer neurites on either MAG-expressing CHO cells or a substrate of purified myelin compared with those expressing a control vector (green fluorescent protein alone) (Fig. 4). In addition, if the polyamine synthesis pathway is pharmacologically blocked, neither neurotrophins nor db-cAMP are able to overcome the inhibition of axonal regeneration by MAG or myelin. However, this effect can be restored by supplemental addition of the downstream polyamine product, putrescine. Finally, the expression levels of Arginase I decrease dramatically after development and coincide temporally with the decrease in cAMP levels noted during the developmental switch from axonal promotion to inhibition by the CNS environment (Cai et al. 2001). These findings suggest yet another step in the induction of axonal regeneration induced by elevation of intracellular cAMP levels. The activation of PKA induces transcription of regeneration-related genes, including the enzyme Arginase I, which in turn catalyses the production of polyamine products (Cai et al. 2002). Precisely how these polyamines facilitate axonal regeneration is not yet known. Polyamines, however, have been suggested to have many roles in the adult nervous system (Slotkin & Bartolome, 1986; Kauppila, 1992; Chu et al. 1995), but one of the most intriguing of their many effects is the regulation of various cytoskeletal events such as regulation of microtubule assembly (Banan et al. 1998; Wolff, 1998) and modulation of the expression of certain major cytoskeleton proteins (Kaminska et al. 1992). Thus one possibility for the mechanism of action of polyamines in regeneration is that they may directly affect cytoskeletal stability and rearrangement and thereby prevent the changes induced by the actions of the myelin-associated inhibitors. One of the ongoing projects in our laboratory is to attempt to elucidate this system.
Fig. 4.
Cerebellar neurons, infected with an adenoviral vector containing the ArgI gene under cyctomegalovirus (CMV)-promoter regulation, overexpress the Arginase I enzyme and are able to overcome the inhibition of axonal regeneration by myelin. (Reproduced from Cai et al. 2002, with permission.)
Conclusions
Although it has long been acknowledged that neurons of the CNS do not regenerate after injury, the reasons for this regenerative failure have, until recently, remained a mystery. In the last few years we have seen both the identification of the major myelin-associated inhibitors of axonal regeneration and the receptor complex that mediates their inhibitory signalling, as well as the elucidation of some of the intracellular signalling pathways active during periods of spontaneous regeneration. These findings represent a giant leap forward in the pursuit of a therapeutic treatment for spinal cord injuries.
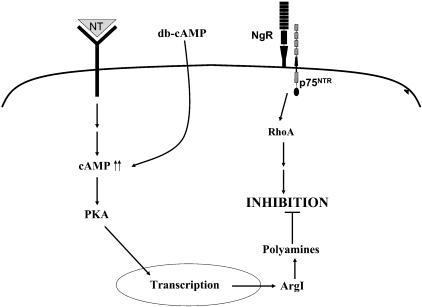
Work from our laboratory has shown that elevation of intracellular cAMP levels either via priming with neurotrophins or via application of an analogue can initiate a signalling cascade that leads to transcription of regeneration-associated genes and, subsequently, the synthesis of polyamines (Fig. 5). This appears to be the determining factor in the spontaneous CNS regeneration observed during development and the elevation of cAMP alone can mimic this system, even in the absence of other cues. Although it is not yet known precisely how this pathway interacts to disrupt the inhibitory signalling of myelin, it appears likely that regulation of cytoskeletal elements will be integral. Together, these findings provide many potential targets for therapeutic intervention in order to block the inhibitory signalling induced by the CNS environment and to facilitate axonal regeneration and functional recovery after CNS injury in vivo.
Fig. 5.
Model for inducing regeneration in the inhibitory CNS environment. Following injury, elevation of cAMP levels can induce a cascade of events that leads to synthesis of polyamines and a subsequent block of the MAG or myelin-induced inhibition of axonal regeneration.
References
- Banan A, McCormack SA, Johnson LR. Polyamines are required for microtubule formation during gastric mucosal healing. Am. J. Physiol. 1998;274:G879–G885. doi: 10.1152/ajpgi.1998.274.5.G879. [DOI] [PubMed] [Google Scholar]
- Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J. Neurocytol. 1996;25:147–170. doi: 10.1007/BF02284793. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS, Patel PN, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Broude E, McAtee M, Kelley MS. Transplants and neurotrophic factors prevent atrophy of mature CNS neurons after spinal cord injury. Exp. Neurol. 1998;149:13–27. doi: 10.1006/exnr.1997.6669. [DOI] [PubMed] [Google Scholar]
- Cai D, Shen Y, De Bellard M, Tang S, Filbin MT. Prior exposure to neurotrophins blocks inhibition of axonal regeneration by MAG and myelin via a cAMP-dependent mechanism. Neuron. 1999;22:89–101. doi: 10.1016/s0896-6273(00)80681-9. [DOI] [PubMed] [Google Scholar]
- Cai D, Qiu J, Cao Z, McAtee M, Bregman BS, Filbin MT. Neuronal cyclic AMP controls the developmental loss in ability of axons to regenerate. J. Neurosci. 2001;21:4731–4739. doi: 10.1523/JNEUROSCI.21-13-04731.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Deng K, Mellado W, Lee J, Ratan RR, Filbin MT. Arginase I and polyamines act downstream from cyclic AMP in overcoming inhibition of axonal growth MAG and myelin in vitro. Neuron. 2002;35:711–719. doi: 10.1016/s0896-6273(02)00826-7. [DOI] [PubMed] [Google Scholar]
- Chen MS, Huber AB, van der Haar ME, Frank M, Schnell L, Spillmann AA, et al. Nogo-A is a myelin-associated neurite outgrowth inhibitor and an antigen for monoclonal antibody IN-1. Nature. 2000;403:434–439. doi: 10.1038/35000219. [DOI] [PubMed] [Google Scholar]
- Chu PJ, Saito H, Abe K. Polyamines promote regeneration of injured axons of cultured rat hippocampal neurons. Brain Res. 1995;673:233–241. doi: 10.1016/0006-8993(94)01419-i. [DOI] [PubMed] [Google Scholar]
- David S, Aguayo AJ. Axonal elongation into peripheral nervous system ‘bridges’ after central nervous system injury in adult rats. Science. 1981;214:931–933. doi: 10.1126/science.6171034. [DOI] [PubMed] [Google Scholar]
- Domeniconi M, Cao Z, Spencer T, Sivasankaran R, Wang K, Nikulina E, et al. Myelin-associated glycoprotein interacts with the Nogo66 receptor to inhibit neurite outgrowth. Neuron. 2002;35:283–290. doi: 10.1016/s0896-6273(02)00770-5. [DOI] [PubMed] [Google Scholar]
- Fournier AE, GrandPre T, Strittmatter SM. Identification of a receptor mediating Nogo-66 inhibition of axonal regeneration. Nature. 2001;409:341–346. doi: 10.1038/35053072. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH. Early polyamine treatment enhances survival of sympathetic neurons after postnatal axonal injury or immunosympathectomy. Brain Res. 1988;466:175–181. doi: 10.1016/0165-3806(88)90042-9. [DOI] [PubMed] [Google Scholar]
- Gilad VH, Tetzlaff WG, Rabey JM, Gilad GM. Accelerated recovery following polyamines and aminoguanidine treatment after facial nerve injury in rats. Brain Res. 1996;724:141–144. doi: 10.1016/0006-8993(96)00287-9. [DOI] [PubMed] [Google Scholar]
- GrandPre T, Nakamura F, Vartanian T, Strittmatter SM. Identification of the Nogo inhibitor of axon regeneration as a Reticulon protein. Nature. 2000;403:439–444. doi: 10.1038/35000226. [DOI] [PubMed] [Google Scholar]
- Ingoglia NA, Sharma SC, Pilchman J, Baranowski K, Sturman JA. Axonal transport and transcellular transfer of nucleosides and polyamines in intact and regenerating optic nerves of goldfish: speculation on the axonal regulation of periaxonal cell metabolism. J. Neurosci. 1982;2:1412–1423. doi: 10.1523/JNEUROSCI.02-10-01412.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B, Kaczmarek L, Grzelakowska-Sztabert B. Inhibitors of polyamine biosynthesis affect the expression of genes encoding cytoskeletal proteins. FEBS Lett. 1992;304:198–200. doi: 10.1016/0014-5793(92)80618-q. [DOI] [PubMed] [Google Scholar]
- Kauppila T. Polyamines enhance recovery after sciatic nerve trauma in the rat. Brain Res. 1992;575:299–303. doi: 10.1016/0006-8993(92)90093-o. [DOI] [PubMed] [Google Scholar]
- Kottis V, Thibault P, Mikol D, Xiao ZC, Zhang R, Dergham P, et al. Oligodendrocyte-myelin glycoprotein (OMgp) is an inhibitor of neurite outgrowth. J. Neurochem. 2002;82:1566–1569. doi: 10.1046/j.1471-4159.2002.01146.x. [DOI] [PubMed] [Google Scholar]
- Lehmann M, Fournier A, Selles-Navarro I, Dergham P, Sebok A, Leclerc N, et al. Inactivation of Rho signaling pathway promotes CNS axon regeneration. J. Neurosci. 1999;19:7537–7547. doi: 10.1523/JNEUROSCI.19-17-07537.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BP, Fournier A, GrandPre T, Strittmatter SM. Myelin-associated glycoprotein as a functional ligand for the Nogo-66 receptor. Science. 2002;297:1190–1193. doi: 10.1126/science.1073031. [DOI] [PubMed] [Google Scholar]
- McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J. Neurosci. 1991;11:3398–3411. doi: 10.1523/JNEUROSCI.11-11-03398.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song HJ, Berninger B, Holt CE, Tessier-Lavigne M, Poo MM. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- Morris SM, Jr, Kepka-Lenhart D, Chen LC. Differential regulation of arginases and inducible nitric oxide synthase in murine macrophage cells. Am. J. Physiol. 1998;275:E740–E747. doi: 10.1152/ajpendo.1998.275.5.E740. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay G, Doherty P, Walsh FS, Crocker PR, Filbin MT. A novel role for myelin-associated glycoprotein as an inhibitor of axonal regeneration. Neuron. 1994;13:757–767. doi: 10.1016/0896-6273(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Nebes VL, Morris SM., Jr Regulation of messenger ribonucleic acid levels for five urea cycle enzymes in cultured rat hepatocytes. Requirements for cyclic adenosine monophosphate, glucocorticoids, and ongoing protein synthesis. Mol. Endocrinol. 1988;2:444–451. doi: 10.1210/mend-2-5-444. [DOI] [PubMed] [Google Scholar]
- Neumann S, Woolf CJ. Regeneration of dorsal column fibers into and beyond the lesion site following adult spinal cord injury. Neuron. 1999;23:83–91. doi: 10.1016/s0896-6273(00)80755-2. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Niederost BP, Zimmermann DR, Schwab ME, Bandtlow CE. Bovine CNS myelin contains neurite growth-inhibitory activity associated with chondroitin sulfate proteoglycans. J. Neurosci. 1999;19:8979–8989. doi: 10.1523/JNEUROSCI.19-20-08979.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinjha R, Moore SE, Vinson M, Blake S, Morrow R, Christie G, et al. Inhibitor of neurite outgrowth in humans. Nature. 2000;403:383–384. doi: 10.1038/35000287. [DOI] [PubMed] [Google Scholar]
- Qiu J, Cai D, Dai H, McAtee M, Hoffman PN, Bregman BS, et al. Spinal axon regeneration induced by elevation of cyclic AMP. Neuron. 2002;34:895–903. doi: 10.1016/s0896-6273(02)00730-4. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Issa VM. Peripheral injury enhances central regeneration of primary sensory neurones. Nature. 1984;309:791–793. doi: 10.1038/309791a0. [DOI] [PubMed] [Google Scholar]
- Richardson PM, Verge VM. The induction of a regenerative propensity in sensory neurons following peripheral axonal injury. J. Neurocytol. 1986;15:585–594. doi: 10.1007/BF01611859. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Bartolome J. Role of ornithine decarboxylase and the polyamines in nervous system development: a review. Brain Res. Bull. 1986;17:307–320. doi: 10.1016/0361-9230(86)90236-4. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ, Trepanier PA, Whitmore WL, Lerea L, Barnes GA, et al. Ornithine decarboxylase and polyamines in tissues of the neonatal rat: effects of alpha-difluoromethylornithine, a specific, irreversible inhibitor of ornithine decarboxylase. J. Pharmacol. Exp. Ther. 1982;222:741–745. [PubMed] [Google Scholar]
- Song HJ, Ming GL, Poo MM. cAMP-induced switching in turning direction of nerve growth cones. Nature. 1997;388:275–279. doi: 10.1038/40864. [DOI] [PubMed] [Google Scholar]
- Song H, Ming G, He Z, Lehmann M, McKerracher L, Tessier-Lavigne M, et al. Conversion of neuronal growth cone responses from repulsion to attraction by cyclic nucleotides. Science. 1998;281:1515–1518. doi: 10.1126/science.281.5382.1515. [DOI] [PubMed] [Google Scholar]
- Wang KC, Kim JA, Sivasankaran R, Segal R, He Z. P75 interacts with the Nogo receptor as a co-receptor for Nogo, MAG and OMgp. Nature. 2002a;420:74–78. doi: 10.1038/nature01176. [DOI] [PubMed] [Google Scholar]
- Wang KC, Koprivica V, Kim JA, Sivasankaran R, Guo Y, Neve RL, et al. Oligodendrocyte-myelin glycoprotein is a Nogo receptor ligand that inhibits neurite outgrowth. Nature. 2002b;417:941–944. doi: 10.1038/nature00867. [DOI] [PubMed] [Google Scholar]
- Wolff J. Promotion of microtubule assembly by oligocations: cooperativity between charged groups. Biochemistry. 1998;37:10722–10729. doi: 10.1021/bi980400n. [DOI] [PubMed] [Google Scholar]
- Wong ST, Henley JR, Kanning KC, Huang KH, Bothwell M, Poo MM. A p75 (NTR) and Nogo receptor complex mediates repulsive signaling by myelin-associated glycoprotein. Nat. Neurosci. 2002;5:1302–1308. doi: 10.1038/nn975. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Higuchi H, Tohyama M. The p75 receptor transduces the signal from myelin-associated glycoprotein to Rho. J. Cell Biol. 2002;157:565–570. doi: 10.1083/jcb.200202010. [DOI] [PMC free article] [PubMed] [Google Scholar]