Abstract
The blood supply of the symphysis pubis is still the subject of some debate. Classic anatomy books state that this joint is avascular, whereas some published works have shown blood vessels in young specimens. As several articular discs such as the knee menisci are known to have blood vessels in their peripheries, we decided to investigate the possible nutrition pathways to the interpubic disc and ligaments. We used 60 Wistar rats, male and female, aged between 28 and 32 days, or between 90 and 100 days. Samples were processed using a variety of techniques: regular histology, immunohistochemestry, India ink injection and corrosion casting. The interpubic disc consisted of an inner bearing portion and an outer fibrous rim. The interpubic ligaments and the fibrous rim were well vascularized in all groups. Marrow contacts between the interpubic disc and the subchondral bone were also observed. Blood vessels formed an authentic arterial circle embracing the joint, from which blood vessels branched into capillary loops facing the avascular inner bearing portion of the disc. These results confirm the need for future studies on the human symphysis pubis, to provide more details on its structure, which would enable clinicians such as physiotherapists to improve prognosis and treatment design. Future studies may also explain the pathways down which the hormone relaxin reaches its targets within this joint.
Keywords: blood supply, connective tissue, fibrocartilage, physical therapy, symphysis pubis
Introduction
Knowledge regarding the degree of vascularization of musculoskeletal tissues is fundamental for physiotherapists and physicians (among other health professionals) for clinical decision-making. In order to clarify this issue, several studies have been conducted to describe the nutrient sources for fibrocartilaginous structures, especially articular discs. Fibrocartilage appears to be well vascularized in the fetal stage; but as soon as extra-uterine life starts, a process of vascular regression begins, until eventually it becomes avascular (Benjamin & Evans, 1990; Messner & Jizong, 1998). In adulthood blood vessels can be located in the periphery of the intervertebral discs, knee menisci, temporomandibular discs and the triangular complex of the wrist, in different mammals including humans (Griffin &Sharpe, 1960; Ghadially, 1983; Benjamin et al. 1990; Mikic, 1992; Rudert & Tillmann, 1993; Grignon et al. 1994; Piette & Lametschwandtner, 1995; Tong & Tideman, 2001). Authors have argued that this is because the periphery of these structures is made up of dense connective tissue, which is vascularized (Benjamin et al. 1990; Peterssen & Tillmann, 1999; Benjamin & Ralphs, 2000). Some works have also addressed another source for nutrition to these tissues. The subchondral bone is porous, permitting exchange of molecules between the marrow cavity and fibrocartilage through the so-called marrow contacts (Maroudas et al. 1975; Grignon et al. 1994).
By contrast, the blood supply of the symphysis pubis has been neglected in the literature. Indeed, classic anatomy books classify it as being avascular, receiving its nutrients through diffusion by neighboring structures (Bertelli et al. 1932; Testut & Latarjet, 1959; Kamina & Francke, 1999; Moore & Dalley, 1999). Gatta (1934) conducted a detailed study on the whole structure of this joint in humans, classifying it according to two separate periods. Gatta stated that between birth and the second decade of life, blood vessels are present in the whole interpubic space; whereas from the second decade of life onwards, no blood vessels can be seen. Baer (1949) disagreed with these observations when he showed that children up to 7 years of age have blood vessels only in the anterior periphery of the interpubic disc. Later, Loreti & Franceschini (1965) inidcated the necessity for more studies on this specific issue. Some of our preliminary results have shown the presence of blood vessels in the periphery of the interpubic disc in rats (Rocha & Chopard, 2002).
In order to obtain more detailed information regarding the nutrition pathways to the interpubic tissues, we decided to conduct a study using light microscopy, immunohistochemestry, India ink injection and corrosion casts, using Wistar rats as an animal model.
Materials and methods
All the experiments were carried in accordance with the Animal Experimentation Ethical Committee of the Institute of Biomedical Sciences, University of São Paulo. Sixty Wistar rats, male and female, 28–32 and 90–100 days of age were used; so for each technique we had four groups: group A (young male 28–32 days old), group B (young female 28–32 days old), group C (adult male 90–100 days old) and group D (adult female 90–100 days old). All animals were killed with an injection of pentobarbital (60 mg kg−1) with heparin (700 U kg−1).
Regular histology
Dissection of specimens was performed with a dissection microscope until the symphysis pubis could be localized. The joint was excised with the medial borders of the pubic bones and with muscle attachments. After fixation in 10% buffered formalin overnight, three samples from each group were processed for 5-μm sagittal sections and the other three for 5-μm coronal sections at 250-μm intervals. Sections were stained with haematoxilin and eosin and Masson's trichrome, and analysed under light microscopy.
Immunohistochemistry
Sections were obtained as described for regular histology of sagittal sections. Samples were then immersed in 20% hydrogen peroxide for 30 min and washed in phosphate-buffered saline (PBS, pH 7.3) three times for 5 min each. Sections were incubated with Anti-collagen type IV primary antibody (Chemicon®), as a specific marker of basement membranes for localization of vascular elements, at a concentration of 1 : 1000 in PBS plus albumin 1%, overnight. After washing in PBS for 5 min, three times, they were incubated in anti-rabbit GG (Vector®), as a secondary antibody at 1 : 1000 in PBS for 30 min. They were rinsed in PBS for 5 min, three times, and incubated in a 1 : 100 dilution of avidin–biotin–peroxidase complex (Vector®) in PBS for 30 min. After rinsing in PBS for 5 min, three times, they were treated with diaminobenzidine (Sigma®) for 3 min and counterstained with a 1% Harris haematoxylin. Sections were then dehydrated and mounted for analysis under light microscopy.
India ink injection
The details of this technique are described elsewhere (Lufti, 1970). Three animals from each group were used for this technique. Animals were killed and then dissected and had their abdominal aorta canulated. Infusion of saline solution at 37 °C was performed in order to flush blood vessels. Then 200 mL of a solution containing two parts of India ink (Acrilex® nanquim escolar preto) in one part distilled water with 1% gelatin at 60 °C was injected with manual pressure until the hind limbs became dark. Then for the last 5 mL of solution injection, the inferior vena cava was clipped in order to promote complete filling of the vascular network. The joints were carefully isolated from muscle attachments using a dissecting microscope, leaving the joint with its osseous insertions. Samples were dehydrated, cleared in methyl salicilate and analysed under light microscopy as whole blocks.
Scanning electron microscopy
The last three animals from each group were used for this technique. Each animal was dissected and had its abdominal aorta canulated. The vascular network was flushed with saline solution at 37 °C. After clearing blood vessels, 25 mL for the 28–32-day-old animals and 40 mL for the 90–100-day-old animals of a low-viscosity resin, Mercox® (Ladd Research) was infused by manual pressure according to the manufacturer's specifications. The last 5 mL of the resin was infused after clipping the inferior vena cava in order to guarantee complete filling of the vascular network. Polymerization of the resin was finished after immersing the animals in hot water (50 °C) for 2 h. Maceration of organic tissues was performed by immersion in 5% KOH and hot water (50 °C), for 2 h in each solution until the relevant vessels became apparent. Samples were not completely macerated because we wanted to maintain the anatomical relation of casts with pubic bones for accurate localization. Generally, blood vessels were classified according to their diameter rather then by analysis of their nuclei imprints, because the latter were not completely clear. Connective tissue junctions between casts and bones were so delicate that we were unable to carry out critical-point drying. Samples were then mounted for scanning electron microscopy.
Results
The interpubic disc has two distinct portions: an inner bearing made up of fibrocartilage (fibrocartilaginous centre), and a peripheral portion made up of dense connective tissue (fibrous rim). Abdominal and adductor muscles attach to the anterior aspect of the symphysis pubis, reinforcing the anterior fibrous rim (Fig. 1). From the pubic branches of the obturatory and inferior epigastric arteries, blood vessels ran over the pubic bones until they formed a ring-like vascular plexus around the symphysis pubis, becoming an authentic articular arterial circle. From this plexus, blood vessels originated, supplying the medial extremity of each pubic bone and penetrating muscle attachments and articular tissues to supply the pubic ligaments and the fibrous rim of the interpubic disc. Ligaments were well vascularized in all groups, generally presenting a vascular plexus at the junction between them and the disc. At the anterior aspect of the disc, muscles attached through a fibro-fatty pad, defining three zones: muscular, fibro-fatty and fibrous, with markedly different vascular patterns (Fig. 1). At the posterior aspect of the disc a pad consisting of connective tissue that is richly vascularized and innervated is attached. Blood vessels penetrate the posterior fibrous rim more deeply until they become loops (Fig. 2).
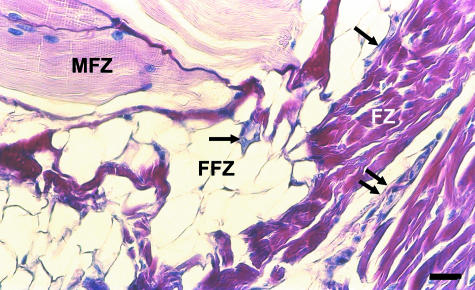
Fig. 1.
Sagittal section of the anterior aspect of the symphysis pubis of an adult male. The three zones are readily distinguished. The muscular zone (MFZ) gives several blood vessels (arrows) that travel through the fibro-fatty zone (FFZ), before reaching the outer lamellae of the fibrous rim (FR) where they terminate in winding spiralling capillaries (double arrows). Stain: haematoxilin and eosin. Scale bar = 25 μm.
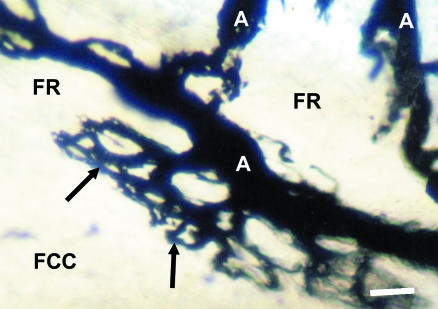
Fig. 2.
Posterior view of the symphysis pubis of a young male. Arterioles (A) within the posterior fibrous rim (FR) give rise to terminal capillary loops (arrows) that face the avascular fibrocartilagionous centre (FCC). Stain: India ink. Scale bar = 300 μm.
On the anterior surface of the disc, small-calibre arteries within the muscular zone give rise to arterioles that penetrate the fibro-fatty zone before reaching the outer lamellae of the fibrous rim, where capillaries become winding spiralling structures (Fig. 3). From the arterial circle, other blood vessels penetrate the cortical bone of the medial pubis and give rise to several capillaries that form a complex vascular plexus at the interface between the subchondral bone and the calcified fibrocartilage zone of the interpubic disc (Fig. 4). The subchondral bone shows several marrow contacts, linking the marrow spaces of the pubic bones with the interpubic disc (Fig. 5). Qualitatively, the only difference observed between groups was that in young rats the blood vessels penetrate the anterior fibrous rim more deeply, reaching the periphery of the fibrocartilagenous centre.
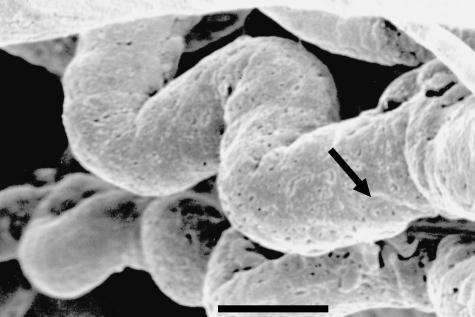
Fig. 3.
Highly magnified view of two capillaries of the anterior portion of the arterial circle of an adult male. Note the winding and spiralling pattern that increases the contact area and decreases blood flow. Nuclei imprints are recognizable (arrow). Corrosion cast. Scale bar = 10 μm.
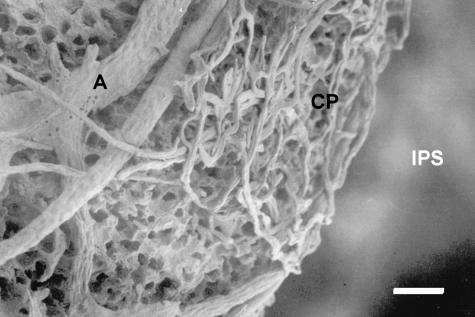
Fig. 4.
Medial view of the subchondral bone of the right pubis in an adult female. Arterioles (A) originating from the arterial circle penetrate the bone and branch into capillaries that form a plexus (CP) at the interface between the subchondral bone and the calcified fibrocartilaginous portion of the interpubic disc. ISP, interpubic space. Corrosion cast. Scale bar = 48 μm.
Fig. 5.
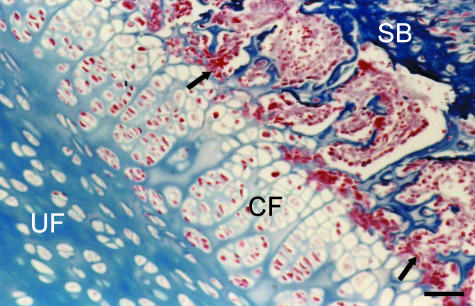
Coronal section of the interpubic disc of an adult male. The subchondral bone (SB) exhibits several marrow contacts (arrows) that communicate the highly vascularized marrow spaces with the calcified fibrocartilaginous portion (CF) of the interpubic disc, whereas the uncalcified portion (UF) has no blood vessels. Stain: Masson's trichrome. Scale bar = 50 μm.
Discussion
Articular discs constitute structures differentiated from the mesenchyme. Where these structures have suffered compressive and tensional forces they have differentiated into fibrocartilage, whereas pure tensional forces gave rise to differentiation into dense connective tissue (Crelin & Koch, 1965; Benjamin & Evans, 1990; Bruehlmann et al. 2002). Because of the anatomical relationship between articular discs and bones, fibrocartilage is usually present in the central portion of these structures, whereas dense connective tissue becomes a peripheral rim encircling the fibrocartilaginous portion (Piette & Lametschwandtner, 1995; Bogduk, 1997). Our results support these findings, because we were able to find a central bearing portion made up of fibrocartilage (fibrocartilaginous centre) that was encircled by a dense connective tissue rim (fibrous rim) where collagen was arranged in lamellae for optimal tension stress management (Zambrano et al. 1982). Future experiments using immunolocalization of collagen types II and I should be performed to reinforce this observation. Cartilaginous tissue must express angiogenic inhibitors in order to guarantee its development and differentiation (Moses et al. 1990, 1999; Yin & Pacifini, 2001). This concept may help to explain why fibrocartilage becomes avascular (Benjamin & Evans, 1990; Peterssen et al. 2002). By contrast, some works have described the presence of blood vessels in the fibrocartilaginous portions of fetal discs (e.g. Messner & Jizong, 1998). In the samples analysed in this experiment, blood vessels were present only in the fibrous rim, leaving the whole fibrocartilaginous centre avascular. One possible reason why fibrocartilage does become avascular is that compressive forces may lead to vascular diseases, so the presence of blood vessels inside fibrocartilage parenchyma could be harmful (Pereira et al. 1996b). The poor vascularization of articular discs explains the low cellularity (Pereira et al. 1996a). In fact, Maroudas et al. (1975) stated that cells increase in density near vascular regions in lumbar intervertebral discs. Discal cells (fibrocytes and chondrocytes) have to organize the extracellular matrix during development (Rufai et al. 1995; Hayes et al. 1999) and maintain the turnover of extracellular matrix molecules (Benjamin & Evans, 1990). This raises the question of how these cells can be optimally supplied without putting blood vessels inside the discs themselves. Blood vessels around the anterior aspect of the interpubic disc go through three zones – muscular, fibro-fatty and fibrous – before returning to venous circulation. Inside the muscular zone, contraction of the tissue leads to increased blood flow, but as soon as the blood reaches the fibro-fatty region, blood flow decreases. In the fibrous zone, because blood vessels become winding and spiralling capillaries, blood flow decreases even further. We may conclude that this muscular/fibro-fatty pressure system helps maintain blood cells for a longer time inside terminal capillaries to optimize exchanges while the structure of the terminal capillaries enhances the contact area (Fig. 6). This model is also strengthened by the fact that, posteriorly, capillary loops penetrated more deeply into the substance of the fibrous rim, perhaps because there are no muscle fibres attached to the retrodiscal pad. The fact that capillaries penetrated deeper into the anterior fibrous rim of young animals may be explained by the increased development demands. The presence of arterial circles culminating in capillary loops has also been addressed in experiments concerning the temporomandibular disc and the triangular complex of the wrist (Mikic, 1992; Piette & Lametschwandtner, 1995). Marrow contacts constituted a second pathway of nutrients to the interpubic disc. The high levels of oxygen and molecular interactions between bone and fibrocartilage may explain the presence of calcified fibrocartilage at the attachment of the disc, where cellularity is also higher. Corrosion casts showed a complex plexus of capillaries that lacked loops at the interface between subchondral bone and calcified fibrocartilage. In this context, we may consider that this pattern is different from that found in intervertebral discs, where capillaries show circumvolutions to increase the vascular contact area (Oki et al. 1996). The presence of blood vessels in the pubic ligaments found in all groups studied is in agreement with observations on the blood supply of ligaments in general. Interestingly, blood vessels were commonly located within loose connective tissue sheaths as in tendons (Benjamin & Ralphs, 2000).
Fig. 6.
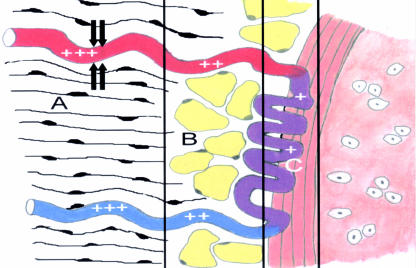
Schematic model of the anterior portion of the arterial circle in contact with the interpubic disc. Blood flow is increased in the muscular zone (A) through the contraction of muscular tissue (+++). When blood reaches the fibro-fatty zone (B), the flow decreases (++) because of lack of contraction and compliance of the tissue. At the interface between this zone and the fibrous zone (C), which corresponds to the fibrous rim, capillaries adopt a winding and spiralling morphology that decreases the blood flow even further (+) and increases the contact area to optimize supply. Further contraction of the muscular zone guarantees blood drainage (+++) through compression of the venous system.
Another issue that has to be addressed is whether the results obtained in this experiment are in agreement with our current knowledge of human anatomy. Our results disagree with the observations of Gatta (1934) and Baer (1949). Whereas these authors conducted studies aimed at the description of the whole structure of the symphysis pubis, to our knowledge, this is the first entirely dedicated to the description of blood vessels and marrow contacts within this joint, using light and electron microscopy (Rocha & Chopard, 2002).
However, our study clearly shows several weaknesses. Rats are quadruped whereas humans are biped animals, which may lead to striking biomechanical and consequent morphological differences between them. For example, the knee menisci show important morphological differences between rats (Hildebrand et al. 1991) and humans (Messner & Jizong, 1998).
Although these problems cannot be solved until human samples are analysed, we believe that our experiments do have merit. The intervertebral disc, for example, is so similar in its structure and blood supply among different mammals that several studies have been conducted in rats and rabbits for physiological and bioengineering purposes even with all the inherent biomechanical differences (Konerding & Blank, 1987; Hayes et al. 1999; Bruehlmann et al. 2002). Animal models and human samples have also been used for the study of diseased discs, where blood vessels penetrate the fibrocartilaginous parenchyma in order to permit tissue regeneration (Pereira et al. 1996b; Johnson et al. 2001; Melrose et al. 2002). Future studies are required in order to clarify whether the blood vessels described in this work may contribute to the regeneration of lesions within the symphysis pubis.
During pregnancy, ovaries secrete the hormone relaxin, which changes the structure of the symphysis pubis in order to permit a safe delivery (Benjamin & Evans, 1990; Samuel et al. 1996, 1998). The nutrition pathways described here probably constitute the medium through which relaxin reaches its targets within the symphysis pubis. Although we could not observe any differences between males and females, it is possible that pregnancy could induce microvascular changes within this joint.
The symphysis pubis can become the site of innumerable dysfunctions such as osteitis pubis and pubalgia post partum (Walheim et al. 1984; Sgambati et al. 1996; Williams et al. 2000). In fact statistics show that one in 37 women suffers from pubalgia post partum for at least 6 months after a regular delivery in the UK (Owens et al. 2002).
Based on the current state of knowledge of human anatomy, we have developed a new clinical approach to treat mechanical pubic disorders in our clinical physiotherapy division (unpublished data). This approach includes anteroposterior pressures on the iliac bone for mobilization, and mobilization with movements as manipulative techniques and specific therapeutic exercises aimed at the transversus abdominis in co-contraction with the lumbar multifidus and adductor muscles as a variation of the technique proposed by Richardson et al. (1999).
Our results strongly support the need for future studies in humans for the purposes of comparative anatomy. Possible similarities between rats and humans may go some way to explaining the positive outcomes of our clinical approach, because manipulative and specific exercise therapy cause increases in blood flow, among other biological responses, that may help tissues to heal.
Acknowledgments
We would like to thank Professor Mike Benjamin (Cardiff University) for his kind comments and suggestions. We would also like to thank Kim Robinson and Toby Hall (Manual Concepts, Curtin University of Technology) for tuition in musculoskeletal physiotherapy and for the encouragement to combine anatomical knowledge with physiotherapy practice.
References
- Baer JP. Der funktionelle bau der symphyse im embrional und kindesalter auf grund von von untersuchungen im polarisierten licht. Acta Anat. 1949;7:273–301. [PubMed] [Google Scholar]
- Benjamin M, Evans EJ. Fibrocartilage. J. Anat. 1990;171:1–15. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Pemberton DJ. Histological studies on the fibrocartilage complex of the wrist. J. Anat. 1990;172:59–67. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Ralphs JR. The cell and developmental biology of tendons and ligaments. Int. R. Cytol. 2000;196:85–130. doi: 10.1016/s0074-7696(00)96003-0. [DOI] [PubMed] [Google Scholar]
- Bertelli D, Balli R, Bruni AC, Gianelli L, Luna E, Pende N, et al. Trattato Di Anatomia Umana. 2. Vol. 2. Milan: Dottor Francesco Vallardi.; 1932. [Google Scholar]
- Bogduk N. Clinical Anatomy of the Lumbar Spine and Sacrum. 3. Toronto: Churchill Livingstone; 1997. [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J. Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crelin ES, Koch WE. Development of mouse pubic joint in vivo following initial differentiation in vitro. Anat. Rec. 1965;153:161–172. doi: 10.1002/ar.1091530206. [DOI] [PubMed] [Google Scholar]
- Gatta R. Osservazioni sulla struttura e sullo sviluppo della sinfisi pubica. Arch. Ital. Anat. Embriol. 1934;33:813–834. [Google Scholar]
- Ghadially FN. Fine Structure of Synovial Joints. London: Butterworth; 1983. [Google Scholar]
- Griffin CJ, Sharpe CJ. The structure of the human adult human temporomandibular meniscus. Aust. Dent.J. 1960;5:190–195. [Google Scholar]
- Grignon B, Roland J, Gross S, Floquet J, Membre H, Braun M. Le disque intervertébral lombaire. Voies de passage disque intervertébral-corps vertebral et vascularisation discale. BullL’Assoc. Anat. 1994;78:53–57. [PubMed] [Google Scholar]
- Hayes AJ, Benjamin M, Ralphs JR. Role of actin stress fibres in the development of the intervertebral disc: cytoskeletal control of extracellular matrix assembly. Dev. Dyn. 1999;215:179–189. doi: 10.1002/(SICI)1097-0177(199907)215:3<179::AID-AJA1>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Hildebrand C, Oqvist G, Brax L, Tuisku F. Anatomy of the rat knee joint and fibre composition of a major articular nerve. Anat. Rec. 1991;229:545–555. doi: 10.1002/ar.1092290415. [DOI] [PubMed] [Google Scholar]
- Johnson WEB, Evans H, Menage J, Eisenstein SM, Haj AE, Roberts S. Immunohistochemical detection of schwann cells in innervated and vascularized human intervertebral discs. Spine. 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- Kamina P, Francke JP. Anatomie: Introduction a la Clinique. Vol. 4. Paris: Maloine; 1999. [Google Scholar]
- Konerding MA, Blank M. Vascularization of the vertebral column of rats. Scanning Microsc. 1987;1:1727–1732. [PubMed] [Google Scholar]
- Loreti F, Franceschini M. Modificazioni architettoniche del collagene correlative alla etá ed alla funzione nelle fibrocartilagini intrarticolari umane. G. Gerontol. 1965;13:11–30. [Google Scholar]
- Lufti AM. Mode of growth, fate and functions of cartilage canals. J. Anat. 1970;106:135–145. [PMC free article] [PubMed] [Google Scholar]
- Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J. Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- Melrose J, Roberts S, Smith S, Menage J, Ghosh P. Increased nerve and blood vessel ingrowth associated with proteoglican depletion in an ovine annular lesion model of experimental disc degeneration. Spine. 2002;27:1278–1285. doi: 10.1097/00007632-200206150-00007. [DOI] [PubMed] [Google Scholar]
- Messner K, Jizong G. The menisci of the knee joint. Anatomical and functional characteristics, and a rationale for clinical treatment. J. Anat. 1998;193:161–178. doi: 10.1046/j.1469-7580.1998.19320161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikic ZD. Blood supply of the articular disc of the antebrachiocarpal joint in dogs. J. Anat. 1992;181:447–453. [PMC free article] [PubMed] [Google Scholar]
- Moore KL, Dalley AF. Clinically Oriented Anatomy. 4. Philadelphia: Lippincott, Williams & Wilkins; 1999. [Google Scholar]
- Moses MA, Sudhalter J, Langer R. Identification of an inhibitor of neovascularization from cartilage. Science. 1990;248:1408–1410. doi: 10.1126/science.1694043. [DOI] [PubMed] [Google Scholar]
- Moses MA, Wiederschain D, Wu I, Fernandez CA, Ghazizadeh V, Lane WS, et al. Troponin I is present in human cartilage and inhibits angiogenesis. Proc. Natl Acad. Sci. USA. 1999;96:2645–2550. doi: 10.1073/pnas.96.6.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oki S, Matsuda Y, Shibata T, Okumura H, Desaki J. Morphologic differences of the vascular buds in the vertebral endplate: scanning electron microscopy study. Spine. 1996;21:174–177. doi: 10.1097/00007632-199601150-00003. [DOI] [PubMed] [Google Scholar]
- Owens K, Pearson K, Mason G. Symphysis pubis dysfunction – a cause of significant obstetric morbidity. Eur. J. Obstet. Gynecol. Reprod. Biol. 2002;15(105(2)):143–146. doi: 10.1016/s0301-2115(02)00192-6. [DOI] [PubMed] [Google Scholar]
- Pereira FJ, Jr, Lundh H, Westesson P. Age-related changes of the retrodiscal tissues in the temporomandibular joint. J. Oral Maxillofac. Surg. 1996a;54:55–56. doi: 10.1016/s0278-2391(96)90305-5. [DOI] [PubMed] [Google Scholar]
- Pereira FJ, Jr, Lundh H, Erikson L, Westesson P. Microscopic changes in the retrodiscal tissues of painful temporomandibular joints. J. Oral Maxillofac. Surg. 1996b;54:461–468. doi: 10.1016/s0278-2391(96)90122-6. [DOI] [PubMed] [Google Scholar]
- Peterssen W, Pufe T, Kurz B, Mentlein R, Tillmann B. Angiogenesis in fetal tendon development: spatial and temporal expression of the angiogenic peptide vascular endothelial cell growth factor. Anat. Embriol. 2002;205:263–270. doi: 10.1007/s00429-002-0241-1. [DOI] [PubMed] [Google Scholar]
- Peterssen W, Tillmann B. Structure and vascularization of the knee joint menisci. Z. Orthop. Ihre Grenzgeb. 1999;137:31–37. doi: 10.1055/s-2008-1037032. [DOI] [PubMed] [Google Scholar]
- Piette E, Lametschwandtner A. The angioarchitecture of the rat mandibular joint bilaminar zone. Arch. Oral Biol. 1995;40:499–505. doi: 10.1016/0003-9969(95)00007-c. [DOI] [PubMed] [Google Scholar]
- Richardson C, Jull G, Hodges P, Hides J. Therapeutic Exercise for Spinal Segmental Stabilization in Low Back Pain. Scientific Basis and Clinical Approach. Edinburgh: Churchill Livingstone; 1999. [Google Scholar]
- Rocha RCG, Chopard RP. Blood supply of the symphysis pubis. Microsc. Microanal. 2002;8(Suppl. 2):232–233. [Google Scholar]
- Rudert M, Tillmann B. Detection of lymph and blood vessels in the human intervertebral disc by histochemical and immunohistochemical methods. Ann. Anat. 1993;175:237–242. doi: 10.1016/s0940-9602(11)80009-9. [DOI] [PubMed] [Google Scholar]
- Rufai A, Benjamin M, Ralphs JR. The development of fibrocartilage in the rat intervertebral disc. Anat. Embriol. 1995;192:53–62. doi: 10.1007/BF00186991. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Butkus A, Coghlan JP, Bateman JF. The effect of relaxin on collagen metabolism in the nonpregnant rat pubic symphysis: the influence of estrogen and progesterone in regulating relaxin activity. Endocrinology. 1996;137:3884–3890. doi: 10.1210/endo.137.9.8756561. [DOI] [PubMed] [Google Scholar]
- Samuel CS, Coghlan JP, Bateman JF. Effects of relaxin, pregnancy and parturation on collagen metabolism in the rat pubic symphysis. J. Endocrinol. 1998;159:117–125. doi: 10.1677/joe.0.1590117. [DOI] [PubMed] [Google Scholar]
- Sgambati E, Stecco A, Capaccioli L, Brizzi E. Morphometric evaluation of the symphysis pubis joint. Ital. J. Anat. Embriol. 1996;101:195–201. [PubMed] [Google Scholar]
- Testut L, Latarjet A. Tratado de Anatomia Humana. Vol. 1. Barcelona: Saluat Editores S.A.; 1959. [Google Scholar]
- Tong AC, Tideman H. The microanatomy of the rhesus monkey temporomandibular joint. J. Oral Maxillofac. Surg. 2001;59:46–52. doi: 10.1053/joms.2001.19284. [DOI] [PubMed] [Google Scholar]
- Walheim GG, Olerud S, Ribbe T. Motion of the pubic symphysis in pelvic instability. Scand. J. Rehabil. Med. 1984;16:163–169. [PubMed] [Google Scholar]
- Williams PR, Thomas DP, Downes EM. Osteitis pubis and instability of the pubic symphysis. Am. J. Sports Med. 2000;28:350–355. doi: 10.1177/03635465000280031101. [DOI] [PubMed] [Google Scholar]
- Yin M, Pacifini M. Vascular regression is required for mesenchymal condensation and chondrogenesis in the developing limb. Dev. Dyn. 2001;222:522–533. doi: 10.1002/dvdy.1212. [DOI] [PubMed] [Google Scholar]
- Zambrano NZ, Montes GS, Shigihara KM, Sanchez EM, Junqueira LCU. Collagen arrangement in cartilages. Acta Anat. 1982;113:26–38. doi: 10.1159/000145534. [DOI] [PubMed] [Google Scholar]