Abstract
The topography and phenotype of mast cells in the human area postrema, together with correlation between mast-cell density and microvessel density (MVD), were analysed in 16 brains. Transverse serial sections of formalin-fixed, paraffin-embedded brainstems were stained with toluidine blue and alcian blue/safranin stainings, and with anti-tryptase and anti-CD31 monoclonal antibodies. The mean (± SD) numbers of mast cells per section were 1.3 ± 0.8 and 1.2 ± 0.7 with toluidine blue and alcian blue/safranin, respectively, whereas anti-tryptase monoclonal antibody showed a mean of 5.1 ± 2.4 cells. Mast cells were alcian blue- and safranin-positive in 56%, because of the coexistence of low-sulphated (blue-staining) and high-sulphated (red-staining) granules. No significant linear correlation between mast-cell density (4.9 mm−2) and MVD (114.5 mm−2) was found (r2 = 0.19, P = 0.09). Mast cells were frequently located close to blood vessels (55%) (33% to venules, 22% to arterioles), indicating that their products play a role in the regulation of blood flow and in vessel permeability in the area postrema. Mast cells were located subependymally in 44% and close to the dorsal aspect of the nucleus of the tractus solitarius in 31%, suggesting a subregional distribution.
Keywords: alcian blue/safranin, anti-tryptase, human, microvessel density, medulla
Introduction
The area postrema is important as a relay in mammalian brain for taste aversion and vomiting reflexes, through µ opioid, 5HT3 and H2 receptor binding (Bhargava et al. 1976; Gutstein & Akil 2001; Japundzic-Zigon et al. 1997). Moreover, many peptides, such as angiotensin, adrenomedullin, endothelin and endorphin, regulate cardiovascular function (Szilagyi & Ferrario, 1981; Sander et al. 1989; Allen et al. 1997; Nakayama et al. 1997) by binding on receptors located in the area postrema, the regulatory function of which is mediated through efferent projections towards other medullary centres (Cai et al. 1996; Tian & Hartle, 1994; Chen & Bonham, 1998).
The area postrema is located in the caudal fourth ventricle floor (Fig. 1a–e). In rodents, it is a single midline structure at the apex of the calamus scriptorius; in humans, it is composed of bilateral areas joining in the midline of the caudal levels. The body of the area postrema consists of a loose network of neuroglia, with small sparse neurons (Brizee & Klara, 1984). Microcirculation in the area postrema is characterized by high capillary density (526 mm−2 in the rat; Shaver et al. 1991), large pericapillary spaces and lack of a tight blood–brain barrier (Brizee & Klara, 1984; Gross, 1991). Cytoarchitectonic and neurochemical studies in rat indicate the existence of distinct anatomical and functional compartments within the area postrema, and even topographical diversity in its capillary organization (Gross et al. 1991; Shaver et al. 1991).
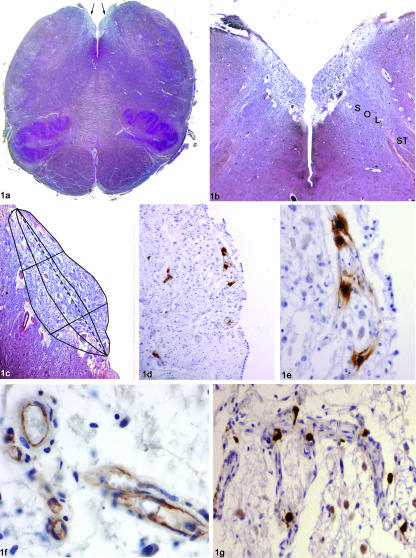
Fig. 1.
(a) Transverse section of the caudal medulla showing the separation of the right and left parts of the area postrema (arrows) as a result of the interposition of the caudal extremity of the IV ventricle floor (azan-Mallory, ×1). (b) Higher magnification of the medullary tegmentum shown in (a), demonstrating the relationship of the area postrema with the dorsal part of the nucleus of the tractus solitarius (azan-Mallory, ×2.5) (ST: solitary tract; Sol: nucleus of the tractus solitarius). (c) Schematic diagram of the subdivision of the area postrema (higher magnification of b) in the three subregions (subependymal, central and close to the nucleus of the tractus solitarius). The dotted line represents the main axis of the area postrema in the transverse section; the perpendicular lines have been subdivided into three segments of the same length corresponding to the extension of the three subregions. (d) Topography of mast cells in the area postrema, showing the possible superficial and deep (near nucleus of the tractus solitarius) location (anti-tryptase, ×4). (e) Detail of (d), showing the perivascular location of four mast cells in subependymal subregion (anti-tryptase, ×20). (f) Section stained immunohistochemically for CD31, showing positive reaction at the level of endothelial cells of small vessels in the area postrema (anti-CD31, ×40). (g) Perivascular location of mast cells along the course of small arterioles (anti-tryptase, ×10).
Mast cells in the brain have been described in particular around the third ventricle, in the thalamus, hypothalamus and leptomeninges (Dropp, 1979; Theoharides, 1990), where they are found mainly close to the blood vessels. In addition, they have been described at the level of the circumventricular organs. They may be stimulated by various neuropeptides such as somatostatin (Theoharides & Douglas, 1978), neurotensin (Rioux et al. 1985), NGF (Florenzano & Bentivoglio, 2000), IFNα (Crivellato et al. 2002), neurotransmitters such as acetylcholine (Fantozzi et al. 1978) and pharmacological compounds such as C48/80 (Ibrahim et al. 1976) to release vasoactive molecules such as histamine, serotonin, prostaglandins and leucotrienes (Theoharides, 1990; Marathias et al. 1991; Manning et al. 1994; Zhuang et al. 1996). Mast cells also secrete vascular endothelial growth factor/vascular permeability factor (VEGF/VPF), which both enhances vascular permeability and stimulates proliferation of endothelial cells (Boesiger et al. 1998; Grutzkau et al. 1998).
The aim of the present report is to provide information about topographical distribution, vascular relationships and phenotypic characteristics of mast cells in the human area postrema. The correlation between mast-cell density and microvessel density (MVD) is also analysed.
Materials and methods
We selected 16 subjects (nine male, seven female, mean age: 46 years), who had died of various causes but whose medical history was free of allergic reactions, and neurological and neurovascular pathologies. Autopsy was performed within 24 h of death. The brain was cut after fixation in 10% formalin for 15 days. Histological examination was performed on transverse serial sections, 5 µm thick, of the medulla stained with haematoxylin–eosin, Klüver-Barrera and azan-Mallory. In each case, to detect the topography and phenotype of mast cells, a total of 12 sections, collected at various levels, were stained with toluidine blue, 12 with anti-tryptase and 12 with alcian blue/safranin. Additionally, 12 sections were stained with anti-CD31 for detecting small blood vessels (Fig. 1f).
Toluidine blue staining was performed as follows: the sections were soaked for 20 min in a solution of 0.1% toluidine blue (Croma, Gesellschaft, Schmid Gmbh & Co, Germany), in a solution of Veronal (5 mL solution of sodium citrate 9.7 g and sodium diethylbarbiturate 14.7 g in deionized H2O to 500 mL + 16 mL HCl 0.1 n + 2 mL deionized H2O) at pH 2.5, and then drained from the staining solution, dehydrated in 100% ethanol for 20–40 s, dipped in xylene and cover-slipped.
Alcian blue/safranin staining was performed as follows: the sections were first stained in alcian blue (Alcian Blue 8GS, Gesellschaft, Schmid Gmbh & Co; 10 mg mL−1 in 0.7 n HCl, pH 0.3, for 40 min at room temperature), followed by counterstaining with safranin (Safranin, Merck, Germany; 5 mg mL−1 in 0.125 n HCl, 2.5 min at room temperature) (Zhuang et al. 1999). The percentages of differential histochemical characteristics of mast cells in the area postrema were calculated. To evaluate different staining properties with alcian blue/safranin, one-way anova and Tukey's multiple comparison tests were performed.
For immunohistochemical study, sections were hydrated gradually through decreasing concentrations of ethanol and then washed in deionized H2O. Antigen unmasking was performed through trypsin treatment. Sections were incubated in 1% hydrogen peroxide in deionized H2O to remove endogenous peroxidase activity and enhance antibody penetration into the tissue, and then washed in 0.01 m phosphate-buffered saline (PBS). Sections were then incubated for 30 min in blocking serum to eliminate non-specific binding. To detect tryptase, a monoclonal antibody raised against human tryptase (DAKO, Italy) was used, together with blocking serum, at a dilution of 1 : 200 in PBS. To detect CD31, a monoclonal antibody (DAKO) was used, at a dilution of 1 : 50 in PBS. After primary antibody incubation, sections were incubated with anti-mouse serum (Envision, DAKO) and placed in 3,3′-diaminobenzidine (DAB, Sigma, Italy) containing H2O2. Finally, they were counterstained with haematoxylin. Sections incubated without the primary antibody showed no immunoreactivity, confirming the specificity of the immunostaining.
Mast cells were counted in each section, and then the mean number of mast cells per section was calculated in each case. The mean number of mast cells per section and the standard deviation were calculated for all the individuals and, separately, for the male and female individuals. An unpaired Student's t-test was performed to evaluate if there was any relation to gender.
Mast cell density in the area postrema was calculated by dividing the number of mast cells by the area, obtained with the help of image analysis software (Qwin Leica Imaging System, Cambridge, UK). For each section, vascular structures with a defined lumen or well-defined linear vessel shape were counted and then divided by the area to determine MVD. The mean MVD was calculated for each case and for the entire sample. Statistical analysis of the linear correlation between mast-cell density and MVD was also performed (P < 0.05 was considered to be statistically significant).
The topographical relationship of mast cells with vasculature was analysed – in each case and then for all cases (Fig. 1g). Topographic relationship with vessels was analysed by placing, using image analysis software (Qwin Leica Imaging System), around each cell a circle of diameter corresponding to three diameters of the cell itself – if a vascular structure was present inside the circle the mast cell was considered perivascular. The percentages of mast cells located near vessels and with no relationships with them were calculated. Differences between perivascular and non-perivascular locations were compared using a paired Student's t-test and considering P < 0.05 as statistically significant. The vascular structures near mast cells were then classified as venules or arterioles on the basis of the higher ratio between diameter of the lumen and thickness of the vessel wall and lack of muscle cells in the post-capillary venules compared with arterioles. The percentages of mast cells close to venules or arterioles were calculated. To evaluate differences in vascular topographical relationships (near arterioles, venules or with no relationships) one-way anova and Tukey's multiple comparison tests were performed.
In addition, in each section, the subregional location of mast cells in the area postrema (i.e. subependymal, central or close to the nucleus of the tractus solitarius (NTS) was evaluated. In order to subdivide the area postrema into three regions from transverse sections, a contour of the area postrema was delineated and a reference axis was obtained connecting the anteromedial and posterolateral extremities of its ependymal aspect. Lines perpendicular to this axis were subdivided into three segments of the same length and the surface of the area postrema was subdivided into three subregions by two lines passing through the identified points (Fig. 1c–e). The percentages of mast cells located in the different subregions were calculated in each case and in the series. To evaluate differences in topographical location, one-way anova and Tukey's multiple comparison tests were performed. P < 0.05 was considered to be statistically significant.
Results
In toluidine-blue-stained sections, mast cell granules were light purple in colour, and extruded granules were not visible, so that only fully granulated mast cells were detectable. In some sections, no mast cells were visible. The mean number of mast cells per section of area postrema in the series was 1.3 ± 0.8 (1.3 ± 0.8 in males, 1.3 ± 0.8 in females). There were no mast cells visible in 31% of the sections.
The mean number of mast cells per section identified by alcian blue/safranin was 1.2 ± 0.7 (1.2 ± 0.7 in males, 1.1 ± 0.7 in females). Fifty-six per cent of mast cells were mixed alcian-blue-positive and safranin-positive with the coexistence of red and blue granules (Fig. 2a), 25% were safranin-positive and alcian-blue-negative and 19% were alcian-blue-positive and safranin-negative. One-way anova confirmed that the differences in staining properties were statistically significant (P < 0.0001). Tukey's test revealed a significant difference when comparing alcian-blue- and safranin-positive mast cells with alcian-blue-positive and safranin-negative (P < 0.001) and with alcian-blue-negative and safranin-positive (P < 0.001).
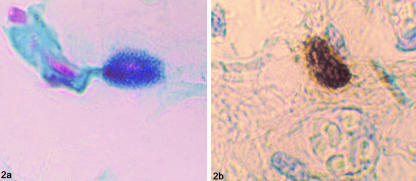
Fig. 2.
(a) Coexistence of alcian-blue-(low-sulphated glycosaminoglycans) and safranin-positive (high-sulphated glycosaminoglycans) granules giving a dark violet colour to the mast cell cytoplasm. Note the proximity with a small pale-blue stained vessel on the left (alcian blue/safranin, ×100). (b) Intense anti-tryptase reactivity of mast cell cytoplasmic granules (anti-tryptase, ×100).
With anti-tryptase monoclonal antibody, there was intense immunoperoxidase staining of mast cell granules (Fig. 2b). Mast cells appeared as round or oval-shaped cells, the stained granules of which often masked the centrally located nucleus, weakly counterstained with haematoxylin. In some specimens, partially degranulated mast cells were seen with tryptase-staining extruded granules. No background staining was seen in the sections. The mean number of mast cells per section in the series was 5.1 ± 2.4 (5.1 ± 2.5 in males, 5.1 ± 2.3 in females). There were no mast cells visible in 10% of the sections. The mean number of mast cells per section with the three staining techniques was not related to gender.
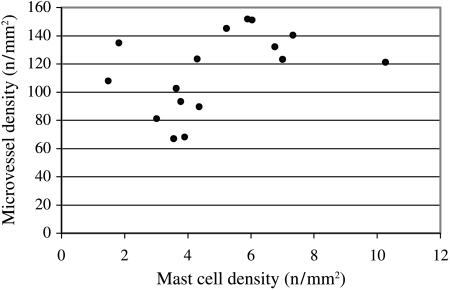
The mean mast-cell density and MVD for single cases are shown in Fig. 3. The mean mast-cell density in the entire sample was 4.9 mm−2. The mean MVD was 114.5 mm−2. No significant statistical correlation between mast-cell density and MVD was found (r2 = 0.19, P = 0.09).
Fig. 3.
Diagram showing the mean mast cell density and microvessel density in the single cases. Analysis of linear correlation revealed no statistical significance (r2 = 0.19, P = 0.09).
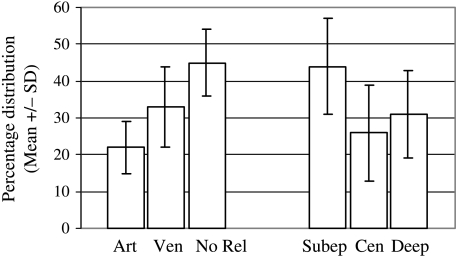
Mast cells were frequently located just outside the basement membrane of the small vessels (55 ± 9%), close to the venules (33 ± 11%) and close to the arterioles (22 ± 7%). No evident relationship with a vessel was detected in 45 ± 9% (Fig. 4). A paired Student's t-test confirmed the preferential location of mast cells near blood vessels (P = 0.029). One-way anova revealed that the differences in topographical distribution (near arterioles, venules or without vascular relationships) were statistically significant (P < 0.0001). Tukey's test revealed significant differences when comparing all the groups.
Fig. 4.
Diagram showing the topographic distribution of mast cells with reference to vascular relationships, i.e. close to arterioles (Art), venules (Ven) or with no vascular relationships (No Rel), and with reference to subregional division – subependymal (Subep), central (Cen) and close to the nucleus of the tractus solitarius (Deep). Values are expressed as mean ± SD.
With regard to topographical distribution, mast cells were located in the subependymal zone in 44 ± 13%, close to the NTS in 31 ± 12%, and in the central zone of the area postrema in 26 ± 13% (Fig. 4). One-way anova confirmed that the differences in topographical distribution were statistically significant (P = 0.001). Tukey's test revealed significant differences when comparing mast cells located subependymally with those located centrally (P < 0.001) and with those located close to the NTS (P < 0.01). No differences were found in the distribution of mast cells between the rostral and caudal levels of the area postrema.
Discussion
In the central nervous system, mast cells have been identified in the thalamus, hypothalamus, hypophysis, epiphysis, meninges and circumventricular organs (Dropp, 1979). In sections of the area postrema stained with cresyl violet, Dropp (1979) found up to five mast cells per section. In our study, although no mast cells were visible in some sections (10% with anti-tryptase, 31% with toluidine blue), the mean number per section was 1.3 ± 0.8 with toluidine blue, 1.2 ± 0.7 with alcian blue/safranin, and 5.1 ± 2.4 with anti-tryptase. Thus, the three different identifying stains produced a discrepancy in the detection of mast cells. With toluidine blue and alcian blue/safranin stains, only fully granulated mast cells are detectable because staining is less intense and extruded granules are not visible. Anti-tryptase immunohistochemical staining is the most sensitive method, because it also reveals partially degranulated mast cells with extruded granules, and it is also highly specific in that tryptase is a serine protease selectively expressed in significant amounts only in mast cells, whereas in blood basophils it is present only in trace amounts (Samorapoompichit et al. 2001).
The presence of mast cells in the area postrema may be ascribed to the fact that they migrate in and out through the incomplete blood–brain barrier, as demonstrated by Silverman et al. (2000) at the level of the rat thalamus.
The functions of mast cells in the area postrema are not yet fully understood but are suggested by their histochemical characteristics and local topographical distribution. Based on different tissue location, staining properties and functional characteristics, two types of mast cells have been described, mucosal and serosal (Dimitriadou et al. 1990). Mucosal mast cells are present in the skin and respiratory or gastrointestinal mucoses; serosal mast cells have been described in peritoneal and pleural folds. Mast cells may have differing granular contents, which result in heterogeneous histochemical characteristics when stained with alcian blue/safranin. The combination of these two dyes can distinguish mucosal (blue-staining) mast cells, containing low-sulphated glycosaminoglycans, from serosal (red-staining) mast cells, containing high-sulphated glycosaminoglycans (Zhuang et al. 1999). Mucosal mast cells contain both tryptase and chimase, and have large amounts of lipids in the granules, whereas serosal mast cells do not contain chimase and have only a few lipids (Irani, 1986; Fineschi et al. 2001). Little is known about the histochemical and functional phenotype of brain mast cells. Rat thalamic mast cells are characterized by red granules when stained with alcian blue/safranin, but they do not share all the histochemical characteristics of serosal mast cells, in that they also contain considerable amounts of lipids (Dimitriadou et al. 1990). Variations in the staining properties of mast cells with ageing have also been described in studies conducted in ring dove forebrain. Zhuang et al. (1999) found a switch from alcian-blue granules in young animals to mixed alcian-blue and safranin granules in older animals.
In our study, although most mast cells contained both low-sulphated and high-sulphated granules (56%) (Fig. 2a), in 25% only high-sulphated and in 19% only low-sulphated granules were present, indicating a certain histochemical heterogeneity.
Mast-cell density and MVD were analysed and correlated, as there is some evidence that mast cells play a crucial role in angiogenesis, for instance through the secretion of VEGF/VPF; on the other hand, mast cell migration could be favoured by high MVD. In our study, however, no statistically significant linear correlation was found between mast-cell density and MVD. This lack of correlation may be due to an additive action of other unknown factors on mast-cell density and MVD.
Brain mast cells are located mainly around the blood vessels in both animals (Ibrahim et al. 1996; Florenzano & Bentivoglio, 2000) and humans (Dropp, 1979). Our study confirms the close proximity of mast cells to blood vessels in the human area postrema (Fig. 1g). Mast cells were close to venules in 33% and to arterioles in 22%. In 45%, mast cells were not close to visible vessels, but appeared to be related to neurons, suggesting the presence of two mast-cell populations with possibly different functions.
The perivascular location of mast cells suggests that their vasoactive molecules (histamine, serotonin, prostaglandins, leucotrienes) play a role in the regulation of blood flow in this region. The physiological characteristics of the microcirculation of the area postrema, i.e. elevated vascular volume and slow blood flow, favour the local action of cardiovascular neuropeptides, prolonging the time of interaction with specific receptors (Gross, 1991), so that mast-cell degranulation products may intervene, affecting blood flow, and also the local action of these neuropeptides.
Mast cells are known to regulate the permeability of blood vessels through VEGF/VPF (Boesiger et al. 1998; Grutzkau et al. 1998) and their close relationship with vessels in the area postrema, which is outside the blood–brain barrier, suggests that their products also play a critical role in regulating the exchanges between blood and nervous tissue.
The dorsal and medial zones of the area postrema in rats show higher capillary density with respect to the rostral ventral zone, and it has been suggested that morphological specialization limits blood-tissue communication (Shaver et al. 1991). In our study, mast cells were mainly found in the subependymal zone (44%) with respect to the central subregion (26%) and to the subregion close to the NTS (31%) (Figs 1d,e and 4), suggesting subregional specialization in the human area postrema as well.
The subependymal location of mast cells has also been described in the thalamus of ring dove after courtship (Silverman et al. 1994), and therefore mast cells may play a part in controlling the ependyma in exchanges between cerebrospinal fluid and nervous tissue. The particular topographical distribution of mast cells in the area postrema, and their relationship with the specialized structures there (specialized ependyma and vessels without a blood–brain barrier), indicates that these cells play a critical role in the physiology of the area postrema.
Acknowledgments
We are grateful to Giuliano Carlesso for his skilful technical assistance.
References
- Allen MA, Smith PM, Ferguson AV. Adrenomedullin microinjection into the area postrema increases blood pressure. Am. J. Physiol. 1997;272:R1698–R1703. doi: 10.1152/ajpregu.1997.272.6.R1698. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Bhargava KP, Dixit KS, Palit G. Nature of histamine receptors in the emetic chemoreceptor trigger zone. Br. J. Pharmacol. 1976;57:211–213. doi: 10.1111/j.1476-5381.1976.tb07469.x. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, et al. Mast cells can secrete vascular permeability factor/vascular endothelial cell growth factor and exhibit enhanced release after Immunoglobulin E-dependent upregulation of Fcɛ receptor I expression. J. Exp. Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizee KR, Klara PM. The structure of the mammalian area postrema. Fed. Proc. 1984;43:2944–2948. 10.1111/j.1469-7580.2004.00256.x. [PubMed] [Google Scholar]
- Cai Y, Hay M, Bishop VS. Synaptic connections and interactions between area postrema and nucleus tractus solitarius. Brain Res. 1996;724:121–124. doi: 10.1016/0006-8993(96)00282-x. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Chen CY, Bonham AC. Non-NMDA and NMDA receptors transmit area postrema input to aortic baroreceptor neurons in NTS. Am. J. Physiol. 1998;275:1695–1706. doi: 10.1152/ajpheart.1998.275.5.H1695. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Crivellato E, Candussio L, Mallardi F, Ribatti D. Recombinant human alpha-2a interferon promotes an atypical process of mast cell secretion with ultrastructural features suggestive for piecemeal degranulation. J. Anat. 2002;201:507–512. doi: 10.1046/j.1469-7580.2002.00116.x. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitriadou V, Lambracht-Hall M, Reichler J, Theoharides TC. Histochemical and ultrastructural characteristics of rat brain perivascular mast cells stimulated with compound 48/80 and carbachol. Neuroscience. 1990;39:209–224. doi: 10.1016/0306-4522(90)90234-u. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Dropp JJ. Mast cells in the human brain. Acta Anat. 1979;105:505–513. doi: 10.1159/000145157. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Fantozzi R, Masini E, Blandina P, Mannaioni PF, Bani-Sacchi T. Release of histamine from rat mast cells by acetylcholine. Nature. 1978;273:473–474. doi: 10.1038/273473a0. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Fineschi V, Cecchi R, Centini F, Reattelli LP, Turillazzi E. Immunohistochemical quantification of pulmonary mast-cells and post-mortem blood dosages of tryptase and eosinophil cationic protein in 48 heroin-related deaths. Forensic Sci. Int. 2001;120:189–194. doi: 10.1016/s0379-0738(00)00469-2. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Florenzano F, Bentivoglio M. Degranulation, density, and distribution of mast cells in the rat thalamus: a light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J. Comp. Neurol. 2000;424:651–669. 10.1111/j.1469-7580.2004.00256.x. [PubMed] [Google Scholar]
- Gross PM. Morphology and physiology of capillary systems in subregions of the subfornical organ and area postrema. Can. J. Physiol. Pharmacol. 1991;69:1010–1025. doi: 10.1139/y91-152. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Gross PM, Wall KM, Wainman DS, Shaver SW. Subregional topography of capillaries in the dorsal vagal complex of rats. II. Physiological properties. J. Comp. Neurol. 1991;306:83–94. doi: 10.1002/cne.903060107. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwartz C, Kogel H, Welker P, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol. Biol. Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutstein H, Akil H. Opioid analgesics. In: Hardman JG, Limbird LE, Gilman AG, editors. Goodman and Gillman's the Pharmacological Basis of Therapeutics. New York: McGraw-Hill Professional; 2001. pp. 569–621. 10.1111/j.1469-7580.2004.00256.x. [Google Scholar]
- Ibrahim MZ, Reder AT, Lawand R, Takash W, Sallouh-Khatib S. The mast cells of the multiple sclerosis brain. J. Neuroimmunol. 1996;70:131–138. doi: 10.1016/s0165-5728(96)00102-6. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Ibrahim MZ, Tenekjian V, Ulthman MAA. The effect of compound 48/80 on the perivascular granular cells (neurolipomastocytes of the rabbit brain) Anat. Rec. 1976;184:434. [Google Scholar]
- Irani AA. Two types of human mast cells subsets that have distinct neutral protease compositions. Proc. Natl-Acad. Sci. USA. 1986;83:4464–4468. doi: 10.1073/pnas.83.12.4464. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Japundzic-Zigon N, Samardzic R, Beleslin DB. Clonidine-induced emesis: a multitransmitter pathway. Pharmacol. Res. 1997;35:287–297. doi: 10.1006/phrs.1997.0133. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Manning KA, Pienkowski TP, Uhlrich DJ. Histaminergic and non-histamine-immunoreactive mast cells within the cat lateral geniculate complex examined with light and electron microscopy. Neuroscience. 1994;63:191–206. doi: 10.1016/0306-4522(94)90016-7. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Marathias K, Lambracht-Hall M, Savala J, Theoharides TC. Endogenous regulation of rat brain mast cell serotonin release. Int. Arch. Allergy Appl. Immunol. 1991;95:332–340. doi: 10.1159/000235470. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Nakayama Y, Takano Y, Eguchi K, Migita K, Saito R, Tsujimoto G, et al. Modulation of the arterial baroreceptor reflex by the vasopressin receptor in the area postrema of the hypertensive rats. Neurosci. Lett. 1997;226:179–182. doi: 10.1016/s0304-3940(97)00274-7. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Rioux F, Kerouac R, St-Pierre S. Release of mast cell mediators, vasoconstriction and edema in the isolated perfused head of the rat following intracarotid infusion of neurotensin. Neuropeptides. 1985;6:1–12. doi: 10.1016/0143-4179(85)90125-8. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Samorapoompichit P, Kiener HP, Schernthaner GH, Jordan JH, Agis H, Wimazal F, et al. Detection of tryptase in cytoplasmatic granules of basophils in patients with chronic myeloid leukemia and other myeloid neoplasms. Blood. 2001;98:2580–2583. doi: 10.1182/blood.v98.8.2580. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Sander GE, Lowe RF, Given MB, Giles TD. Interaction between circulating peptides and the central nervous system in hemodynamic regulation. Am. J. Cardiol. 1989;64:44–50. doi: 10.1016/0002-9149(89)90683-8. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Shaver SW, Pang JJ, Wall KM, Sposito NM, Gross PM. Subregional topography of capillaries in the dorsal vagal complex of rats. II. Morphometric properties. J. Comp. Neurol. 1991;306:73–82. doi: 10.1002/cne.903060106. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Silverman AJ, Miller RP, King JA, Zhuang X, Silver R. Mast cells with gonadotropin-releasing hormone-like immunoreactivity in the brain of doves. Proc. Natl-Acad. Sci. USA. 1994;91:3695–3699. doi: 10.1073/pnas.91.9.3695. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Sutherland AK, Wilhelm M, Silver R. Mast cells migrate from blood to brain. J. Neurosci. 2000;20:401–408. doi: 10.1523/JNEUROSCI.20-01-00401.2000. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szilagyi JE, Ferrario CM. Central opiate system modulation of the area postrema pressor pathway. Hypertension. 1981;3:313–317. doi: 10.1161/01.hyp.3.3.313. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Theoharides TC, Douglas WW. Somatostatin induces secretion from rat peritoneal mast cells. Endocrinology. 1978;102:1143–1145. doi: 10.1210/endo-102-5-1637. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Theoharides TC. Mast cells: the immune gate to the brain. Life Sci. 1990;46:607–617. doi: 10.1016/0024-3205(90)90129-f. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Tian B, Hartle DK. Cardiovascular effects of NMDA and MK-801 infusion at area postrema and mNTS in rat. Pharmacol. Biochem. Behav. 1994;49:489–495. doi: 10.1016/0091-3057(94)90060-4. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Brain mast cell degranulation regulates blood–brain barrier. J. Neurobiol. 1996;31:393–403. doi: 10.1002/(SICI)1097-4695(199612)31:4<393::AID-NEU1>3.0.CO;2-4. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]
- Zhuang X, Silverman AJ, Silver R. Distribution and local differentiation of mast cells in the parenchyma of the forebrain. J. Comp. Neurol. 1999;408:477–488. doi: 10.1002/(sici)1096-9861(19990614)408:4<477::aid-cne3>3.0.co;2-o. 10.1111/j.1469-7580.2004.00256.x. [DOI] [PubMed] [Google Scholar]