Abstract
The modern human bony labyrinth is morphologically distinct from that of all other primates, showing derived features linked with vestibular function and the overall shape of the cranial base. However, little is known of how this unique morphology emerges prenatally. This study examines in detail the developing fetal human labyrinth, both to document this basic aspect of cranial biology, and more specifically, to gain insight into the ontogenetic basis of its phylogenetically derived morphology. Forty-one post-mortem human fetuses, ranging from 9 to 29 weeks gestation, were investigated with high-resolution magnetic resonance imaging. Quantitative analyses of the labyrinthine morphology revealed a number of interesting age-related trends. In addition, our findings show that: (1) the prenatal labyrinth attains an adult equivalent size between 17 and 19 weeks gestation; (2) within the period investigated, shape changes to all or most of the labyrinth cease after the 17–19-week size maturation point or after the otic capsule ossifies; (3) fetal cochlea development correlates with the surrounding petrosal morphology, but not with the midline basicranium; (4) gestational age-related rotations of the ampullae and cochlea relative to the lateral canal, and posterior canal torsion are similar to documented phylogenetic trends whereas other trends remain distinct. Findings are discussed in terms of the ontogenetic processes and mechanisms that most likely led, in part, to the emergence of the phylogenetically derived adult modern human labyrinth.
Keywords: basicranium, cochlea, ontogeny, phylogeny, semicircular canals
Introduction
The bony labyrinth inside the petrous temporal bone houses the inner ear, including the sense organ of hearing in the cochlea, and that of balance in the vestibule and the semicircular canals. Comparative analyses have demonstrated that the bony labyrinth of modern humans is morphologically distinct from that of other primates (Hyrtl, 1845; Gray, 1907; Delattre & Fenart, 1960, 1961, 1962; Spoor, 1993; Spoor & Zonneveld, 1998). Differences in the size of the semicircular canals are thought to relate functionally to sensory control of modern human bipedal locomotion, whereas general shape differences appear to reflect aspects of the strongly derived human cranial base (Spoor & Zonneveld, 1998; Spoor, 2003). The evolutionary history of these derived labyrinthine features has become increasingly well understood through the radiological examination of fossil hominid temporal bones (Spoor, 1993; Spoor & Zonneveld, 1994; Spoor et al. 1994, 1996, 2003; Hublin et al. 1996).
Studies assessing the human labyrinth from a comparative and evolutionary perspective suggest that it attains its adult morphology before birth, so that direct comparison can be made between adult and juvenile specimens (Spoor, 1993; Hublin et al. 1996; Spoor et al. 2002b, 2002c). Moreover, it is argued that the labyrinth may constitute a better representation of the genotypic make-up of an individual than do most other skeletal parts, because postnatal influences on the morphology by environmental or behavioural factors are minimal or absent. The notion that the human labyrinth matures prenatally is largely based on the observation that ossification of the surrounding otic capsule is completed during the sixth month in utero (Bast, 1930). In addition, some studies report that late fetal and neonatal labyrinths are morphologically similar to adult ones (Siebenmann, 1890; Schönemann, 1906). Others nevertheless maintain that temporal changes do occur well into adulthood (Hyrtl, 1845; Tremble, 1929; Sercer & Krmpotic, 1958). These contradictory views are based on samples that are limited in size and age range, and observations that are not tested statistically. Consequently, it can be concluded that little is known of any significant morphological changes after the labyrinth is formed in the embryonic phase of development. We therefore performed a comprehensive morphometric analysis of the developing fetal human labyrinth, both to document this basic aspect of cranial biology, and more specifically, to gain insight into the ontogenetic basis of its phylogenetically derived morphology. Whereas previous studies are based on corrosion casts and histological sections, we used high-resolution magnetic resonance imaging (hrMRI) to visualize and quantify the fetal morphology (Jeffery & Spoor, 2002; Jeffery, 2002). Before reviewing earlier reports on ontogenetic change of the human labyrinth, and outlining the specific questions that will be addressed in this study, we will briefly summarize the features of the adult bony labyrinth that distinguish humans from other primates (see Spoor & Zonneveld, 1998, for a full account).
Characteristics of the human labyrinth
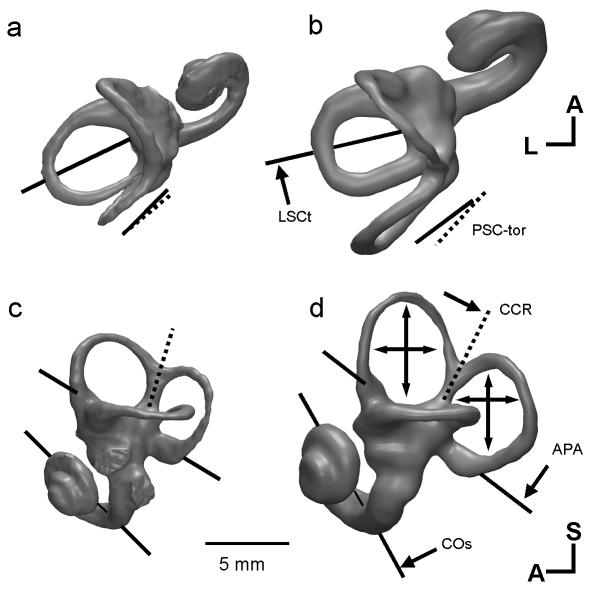
Comparison will be made primarily with the extant great apes (Fig. 1), as these are the human's phylogenetically closest living relatives. However, most characters distinguish humans from other primates as well. Among species, there is a small, negative allometric increase of the adult size of the labyrinth with body mass (Jones & Spells, 1963; Spoor & Zonneveld, 1998; Spoor et al. 2002b). With this scaling effect taken into account, modern humans have anterior and posterior semicircular canals that have a larger arc size than the great apes (Fig. 1). By contrast, the arc of the lateral canal of humans is marginally smaller, and their cochlea is similar in size.
Fig. 1.
The major differences between the labyrinth of humans and great apes: CT-based 3D reconstructions, showing superior (a,b) and lateral (c,d) views of the left bony labyrinth of a female gorilla (a,c) and a modern human (b,d). In lateral view the planes of the lateral canal are aligned. Differences of orientation are indicated by single-headed arrows and differences of canal size are shown by double-headed arrows. The gorilla labyrinth is shown at 97% of its actual size to compensate for the difference in body mass (scaling follows Spoor et al. 2002b). Measurement codes as listed in Table 1 and shown in Fig. 2. A, anterior; L, lateral; S, superior.
Seen in superior view (Fig. 1a,b), the planar orientations of the anterior and posterior canals are similar in humans and other primates. However, the axis of symmetry of the arc of the lateral canal is orientated more coronally in humans, so that the vertex of this canal is directed more laterally. This morphology appears to follow the more coronal orientation of the petrous temporal bone in humans than in other primates. The superior view also demonstrates that the torsion of the posterior canal differs in humans and the great apes. In humans, the superior limb of this canal is orientated more coronally than the inferior limb, whereas this is the reverse in great apes.
In lateral view (Fig. 1c,d), aspects of the human labyrinth, including the common crus and the cochlea, have a rotated orientation relative to the plane of the lateral canal. This rotation is clockwise when viewing the lateral aspect of the left labyrinth. Among primate species, the plane of the lateral canal tends to align itself with the anterior cranial base, whereas the other parts follow the orientation of aspects of the posterior cranial base, including the basioccipital, foramen magnum and the posterior surface of the petrous temporal bone. Hence, in humans the typically rotated morphology of the labyrinth in lateral view appears to mirror the strongly rotated, i.e. flexed, cranial base.
Spoor (1993) and Spoor & Zonneveld (1998) have suggested that the unique shape of the human labyrinth is the consequence of phylogenetic remodelling of the cranial base to accommodate the greatly expanded human brain. In this process the petrous bones reorientate coronally, and rotate (pitch) with the increased flexion of the posterior cranial base. The labyrinth follows these changes to the petrous bone, with the important exception that the planar orientations of the semicircular canals are conserved, because of functional constraints. Hypothetically, this model could have an ontogenetic basis, with species-specific differential patterns of brain growth influencing the fetal cranial base, including the labyrinth up to the moment the otic capsule ossifies (Spoor, 1993; Spoor & Zonneveld, 1998).
Ontogeny of the human labyrinth
There has been a marked advance in recent decades in our understanding of the early development of the labyrinth, from the underlying molecular signalling involved to cellular differentiation and to actual morphogenesis (Van de Water & Represa, 1991; Noden & Van de Water, 1992; Martin & Swanson, 1993; Fritzsch et al. 1998; Mazan et al. 2000; Boyl et al. 2001). Derived from the otic placodes situated on either side of the embryonic hindbrain (Anniko, 1983), the otic vesicles each differentiate into a superior vestibular part and an inferior cochlear part. The cochlear part gradually winds around its own axis towards the vestibular part, typically completing 2½ windings by the tenth week in utero (Streeter, 1917). The vestibular apparatus develops earlier than the cochlear part and grows at a faster rate. The semicircular canals rapidly emerge as disc-like out-pocketings from the vestibular part (Arnold & Lang, 2001), and attain their general form by about the eighth week in utero (Streeter, 1918). Growth differences are evident among the three canals. In particular, the lateral canal arises later and at a slower rate than the anterior or posterior canal (Rinkwitz et al. 2001).
Comparisons of the actual morphology of the fetal, juvenile and adult labyrinth in humans have been made largely based on corrosion casts, a technique that limits the fetal period that is assessed to after the ossification of the otic capsule. Hyrtl (1845) concluded that the semicircular canals show a constant increase in length, even in later periods of adult life, and Tremble (1929) came to the same conclusion for the postnatal period. By contrast, Siebenmann (1890) and Schönemann (1906) did not find any postnatal changes in labyrinthine morphology, including the size of the canals. Neither Turkewitsch (1930) nor Sato (1903) observed size differences between third trimester fetal, neonatal and adult canals either. However, the former study reports a small increase in cochlea size, and the latter that the lateral canal rotates laterally in association with the growth of external acoustic meatus and mastoid process. Sercer (1958) suggests that, compared with neonates, the adult labyrinth has canals with increased torsion, and a cochlea that is more ‘rolled-up’. Moreover, Sercer & Krmpotic (1958) explicitly state that the labyrinth changes continuously from fetus until after adolescence, and that these ontogenetic changes are a recapitulation of a similar phylogenetic transformation. By contrast, Sato et al. (1991, 1993), using histological sections, found no postnatal changes in the length of the cochlear turns, and the torsion or angles of the canal planes. Finally, Spoor (1993) used computer tomography (CT) scans to make a preliminary comparison between the labyrinths of a few fetal specimens with ossified otic capsules, some infants and a large sample of adults. He found a small, but statistically significant difference between the fetal and adult samples in the orientation of the cochlea relative to the vestibule, when viewed superiorly. By contrast, no differences were observed between the infant and adult labyrinths.
Apart from the morphology of the labyrinth itself, a few studies have also investigated its spatial orientation in the cranium during ontogeny. Bossy & Gaillard de Collogny (1965) studied changes of the labyrinth in the transverse plane in 12 fetuses of between 11 weeks and birth, and report on the reorientations of the long axis of the labyrinth, the anterior canal and the cochlea. Spoor (1993) compared angles between the planar orientation of the lateral canal and aspects of the cranial base in small numbers of late fetal and infant specimens with those in substantial adult samples, based on original measurements as well as values reported in Cousin (1969) and Pellerin (1983). No difference was found between these samples for the angle to the anterior cranial base, whereas angles to the posterior cranial base did differ with age. Spoor (1993) therefore suggested that the lateral canal has a stable orientation to the anterior base, not only phylogenetically among primates, but also in ontogeny (at least for the fetal period after ossification of the otic capsule). Whether other parts of the labyrinth, such as the common crus and the cochlea, correlate with the posterior cranial base during ontogeny, as has been found interspecifically for adult primates (Spoor & Zonneveld, 1998), has not been investigated. In all, insufficient empirical evidence is currently available to assess the ontogenetic model that differential integration of the cartilagenous otic capsule and the surrounding fetal cranial base underlies the unique shape of the human labyrinth, and forms a mechanism of phylogenetic change.
Questions
This study primarily aims to document general, growth-related changes of the human fetal labyrinth. In addition, by adjusting for the impact of growth, the study also aims to resolve distinct ontogenetic patterns of change relating to the process of otic capsule ossification and to restructuring of the basicranium. With regard to the latter, the present study examined its potential role as a proximate mechanism driving the pattern of change in the fetal labyrinth. However, it is worth noting that basicranial restructuring is in itself a pattern of change driven by a process, possibly brain enlargement (Spoor, 1997; Jeffery & Spoor, 2002; Jeffery, 2003). Lastly, the study proposes to draw together the ontogenetic evidence to gain insight into the factors underlying the emergence of the phylogenetically derived adult modern human labyrinth. Specific questions addressed are:
At what point in fetal growth does the labyrinth attain its adult size and shape, and is the rate of maturation the same for each part (i.e. the cochlea and each of the semicircular canals)?
What is the temporal relationship between completion of otic capsule ossification and the cessation of changes in labyrinthine morphology? Do they coincide, is morphological change halted before ossification, or does change occur after ossification? The underlying question is whether it is the process of ossification that arrests further change.
Are changes of the fetal labyrinth interrelated with those of the surrounding skull base?
Is the human labyrinth laid down as a small version of the phylogenetically derived adult morphology? If not, do fetal changes reflect the phylogenetic trend from the primitive hominin condition, as also found in the great apes, to the derived modern human condition?
Materials and methods
Sample and imaging
The sample consists of 41 late first, second and early third trimester human fetuses from the D.V. Davies museum collection at the Department of Anatomy and Developmental Biology, University College London, and collections at the Department of Anatomy, Queen Mary and Westfield College, London. The ethnic affinities of these fetuses are unknown, but are likely to reflect the populations living in the London area in the early to mid-twentieth century. The head region of each specimen was scanned with hrMRI. Details of the imaging gradients, probes and pulse sequence employed are given in detail elsewhere (Jeffery, 1999, 2002; Jeffery & Spoor, 2002). For each specimen the hrMRI acquisition matrix was maintained at 256 × 256. Imaging field of view (FOV) was adjusted according to the size of each specimen and varied from 19.2 to 115.2 mm2, giving pixel sizes of 0.075–0.45 mm. Images were acquired contiguously along the transverse (axial) plane with a slice thickness of between 0.15 and 0.90 mm, i.e. twice pixel resolution, and interpolated with a bi-cubic spline function (3Dviewnix; J. K. Udupa, Pennsylvania State University) to create isometric voxels. Voxels were reformatted into arbitrary slice planes with the Align3D plugin (J. A. Parker, Harvard University) for ImageJ (W. Rusden, National Institutes of Health). These applications were also used to acquire coordinate data for landmarks and take measurements.
To provide a more readily interpreted axis against which to assess prenatal growth and development, estimates of gestational age (EGA) were computed for each specimen with reference to biparietal diameter (BPD). Other common age proxy measures, such as crown–rump length, were not used because part of the sample consisted of only the head region. The sample ranges over the fetal period and because there are no individual studies of BPD that encompass both early first and late second trimester fetuses, ages were computed with reference to data from two separate but technically comparable studies. Ages for the larger fetuses were computed with the outer − outer BPD equation given in Chitty et al. (1994), which covers diameters of 20.8–99.3 mm (12–42 weeks). Ages for the smaller specimens not included in the Chitty range were computed with reference to the equation of Kustermann et al. (1992), which covers diameters of 1.9–24.7 mm (6–15 weeks). Ages for the few specimens in the overlap between these data (i.e. 20.8–24 mm; 12–15 weeks) were calculated as means. In each case, estimated gestational ages were computed to the nearest tenth of a week in order to preserve the distinction between specimens given by BPD. In this study, which explores a period of considerable biological variance, the descriptive value gained in employing this somewhat artificial variable outweighs the limitations of increased overall sample noise. The relative position of datum points remain the same, and thus rank correlation coefficients are also the same for both values of BPD and computed age estimates. Specimens range from 9 to 29 weeks gestation.
The fetal specimens were compared with the adult sample of 54 individuals quantitatively described in Spoor (1993) and Spoor & Zonneveld (1998). This sample was compiled to best reflect worldwide geographical variation, and the region of origin and collection numbers of the specimens are listed in appendix 5.1 of Spoor (1993).
Measurements
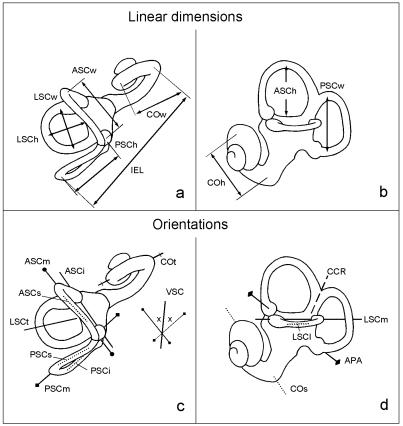
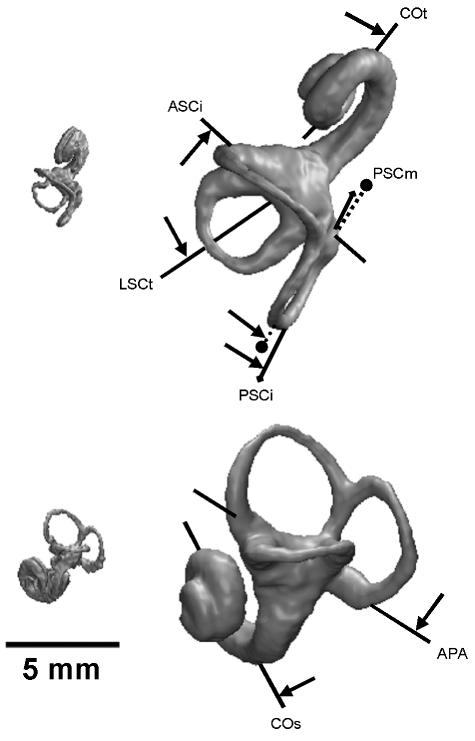
The measurements of the labyrinth are summarized in Table 1, shown in Fig. 2, and detailed definitions are given in Spoor & Zonneveld (1995). Linear measurements were taken to the nearest tenth of a millimetre, and angles to the nearest degree.
Table 1. The abbreviations in alphabetical order of the measurements of the labyrinth and the cranium.
| Linear dimensions and their derivatives | |
| ASCh | Height of the anterior semicircular canal (Fig. 2b) |
| ASCw | Width of the anterior semicircular canal (Fig. 2a) |
| COh | Height of the basal turn of the cochlea (Fig. 2b) |
| COw | Width of the basal turn of the cochlea (Fig. 2a) |
| h/w | Shape index of the arc of a semicircular canal or of the basal turn of the cochlea (height/width × 100) |
| IEL | Inner ear length measured from the posterior-most point of the arc of the posterior canal at its greatest width, to the centre of the lumen of the basal turn of the cochlea |
| LSCh | Height of the lateral semicircular canal (Fig. 2a) |
| LSCw | Width of the lateral semicircular canal (Fig. 2a) |
| PSCh | Height of the posterior semicircular canal (Fig. 2a) |
| PSCw | Width of the posterior semicircular canal (Fig. 2b) |
| R | Radius of curvature of a semicircular canal or the cochlear basal turn, measured to the centre of the lumen (R = 0.5 × [h + w]/2) |
| Orientations | |
| APA | The ampullar line, connecting the centres of the anterior and posterior ampullae, and projected onto the sagittal plane (Fig. 2d). Reflects how the vertical canals are set on to the vestibule in lateromedial view |
| ASCi | The inferior-most part of the anterior semicircular canal, defined by the line in the transverse plane connecting the apertures of the anterior ampulla and the common crus into the vestibule (Fig. 2c). Labelled as ‘V’ inSpoor & Zonneveld (1995) |
| ASCm | The arc of the anterior semicircular canal at its greatest width in the transverse plane (Fig. 2c) |
| ASCs | The superior-most part of the anterior semicircular canal in the transverse plane (Fig. 2c) |
| CCR | The common crus in the sagittal plane (Fig. 2d). |
| COs | The basal turn of the cochlea in the sagittal plane (Fig. 2d) |
| COt | The basal turn of the cochlea in the transverse plane (Fig. 2c) |
| LSCl | The lateral most part of the lateral semicircular canal in the sagittal plane (Fig. 2d) |
| LSCm | The arc of the lateral semicircular canal at its greatest width in the sagittal plane (Figs 2d and 7) |
| LSCt | The axis of symmetry of the lateral semicircular canal in transverse plane (Fig. 2c) |
| PPip | The posterior petrosal surface in the transverse plane at the level of the lateral semicircular canal |
| PSCi | The inferior limb of the posterior semicircular canal in the transverse plane (Fig. 2c) |
| PSCm | The arc of the posterior semicircular canal at its greatest width in the transverse plane (Fig. 2c) |
| PSCs | The superior limb of the posterior semicircular canal in the transverse plane (Fig. 2c) |
| SG | Intersection of the midsagittal plane of the cranium in the transverse plane |
| VSC | Reference line in the transverse plane based on the vertical semicircular canals. It bisects the angle between the arc orientations of these two canals that opens anteriorly or posteriorly (ASCm, PSCm; Fig. 2c) |
Fig. 2.
Superior (a,c) and lateral (b,d) views of the left human labyrinth, showing the measurements used in the present study (refer to Table 1).
The linear measurements of the labyrinth include the height and width of the arc of each semicircular canal and of the basal turn of the cochlea (Fig. 2a,b). The height of each canal arc is measured to the point furthest from the vestibule (the vertex), and the width perpendicular to the height. The radius of curvature (R) of each canal arc and of the cochlear basal turn was calculated by taking half the average of the height and width measurements (0.5[h + w]/2).
Angles were taken to quantify the spatial orientation of labyrinthine structures in relation to each other and in relation to aspects of the cranium. All orientations are defined in the transverse or sagittal plane (Fig. 2c,d), and angles calculated between two such orientations are therefore projected onto either of these planes. Angles are indicated by the abbreviations of the two orientations on which they are based, separated by the ‘<’ symbol. For example, ‘CCR<LSCm’ is the angle between the common crus and the lateral canal in the sagittal plane (Fig. 2d; Table 1).
Two aspects of each of the three semicircular canals are quantified using angles, the degree of torsion and the planar orientation. The arc of a semicircular canal is rarely entirely planar; rather it is nearly always somewhat twisted, showing a degree of torsion. Here the torsion of the anterior and posterior canals is quantified as the difference between the orientations of their superior-most and inferior-most parts (Fig. 2c: ASCs<ASCi; PSCs<PSCi), and that of the lateral canal as the difference between orientations of the lateral-most part and at the greatest arc width (Fig. 2d: LSCl<LSCm). The planar orientation of each canal is approximated by measuring the arc at its widest part, for the vertical semicircular canals in the transverse plane (Fig. 2c: ASCm, PSCm), and for the lateral canal in the sagittal plane (Fig. 2d: LSCm). These three canal orientations constitute the most stable, i.e. least variable, feature of the labyrinth among extant primate species, most likely because they quantify the functionally important physiological plane of optimum perceptive sensitivity (Spoor & Zonneveld, 1998). They are therefore used as reference orientations in the comparison of more diverse aspects of labyrinthine shape. The measurements of the anterior and posterior canals in the transverse plane are combined into a single reference orientation by taking the line that bisects the angle between the two canal orientations (Fig. 2c: VSC, the bisector of the angle ASCm<PSCm opening anteriorly and posteriorly). Other aspects of the labyrinth for which the orientation is measured are the axis of symmetry of the lateral canal, the common crus, the ampullar line and the cochlea (Fig. 2, Table 1: LSCt, CCR, APA, COt and COs, respectively). The vestibulocochlear line (VC), used in Spoor & Zonneveld (1998), has not been measured here because the second turn of the cochlea is not yet developed in the younger specimens of the sample, so that one of the landmarks cannot be registered.
To examine the orientation of the labyrinth in relation to the cranium, the orientation of the midsagittal plane in transverse hrMRI scans was recorded. Midsagittal images were used to measure the orientations of the lines sella to foramen caecum (S-Fc), and basion to sella (Ba-S), representing the orientations of the anterior cranial base and the basioccipital, respectively. The cross-sectional orientation of the posterior surface of the petrous pyramid was measured in a transverse scan at the level of the lateral semicircular canal (Spoor & Zonneveld, 1995, 1998: PPip). Its orientation in the sagittal plane (Spoor & Zonneveld, 1995, 1998: PPp) could not be measured in the fetal sample because its landmarks are not yet developed.
Statistics
Comparisons between means were made with Welch's approximate t-test of equality, which works well for both homoscedastic and heteroscedastic samples (Sokal & Rohlf, 1995). Associations between variables were assessed using Spearman's rank correlation coefficients with adjustment for tied ranks (PAST v1.01; Ø. Hammer & and D. A. T. Harper, University of Oslo). Partial correlation coefficients were computed with SPSS v11 (Lead Technologies Inc.). Partial coefficients of two uncorrelated variables can give false positive significance if the third, controlled, variable removes sufficient noise from the analyses. Thus, partial correlations were only carried out on variables with a significant product moment correlation. The significance (P) level of rank, product-moment and partial correlation coefficients were determined using t-tests (Sokal & Rohlf, 1995). In all statistical tests, a significance level of P = 0.05 was used to reject the null hypothesis.
The relationship between two variables was modelled with either polynomial regression or reduced major axes (RMA) regression-line fittings. Linear trends were modelled with RMA. However, when the distribution of datum points appeared curvilinear, values of R2 were computed as indicators of goodness of fit in order to determine if polynomial fittings were more appropriate than RMA.
Directly investigating temporal changes of fetal shape is only possible based on longitudinal growth series of individuals, using computed tomography or magnetic resonance imaging to document repeatedly the changing morphology over time. In practice, all human fetal studies are based on cross-sectional series, using multiple individuals of different stages of maturity. Hence, what is really assessed in this and other studies is shape variation within the cross-sectional sample as a way of inferring shape changes. The terminology of shape variations and shape changes is used here accordingly, referring to the actual observations and the inferred pattern, respectively.
Results
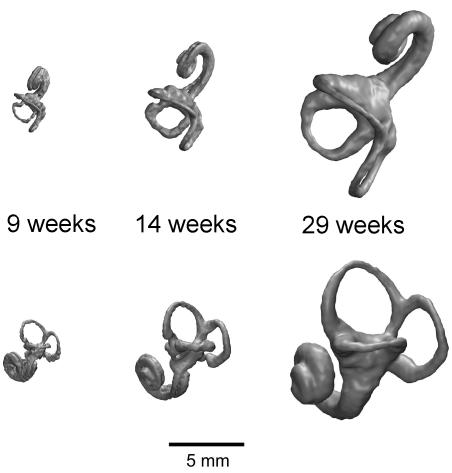
Three-dimensional renderings of the labyrinth at around 9, 14 and 29 weeks gestation are shown in Fig. 3 to illustrate the general variation in morphology that is found for the fetal period studied here. Sample statistics for all raw measurements are given in Table 2. The range and standard variation mark the size and shape variations that occur in the cross-sectional sample, thus giving an indication of the degree of ontogenetic changes. The mean is given to facilitate comparison of the fetal and adult samples. The analyses of these data are divided into age-related variation and maturation of size and shape in which the term shape refers to dimensionless variables such as ratios and angles. Table 3–5 detail the regression and correlation statistics for all of the analyses.
Fig. 3.
Superior (top row) and lateral (bottom row) hrMRI 3D volume reconstructions of the left human fetal labyrinth at 9, 14 and 29 weeks gestation.
Table 2. Measurement statistics, giving the range (min., max.), mean and standard variation (SD).
| Fetal | Adult‡ | Difference of means§ | |||||
|---|---|---|---|---|---|---|---|
| Measure | Min. | Max. | Mean | SD | Mean | SD | |
| BPD (mm) | 11 | 77 | 40.4 | 18.5 | – | – | – |
| Age (weeks) | 9.4 | 29.2 | 17.8 | 5.3 | – | – | – |
| Size (mm) | |||||||
| IEL | 3.7 | 17.5 | 11.78 | 3.92 | – | – | – |
| ASC-R | 0.8 | 3.8 | 2.41 | 0.83 | 3.2 | 0.24 | – |
| PSC-R | 0.7 | 3.6 | 2.24 | 0.85 | 3.1 | 0.30 | – |
| LSC-R | 0.5 | 2.9 | 1.70 | 0.67 | 2.3 | 0.21 | – |
| CO-R | 0.6 | 2.7 | 1.96 | 0.57 | 2.3 | 0.13 | – |
| Ratios | |||||||
| ASCh/w | 72 | 104 | 88.1 | 7.4 | 87 | 4.6 | ns |
| PSCh/w | 83 | 126 | 109.6 | 9.1 | 107 | 7.6 | ns |
| LSCh/w | 73 | 113 | 88.9 | 8.9 | 89 | 7.0 | – |
| COh/w | 93 | 152 | 134.3 | 12.3 | 136 | 8.6 | ns |
| Angles (°)† | |||||||
| Labyrinth | |||||||
| ASC-tor | −5 | 21 | 6.5 | 7.4 | 16 | 5.3 | – |
| PSC-tor | −13 | 8 | −1.0 | 4.6 | −9 | 5.4 | – |
| LSC-tor | 2 | 26 | 14.1 | 5.7 | 4 | 4.1 | *** |
| ASCm<PSCm | 81 | 113 | 98.1 | 6.0 | 104 | 5.2 | – |
| LSCt<VSC | 98 | 121 | 112.1 | 5.6 | 112 | 5.0 | ns |
| COt<VSC | 105 | 147 | 117.3 | 8.6 | 116 | 4.9 | – |
| CCR<LSCm | 105 | 127 | 117.7 | 5.4 | 121 | 4.0 | ** |
| APA<LSCm | 15 | 54 | 32.3 | 7.9 | 41 | 4.7 | – |
| COs<LSCm | 32 | 66 | 48.4 | 8.8 | 59 | 5.1 | – |
| Labyrintho-midline | |||||||
| ASCm<SG | 20 | 49 | 39.8 | 5 | 36 | 4.9 | *** |
| ASCs<SG | 31 | 55 | 45.8 | 4.5 | 45 | 5.3 | ns |
| ASCi<SG | 23 | 50 | 39.2 | 6.5 | 29 | 4.7 | – |
| PSCm<SG | 121 | 153 | 137.9 | 6.6 | 139 | 5.4 | – |
| PSCs<SG | 125 | 146 | 133.8 | 4.1 | 127 | 5.7 | *** |
| PSCi<SG | 124 | 150 | 134.8 | 6.5 | 136 | 5.1 | – |
| LSCt<SG | 92 | 126 | 111.0 | 7.4 | 110 | 5.7 | – |
| COt<SG | 105 | 128 | 116.1 | 5.7 | 113 | 5.4 | – |
| Labyrintho-cranial | |||||||
| LSCm<S-Fc | 2 | −44 | −22.1 | 12.3 | −3 | 5.9 | – |
| APA<S-Fc | −37 | −85 | −54.4 | 9.6 | −44 | 6.8 | – |
| COs<S-Fc | −55 | −95 | −70.5 | 9.5 | −63 | 6.7 | – |
| CCR<S-Fc | −115 | −162 | −139.7 | 13.0 | −124 | 6.5 | – |
| LSCm<Ba-S | 15 | 39 | 26.2 | 6.3 | 39 | 6.1 | – |
| APA<Ba-S | 4 | −33 | −6.1 | 6.3 | −1 | 6.0 | *** |
| COs<Ba-S | −12 | −44 | −22.2 | 6.4 | −20 | 6.3 | ns |
| CCR<Ba-S | −79 | −105 | −91.4 | 7.3 | −82 | 5.5 | – |
| Basicranial | |||||||
| CBA | 116 | 147 | 131.6 | 7.3 | 137 | 4.9 | – |
| PPip<SG | 145 | 165 | 154.6 | 5.6 | 125 | 4.4 | – |
Angle classification: ASC-tor positive when ASCs is more coronally orientated than ASCi; PSC-tor positive when PSCs is more sagittally orientated than PSCi; the 5th & 6th labyrinth angles open anterolaterally whilst the last three open anterior-superiorly; all labyrintho-midline angles open anterolaterally; all labyrintho-cranial angles open anterosuperiorly, and are positive when the basicranial line segment (e.g. S-Fc) is anterosuperiorly to the labyrinth line segment (e.g. LSCm).
Adult values from Spoor (1993), Spoor & Zonneveld (1998), and newly calculated (n = 53 for angles of labyrinth, n = 50 for angles to SG, and n = 48 for angles to midline cranial base). The signs of labyrintho-cranial angles reported by Spoor (1993) were changed according to the conventions set by Spoor & Zonneveld (1998).
For those variables not correlated with EGA (Table 3) significant difference from the adult mean is indicated by * P ≤ 0.05, ** P ≤ 0.01, *** P ≤ 0.001; ns, not significantly different.
Table 3. Rank correlation coefficients, and line fittings†.
| Comparisons against EGA | rrank | a2 | a | 95% Conf. Int. for a | b | R2 |
|---|---|---|---|---|---|---|
| Size (mm) | ||||||
| IEL | 0.91*** | −0.059 | 2.885 | – | −19.283 | 0.96 |
| ASC-R | 0.91*** | −0.012 | 0.592 | – | −3.992 | 0.94 |
| PSC-R | 0.93*** | −0.010 | 0.518 | – | −3.584 | 0.93 |
| LSC-R | 0.92*** | −0.008 | 0.425 | – | −3.030 | 0.91 |
| CO-R | 0.86*** | −0.011 | 0.498 | – | −3.158 | 0.91 |
| Ratios | ||||||
| ASCh/w | 0.19 ns | – | – | – | – | – |
| PSCh/w | −0.27 ns | – | – | – | – | – |
| LSCh/w | 0.46** | – | 1.559 | 1.107 > 2.011 | 60.170 | 0.22 |
| COh/w | −0.13 ns | – | – | – | – | – |
| Angles (°) | ||||||
| Labyrinth | ||||||
| ASC-tor | 0.67*** | – | 1.399 | 1.069 > 1.729 | −18.363 | 0.47 |
| PSC-tor | −0.44** | – | −0.877 | −1.122 > −0.632 | 14.613 | 0.26 |
| LSC-tor | −0.19 ns | – | – | – | – | – |
| ASCm<PSCm | 0.38* | – | 1.136 | 0.797 > 1.475 | 77.897 | 0.15 |
| LSCt<VSC | −0.04 ns | – | – | – | – | – |
| COt<VSC | −0.61*** | – | −1.568 | −1.991 > −1.447 | 111.611 | 0.31 |
| CCR<LSCm | −0.04 ns | – | – | – | – | – |
| APA<LSCm | 0.57*** | – | 1.497 | 1.083 > 1.912 | 5.647 | 0.27 |
| COs<LSCm | 0.43** | – | 1.666 | 1.176 > 2.156 | 18.766 | 0.18 |
| Labyrintho-midline | ||||||
| ASCm<SG | 0.22 ns | – | – | – | – | – |
| ASCs<SG | 0.30 ns | – | – | – | – | – |
| ASCi<SG | −0.54*** | – | −1.222 | −1.571 > −0.873 | 60.943 | 0.22 |
| PSCm<SG | 0.56*** | – | 1.248 | 0.915 > 1.582 | 115.713 | 0.32 |
| PSCs<SG | 0.28 ns | – | – | – | – | – |
| PSCi<SG | 0.58*** | – | 1.220 | 0.904 > 1.537 | 113.098 | 0.36 |
| LSCt<SG | 0.36* | – | 1.398 | 0.975 > 1.820 | 86.173 | 0.13 |
| COt<SG | −0.48** | – | −1.069 | −1.380 > −0.759 | 135.163 | 0.20 |
| Labyrintho-cranial | ||||||
| LSCm<S-Fc | 0.77*** | – | 2.333 | 1.885 > 2.781 | −63.645 | 0.65 |
| APA<S-Fc | 0.64*** | – | 1.821 | 1.351 > 2.291 | −86.820 | 0.37 |
| COs<S-Fc | 0.66*** | – | 1.792 | 1.357 > 2.227 | −102.390 | 0.44 |
| CCR<S-Fc | 0.69*** | – | 2.46 | 2.031 > 2.987 | −183.650 | 0.49 |
| LSCm<Ba-S | 0.71*** | – | 1.184 | 0.936 > 1.432 | 5.120 | 0.58 |
| APA<Ba-S | −0.04 ns | – | – | – | – | – |
| COs<Ba-S | 0.05 ns | – | – | – | – | – |
| CCR<Ba-S | 0.53*** | – | 1.368 | 1.147 > 1.638 | −115.880 | 0.26 |
| Basicranial | ||||||
| CBA | 0.71*** | – | 1.373 | 1.061 > 1.685 | 107.121 | 0.51 |
| PPip<SG | −0.82*** | – | −1.055 | −1.263 > −0.848 | 173.344 | 0.63 |
Polynomial fittings of the order y = ax2 + ax + b; reduced major axes fittings of the type y = ax + b.
P ≤ 0.05;
P ≤ 0.01;
P ≤ 0.001; ns, not significant.
Table 5. Partial correlation coefficients: CBA or EGA held constant for PPip comparisons, and PPip or EGA held constant for CBA comparisons (* P ≤ 0.05; **P ≤ 0.01; *** P ≤ 0.001; ns, correlation not significant).
| Measure | CBA rproduct-moment | CBA rpartial (PPip) | CBA rpartial (EGA) | PPip<SG rproduct-moment | PPip<SG rpartial (CBA) | PPip<SG rpartial (EGA) |
|---|---|---|---|---|---|---|
| Labyrinth | ||||||
| ASC-tor | 0.59*** | 0.28 ns | 0.20 ns | −0.63*** | −0.39* | −0.20 ns |
| PSC-tor | 0.29 ns | – | – | 0.42** | 0.32* | 0.04 ns |
| LSC-tor | −0.15 ns | – | – | 0.13 ns | – | – |
| ASCm<PSCm | 0.33* | 0.12 ns | 0.07 ns | −0.36* | −0.18 ns | −0.08 ns |
| LSCt<VSC | 0.06 ns | – | – | 0.15 ns | – | – |
| COt<VSC | −0.44** | −0.12 ns | −0.03 ns | 0.54*** | 0.36* | 0.13 ns |
| CCR<LSCm | 0.01 ns | – | – | −0.17 ns | – | – |
| APA<LSCm | 0.54*** | 0.25 ns | 0.27 ns | −0.55*** | −0.31 ns | −0.26 ns |
| COs<LSCm | 0.51** | 0.17 ns | 0.33* | −0.59** | −0.38* | −0.47** |
| Labyrintho-midline | ||||||
| ASCm<SG | 0.11 ns | – | – | −0.06 ns | – | – |
| ASCs<SG | 0.29 ns | – | – | −0.33* | −0.19 ns | 0.04 ns |
| ASCi<SG | −0.48** | −0.22 ns | −0.23 ns | 0.49** | 0.26 ns | 0.22 ns |
| PSCm<SG | 0.37* | 0.18 ns | −0.05 ns | −0.37* | −0.16 ns | 0.16 ns |
| PSCs<SG | 0.27 ns | – | – | −0.29 ns | – | – |
| PSCi<SG | 0.39* | 0.08 ns | −0.07 ns | −0.49** | −0.34* | −0.03 ns |
| LSCt<SG | 0.26 ns | – | – | −0.07 ns | – | – |
| COt<SG | −0.40* | −0.02 ns | −0.13 ns | 0.55** | 0.43** | 0.36* |
| Labyrintho-cranial | ||||||
| LSCm<S-Fc | 0.93*** | 0.85*** | 0.84*** | −0.78*** | −0.53** | −0.39* |
| APA<S-Fc | 0.75*** | 0.61*** | 0.57*** | −0.55*** | −0.07 ns | −0.15 ns |
| COs<S-Fc | 0.73*** | 0.63*** | 0.49** | −0.47** | –0.06 ns | 0.11 ns |
| CCR<S-Fc | 0.88*** | 0.77*** | 0.74*** | −0.69*** | −0.24 ns | −0.17 ns |
| LSCm<Ba-S | 0.68*** | 0.31* | 0.28 ns | −0.76*** | −0.56*** | −0.41* |
| APA<Ba-S | −0.01 ns | – | – | −0.06 ns | – | – |
| COs<Ba-S | 0.05 ns | – | – | 0.07 ns | – | – |
| CCR<Ba-S | 0.58*** | 0.35* | 0.18 ns | −0.53** | −0.22 ns | −0.02 ns |
Size variations with age
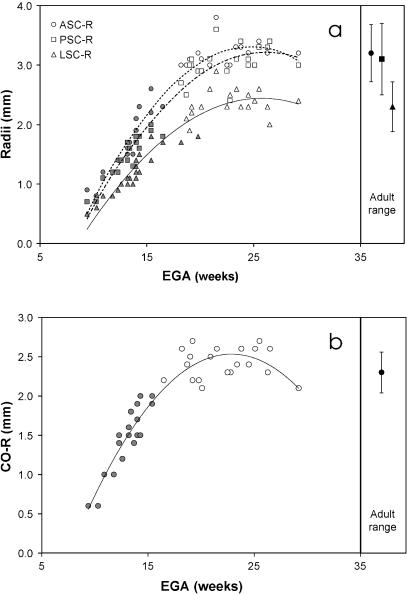
Overall growth of the labyrinth was evaluated by plotting its length (IEL) against estimated gestational age (EGA). The bivariate plot, shown in Fig. 4, reveals a highly significant correlation with a polynomial, biphasic variation of labyrinth length (Table 2). This trend describes a three-fold increase of length during an early gestational growth phase from 9 to 18 weeks after which it tapers off to a plateau, and no further age-related increase in IEL is evident.
Fig. 4.
Bivariate plot of inner ear length (IEL) against estimated gestational age (EGA). Datum points for older fetuses are shaded according to the amount of bone formation around the anterior semicircular canal (ASC), posterior canal (PSC), lateral canal (LSC) and cochlea (CO).
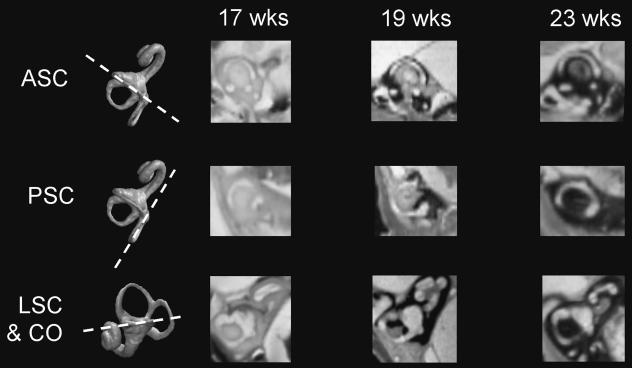
Figure 4 also shows scores of endochondral ossification of the otic capsule around the developing labyrinth of each individual. Example images showing the progression of bone formation to encapsulate the anterior semicircular canal (ASC), posterior semicircular canal (PSC), lateral semicircular canal (LSC) and cochlea (CO) are given in Fig. 5. These figures demonstrate that the labyrinth is rapidly enveloped in bone over a 5-week period; CO and ASC are first surrounded by bone on all sides by approximately 19 weeks gestation, followed by PSC at 21–22 weeks gestation and lastly LSC at 21–23 weeks. Figure 5 shows that bone formation around the canals tends to progress from around the vestibule towards the canal vertices and that the last region of the labyrinth to be surrounded is the posterolateral part of the lateral canal.
Fig. 5.
Resampled hrMRI scans showing the progress of bone formation around the: anterior semicircular canal (ASC; top row); posterior semicircular canal (PSC; middle row); lateral canal and cochlear (LSC & CO; bottom row) at 17, 19 and 23 weeks gestation. The first column shows 3D reconstructions and the plane of resampled images.
To further explore age-related growth variations, radius measures of each semicircular canal and the basal turn of the cochlea were plotted against EGA (Fig. 6a,b). These plots show highly significant curvilinear increases of size, best described by polynomial regression fittings (Table 3). The regression statistics (see a and a2, Table 3) and the growth trajectories depicted in Fig. 6(a) indicate that the anterior and posterior canals grow at similar rates whereas the lateral canal grows at a slower rate and is generally much smaller. Despite these differences, the age at which the polynomial trends inflect (i.e. no further discernible size increase) is about the same for all three canals and the cochlea (∼18 weeks). This is at least a week before the first parts of the labyrinth (ASC, CO) are fully embedded in bone.
Fig. 6.
Plots of fetal labyrinth size measurements against estimated gestational age (EGA): (a) plots of anterior semicircular canal radius (ASC-R), posterior canal radius (PSC-R), and lateral canal radius (LSC-R) against EGA. Adult ranges of variation for these radii are shown in the right-hand column and fetal datum points that fall below these ranges are shaded; (b) plot of cochlear radius against EGA. Adult range of variation is shown in the right-hand column and fetal datum points that fall below this range are shaded. Polynomial regression lines are also shown for both graphs.
Size maturation
To determine whether the fetal labyrinth achieves adult size during the period under investigation, individual fetal size measures were compared statistically (t-test) with the adult sample means given by Spoor & Zonneveld (1998). Those fetuses with size measures significantly smaller than the adult range of variation are shaded grey in Fig. 6(a,b). The youngest individuals that fall within the adult range for the three semicircular canals are between 18 and 19 weeks old (Fig. 6a). A younger individual, aged 16.5 weeks gestation, falls within the adult range for the cochlea (Fig. 6b). These findings indicate that the size of the labyrinth at 17–19 weeks gestation and older is commensurate with that in adulthood. Moreover, results also suggest that despite the notable differences of growth rate, all four measured regions of the labyrinth reach an adult-equivalent size at roughly the same point in time, from 17 to 19 weeks gestation. Indeed, the subset of specimens in the range of 18–29.2 weeks have mean canal and cochlea radii that are not significantly different from the adult samples (Table 4).
Table 4. Subsample comparisons of ossified and adult sized fetal labyrinths against estimated gestational age (EGA) and against reported adult means (Welch's test of equality). Measures included show significant intralabyrinth change over the fetal period investigated (Table 3).
| Fetal subsamples | ASC-R | PSC-R | LSC-R | CO-R | LSCh/w | ASC-tor | PSC-tor | ASCm<PSCm | COt<VSC | APA<LSCm | COs<LSCm |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Adult sized | |||||||||||
| n | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 | 18 |
| Mean | 3.19 | 3.08 | 2.34 | 2.42 | 92.3 | 13.1 | −3.7 | 100.8 | 113.2 | 36.6 | 53.0 |
| SD | 0.20 | 0.26 | 0.27 | 0.19 | 7.1 | 4.2 | 5.0 | 5.7 | 5.3 | 5.5 | 5.7 |
| Ossified | |||||||||||
| n | 19 | 12 | 11 | 19 | 11 | 19 | 12 | 12 | 19 | 11 | 11 |
| Mean | 3.18 | 3.08 | 2.39 | 2.42 | 93.4 | 13.4 | −4.1 | 102.7 | 113.1 | 38.2 | 54.2 |
| SD | 0.20 | 0.27 | 0.18 | 0.19 | 7.7 | 4.2 | 5.5 | 6.1 | 5.2 | 6.1 | 4.6 |
| vs. EGA† | |||||||||||
| Adult sized (rank) | 0.29 ns | 0.36 ns | 0.35 ns | 0.12 ns | 0.03 ns | −0.44 ns | −0.28 ns | 0.51* | −0.59** | 0.65 ns | 0.27 ns |
| Ossified (rank) | 0.34 ns | 0.34 ns | −0.18 ns | 0.08 ns | 0.26 ns | −0.51* | −0.13 ns | −0.04 ns | −0.51* | 0.08 ns | 0.57 ns |
| vs. Reported adult means†‡ | |||||||||||
| Adult sized (Welch's test) | ns | ns | ns | ns | ns | <* | <*** | <* | ns | <** | >*** |
| Ossified (Welch's test) | ns | ns | ns | ns | ns | <* | <* | ns | ns | ns | >* |
*P ≤ 0.05
P ≤ 0.01
P ≤ 0.001; ns, no significant difference between means.
> fetal mean significantly greater than adult; < fetal mean significantly less than adult.
Shape variations with age
Indices
Temporal variations in the shapes of the semicircular canal arcs and cochlear basal turn were explored by plotting their width/height indices against EGA. Findings show that there are no significant age-related variations in the indices of the anterior canal (rrank = 0.19, ns), the posterior canal (rrank = −0.27, ns) and even the cochlea (rrank = −0.13, ns). However, there is a small significant correlation between variations in the index of the lateral canal and EGA (Table 3). This suggests that the height of the lateral canal increases relative to its width during fetal life, although these variations fall within the adult range of variation (75–103). At the beginning of the period investigated the lateral canal is wider than it is tall, but with gradual increases of height a more circular lateral canal develops.
Angles
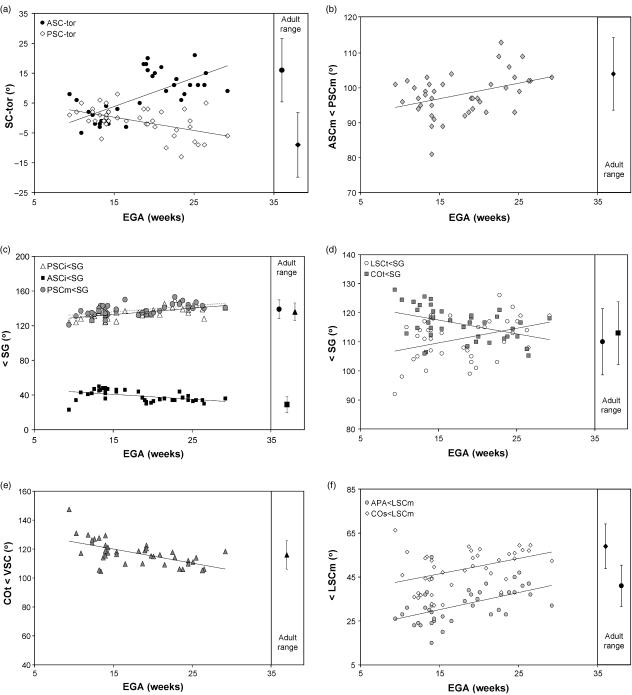
Angular variations of the arc plane of each semicircular canal were explored with plots of their degree of torsion against EGA. Findings show no significant shift in the torsion of the lateral canal (rrank = −0.19, ns), suggesting that the lateral-most margin of the lateral canal remains inclined relative to the medial-most part throughout the period investigated. Note, however, that there is a significant 10° difference in comparison with the adult mean given in Table 2. Torsion of the anterior canal and posterior canal increases positively and negatively with age, respectively (Fig. 7a; Table 3). These findings indicate that the canal arcs are nearly flat during early fetal life, and subsequently show an increasing degree of torsion. Plots of the angular variations between the functionally important planes of the anterior and posterior canals, as measured through the median axes in the transverse plane (ASCm<PSCm), show a small but significant correlation with age (Fig. 7b; Table 3). This suggests that the angle between the planes of ASC and PSC opens-out laterally during fetal life.
Fig. 7.
Plots of fetal labyrinth angular measures against estimated gestational age (EGA): (a) plots of anterior and posterior canal torsions (ASC-tor & PSC-tor, respectively) against EGA; (b) plot of the angle between the median axes of the anterior and posterior canals (ASCm<PSCm) against EGA; (c) plots of inferior-most aspects of anterior and posterior canals and median axis of posterior canal measured to the midline (ASCi<SG, PSCi<SG and PSCm<SG, respectively) against EGA; (d) plots of the lateral canal axis and cochlear transverse axis measured to the midline (LSCt<SG & COt<SG, respectively) against EGA; (e) plot of cochlear transverse axis measured to a reference line derived from the orientation of the anterior and posterior canals (COt<VSC) against EGA; (f) plots of the ampullae line and cochlear sagittal axis measured to the lateral canal (APA<LSCm & COs<LSCm, respectively) against EGA. Adult ranges and reduced major axes line fittings are also shown for each measurement.
To resolve what parts of the anterior and posterior canals contribute to their increased torsion and to the opening-out of the median planes, their superior, median and inferior orientations relative to the midline (SG) were plotted against gestational age. No significant correlations were observed for variations of either of the superior-most orientations relative to the midline and gestational age (ASCs<SG & EGA, rrank = 0.30, ns; PSCs<SG & EGA, rrank = 0.28, ns). However, the inferior-most orientations relative to the midline decrease (ASCi) and increase (PSCi) significantly with age (Fig. 7c; Table 3). This shows that the increased torsions of these canals follow from the divergence of the inferior-most line segments ASCi and PSCi toward the midline, whereas the superior-most parts remain stable. No significant correlation was observed for the median axis of the anterior canal (ASCm<SG, rrank = 0.22, ns), but that of the posterior canal relative to the midline increases with age (Fig. 7c; Table 3). Thus, the plane of the posterior canal (PSCm) tracks with changes to its inferior-most part (PSCi), gradually reorientating toward the sagittal axis by some 15° during the period investigated, and opening the angle between the two vertical canals (ASCm<PSCm).
The axis of symmetry orientation of the lateral canal (LSCt) relative to the planar orientations of the anterior and posterior canals, as represented by the reference line VSC, is not significantly related with gestational age (LSCt<VSC & EGA, rrank = −0.04, ns). However, when considered relative to the midline it shows a small significant correlation, suggesting that LSCt rotates more sagittally with age (Fig. 7d; Table 3). The orientation of the cochlear basal turn (COt), relative to both VSC and the midline (SG), shows a significant age-related decrease (Fig. 7d,e; Table 3). Thus, it rotates coronally, so that the apex of the cochlea faces increasingly anteriorly.
Turning to the labyrinth in lateral view, the orientation of the common crus, measured relative to the plane of the lateral canal, shows no significant correlation with age (CCR<LSCm & EGA, rrank = −0.04, ns). There are, however, significant age-related variations in the sagittal orientations of the ampullar line (APA) and cochlea (COs) relative to LSCm (Fig. 7f; Table 3). These amount to clockwise rotations when viewing the lateral aspect of the left labyrinth.
The age-related angular changes of the labyrinth during the fetal period investigated are summarized in Fig. 8. When viewing the superior aspect of the left labyrinth, antero-inferior parts of the labyrinth (ASCi, COt) rotate clockwise relative to the midline, whereas postero-inferior parts (PSCm, PSCi, LSCt) rotate anticlockwise. The superior-most parts (ASCs, ASCm, PSCs) remain stable. In lateral view, the antero-inferior parts (APA, COs) rotate clockwise relative to the lateral canal LSCm.
Fig. 8.
Superior (a,b) and lateral (c,d) views of the left human fetal labyrinth at 9 (a,c) and 29 (b,d) weeks of gestation. Observed age-related angular variations are summarized by arrows pointing clockwise or anticlockwise (see main text).
Shape maturation
Establishing whether the fetal labyrinth attains adult shape when it reaches adult size, when the surrounding otic capsule is ossified, or whether it continues to change subsequently, is more difficult to assess than size maturation. The degree of shape changes observed for the fetal period is small compared with the range of variation in adults, which makes it difficult to establish that a plateau is reached in the plots. Here we specifically assess continuing shape changes in two later fetal subsamples. These include (1) those specimens with labyrinth sizes within the adult range of variation (n = 18; see Fig. 6a,b), and (2) those specimens in which the morphology of interest is fully encapsulated by bone (see Figs 4 and 5). For example, the subsample for comparisons with ASC measures contains 19 fetuses in which the anterior canal is surrounded by bone; PSC measures or PSC to ASC measures, n = 12; LSC measures, LSC to PSC or LSC to ASC measures, n = 11. These subsamples were re-analysed for age-related shape variations and tested for equality with the adult means (Table 4).
No significant differences were noted for canal radii or proportions of the lateral canal. The angle between the anterior and posterior canals (ASCm<PSCm) continues to change after adult canal size is reached, but stabilizes and attains the adult value when the relevant parts are embedded in bone. The transverse orientation of the cochlea relative to the vertical canals (COt<VSC) appears to change after adult size is reached and ossification is completed. However, the mean values are not significantly different from the adult mean. By contrast, the inclination of the cochlea and the ampullar line, relative to the planar orientation of the lateral canal (COs<LSCm, APA<LSCm) do differ from the adult mean values, but no change is evident after adult size is reached (measured against EGA). Finally, the mean torsion angles of the anterior and posterior canal (ASC-tor, PSC-tor) are smaller than in the adult sample, with no evidence for further increase after adult canal size is reached. In fact, the significant correlation coefficient obtained for ASC-tor suggests that the torsion of this canal decreases after ossification (i.e. opposite to the trend during the full fetal period). In all, these results hint at the possibility that a few of the observed trends of labyrinthine shape changes could continue after ossification of the otic capsule, but the evidence is ambiguous.
Basicranial integration
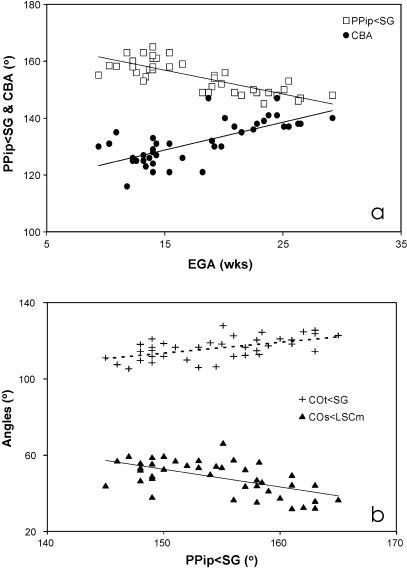
Two main aspects of basicranial shape, the transverse orientation of the petrous pyramids relative to the midline (PPip<SG) and the cranial base angle (CBA), are both strongly correlated with EGA (Table 3). This implies that the petrous bones reorientate coronally by approximately 15° during the period investigated and the cranial base flattens out by some 15–20° (Fig. 9a). To evaluate if the observed temporal changes of the fetal labyrinth are interrelated with these variations of basicranial architecture, all labyrinthine angles were compared with petrous orientation and cranial base angle (Table 5). Correlations were calculated while holding PPipw;SG constant for CBA comparisons and CBA constant for PPip<SG comparisons to filter out the influence of the strong background correlation between PPip and CBA (rrank = −0.70, P < 0.001). Moreover, correlations were also calculated while holding the growth-related variable EGA constant, because PPip<SG and CBA, and many labyrinthine angles correlate closely with this variable (Table 3). The results of the partial correlation analysis are given in Table 5.
Fig. 9.
Plots of basicranial and labyrinth measures: (a) plot of petrosal angle (PPip<SG) and cranial base angle (CBA) against estimated gestational age (EGA); (b) cochlear measures (COt<SG and COS<LSCm) against PPip<SG. Reduced major axes regression lines are shown for all plots (see Table 6 and main text).
The findings provide evidence for an association between the orientations of the fetal cochlea and petrous bone, independent of EGA or CBA. The basal turn of the cochlea rotates coronally (COt<SG) and becomes more inclined relative to the lateral canal (COs<LSCm), with a more coronal petrous orientation (Fig. 9b). There is no evidence for any association between fetal labyrinth morphology and the increase in cranial base angle (referred to here as retroflexion). The cochlear angle COs<LSCm appears to be correlated with CBA, independent of EGA. However, this apparent link is not significance when PPip is kept constant, and thus indirectly follows from the underlying correlation between PPip and CBA.
To assess whether individual parts of the labyrinth are correlated with either the anterior or posterior midline cranial base the angles of these parts to Ba-S and S-Fc were analysed (Table 5, bottom eight rows: note that CBA equals Ba-S<S-Fc). All labyrinthine angles to S-Fc are significantly correlated with CBA, with or without holding EGA and PPip constant. Hence, reorientation of all labyrinthine parts relative to S-Fc positively correlates with the reorientation of Ba-S to S-Fc. By contrast, none of the angles relative to Ba-S are significantly correlated with CBA when holding EGA constant. This pattern implies that all parts of the labyrinth follow the midline posterior cranial base (i.e. the basioccipital), and not the anterior cranial base. As the midline retroflexes, the labyrinth pitches antero-inferiorly.
The labyrinthine angles to the midline cranial base were also assessed against petrous bone orientation. Only the angles of LSCm to both S-Fc and Ba-S correlate with PPip<SG, with or without holding EGA and CBA constant. Hence, the lateral canal orientation shows antero-inferior pitch relative to the entire midline cranial base, in association with the coronal reorientation of the petrous bones. This is independent of the association of the entire labyrinth with the posterior cranial base.
Discussion
This paper set out to document developmental changes of the fetal labyrinth during human fetal life. We found considerable size increases of the labyrinth during the early part of the period investigated, which gradually wane, and cease later in fetal life. Age-related shape changes of the labyrinth in superior view comprise an opening-out, or unfolding, about an origin in the region of the vestibule (Fig. 8). Antero-inferior parts (ASCi and COt) rotate clockwise, when considering the left labyrinth, and postero-inferior parts (LSCt, PSCm and PSCi) rotate anticlockwise. The more superior parts of the labyrinth (ASCm, ASCs and PSCs) remain stable. In lateral view, both the ampullar line segment and the cochlea axis rotate to a more upright orientation (clockwise for a left labyrinth; Fig. 8). These and other findings will now be discussed in more detail in relation to the four specific questions formulated in the Introduction.
Size maturation
The first question asked ‘at what age does the fetal labyrinth attain adult size’. Our findings show different rates of growth among the canals and cochlea, indicating that most components of the labyrinth, in particular the cochlea and lateral canal, follow distinct growth trajectories. It seems likely that the slower growth of the lateral canal corresponds to the proportional readjustments of its height in relation to its width. However, despite the growth lag of the lateral canal, we find that increases of all size measures, including overall size and that of the lateral canal, taper off to a plateau at around the same time, between 17 and 19 weeks gestation. Moreover, these plateaus of size are commensurate with the adult values reported by Spoor & Zonneveld (1998). Thus, our findings suggest that there is no evidence for growth of the labyrinth during the late fetal and postnatal period. These results confirm similar observations reported by Siebenmann (1890), Schönemann (1906) and Sato et al. (1991, 1993), but contradict previous suggestions that canal size and cochlea height increase postnatally (Hyrtl, 1845; Tremble, 1929; Turkewitsch, 1930). In fact, with respect to cochlear growth we find that it is this structure which perhaps first attains adult size at around 16–17 weeks gestation.
Shape maturation
The second question asked whether labyrinthine morphology continues to change in shape after attaining adult size and after the otic capsule has ossified. The results indicate that the angle between the anterior and posterior canals continues to open laterally after the canals reach adult size, but stabilizes with the ossification of the surrounding capsule. Two angles, describing the torsion of the anterior canal (ASC-tor) and the orientation of the cochlea (COt<VSC), appear to change after ossification. However, the correlations with age are not strong, and after reaching adult size the cochlea angle is not different from that of adults. Moreover, the suggested direction of change for the small ASC-tor subset (Table 4) is opposite to that of the overall trend during the full fetal period investigated (Table 3). This would suggest a more complex pattern of shape changes, marked by reversals in direction. Figure 7(a), showing the full trend for the torsion angle, does not support such a pattern, and a larger post-ossification sample would need to be analysed to reject the null hypothesis of a single trend towards the adult morphology.
Sato (1903) reported that adult labyrinths show a 13° wider angle between the posterior limb of the lateral canal and the plane of the posterior canal than fetal labyrinths. Of all labyrinthine measurements used in our study, this phenomenon should best be expressed by the orientation of the axis of symmetry of the lateral canal (LSCt<VSC), but this angle is not correlated with age, and is not significantly different in the fetal and adult samples (Table 2). Moreover, relative to the midsagittal orientation the axis of symmetry does change over fetal time (Table 3), but in the opposite direction to that indicated by Sato, i.e. we find that LSCt turns more sagitally, with the vertex moving posteriorly rather than laterally.
Bossy & Gaillard de Collogny (1965) described that the long axis of the fetal labyrinth turns to a more coronal orientation by about 15°. Whereas the cochlea partly follows this rotation, the anterior canal rotates in the opposite direction by about 10°, and stabilizes at about 5 months. Although the age when shape changes cease approximately corresponds with our results, we do not find any age-related change of the equivalent measurement of the anterior canal (ASCm<SG). As Bossy & Gaillard de Collogny (1965) cover the time period from 2.5 to 9 months with a sample of just 12 fetuses, it may well be that the reported 10° change falls well within the normal interindividual variation, and does not represent a temporal trend (for a shorter period the variation in our sample is 29°).
Without specifically quantifying the difference, Sercer (1958) reports that adult semicircular canals show more torsion than those of newborns. Our study shows no evidence for increasing torsion after the fetal labyrinth attains adult size (Table 4). However, we did observe significant differences in comparisons with the adult means, suggesting that torsion of the anterior and posterior canals is greater in the adults. This could be seen as support for Sercer's general observation, except that we find the reverse pattern for the lateral canal, which shows more torsion in our fetal sample than in the adults (Table 2).
In addition to the torsion angles, two further angles of the labyrinth are significantly different in the adult sample and either the post-ossification fetal sample (Table 4) or the full fetal sample (Table 2). These are, respectively, the cochlear angle COs<LSCm, and the common crus angle CCR<LSCm, which is spatially linked with torsion of the vertical canals. Such differences probably reflect the distinct geographical compositions of the fetal and adult samples. The adults represent a worldwide sample, drawn from indigenous populations from all continents, whereas the fetal specimens represent the London (UK) area only. Given that Spoor (1993) observed population differences in adult labyrinthine morphology, this thus seems the most plausible explanation. However, it cannot be fully excluded that these morphological differences represent changes in labyrinthine shape after ossification of the otic capsule. This process would require local remodelling through resorption and deposition of bone, and this possibility should be examined through histological studies of the otic capsule. If it were to be demonstrated that angles with functional importance, canal torsion in particular, do change after capsule ossification, such an additional phase of plasticity could possibly be linked with fine-tuning of the vestibular apparatus.
Overall, our findings suggest that the fetal labyrinth shows little or no changes in shape after it has attained adult size and, in particular, once it is embedded in the ossified otic capsule.
Basicranial integration
The third question asked if the observed changes of the human fetal labyrinth are in any way interrelated with larger scale changes of the fetal cranial base. The partial correlation analyses did not reveal any link between shape changes of the labyrinth and the retroflexion of the midline cranial base. All parts of the labyrinth appear to follow the posterior cranial base, and not the anterior cranial base, so that the labyrinth pitches antero-inferiorly when the midline retroflexes. This appears to contrast with the observation of Spoor (1993) that the planar orientation of the lateral canal (LSCm) maintains a constant orientation relative to the anterior cranial base during human ontogeny. However, not only are these observations based on different measurements of the anterior cranial base (Spoor, 1993, considered the orientation sella to nasion, rather than to foramen caecum), they also cover dissimilar time periods. The eight fetal specimens considered by Spoor (1993) are between 24 weeks and full term. In the current study the eight equivalent specimens have an angle LSCm<S-Fc (mean = −5.5°, SD = 3.6°) that is very similar and not significantly different from the value for adults (Table 2). Hence, results indicate that the plane of the lateral canal pitches antero-inferiorly relative to the anterior cranial base up to about 24 weeks, and subsequently maintains a stable orientation into adulthood.
The partial correlation analyses do reveal an association between the increasingly coronal orientation of the petrous bones (PPip<SG) and changes to the cochlea. The basal turn of the latter both rotates coronally (COt<SG) and becomes more inclined (COs<LSCm), so that the cochlear apex faces more anteriorly. The coronal reorientation of the cochlea thus appears to follow the surrounding petrous bone, whereas other parts of the labyrinth do not. In the case of the vertical canal orientations, this may be because of inherent functional constraints, given that canal orientation directly affects its plane of optimum perceptive sensitivity. Functionally, all four vertical canals are integrated as contralateral synergistic push–pull pairs, and the adult configuration must be present when the planes become fixed by the end of the second trimester. However, this argument of functional constraint does not apply to the symmetry axis of the lateral canal (LSCt), because changes in its orientation do not affect the plane of the canal. Interestingly, LSCt rotates more sagitally with age (Fig. 8), as the petrous bone rotates coronally, but these movements are not correlated.
Whereas the joint coronal reorientation of the cochlea with the surrounding petrous bone is easily understandable, this is not the case for the increased cochlear inclination, measured in the sagittal plane. This correlation could be an indirect consequence of the spiral structure of the cochlea, so that any reorientation in the transverse plane (COt) affects the landmarks of the orientation in the sagittal plane (COs). The angles COt<SG and COs<LSCm are indeed significantly correlated (rproduct-moment −0.44**). However, when petrous orientation PPip is held constant the correlation is no longer significant (rpartial −0.16, ns), and the increased inclination of the cochlear basal turn is clearly not a simple consequence of its coronal reorientation.
Another unexpected finding is that, relative to the entire midline cranial base (S-Fc and Ba-S), the lateral canal (LSCm) pitches antero-inferiorly with coronal petrous reorientation. A specific explanation for this link is not evident, but it is interesting to note that it only applies to LSCm, and not to other labyrinthine orientations measured in the sagittal plane (Table 5). Among these, LSCm is also the only functionally relevant orientation, as it quantifies the plane of the lateral canal. A potential functional constraint on lateral canal orientation could be related to its input into the vestibulo-ocular reflex, working on the medial and lateral rectus muscles of the eye. Hence, a degree of alignment of these muscles, the bony orbit and the lateral canal planes is a possibility that remains to be explored (Spoor & Zonneveld, 1998). However, even if such a spatial association were found, it is not clear how this would relate to the orientation of the petrous bone.
Finally, the fact that only LSCm, and not COs, shows antero-inferior pitching in correlation with petrous reorientation may give new light to the poorly understood link between the cochlear inclination angle and petrous reorientation. Indeed, the correlation between the angles COs<LSCm and PPip<SG (Table 5) is no longer significant when the angles LSCm<Fs-S or LSCm<Ba-S are held constant (rpartial−0.17, ns, and −0.11 ns, respectively). This implies that the cochlear inclination angle is seen to co-vary with petrous reorientation not because of true inclination changes in COs but because of changes in the reference orientation LSCm.
In summary, the observed labyrintho–basicranial associations during fetal development reveal the following pattern: (1) a joint coronal reorientation of the petrous bone and the cochlea, not followed by other parts of the labyrinth; (2) the entire labyrinth follows the posterior cranial base, so that the former pitches antero-inferiorly, when the latter retroflexes; and (3) antero-inferior pitching of the lateral canal, relative to the anterior and posterior cranial base in association with coronal reorientation of the petrous bone.
Ontogenetic and phylogenetic trends
The fourth and final question asked if the human labyrinth is laid down as a small version of the phylogenetically derived adult morphology, and, if not, whether fetal changes reflect the phylogenetic trend observed for modern humans. The age-related shape changes observed here clearly demonstrate that the early fetal labyrinth does not show the adult morphology. Among these ontogenetic changes, some do indeed follow the phylogenetic trends observed when modern humans are compared with the extant great apes and Plio-Pleistocene hominids, the South African australopithecines in particular (Spoor, 1993; Spoor et al. 1994). Comparison of Figs 1 and 8 shows similar rotations of the ampullar line and the cochlea relative to the lateral canal (APA<LSCm, COs<LSCm). The ontogenetic and phylogenetic trends both also share a shape change of the posterior canal from a flat arc to one with negative torsion. However, the ontogenetic change appears to be caused by the increasingly sagittal orientation of the inferior part of the posterior canal, whereas the phylogenetic trend follows from an increasingly coronal orientation of its superior part (Spoor, 1993; Spoor & Zonneveld, 1998).
Several phylogenetic trends are not seen during fetal development. For example, the axis of symmetry of the lateral canal (LSCt) changes phylogenetically to a more coronal position (Fig. 1), but ontogenetically it remains unchanged (when measured against VSC) or even takes a more sagittal position (when measured against the midline SG). In addition, phylogenetically the common crus (CCR) rotates to a more posteriorly tilted orientation (Fig. 1), whereas no ontogenetic change was observed here.
The findings of the present study can now be used to evaluate the model that it is differential integration of the cartilagenous otic capsule and the surrounding fetal cranial base that results in the unique shape of the human labyrinth, as an ontogenetic process which could also form the basis of phylogenetic change (Spoor, 1993; Spoor & Zonneveld, 1998). The mechanism essential to this model is that parts of the labyrinth reorientate in correlation with the cranial base, whereas others remain stable or change independently. If the entire labyrinth simply follows the surrounding cranial base no shape change occurs, a situation shown by the antero-inferior pitch of the labyrinth in association with the retroflexion of the posterior midline cranial base. However, the mechanism is indeed demonstrated here by the exclusive reorientation of the cochlea and lateral canal plane in correlation with the petrous bones.
A second aspect of the model, that the differential link with the cranial base results in the derived, typically human shape of the labyrinth, is clearly not supported here. Most strikingly perhaps, the cochlea orientation COt follows the petrous bone ontogenetically, but shows no change phylogenetically, whereas the symmetry axis of the lateral canal LSCt reorientates with the petrous bone phylogenetically, but not ontogenetically (compare Figs 1b and 8b). The interpretation of the results with respect to the angles measured in the sagittal plane (Figs 1d and 8d) is more complex. Comparison could only be made with the midline cranial base, and not with the local inclination of the petrous surface, because the landmarks of the orientation PPp, used in adult comparisons, are not yet developed.
A third aspect of the model is the proposal that the process of fetal brain development affects both the pattern of change in the labyrinth and the pattern of basicranial variation, and that this common influence accounts for the link between the labyrinth and basicranium (Spoor, 1993; Spoor & Zonneveld, 1998). The potential impact of the brain on the cartilaginous otic capsules and the surrounding petrous bones is evident, as they are wedged between the cerebral temporal lobes and the cerebellum, and form a major line of attachment of the tentorium cerebelli. Moreover, phylogenetically, both midline cranial base flexion and coronal reorientation of the petrous bones have been shown to correlate with increased relative brain size (Ross & Ravosa, 1993; Ross & Henneberg, 1995; Spoor, 1997; Lieberman et al. 2000; McCarthy, 2001). However, analyses of the human fetal cranial base covering the same period as the present study indicate that these correlations do not occur ontogenetically (Jeffery & Spoor, 2002; Jeffery, 2003). Hence, whereas fetal brain growth may affect the labyrinthine shape locally, it is less likely to be the dominant factor integrating the labyrinth and the rest of the cranial base.
Overall, it can be concluded that there is evidence for the envisaged mechanism that the shape of the fetal labyrinth changes because parts follow the petrous bones but others do not. However, independent shape changes of the fetal labyrinth play at least as important a role in establishing the adult morphology. Moreover, ontogenetic patterns of shape change are not necessarily the basis for phylogenetic change of the labyrinth.
These and previous findings clearly highlight the complex nature of changes of the labyrinth and surrounding otic capsule during both development and evolution. A confounding factor is that studies thus far have only considered two-dimensional rotations of the labyrinth and the surrounding petrous bone, as a limited representation of complex three-dimensional changes. Although every attempt has been made here to cross-reference measures between the perpendicular transverse and sagittal planes, this is probably the reason for difficulties in understanding some results, such as the link between petrous reorientation in the transverse plane and rotation of the lateral canal in the sagittal plane. This study has been able to answer the basic questions regarding the ontogeny of the human labyrinth, but further advancing our understanding of its complexities will require full three-dimensional morphometric analysis.
Conclusions
The human labyrinth undergoes a number of complex changes during early fetal life, including a general ‘unfolding’ of the vestibular apparatus and reorientation of the cochlea.
Despite the different growth rates of its subcomponents, the human fetal labyrinth as a whole attains an adult equivalent size between 17 and 19 weeks gestation and is fully encapsulated by bone a few weeks later.
The shape changes of all or most of the labyrinth ceases after the otic capsule has ossified, though there remain significant differences with adult means, indicating either population differences between the fetal and adult samples or, potentially, that change of the labyrinth may recur later in life.
Shape changes to the human fetal labyrinth are not associated with contemporary retroflexion of the midline cranial base. However, changes in labyrinthine shape do correlate with the reorientation of the surrounding petrous bone, because some of its parts do follow this reorientation, whereas others do not.
Some ontogenetic changes are similar in nature to the phylogenetic trends that mark the adult human labyrinth. However, the overall pattern of change in fetal shape and of correlations with the surrounding cranial base does not provide a clear ontogenetic basis for the evolutionary history of the human labyrinth.
Acknowledgments
We would like to thank Drs K. H. Höhne and A. Pommert (University of Hamburg) for allowing us to use the rendering package VOXELMAN. We also thank Dr W. Rasband (NIH of Mental Health) and Dr A. Parker (Harvard University) for developing the freeware packages ImageJ and plugin Align3D, respectively (see http://rsb.info.nih.gov/ij/). The helpful and constructive comments provided by an anonymous referee are acknowledged. This research was supported by a Bioarchaeology Research Grant (No. 057210), awarded by the Wellcome Trust, and the University of London intercollegiate NMR research service at Queen Mary and Westfield College, London.
References
- Anniko M. Embryonic development of the vestibular sense organs and their innervation. In: Romand R, editor. Development of Auditory and Vestibular Systems. New York: Academic Press; 1983. pp. 375–423. [Google Scholar]
- Arnold WH, Lang T. Development of the membranous labyrinth of human embryos and fetuses using computer aided 3D-reconstruction. Ann. Anat. 2001;183:61–66. doi: 10.1016/S0940-9602(01)80014-5. [DOI] [PubMed] [Google Scholar]
- Bast TH. Ossification of the otic capsule in human fetuses. Contribut. Embryol. 1930;121(21):55–82. [Google Scholar]
- Bossy J, Gaillard de Collogny L. Orientation comparée de la cochlea et du canal semi-circulaire anterieur chez les foetus. J. Franc. Oto-Rhino-Laryng. 1965;14:727–725. [PubMed] [Google Scholar]
- Boyl PP, Signore M, Annino A, Barbera JP, Acampora D, Simeone A. Otx genes in the development and evolution of the vertebrate brain. Int. J. Dev. Neurosci. 2001;19:353–363. doi: 10.1016/s0736-5748(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Chitty LS, Altman DG, Henderson A, Campbell S. Charts of fetal size: part 2. Head measurements. Br. J. Obstet. Gynaecol. 1994;101:35–43. doi: 10.1111/j.1471-0528.1994.tb13007.x. [DOI] [PubMed] [Google Scholar]
- Cousin RP. Étude en projection sagittale de crânes d'enfants orientés dans les axes vestibulaires. Paris: Thése Fac. Méd.; 1969. [Google Scholar]
- Delattre A, Fenart R. L'Hominisation du Crâne. Paris: CNRS; 1960. [Google Scholar]
- Delattre A, Fenart R. Evolution des fenêtres du vestibule des mammifères a l'homme. Bull. Mém. Soc. Anthr. Paris. 1961;2:19–20. 11s. [Google Scholar]
- Delattre A, Fenart R. Formation des orifices de la face posterieure du rocher humaine. Étude par la méthode vestibulaire au cours de la phylogenese des mammifères et de l'ontogenese humaine. Mammalia. 1962;26:214–279. [Google Scholar]
- Fritzsch B, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer-Verlag; 1998. pp. 80–145. [Google Scholar]
- Gray AA. The Labyrinth of Animals. Vol. 2. London: Churchill; 1907. [Google Scholar]
- Hublin JJ, Spoor F, Braun M, Zonneveld F, Condemi S. A late Neanderthal from Arcy-sur-Cure associated with Upper Palaeolithic artefacts. Nature. 1996;381:224–226. doi: 10.1038/381224a0. [DOI] [PubMed] [Google Scholar]
- Hyrtl J. Vergleichend-Anatomische Untersuchungen Über das Innere Gehörorgan Des Menschen und der Säugethiere. Prague: F.Ehrlich; 1845. [Google Scholar]
- Jeffery N. PhD thesis. University College London; 1999. Fetal Development and evolution of the human cranial base. [Google Scholar]
- Jeffery N. A high-resolution MRI study of linear growth of the human fetal skull base. Neuroradiology. 2002;44:358–366. doi: 10.1007/s00234-001-0753-z. [DOI] [PubMed] [Google Scholar]
- Jeffery N, Spoor CF. Brain size and the human cranial base: a prenatal perspective. Am. J. Phys. Anthropol. 2002;118:324–340. doi: 10.1002/ajpa.10040. [DOI] [PubMed] [Google Scholar]
- Jeffery N. Brain expansion and comparative prenatal ontogeny of the non-hominoid primate cranial base. J. Hum. Evol. 2003;45:263–284. doi: 10.1016/j.jhevol.2003.08.002. [DOI] [PubMed] [Google Scholar]
- Jones GM, Spells KE. A theoretical and comparative study of the functional dependence of the semicircular canal upon its physical dimensions. Proc. Roy. Soc., Lond. 1963;B157:403–419. doi: 10.1098/rspb.1963.0019. [DOI] [PubMed] [Google Scholar]
- Kustermann A, Zorzoli A, Spagnolo D, Nicolini U. Transvaginal sonography for fetal measurement in early pregnancy. Br. J. Obstet. Gynaecol. 1992;99:38–42. doi: 10.1111/j.1471-0528.1992.tb14389.x. [DOI] [PubMed] [Google Scholar]
- Lieberman DE, Ross CF, Ravosa MJ. The primate cranial base: ontogeny, function and integration. Year Book Phys. Anth. 2000;30:117–170. doi: 10.1002/1096-8644(2000)43:31+<117::aid-ajpa5>3.3.co;2-9. [DOI] [PubMed] [Google Scholar]
- Martin P, Swanson GJ. Descriptive and experimental analysis of the epithelial remodellings that control semicircular canal formation in the developing mouse inner ear. Dev. Biol. 1993;159:549–558. doi: 10.1006/dbio.1993.1263. [DOI] [PubMed] [Google Scholar]
- Mazan S, Jaillard D, Baratte B, Janvier P. Otx1 gene-controlled morphogenesis of the horizontal semicircular canal and the origin of the gnathostome characteristics. Evol. Dev. 2000;2:186–193. doi: 10.1046/j.1525-142x.2000.00062.x. [DOI] [PubMed] [Google Scholar]
- McCarthy RC. Anthropoid cranial base architecture and scaling relationships. J. Hum. Evol. 2001;40:41–66. doi: 10.1006/jhev.2000.0446. [DOI] [PubMed] [Google Scholar]
- Noden DM, Van de Water TR. Genetic analyses of mammalian ear development. Trends Neurosci. 1992;15:235–237. doi: 10.1016/0166-2236(92)90056-e. [DOI] [PubMed] [Google Scholar]
- Pellerin C. Thèse. Paris V: Université René Descartes; 1983. Le crâne humain en orientation vestibulaire. Étude de neuf populations recentes. [Google Scholar]
- Rinkwitz S, Bober E, Baker R. Development of the vertebrate inner ear. Ann. NY. Acad. Sci. 2001;942:1–14. doi: 10.1111/j.1749-6632.2001.tb03730.x. [DOI] [PubMed] [Google Scholar]
- Ross CF, Ravosa MJ. Basicranial flexion, relative brain size, and facial kyphosis in nonhuman primates. Am. J. Phys. Anthropol. 1993;91:305–324. doi: 10.1002/ajpa.1330910306. [DOI] [PubMed] [Google Scholar]
- Ross CF, Henneberg M. Basicranial flexion, relative brain size, and facial kyphosis in homo sapiens and some fossil hominids. Am. J. Phys. Anthropol. 1995;98:575–593. doi: 10.1002/ajpa.1330980413. [DOI] [PubMed] [Google Scholar]
- Sato H, Sando I, Takahashi H. Sexual dimorphism and development of the human cochlea. Acta Otolaryngol. 1991;111:1037–1040. doi: 10.3109/00016489109100753. [DOI] [PubMed] [Google Scholar]
- Sato H, Sando I, Takahasi H, Fujita S. Torsion of the human semicircular canals and its influence on their angular relationship. Acta Otolaryngol. (Stockh.) 1993;113:171–175. doi: 10.3109/00016489309135787. [DOI] [PubMed] [Google Scholar]
- Sato T. Vergleichende Untersuchungen über die bogengänge des Labyrinthes beim neugeborenen und beim erwachsenen Menschen. Z. Ohrenheilk. 1903;42:137–156. [Google Scholar]
- Schönemann A. Schläfenbein und Schädelbasis, eine anatomisch-otiatrische Studie. N. Denkschr. algem. Schweizer. Gesellsch. gesamt. Naturwiss. 1906;40:95–160. [Google Scholar]
- Sercer A. L'étiopathologá, ánie de l'otospongiose et les facteurs anthropologiques. Arch. Ital. Otol. Rinol. Laryngol. 1958;69(34):1–92. [PubMed] [Google Scholar]
- Sercer A, Krmpotic J. Further contributions to the development of the labyrinthine capsule. J. Laryngol. Otol. 1958;72:688–698. [PubMed] [Google Scholar]
- Siebenmann F. Die Korrosions-Anatomie Des Knöchernen Labyrinthes Des Menschlichen Ohres. Wiesbaden: J.F. Bergmann; 1890. [Google Scholar]
- Sokal RR, Rohlf FJ. Biometry. 3. New York: W.H. Freeman; 1995. [Google Scholar]
- Spoor CF. PhD thesis. Utrecht University; 1993. The comparative morphology and phylogeny of the human bony labyrinth. [Google Scholar]
- Spoor F, Wood B, Zonneveld F. Implications of early hominid labyrinthine morphology for the evolution of human bipedal locomotion. Nature. 1994;369:645–648. doi: 10.1038/369645a0. [DOI] [PubMed] [Google Scholar]
- Spoor F, Zonneveld F. The bony labyrinth in Homo erectus, a preliminary report. Cour. Forschsinst. Senckenberg. 1994;171:251–256. [Google Scholar]
- Spoor F, Zonneveld F. Morphometry of the primate bony labyrinth: a new method based on high resolution computed tomography. J. Anat. 1995;186:271–286. [PMC free article] [PubMed] [Google Scholar]
- Spoor F, Wood B, Zonneveld F. Evidence for a link between human semicircular canal size and bipedal behaviour. J. Hum. Evol. 1996;30:183–187. [Google Scholar]
- Spoor F. Basicranial architecture and relative brain size of Sts 5. (Australopithecus africanus) and other Plio-Pleistocene hominids. South African J. Sci. 1997;93:182–187. [Google Scholar]
- Spoor F, Zonneveld F. A comparative review of the human bony labyrinth. Yearbook Phys. Anthrop. 1998;41:211–251. doi: 10.1002/(sici)1096-8644(1998)107:27+<211::aid-ajpa8>3.3.co;2-m. [DOI] [PubMed] [Google Scholar]
- Spoor F, Bajpai S, Hussain ST, Kumar K, Thewissen JGM. Vestibular evidence for the evolution of aquatic behaviour in early cetaceans. Nature. 2002a;417:163–166. doi: 10.1038/417163a. [DOI] [PubMed] [Google Scholar]
- Spoor F, Esteves F, Tecelão Silva F, Pacheco Dias R. The bony labyrinth of Lagar Velho 1. In: Zilhão J, Trinkaus E, Duarte C, editors. The Lapedo Child, a Gravettian Human Skeleton from the Abrigo Do Lagar Velho. Lisbon: Instituto Portugus de Arqueologia; 2002b. pp. 287–292. Chapter 18, Trabalhos de Arqueologia, 22. [Google Scholar]
- Spoor F, Hublin JJ, Kondo O. The bony labyrinth of the Dederiyeh child. In: Akazawa T, Muhesen S, editors. Neanderthal Burials. Excavations of the Dederiyeh Cave, Afrin, Syria. Tokyo: The Tokyo University Press; 2002c. pp. 215–220. Chapter 7. [Google Scholar]
- Spoor F, Hublin JJ, Braun M, Zonneveld F. The bony labyrinth of Neanderthals. J. Hum. Evol. 2003;44:141–165. doi: 10.1016/s0047-2484(02)00166-5. [DOI] [PubMed] [Google Scholar]
- Spoor F. The semicircular canal system and locomotor behaviour, with special reference to hominin evolution. Cour. Forschsinst. Senckenberg. 2003;243:93–104. [Google Scholar]
- Streeter GL. The factors involved in the excavation of the cavities in the cartilaginous capsule of the ear in the human embryo. Am. J. Anat. 1917;22:1–25. [Google Scholar]
- Streeter GL. The histogenesis and growth of the otic capsule and its contained periotic tissue-spaces in the human embryo. Contrib. Embryol. 1918;7:5–53. [Google Scholar]
- Tremble GE. The bony labyrinth of the new-born infant and of the adult. Arch. Otolaryngol. 1929;9:175–180. [Google Scholar]
- Turkewitsch BG. Alters- und Geslechtseigenschaften de anatomischen Baues des menschlichen knöchernen Labyrinthes. Anat. Anz. 1930;70:225–234. [Google Scholar]
- Van de Water TR, Represa J. Tissue interactions and growth factors that control development of the inner ear. Neural tube–otic anlage interaction. Ann. NY Acad. Sci. 1991;630:116–128. doi: 10.1111/j.1749-6632.1991.tb19580.x. [DOI] [PubMed] [Google Scholar]