Abstract
Cytochrome P450 aromatase is a terminal enzyme that catalyses the conversion of androgens into oestrogens. This study investigated the immunohistochemical localization of aromatase in human efferent ductules and proximal ductus epididymis using a mouse anti-human monoclonal P450arom IgG as primary antibody and a goat anti-mouse biotinylated IgG as secondary antibody. A strong immunoreaction was observed in the epithelial cell cytoplasm of both ductuli efferentes and proximal ductus epididymis, whereas the smooth muscle cells were immunonegative in the two regions. The results show, for the first time in humans, that epithelial cells of ductuli efferentes and proximal caput epididymis express aromatase, suggesting that locally produced oestrogens may have a role in epididymal function.
Keywords: caput epididymis, oestrogens, human genital tract, male reproduction, P450arom
Introduction
It is now accepted that oestrogens play a physiological role in male reproduction (O’Donnel et al. 2001; Carreau et al. 2003). Cytochrome P450 aromatase is a terminal enzyme catalysing the conversion of androgens to oestrogens (Simpson et al. 1994), and its expression in male genital tract tissues suggests local oestrogen biosynthesis. In several mammalian species, aromatase has been revealed in Leydig cells, Sertoli cells and germ cells, suggesting oestrogen involvement in gonadal development and in sperm maturation (Nitta et al. 1993; Almadhidi et al. 1995; Levallet et al. 1998; Carpino et al. 2001). In humans, P450arom expression has been demonstrated in prostatic cells (Matzkin & Soloway, 1992), in Leydig cells (Inkster et al. 1995), in elongated spermatids (Turner et al. 2002) and in ejaculated sperm (Aquila et al. 2002; Rago et al. 2003) but the pattern of cellular aromatase distribution in human epididymis is unknown. The aim of this study was the immunolocalization of P450arom in human ductuli efferentes and proximal ductus epididymis.
Materials and methods
Ductuli efferentes and proximal ductus epididymis were obtained, after informed consent, from six patients (aged 37–40 years) with spermatocytic seminoma, undergoing therapeutic orchidectomy. The members of the ethical committee of the University of Calabria approved the investigation programme. Tissues were fixed in formalin (4%), dehydrated in a series of ethanol concentrations and paraffin-embedded. The sections (5 μm thick) were mounted on slides precoated with polylysine, deparaffinized and rehydrated (7–8 serial sections for each sample). Morphological analysis was carried out by haematoxylin and eosin staining. Immunohistochemistry was performed after heat-mediated antigen retrieval. Hydrogen peroxide (3% in distilled water for 30 min) was used to inhibit endogenous peroxidase activity. Normal goat serum (10% for 30 min) was used to block non-specific binding sites. P450arom immunodetection was carried out using a mouse monoclonal antiserum generated against human P450 aromatase (Serotec, Oxford, UK), (1 : 50) at 4 °C overnight. A biotinylated goat–anti-mouse IgG (Vector Laboratories, CA, USA) was applied (1 : 600) for 1 h at room temperature, followed by the avidin–biotin–horseradish peroxidase complex (ABC/HRP) (Vector Laboratories). Reactivity was visualized using the 3-amino-9-ethylcarbazole chromogen (AEC) (Zymed Laboratories, CA, USA), and sections were counterstained with haematoxylin. Controls involved (1) the primary antibody replaced by normal mouse serum and (2) a primary antibody pre-absorbed with an excess of purified aromatase protein (Hauptman-Woodward Medical Research Institute, Buffalo, NY, USA) (4 °C for 48 h).
Results
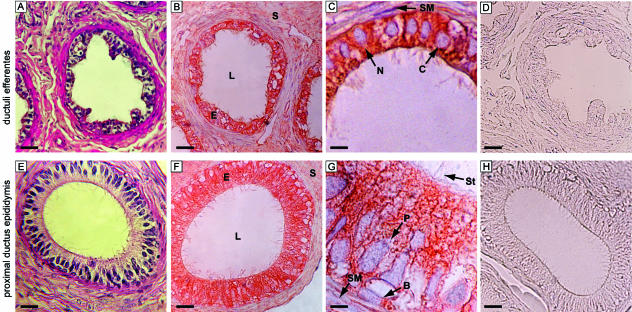
Haematoxylin and eosin staining showed a well-preserved morphology of both the ductuli efferentes (Fig. 1A) and the proximal ductus epididymis (Fig. 1E). The typical epithelium of ductuli efferentes, with alternating groups of columnar and cuboidal cells (ciliated and non-ciliated cells), and also with circular smooth muscle cell layer, was present. Ductus epididymis had a more regular lumen with epithelial basal and principal cells and a smooth muscle cell coat. Ductuli efferentes showed a strong immunoreaction in the cytoplasm of ciliated and non-ciliated cells, but the nuclei of the same cells were not stained (Fig. 1B,C). The smooth muscle cells surrounding the ductuli were also immunonegative. Proximal ductus epididymis showed intense immunostaining in the cytoplasm of the basal and principal cells, but again their nuclei were unstained (Fig. 1F,G). No immunoreactivity was observed in the smooth muscle cells. Control sections (Fig. 1D,H) and absorption control sections (data not shown) were all immunonegative, confirming the immunostaining specificity.
Fig. 1.
Morphology and immunohistochemistry of representative human ductuli efferentes (A–D) and proximal ductus epididymis (E–H). (A) Haematoxylin and eosin staining. (B,C) Strong aromatase immunoreactivity in cytoplasm of epithelial cells and immunonegative smooth muscle cells. (D) No immunostaining in control section. (E) Haematoxylin and eosin staining. (F,G) Strong aromatase immunostaining in cytoplasm of epithelial cells and unstained smooth muscle cells. (H) No immunoreactivity in control section. B, basal cells; C, ciliated cells; E, epithelial cells; L, lumen; N, non-ciliated cells; P, principal cells; S, stromal cells; SM, smooth muscle cells; St, stereocilia. Scale bars = A, B, D, 20 μm; E, F, H, 12.5 μm; C, 8 μm; G, 5 μm.
Discussion
These data show, for the first time, ductuli efferentes and proximal ductus epididymis as sites of aromatase expression in humans, suggesting oestrogen biosynthesis in these parts of the genital tract. In several non-human species, a functional role of oestrogens on epididymal tissues has been indicated by oestrogen receptor expression (Nie et al. 2002; Hess, 2003). The primary function of the ductuli efferentes is the transport of sperm from the rete testis to the epididymal duct and the reabsorption of the luminal fluid (∼90%) to increase sperm concentration. In the ductuli efferentes of rat and mouse, it has been demonstrated that oestrogens, via oestrogen receptors, can regulate Na+ re-absorption and passive water transport, and are also responsible for maintenance of the apical cytoarchitecture in epithelial cells (Hess et al. 1997a,b, 2000; Lee et al. 2000; Zhou et al. 2001).
The proximal ductus epididymis provides a physiological environment for maturation of sperm and supports its progression toward the vas deferens. Oxytocin influences contractile activity of the epididymis through oxytocin receptors, which have been detected in epithelial and smooth muscle cells of the epidyidymis in different species (Maggi et al. 1987; Einspanier & Ivell, 1997; Filippi et al. 2002a). Recently, it has been demonstrated that oestrogens influence the expression and activity of oxytocin receptors in rabbit epididymis, suggesting the regulation of oxytocin responsiveness as a function of oestrogens in the male (Filippi et al. 2002b).
In humans, oestrogen receptor expression has been demonstrated in the epididymis (Ergun et al. 1997), but the functional role of oestrogens on this genital duct is still not defined. Unfortunately, epididymal tissues were not investigated in the only adult male with an inactivating mutation in the oestrogen receptor α gene (Smith et al. 1994) or in the two male patients with aromatase deficiency (Morishima et al. 1995; Carani et al. 1997).
Considering that oxytocin receptors have been detected in the human epididymal epithelium (Frayne & Nicholson, 1998; Filippi et al. 2002a), our localization of aromatase in the same cells suggests that oestrogen could also influence oxytocin responsiveness in humans.
Until now, the major source of oestrogens in the upper epididymis has been thought to be the conversion of androgens to oestrogens by sperm. Our data suggest that androgen aromatization in ductuli efferentes and proximal ductus epididymis also plays a role. The functional activity of caput epididymis could therefore be influenced by locally produced oestrogens.
Acknowledgments
This work was supported by the Ministero dell’Università e della Ricerca Scientifica e Tecnologica (Murst 60%).
References
- Almadhidi J, Seralini GE, Fresnel J, Silberzahn P, Gaillard JL. Immunohistochemical localization of cytochrome P450 aromatase in equine gonads. J. Histochem. Cytochem. 1995;6:571–577. doi: 10.1177/43.6.7769228. [DOI] [PubMed] [Google Scholar]
- Aquila S, Sisci D, Gentile ML, Middea E, Siciliano L, Andò S. Human ejaculated spermatozoa contain active P450 aromatase. J. Clin. Endocrinol. Metab. 2002;87:3385–3390. doi: 10.1210/jcem.87.7.8633. [DOI] [PubMed] [Google Scholar]
- Carani C, Quin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, et al. Effect of testosterone and estradiol in a man with aromatase deficiency. N. Engl. J. Med. 1997;337:91–95. doi: 10.1056/NEJM199707103370204. [DOI] [PubMed] [Google Scholar]
- Carpino A, Pezzi V, Rago V, Bilinska B, Andò S. Immunolocalization of cytochrome P450 aromatase in rat testis during postnatal development. Tissue Cell. 2001;33:349–353. doi: 10.1054/tice.2001.0186. [DOI] [PubMed] [Google Scholar]
- Carreau S, Lambard S, Delalande C, Denis-Galeraud I, Bilinska B, Bourguiba S. Aromatase expression and role of oestrogens in male gonads: a review. Reprod. Biol. Endocrinol. 2003;1:35–00. doi: 10.1186/1477-7827-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einspanier A, Ivell R. Oxytocin and oxytocin receptor expression in reproductive tissues of the male marmoset monkey. Biol. Reprod. 1997;56:416–422. doi: 10.1095/biolreprod56.2.416. [DOI] [PubMed] [Google Scholar]
- Ergun S, Ungefroren H, Holstein AF, Davidoff MS. Estrogen and progesterone receptors and estrogen receptor-related antigen (ER-D5) in human epididymis. Mol. Reprod. Dev. 1997;47:448–455. doi: 10.1002/(SICI)1098-2795(199708)47:4<448::AID-MRD12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Filippi S, Vannelli GB, Granchi S, Luconi M, Crescioli C, Mancina R, et al. Identification, localization and functional activity of oxytocin receptors in epididymis. Mol. Cell Endocrinol. 2002a;193:89–100. doi: 10.1016/s0303-7207(02)00101-6. [DOI] [PubMed] [Google Scholar]
- Filippi S, Luconi M, Granchi S, Vignozzi L, Bettuzzi S, Tozzi P, et al. Estrogens, but not androgens, regulate expression and functional activity of oxytocin receptors in rabbit epididymis. Endocrinology. 2002b;143:4271–4280. doi: 10.1210/en.2002-220384. [DOI] [PubMed] [Google Scholar]
- Frayne J, Nicholson HD. Localization of oxytocin receptors in the human and macaque monkey male reproductive tracts: evidence for a physiological role of oxytocin in the male. Mol. Hum. Reprod. 1998;4:527–532. doi: 10.1093/molehr/4.6.527. [DOI] [PubMed] [Google Scholar]
- Hess RA, Gist DH, Bunick D, Lubahn DB, Farrel A, Bahr J, et al. Estrogen receptor (α & β) expression in the excurrent ducts of the adult male rat reproductive tract. J. Androl. 1997a;18:602–611. [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lee KH, Bahr J, Taylor Korach KS, Lubahn DB. A role for oestrogens in the male reproductive system. Nature. 1997b;390:509–512. doi: 10.1038/37352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RA, Bunick D, Lubahn DB, Zhou Q, Bouma J. Morphological changes in efferent ductules and epididymis in estrogen receptor-α knockout mice. J. Androl. 2000;21:107–121. [PubMed] [Google Scholar]
- Hess RA. Estrogens in the adult male reproductive tract: a review. Reprod. Biol. Endocrinol. 2003;1:52–00. doi: 10.1186/1477-7827-1-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inkster S, Yue W, Brodie A. Human testiculae aromatase: immunocytochemical and biochemical studies. J. Clin. Endocrinol. Metab. 1995;80:1941–1947. doi: 10.1210/jcem.80.6.7539819. [DOI] [PubMed] [Google Scholar]
- Lee KH, Hess RA, Bahr JM, Lubahn DB, Taylor J, Bunick D. Estrogen receptor alpha has a functional role in the mouse rete testis and efferent ductules. Biol. Reprod. 2000;63:1873–1880. doi: 10.1095/biolreprod63.6.1873. [DOI] [PubMed] [Google Scholar]
- Levallet J, Bilinska B, Mittrè H, Genissel C, Fresnel J, Carreau S. Expression and immunolocalization of functional cytochrome P450 aromatase in mature rat testicular cells. Biol. Reprod. 1998;58:919–926. doi: 10.1095/biolreprod58.4.919. [DOI] [PubMed] [Google Scholar]
- Maggi M, Malozowski S, Guardabasso V, Rodbard D. Identification and characterization of two classes of receptors for oxytocin and vasopressin in porcine tunica albuginea, epididymis, and vas deferens. Endocrinology. 1987;120:986–994. doi: 10.1210/endo-120-3-986. [DOI] [PubMed] [Google Scholar]
- Matzkin H, Soloway MS. Immunohistochemical evidence of the existence and localization of aromatase in human prostatic tissues. Prostate. 1992;21:309–314. doi: 10.1002/pros.2990210407. [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. Aromatase deficiency in male and female sibling caused by a novel mutation and the physiological role of estrogens. J. Clin. Endocrinol. Metab. 1995;80:3689–3698. doi: 10.1210/jcem.80.12.8530621. [DOI] [PubMed] [Google Scholar]
- Nie R, Zhou Q, Jassim E, Saunders PTK, Hess RA. Differential expression of estrogen receptors α and β in the reproductive tract of adult male dogs and cats. Biol. Reprod. 2002;66:1161–1168. doi: 10.1095/biolreprod66.4.1161. [DOI] [PubMed] [Google Scholar]
- Nitta H, Bunick D, Hess RA, Janulis L, Newton SC, Milette CF, et al. Germ cells of the mouse testis express P450 aromatase. Endocrinology. 1993;132:1396–1401. doi: 10.1210/endo.132.3.8440194. [DOI] [PubMed] [Google Scholar]
- O’Donnel L, Robertson KM, Jones ME, Simpson ER. Estrogen and spermatogenesis. Endocr. Rev. 2001;22:289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- Rago V, Bilinska B, Palma A, Andò S, Carpino A. Evidence of aromatase localization in cytoplasmic droplet of human immature ejaculated spermatozoa. Folia Histochem. Cytobiol. 2003;41:23–27. [PubMed] [Google Scholar]
- Simpson ER, Mahendroo MS, Means GD, Kilgore MW, Hinshelwood MM, Graham-Lorence S, et al. Aromatase cytochrome P450, the enzyme responsible for estrogen biosynthesis. Endocr. Rev. 1994;15:342–355. doi: 10.1210/edrv-15-3-342. [DOI] [PubMed] [Google Scholar]
- Smith EP, Boyd J, Frank GR, Takahashi H, Cohen RM, Specker B, et al. Estrogen resistance caused by a mutation in the estrogen-receptor gene in a man. N. Engl. J. Med. 1994;331:1056–1061. doi: 10.1056/NEJM199410203311604. [DOI] [PubMed] [Google Scholar]
- Turner KJ, Macpherson S, Millar MR, McNeilly AS, Williams K, Cranfield M, et al. Development and validation of a new monoclonal antibody to mammalian aromatase. J. Endocrinol. 2002;172:21–30. doi: 10.1677/joe.0.1720021. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Clarke L, Nie R, Carnes K, Lai LW, Lien YH, et al. Estrogen action and male fertility: roles of the sodium/hidrogen exchanger-3 and fluid reabsorption in reproductive tract function. Proc. Natl Acad Sci. USA. 2001;98:14132–14137. doi: 10.1073/pnas.241245898. [DOI] [PMC free article] [PubMed] [Google Scholar]