Abstract
Histological analysis of a growth series of alligator femora tests the correlation between strain milieu and microstructure. From mid-diaphyseal cross-sections of these femora (n = 7), vascular canal orientation and density as well as collagen fibre organization were recorded. Throughout ontogeny, the proportion of transverse–spiral (TS) collagen in the dorsal cortex is significantly greater than it is in the ventral cortex (P = 0.008). This regional difference in the proportion of TS collagen is correlated with a regional difference in the state of peak principal strain (compressive or tensile). Nevertheless, the predominant orientation of collagen fibres is longitudinal, which is inconsistent with biomechanical hypotheses that involve peak principal or shear strains. Although the density and orientation of vascular canals do not show significant regional differences (P = 0.26 and P = 0.26, respectively), as with collagen orientation, the vascular canal orientation is predominantly longitudinal. The longitudinal organization of both the vascular canals and the collagen fibres is probably a consequence of longitudinal shifting of subperiosteal osteoid during femoral lengthening. When taken together, these data suggest that growth dynamics is the dominant influence on the histological organization of primary bony tissues in alligator femora.
Keywords: alligator femur, bone adaptation, bone morphogenesis, bone strain, collagen fibre orientation, vascular canal orientation
Introduction
The correlation of long bone structure to their mechanical environment is well documented and is usually addressed at either the organ or the tissue level of integration (sensuPetersen, 1930). At the organ level, examples include the self-correction of abnormally curved bones (Bassett, 1965) and diaphyseal hypertrophy during overloading (Goodship et al. 1979; Lanyon et al. 1982). The tissue level of integration also reveals a striking relationship between form and function. At this level, several studies report a correlation between histological organization of cortical bony tissue and the strain pattern that tissue experiences (Riggs et al. 1993b; Petrtýl et al. 1996; de Margerie, 2002; Kalmey & Lovejoy, 2002).
Collagen fibre organization and the distribution and orientation of vascular canals are two histological parameters that have been investigated in recent years. Across transverse sections of primate and horse long bones, differential organization of collagen is correlated with the cross-sectional strain distribution produced during locomotion (Portigliatti-Barbos et al. 1984; Riggs et al. 1993b; Martin et al. 1996; Kalmey & Lovejoy, 2002). Regions of cortical bony tissue that experience peak tensile strain contain largely longitudinal–spiral (LS) collagen, whereas regions that experience peak compressive strain contain largely transverse–spiral (TS) collagen. Furthermore, in the mallard (Anas platyrhynchos), long bones that presumably experience predominantly torsional loads have significantly more circumferential vascular canals than bones that experience predominantly bending loads (de Margerie, 2002). However, a major issue that remains largely unaddressed, particularly in the collagen fibre literature, is whether these patterns of organization are consistent throughout ontogeny. Consistent functional organization of bony tissue throughout ontogeny would suggest that bone mechanics is relatively important despite growth demands.
This paper deals with the bony tissue parameters of collagen fibre and vascular canal organization in the context of ontogeny. Femora of Alligator mississippiensis were selected because the relationship between these histological parameters and mechanical function have only been studied in a limited set of mammals and birds, which experience rapid rates of osteogenesis (Riggs et al. 1993a; de Margerie, 2002; Kalmey & Lovejoy, 2002; Skedros et al. 2003). Furthermore, the degree of secondary remodelling in alligator femora is low (Enlow & Brown, 1957; de Ricqlès et al. 1991), so one can test whether collagen fibres and vascular canals in primary bony tissue reflect their bone strain milieu like those in secondary tissues (Lanyon & Bourne, 1979; Riggs et al. 1993b; Petrtýl et al. 1996; Kalmey & Lovejoy, 2002). In vivo strain data of the femur are also available for Alligator (Blob & Biewener, 1999). Throughout the stance phase, peak compressive principal strain occurs in the dorsal cortex whereas peak tensile principal strain occurs in the ventral cortex. Additionally, the maximum principal strain direction is aligned significantly away from the femoral axis (47° dorsal and 29° ventral). This suggests that both cortices experience substantial shear strain during locomotion; in fact, average peak shear strains are 39% greater than peak principal strains (Blob & Biewener, 1999). If the functional organization of bony tissue is consistent throughout ontogeny, then (1) the histological organization should be predominantly transverse and (2) the dorsal cortices should have significantly more TS collagen and transversely orientated canals than the ventral cortices.
Methods and materials
An ontogenetic series of alligator (A. mississippiensis) femora was provided by the Rockefeller Wildlife Refuge (Grand Chenier, Louisiana, USA). The series (n = 7) sampled individuals from prehatching to adult stages of development (Table 1). Femora were manually defleshed, and their lengths were measured from the most proximal surface of the femoral head to the proximal surface of the intercondylar groove. The cartilaginous caps were not removed so each femur length was a combined length of bone and cartilage. The femora were then fixed in 10% neutral buffered formalin for 1 week.
Table 1.
Histological parameters of alligator femora
| ID | Femoral length (mm) | Snout–tail length (mm) | % Dorsal TS collagen | Dorsal vascular density | % Dorsal trans canals | % Ventral TS collagen | Ventral vascular density | % Ventral trans canals |
|---|---|---|---|---|---|---|---|---|
| 1 | 25 | 304.8 | 19.4 | 34 | 15 | 2.1 | 53 | 17 |
| 2 | 35 | 444.5 | 0.2 | 19 | 7 | 0.0 | 47 | 3 |
| 3 | 40 | 558.8 | 2.1 | 30 | 3 | 1.8 | 35 | 12 |
| 4 | 72.5 | 1092 | 7.5 | 19 | 9 | 1.0 | 26 | 9 |
| 5 | 160 | 2235 | 2.1 | 8 | 10 | 1.5 | 7 | 14 |
| 6 | 165 | 2286 | 3.7 | 12 | 7 | 3.1 | 12 | 19 |
| 7 | 171 | 2286 | 1.3 | 12 | 10 | 1.2 | 12 | 10 |
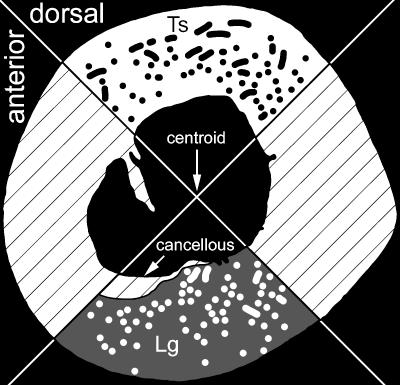
After fixation, a 1-cm-thick block was cut from each femur. To minimize the influence of large muscle insertions such as that of the insertion for the m. caudofemoralis, blocks were taken at mid-diaphysis, approximately 60% of the femoral length from the head to the intercondylar groove. The mid-diaphyseal blocks were vacuum embedded in polymethyl methacrylate (PMMA). From these blocks, an undecalcified, transverse thick section was cut from each femur, mounted on to a slide, and ground and polished to 100 ± 10 μm. Under circularly polarized light microscopy (Boyde & Riggs, 1990) for each section, greyscale images were captured by a CCD (Polaroid) at 40× magnification. Because light intensity affects the birefringence of collagen, the same illumination and CCD exposure time were used for each section. The images were then montaged in Photoshop (Adobe, ver. 6.0). Each map was then divided into quadrants based on anatomical orientation as defined by Romer (1956) and Blob & Biewener (1999) (Fig. 1). When the femoral condyles are resting on a horizontal surface and the femur is aligned horizontally, the dorsal quadrant faces upwards and the anterior quadrant faces the direction of femoral protraction.
Fig. 1.
Location of quadrants in a stylized femoral cross-section and method of distinguishing orientation of vascular canals. Canals that are parallel to the long axis of the femur are longitudinal (Lg), and those that are more perpendicular to the long axis are transverse (Ts). Hatched regions were not analysed.
Vascular canals in each quadrant were counted and their orientation (longitudinal or transverse) was recorded. A vascular canal was considered transverse if its major cross-sectional axis was at least three times greater than its minor axis (Fig. 1). Non-parametric Friedman anovas (SPSS, Systat, ver. 7.0) were performed to test whether there was a significant difference in canal density between the dorsal and ventral quadrants and whether the quadrants differed in the proportion of transverse canals throughout ontogeny. P-values less than 0.05 were considered significant.
To prepare the maps for an analysis of collagen orientation, vascular and medullary spaces as well as the occasional secondary osteon were erased and replaced by black pixels (index value = 0). These black pixels were not analysed further. Under circularly polarized light, LS collagen fibres appear dark (i.e. their index values approach 1). Conversely, TS collagen fibres have greyscale indices that approach 255. Image analysis was performed in ArcView GIS (ESRI, ver. 3.2) with Spatial Analyst (ESRI, ver. 2.0). Pixels from each quadrant were reclassified and colour-coded using a 9-bin scheme similar to the 15-bin scheme in Carando et al. (1991). To prevent counting pixels that represent compacted coarse cancellous and endosteal lamellae, region-of-interest masks were used within ArcView GIS. Consequently, only pixels representing primary cortical tissue were counted. To calculate the proportion of TS collagen in each dorsal and ventral quadrant, pixels in bins 8 and 9 were summed and divided by the total number of pixels within that quadrant. A non-parametric Friedman anova (SPSS, Systat, ver. 7.0) was performed to test whether the proportion of TS collagen between the dorsal and ventral quadrants was significantly different. P-values were significant if they were less than 0.05.
Results
Histological descriptions
Dorsal quadrant
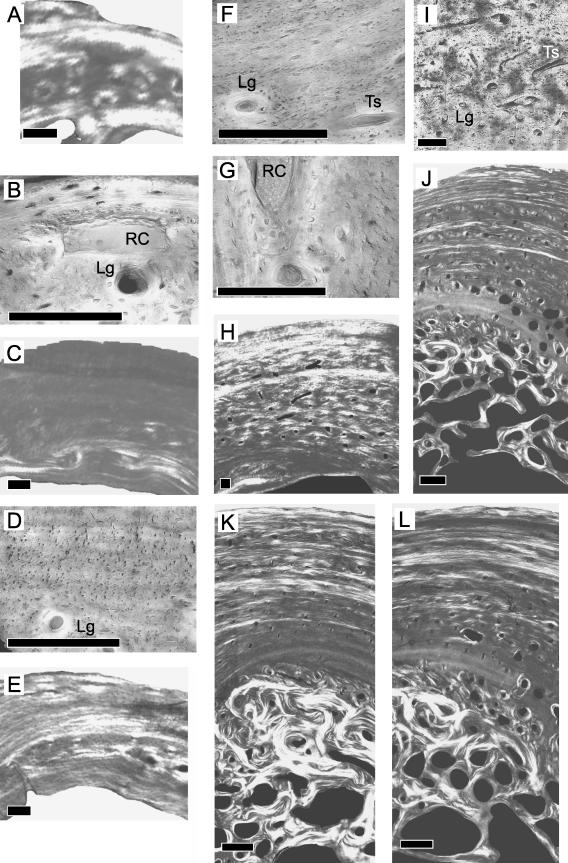
Specimen 1 contains three zones (Fig. 2A). The outermost zone is lamellar and is largely composed of TS collagen fibres. This region lacks vascularization but a large resorption cavity is present between the outermost and middle zone (Fig. 2B). The middle zone is intermediate between lamellar and parallel-fibred tissue, and collagen fibres are generally orientated longitudinally (Fig. 2A). Circumferential lamellae are not clearly visible. Lamellar organization, however, is more visible within the numerous primary osteons. Most of those primary osteons are longitudinal (Fig. 2B). The innermost zone is lamellar and is largely composed of TS collagen. No vascular canals are present in this region.
Fig. 2.
Ontogenetic sampling of the bony tissue from the dorsal quadrant. Scale bars = 100 μm (A–I) and 500 μm (J–L). Superficial is up, deep is down, anterior is to the left, posterior is to the right. Specimens 1–7, general view under circularly polarized light (A,C,E,H,J,K,L). Detailed view under non-polarized light (B,D,F,G,I). Resorption cavity (RC), longitudinal canal (Lg), transverse canal (Ts).
In specimen 2, parallel-fibred lamellar tissue makes up the outer two-thirds of the cortex (Fig. 2C). Collagen fibres are largely LS. Primary osteons are abundant in the inner third of the cortex, and most of them are longitudinal (Fig. 2D). The collagen fibres within the primary osteons are more transverse than the surrounding tissue. Endosteal lamellae are present and have a strong TS collagen component.
Specimen 3 contains parallel-fibred lamellar tissue (Fig. 2E). Lamellae of TS collagen generally alternate with lamellae of LS collagen throughout this quadrant although the lamellae in the middle part tend to contain more transversely orientated collagen (brighter). Vascular canals are found generally in two levels of the cortex: the first within 160 μm of the periosteal surface (Fig. 2F) and the second approximately 400 μm from the periosteal surface at about mid-cortex (Fig. 2G). A large resorption cavity is also present at this latter level.
The cortical tissue of the outermost 300 μm in specimen 4 has parallel-fibred organization (Fig. 2H). Much of that cortical tissue consists of TS collagen fibres. The relatively few canals in this region of the cortex are longitudinal. The remaining part of the cortex consists of several thin packets of lamellar tissue alternating with thick packets of primary osteons. Transverse canals are more abundant deeper in the cortex (Fig. 2I). LS collagen fibres are more prominent internally. On the internal surface of the cortex, thin endosteal lamellae are present.
In the dorsal quadrants of specimens 5–7, the cortex is thinner than it is in the other quadrants (Fig. 2J–L, respectively). Fine lamellar bony tissue comprises the cortical tissue from the periosteal surface to mid-cortex. Groups of lamellae that have more TS collagen alternate with groups of lamellae that have LS collagen, and both are generally thinner than those from the other quadrants. Within the lamellar framework, canals that lack osteonal lamellae (simple, sensude Ricqlès et al. 1991) as well as primary osteons are found. Those canals are generally more abundant towards the mid-cortex and are generally longitudinal. From mid-cortex to the endosteal surface, there is parallel-fibred bony tissue with primary osteons and resorption cavities. Resorption cavities in the dorsal quadrant are less numerous than they are in the other quadrants. Secondary osteons generally form towards the endosteal surface. Deep to the parallel-fibred region, compacted coarse cancellous tissue is found. Collagen fibres in this tissue are generally more transverse than those in other parts of the quadrant.
Ventral quadrant
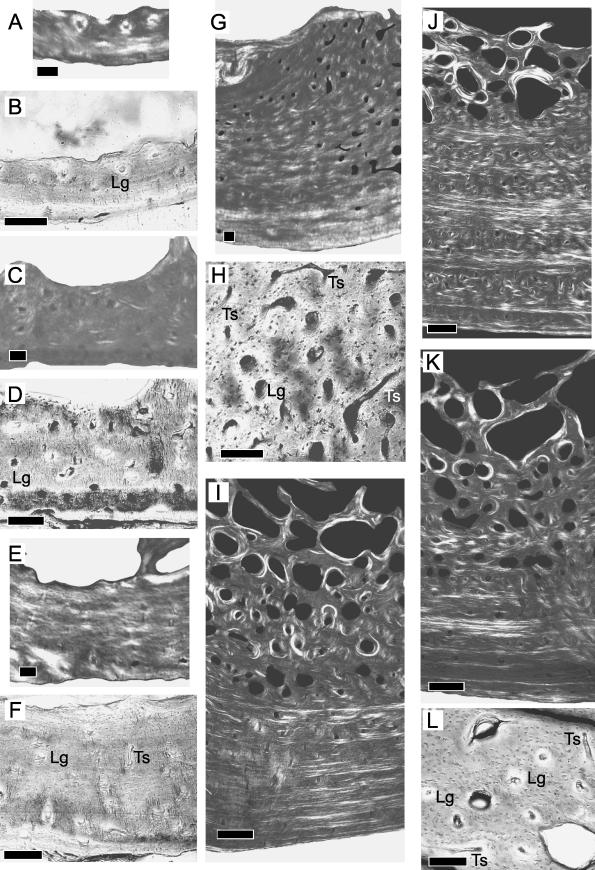
Most of specimen 1 is lamellar tissue (Fig. 3A). Distinct lamellation is often interrupted. Collagen fibres in the outer half of the cortex appear more transverse than in the inner half. Most of the vascular canals are found within primary osteons deep in the cortex (Fig. 3B). Their orientation is generally longitudinal. Resorption occurs along the endosteal surface.
Fig. 3.
Ontogenetic sampling of the bony tissue from the ventral quadrant. Scale bars = 100 μm (A–H) and 500 μm (I–L). Superficial is down, deep is up, anterior is to the left, posterior is to the right. Specimens 1–7, general view under circularly polarized light (A,C,E,G,I,J,K). Detailed view under non-polarized light (B,D,F,H,L). Longitudinal canal (Lg), transverse canal (Ts).
In specimen 2 (Fig. 3C), lamellar tissue is only apparent along the periosteal surface. Most of the cortex appears parallel-fibred. The dark birefringence suggests that the collagen fibres are predominantly LS. Longitudinal primary osteons are abundant throughout the cortex (Fig. 3D). Although resorption occurs along short lengths of the endosteal surface, much of that surface is covered with endosteal lamellae.
Lamellar tissue is found in specimen 3 (Fig. 3E). Lamellae that contain strongly LS collagen fibres alternate with those that contain more transversely orientated fibres. Within this lamellar framework, there are primary osteons. Most are longitudinal, but a few are transverse (Fig. 3F). Resorption is localized to the endosteal surface.
A 300-μm-thick region of the outer cortex in specimen 4 consists of fine lamellar tissue (Fig. 3G). The birefringence of the collagen fibres in that region is brighter than in other regions of the quadrant but is darker than in the corresponding region of the dorsal quadrant (Fig. 2H). Within this quadrant, however, TS collagen is localized to the outermost part of the cortex. Primary osteons are generally longitudinal although some are transverse. Deeper to the outer region, packets of LS-abundant lamellae alternate with packets of primary osteons. The dominant orientation of these primary osteons is longitudinal, although a number are transverse (circumferential or reticular) (Fig. 3H). Towards the endosteal surface, compact coarse cancellous tissue is present but only towards the anterior side of the quadrant. There is resorption on the posterior side.
The outermost region of the cortex to mid-cortex consists of finely lamellar tissue in specimens 5–7 (Fig. 3I–K, respectively). Generally, thin packets of TS-abundant lamellae alternate with thick packets of LS-abundant lamellae. Small primary osteons are prominent closer to the periosteal surface whereas larger primary osteons dominate the mid-cortex. The dominant osteonal orientation is longitudinal, but note the occasional transversely orientated osteon (Fig. 3L). Finely lamellar tissue also occurs from mid-cortex to the endosteal surface. Here, lamellae are also LS-abundant. Some lamellar packets contain primary osteons. Large resorption cavities are also common in this region, and secondary osteons appear within 1 mm from the endosteal surface. Along that surface, compact coarse cancellous tissue is not present.
Quantifying histological parameters
Collagen fibre organization
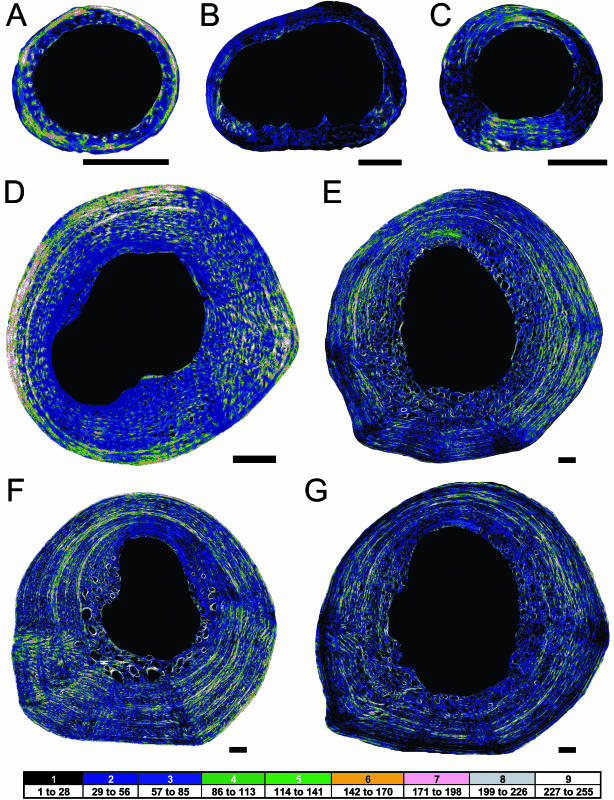
Throughout ontogenetic series, collagen fibres in the femora of alligator are predominantly aligned in the longitudinal direction (Fig. 4). TS collagen fibres are not particularly abundant and appear restricted to relatively thin packets of lamellae in the external cortex of both quadrants. Despite the low proportion of TS collagen throughout the cortex, the results support a weak regional difference in that proportion. The dorsal quadrants contain slightly more TS collagen than the ventral quadrants (Table 1; Fig. 4). Non-parametric statistical testing reveals a significant difference between the proportion of TS collagen fibres in the two quadrants (P = 0.008).
Fig. 4.
Ontogenetic series of collagen fibre distribution of the alligator femur. Scale bar = 1 mm. Specimen 1 (A), specimen 2 (B), specimen 3 (C), specimen 4 (D), specimen 5 (E), specimen 6 (F) and specimen 7 (G). Darker colours represent more longitudinal collagen fibres, and brighter colours represent more transverse collagen fibres. Medullary and vascular spaces are black. Dorsal is up, and anterior is left.
Vascular canal density and orientation
Vascular canals, whether simple or osteonal, are predominantly longitudinal and relatively abundant in the inner half of the cortex. They generally become less abundant towards the periosteal surface. Earlier in ontogeny, the vascular density in the ventral quadrants is slightly greater than it is in the dorsal quadrants. However, differences between the vascular density of the ventral and dorsal quadrants are not consistent or significant throughout ontogeny (P = 0.26; Table 1).
Several of the specimens in this ontogenetic sequence of alligator femora have a greater proportion of transverse canals in their ventral quadrants than in their dorsal quadrants (Table 1). However, this difference in proportion is neither consistent nor significant (P = 0.26; Table 1). Furthermore, the proportion of transverse canals in either quadrant does not show any clear trend during ontogeny.
Discussion
The objective of this study was to test whether bone strain milieu accounts for regional differences in the organization of histological parameters within an ontogenetic series of alligator femora. Across these femora, transverse (TS) collagen fibres occur in significantly greater proportion in the dorsal quadrants than in the ventral quadrants (P = 0.008; Table 1; Fig. 4). Because the dorsal and ventral quadrants, respectively, experience peak compressive and tensile strain during locomotion (Blob & Biewener, 1999), there is, albeit weak, support for the hypothesis that collagen fibre orientation is correlated with the state of principal bone strain. However, insignificant regional differences in the density of vascular canals (P = 0.26) and in the proportion of transverse canals (P = 0.26) do not support a clear relationship between canal orientation and principal bone strain. Furthermore, neither the vascular canal nor the collagen fibre orientation is predominantly transverse as predicted by the high magnitude of femoral shear strain. When taken together, the data from both these parameters suggest that bone strain milieu is not the sole determinant for the organization of collagen fibres and vascular canals during the growth of alligator femora.
Like all long bones, the size of the alligator femur increases during ontogeny through a combination of epiphyseal and subperiosteal osteogenesis (Duhamel, 1740; Horner et al. 2001). The former increases femoral length, and the latter increases femoral width. In subperiosteal osteogenesis, osteoblasts of the subperiosteum initially produce a non-mineralized collagenous matrix, which also incorporates blood vessels from the periosteum (Taylor, 1992). This matrix forms where the cross-sectional bending and shear strains on the femur are maximal and, as a consequence, collagen fibres and blood vessels in that matrix should be prestrained (de Ricqlès et al. 1991).
To an extent, the collagen fibre organization in the alligator femora reflects the state of principal bone strain. The distribution of TS and LS collagen fibres in the respective quadrants that experience peak compressive or tensile principal strain is generally consistent with previous reports on the organization of bone collagen (Riggs et al. 1993b; Martin et al. 1996; Kalmey & Lovejoy, 2002). Interestingly, the post-hatching organization of collagen fibres is also found in the prehatching femoral section (specimen 1; Figs 2A and 3A; Table 1). Whether epigenetics or genetics influences the collagen fibre organization of the prehatching femur cannot be directly tested by the present study because in ovo femoral strain and developmental data are not available for alligators. However, several studies do suggest that bone strain is an important epigenetic regulator. Movements in the embryonic limbs of developing chickens and humans (Carter et al. 1987, 1998; Chambers et al. 1995; Henderson & Carter, 2003) generate bone strains through muscular contractions and shell/uterine reaction forces. In the absence of bone strain, those embryonic limb bones experience reduced subperiosteal osteogenesis and increased endosteal resorption (Rodríguez et al. 1988; Osborne et al. 2002). Consequently, those limb bones have significantly thin cortices. Epiphyseal growth (bone lengthening), however, seems to proceed normally in the absence of bone strain (Rodríguez et al. 1988; Osborne et al. 2002). Because bone lengthening places the periosteum in tension (Taylor, 1992), the products of reduced subperiosteal osteogenesis would have a predominantly longitudinal organization.
Even in the presence of normal bone strains, the orientation of collagen fibres in the alligator femora is predominantly longitudinal. Because the alligator femur is not loaded primarily in axial tension (Blob & Biewener, 1999), the predominantly longitudinal organization of collagen fibres is more likely a reflection of the longitudinal growth dynamics of the femur. Additional support is found in the apparent cyclicity of the thick, LS collagen packets and the thin, TS packets (Fig. 4D–G). The cyclicity may be related to endogenous rhythms that produce seasonal changes in periosteal growth (Castanet et al. 1993). Both the predominantly longitudinal and the somewhat cyclical organization of collagen fibres suggest that, although bone strain milieu does influence the organization of collagen in alligator femora, growth dynamics has a greater influence on that organization.
Regional differences in vascular canal density and orientation are not significant. Furthermore, the vascular canals are predominantly longitudinal. These data are inconsistent with biomechanical hypotheses, which predict that (1) the femoral sections should have predominantly transverse canals and (2) the dorsal rather than the ventral quadrant should have a significantly greater proportion of transverse canals. That transverse canals are not more prominent in the alligator femoral sections is surprising from a biomechanical perspective, particularly if transverse canals are adaptations to shear strains (circular canals: de Margerie, 2002), because the alligator femur experiences average shear strains that are 39% greater than average principal strains (Blob & Biewener, 1999). However, the relative paucity of transverse canals is reasonable if one considers growth strategy. In alligator femora, relatively low rates of subperiosteal osteogenesis produce primary bony tissue that is parallel-fibred or lamellar-zonal. These patterns of tissue organization lack the spacious, woven scaffolding that characterizes rapidly deposited patterns (i.e. fibrolamellar) (Castanet et al. 2000; Horner et al. 2001; de Margerie et al. 2002). Consequently, vascular canals of parallel-fibred and lamellar-zonal tissues are more constrained in their orientation and are less likely to have circular, radial or reticular orientations. Furthermore, subperiosteal blood vessels experience longitudinal shifting as the femur grows lengthwise (Taylor, 1992; Petrtýl et al. 1996). These results suggest that the vascular canal organization in alligator femora primarily reflects growth dynamics.
Bone growth is a potentially confounding factor in understanding the relationship between histological organization and bone strain milieu. Most studies have focused on the secondary bony tissue of adults (Lanyon & Bourne, 1979; Portigliatti-Barbos et al. 1984; Riggs et al. 1993b; Hert et al. 1994; Kalmey & Lovejoy, 2002). To my knowledge, only three studies have reported comparative differences in the collagen fibre or vascular canal organization between secondary and primary bony tissue (Lanyon & Bourne, 1979; Riggs et al. 1993a; Petrtýl et al. 1996). These studies report, in sheep tibiae, horse radii and human femora, respectively, a predominantly longitudinal histological organization in the primary bony tissues. Subsequent remodelling of that primary tissue produces a ‘strain-mode-specificity’ (sensuSkedros et al. 2003) in the secondary osteons. These studies suggest that the relationship between histological organization and bone strain milieu in primary bony tissue may be fundamentally different from that relationship in secondary bony tissue.
The present study suggests that, in primary bony tissue of alligator femora, collagen fibre organization rather than vascular canal organization may be slightly more sensitive to bone strain milieu. Certainly, further testing is required; support for this relationship may vary intraspecifically across different long bones that experience different growth dynamics. If support for this relationship is consistent, however, collagen fibre organization may be an effective proxy for limb posture and movement. Whether different limb postures and kinematics are likely to alter the cross-sectional pattern of strain is currently under investigation. If, for example, sprawling limb kinematics produce a markedly different pattern of strain than do erect limb kinematics, then histological analyses in extinct taxa might provide valuable insights into the diversity and evolution of tetrapod locomotion.
Conclusions
In the primary bony tissue of an ontogenetic series of alligator femora, there is, albeit weak, support for a correlation between collagen fibre organization and peak principal bone strain. The presence of an ‘adult’ pattern of collagen fibre organization in the prehatching femur suggests that the biomechanical regulation of collagen organization begins in ovo. Whether epigenetic or genetic pathways dominate the direction of that organization is not tested because the microstructure of prenatal bones that do not receive biomechanical stimuli needs further study. Interestingly, a high proportion of the collagen fibres do not show ‘strain-mode-specificity’ as related to bone strain (sensuSkedros et al. 2003). LS collagen fibres dominate in all sections and that orientation is probably a consequence of the longitudinal shifting of subperiosteal osteoid during femoral elongation. Apparently, growth dynamics rather than bone strain milieu has a greater influence on the organization of alligator femoral collagen.
The data do not support a biomechanical interpretation for the orientation of vascular canals in the primary tissue of alligator femora. Reduced subperiosteal deposition coupled with longitudinal shifting of the periosteum against the bone surface probably account for the largely longitudinal orientation of the vascular canals. When taken together, the collagen fibre and vascular canal analyses suggest that femoral growth plays a dominant role in the organization of primary tissues in alligator femora.
Acknowledgments
I would like to thank Ruth Elsey of the Rockefeller Wildlife Refuge in Grand Chenier, LA, for contributing the growth series of alligator femora. I also thank William Zinsmeister, Ronald Hullinger and James Farlow for their critiques on the original dissertation chapter that developed into this manuscript. Reto Giere allowed me to adapt and use his photomicrographic system to capture circularly polarized images. Comments from Kenneth Angielczyk, Jonathan Kalmey, Galateia Kazakia, Kevin Padian, James Parham, Nick Pyenson, Marvalee Wake and two anonymous reviewers greatly improved the organization and presentation of this paper. Funding was provided by the Jurassic Foundation and the Paleontological Society. This is UCMP Publication number 1840.
References
- Bassett CAL. Electrical effects in bone. Sci. Am. 1965;213:18–25. doi: 10.1038/scientificamerican1065-18. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Blob RW, Biewener AA. In vivo locomotor strain in the hindlimb bones of Alligator mississippiensis and Iguana iguana: implications for the evolution of limb bone safety factor and non-sprawling limb posture. J. Exp. Biol. 1999;202:1023–1046. doi: 10.1242/jeb.202.9.1023. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Boyde A, Riggs CM. The quantitative study of the orientation of collagen in compact bone slices. Bone. 1990;11:35–40. doi: 10.1016/8756-3282(90)90069-b. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Carando S, Portigliatti-Barbos M, Ascenzi A, Riggs CM, Boyde A. Macroscopic shape of, and lamellar distribution within, the upper limb shafts, allowing inferences about mechanical properties. Bone. 1991;12:265–270. doi: 10.1016/8756-3282(91)90074-s. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Carter DR, Orr TE, Fyhrie DP, Schurman DJ. Influences of mechanical stress on prenatal and postnatal skeletal development. Clin. Orthop. 1987;219:237–250. 10.1111/j.0021-8782.2004.00275.x. [PubMed] [Google Scholar]
- Carter DR, Mikic B, Padian K. Epigenetic mechanical factors in the evolution of long bone epiphyses. Zool. J. Linn. Soc. 1998;123:163–178. [Google Scholar]
- Castanet J, Francillon-Vieillot H, Meunier FJ, de Ricqlès A. Bone and individual aging. In: Hall BK, editor. Bone, Vol. 7: Bone Growth – B. Boca Raton, FL: CRC Press; 1993. pp. 245–283. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Castanet J, Curry Rogers K, Cubo J, Boisard J-J. Periosteal bone growth rates in extant ratites (ostriche and emu). Implications for assessing growth in dinosaurs. C. R. Acad. Sci. Paris, Sci. la Vie. 2000;323:543–550. doi: 10.1016/s0764-4469(00)00181-5. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Chambers SH, Bradley NS, Orosz MD. Kinematic analysis of wing and leg movements for type I motility in E9 chick embryos. Exp. Brain Res. 1995;103:218–226. doi: 10.1007/BF00231708. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Duhamel HL. Observations and experiments with madder root. Phil. Trans. R. Soc. London. 1740;8:420–434. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Enlow DH, Brown SO. A comparative histological study of fossil and recent bone tissues. Part II. Reptilian and bird bone tissues. Texas J. Sci. 1957;2:186–203. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Goodship AE, Lanyon LE, MacFie H. Functional adaptation of bone to increased stress. J. Bone Jt Surg. 1979;61A:539–546. 10.1111/j.0021-8782.2004.00275.x. [PubMed] [Google Scholar]
- Henderson JH, Carter DR. Mechanical induction in limb morphogenesis: the role of growth-generated strains and pressures. Bone. 2003;31:645–653. doi: 10.1016/s8756-3282(02)00911-0. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Hert J, Fiala P, Petrtýl M. Osteon orientation of the diaphysis of the long bones in man. Bone. 1994;15:269–277. doi: 10.1016/8756-3282(94)90288-7. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Horner JR, Padian K, de Ricqlès AJ. Comparative osteohistology of some embryonic and perinatal archosaurs: developmental and behavioral implications for dinosaurs. Paleobiology. 2001;27:39–58. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Kalmey JK, Lovejoy CO. Collagen fiber orientation in the femoral necks of apes and humans: do their histological structures reflect differences in locomotor loading? Bone. 2002;31:327–332. doi: 10.1016/s8756-3282(02)00828-1. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Lanyon LE, Bourn S. The influence of mechanical function on the development and remodeling of the tibia. J. Bone Jt Surg. 1979;61A:263–272. 10.1111/j.0021-8782.2004.00275.x. [PubMed] [Google Scholar]
- Lanyon LE, Goodship AE, Pye CJ, MacFie JH. Mechanically adaptive bone remodeling. J. Biomech. 1982;15:141–154. doi: 10.1016/0021-9290(82)90246-9. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- de Margerie E. Laminar bone as an adaptation to torsional loads in flapping flight. J. Anat. 2002;201:521–526. doi: 10.1046/j.1469-7580.2002.00118.x. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Margerie E, Cubo J, Castanet J. Bone typology and growth rates: testing and quantifying ‘Amprino's rule’ in the mallard (Anas platyrhynchos) C. R. Biol. 2002;325:221–230. doi: 10.1016/s1631-0691(02)01429-4. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Martin RB, Lau ST, Matthews PV, Gibson VA, Stover SM. Collagen fiber organization is related to mechanical properties and remodeling in equine bone. A comparison of two methods. J. Biomech. 1996;29:1515–1522. 10.1111/j.0021-8782.2004.00275.x. [PubMed] [Google Scholar]
- Osborne AC, Lamb KJ, Lewthwaite JC, Dowthwaite GP, Pitsillides AA. Short-term rigid and flaccid paralyses diminish growth of embryonic chick limbs and abrogate joint cavity formation but differentially preserve pre-cavitated joints. J. Musculoskel. Neuron. Interact. 2002;2:448–456. 10.1111/j.0021-8782.2004.00275.x. [PubMed] [Google Scholar]
- Petersen H. Die Organe des Skelettsystems. In: Möllendorf V, editor. Handbuch der Mikroskopische Anatomie der Menschen. Berlin: Springer; 1930. p. 699. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Petrtýl M, Hert J, Fiala P. Spatial organization of the Haversian bone in man. J. Biomech. 1996;29:161–169. doi: 10.1016/0021-9290(94)00035-2. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Portigliatti-Barbos M, Bianco P, Ascenzi A, Boyde A. Collagen orientation in compact bone. II. Distribution of lamellae in the whole of the human femoral shaft with reference to its mechanical properties. Metab. Bone Dis. Rel. Res. 1984;5:309–316. doi: 10.1016/0221-8747(84)90018-3. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- de Ricqlès A, Meunier FJ, Castanet J, Francillon-Vieillot H. Comparative microstructure of bone. In: Hall BK, editor. Bone, Bone Matrix and Bone Specific Products. Vol. 3. Boston, MA: CRC Press; 1991. pp. 1–78. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Riggs CM, Lanyon LE, Boyde A. Functional associations between collagen fibre orientation and locomotor strain direction in cortical bone of the equine radius. Anat. Embryol. 1993a;187:231–238. doi: 10.1007/BF00195760. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Riggs CM, Vaughan LC, Evans GP, Lanyon LE, Boyde A. Mechanical implications of collagen fibre orientation in cortical bone of the equine radius. Anat. Embryol. 1993b;187:239–248. doi: 10.1007/BF00195761. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez JI, Garcia-Alix A, Palacios J, Paniagua R. Changes in long bones due to fetal immobility caused by neuromuscular disease. J. Bone Jt Surg. 1988;70A:1052–1060. 10.1111/j.0021-8782.2004.00275.x. [PubMed] [Google Scholar]
- Romer AS. Osteology of Reptiles. Chicago, IL: University of Chicago Press; 1956. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]
- Skedros JG, Hunt KJ, Hughes PE, Winet H. Ontogenetic and regional morphological variations in the turkey ulna diaphysis: implications for functional adaptation of cortical bone. Anat. Rec. 2003;273A:609–629. doi: 10.1002/ar.a.10073. 10.1111/j.0021-8782.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- Taylor JF. The periosteum and bone growth. In: Hall BK, editor. Bone, Vol. 6: Bone Growth – A. Boca Raton, FL: CRC Press; 1992. pp. 21–52. 10.1111/j.0021-8782.2004.00275.x. [Google Scholar]