Abstract
We performed a light microscope and a computer three-dimensional reconstruction study of serial sections of the molar enamel organ of 3- and 5-day-old rats perfused with Indian ink through the arterial system. The tooth germs were fixed in Bouin's solution, embedded in paraffin, sectioned and stained with haematoxylin and eosin. For the three-dimensional reconstruction, light micrographs of the serial sections were digitized, and aligned using the serial EM Align software downloaded from http://synapses.bu.edu/tools/. After alignment, the boundaries of the India-ink-filled blood vessels were manually traced with a mouse using the software IGL trace (version 1.26b), also downloaded from the above website. After tracing, a three-dimensional representation of the blood vessel contours was generated in a VRML format and visualized with the help of the software Cortona Web3D viewer (version 4.0) downloaded from http://www.parallelgraphics.com/products/cortona/. Our results showed that in regions where ameloblasts are polarized the capillaries are arranged in three distinct levels: (1) penetrating and leaving capillaries in relation to the outer enamel epithelium; (2) capillaries crossing and branching inside the stellate reticulum; and (3) capillaries branching and anastomosing profusely within the stratum intermedium, thereby forming an extensive capillary plexus intimately associated with the cells of the stratum intermedium. The existence of a conspicuous capillary plexus intermingled with cells of the stratum intermedium, as shown in our results, suggests that some molecules produced by cells of the stratum intermedium could be released into the capillary plexus and thereafter carried to the dental follicle.
Keywords: capillaries, computer reconstruction, enamel organ, stellate reticulum, tooth germ
Introduction
Tooth development is characterized by a series of complex events that require the interaction of ectomesenchymal cells of neural crest origin and cells of the oral epithelium. Invagination of the primitive oral epithelium into the subjacent ectomesenchyme leads to the formation of the dental lamina and the enamel organ, the structure responsible for the formation of enamel. The enamel organ develops to form four layers, i.e. the outer and inner enamel epithelia, stratum intermedium and the stellate reticulum (Scott & Symons, 1982; Ten Cate, 1998). Further development of the enamel organ results initially in the expansion of the stellate reticulum and later in its involution, probably by apoptosis (Baratella et al. 1999). Concomitant with these events, blood vessels from the surrounding dental follicle perforate the outer enamel epithelium and invade the reticulum of molar tooth germs (Bernick, 1960, 1966; Tasumi, 1967; Bonnaud, 1984; Sasaki & Garant, 1986; Yoshida et al. 1989) and the papillary layer of rodent incisors (Adams, 1962; Garant & Gillespie, 1969; Sasaki et al. 1984; El-Agroudi et al. 1998). The arrangement and distribution of these intra-epithelial vessels, as well as their function, is poorly understood. We felt therefore that to be able to understand the function of these vessels it would first be necessary to have a three-dimensional (3D) view of their anatomical distribution and relationships within the enamel organ. Thus we performed a light microscope and computer 3D reconstruction study of serial sections of the rat molar enamel organ of animals perfused with Indian ink through the arterial system.
Materials and methods
Three- and 5-day-old Wistar rats, of both sexes, fed ad libitum, were obtained from the Federal University of São Paulo Animal House. Principles of laboratory animal care (NIH publication 85-23, 1985) and national laws on animal use were observed.
The animals were anaesthetized with ether and the thorax opened to expose the heart and lungs. A commercial solution of either black or blue Indian ink was perfused through the left ventricle of the heart, according to the procedure of Bernick (1966). Perfusion was continued until the oral mucosa became intensely coloured and the dye leaked out through a previously sectioned inferior vena cava vein. The animals were subsequently decapitated, and the upper maxilla was removed and fixed in Bouin's solution at room temperature for 48 h. The specimens were dehydrated in graded ethanols and embedded in paraffin. Regions containing the first molar tooth germs were trimmed, and sections 6 μm and 20 μm thick were obtained for morphological and 3D reconstruction studies, respectively. Haematoxylin and eosin (H&E)-stained sections of the Indian-ink-perfused animals were examined and photographed in an Olympus BX-50 light microscope.
For 3D reconstruction, light micrographs were digitized in an HP ScanJet 3400 scanner and saved in as tagged image format files (TIFF). For each image, 3–4 fiducial points present in two consecutive sections were chosen to allow the alignment of the serial sections (Fiala & Harris, 2002). These points were aligned using the serial EM (sEM) Align software (version 1.26b) downloaded from http://synapses.bu.edu/tools/. After alignment, the boundaries of the India-ink-filled blood vessels were manually traced with the mouse using the software IGL trace (version 1.26b), also downloaded from the website cited above. After tracing, a 3D representation of the blood vessel contours was generated in a VRML format and visualized using Cortona Web3D viewer (version 4.0) sofware downloaded from http://www.parallelgraphics.com/products/cortona/.
Results and discussion
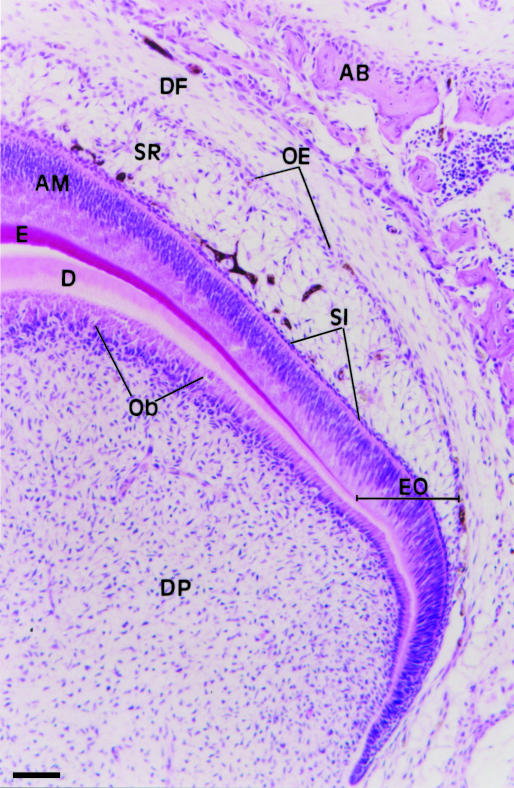
Figure 1 shows a portion of a tooth germ from a 3-day-old rat used in our experiments. The enamel organ with the outer enamel epithelium, stellate reticulum, stratum intermedium and ameloblast layer is clearly observed. A portion of the dental papilla and follicle are also present.
Fig. 1.
Light micrograph of a portion of a molar tooth germ from a 3-day-old rat. The enamel organ (EO) shows the outer epithelium (OE), stellate reticulum (SR), stratum intermedium (SI) and the ameloblast layer (AM). The dental papilla (DP) shows odontoblasts (Ob) adjacent to early dentine (D). E, enamel; DF, dental follicle; AB, alveolar bone. HE. Scale bar = 70 μm.
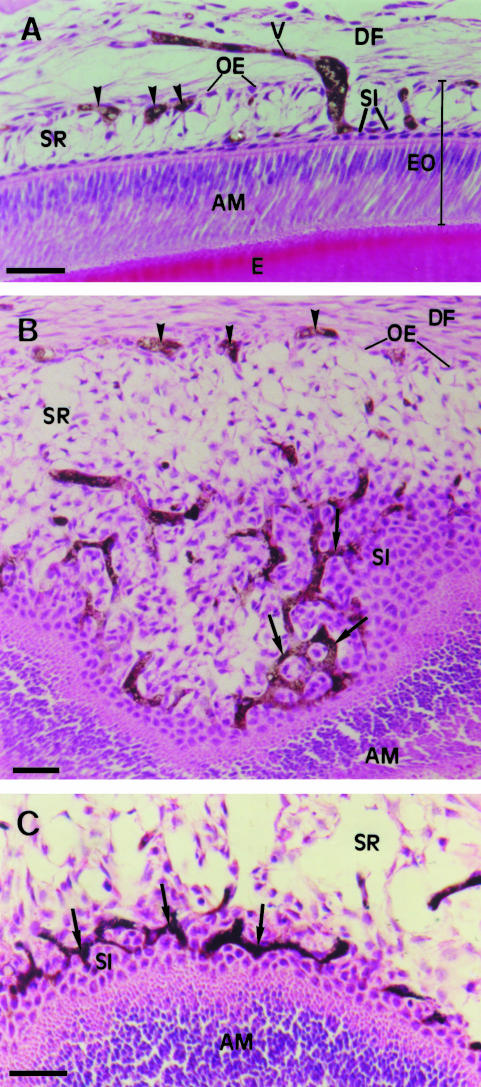
Perfusion of Indian ink through the arterial system of 3- and 5-day-old rats revealed the pathway of blood vessels in the enamel organ. Blood vessels containing Indian ink (black or blue) were observed in the region of the dental follicle close to the enamel organ and often in juxtaposition to the outer enamel epithelium. Conspicuous Indian-ink-filled capillaries were observed inside the stellate reticulum and close to and inside the stratum intermedium. Our images showed that blood vessels from the dental follicle enter the enamel organ through the outer enamel epithelium and cross the interior of the stellate reticulum. Once inside the reticulum some capillaries exhibited dilatations (Fig. 2A). Oblique sections of the enamel organ in the region of the stratum intermedium showed that the capillaries appeared with a reduced diameter and formed a complex and anastomosing network around the cells of the stratum intermedium (Fig. 2B,C).
Fig. 2.
Light micrographs of portions of the enamel organ of molar tooth germs from 5-day-old rats injected with black Indian ink and stained with H&E. (A) A blood vessel (V) containing black Indian ink is either penetrating or leaving the lateral region of the enamel organ (EO), passing thorough the stellate reticulum (SR). Inside the stellate reticulum (SR), the vessel (V) appears dilated and seems to reach the stratum intermedium (SI). Cross-sections of blood vessels (arrowheads) containing black Indian ink are observed close to the outer enamel epithelium (OE). DF, dental follicle; E, enamel; AM, ameloblast layer. Scale bar = 40 μm. (B) An oblique section of a portion of the occlusal region of the enamel organ shows an intricate network of anastomosing blood vessels containing black Indian ink (arrows) between the cells of the stratum intermedium (SI). Blood vessels containing black Indian ink (arrowheads) are also observed in the region of the outer enamel epithelium (OE). AM, ameloblast layer; SR, stellate reticulum; DF, dental follicle. Scale bar = 50 μm. (C) Another angle of an oblique section of a tooth germ (occlusal region), similar to B, shows the complex network of vessels (arrows) containing black Indian ink in close association with cells of the stratum intermedium (SI). SR, stellate reticulum; AM, ameloblast layer. Scale bar = 40 μm.
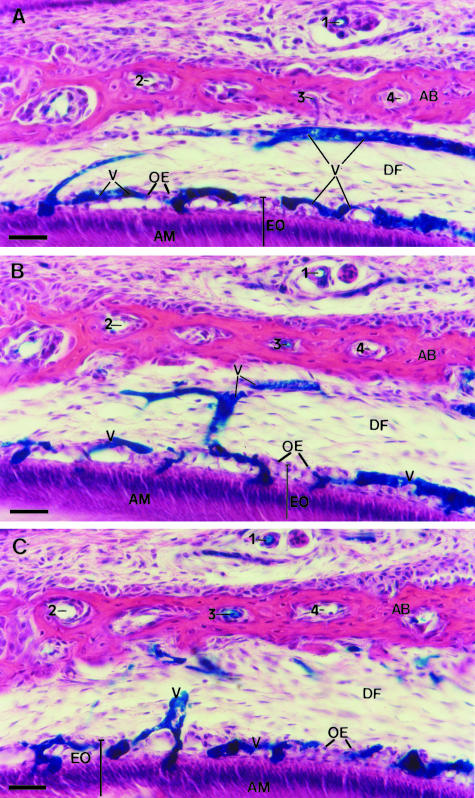
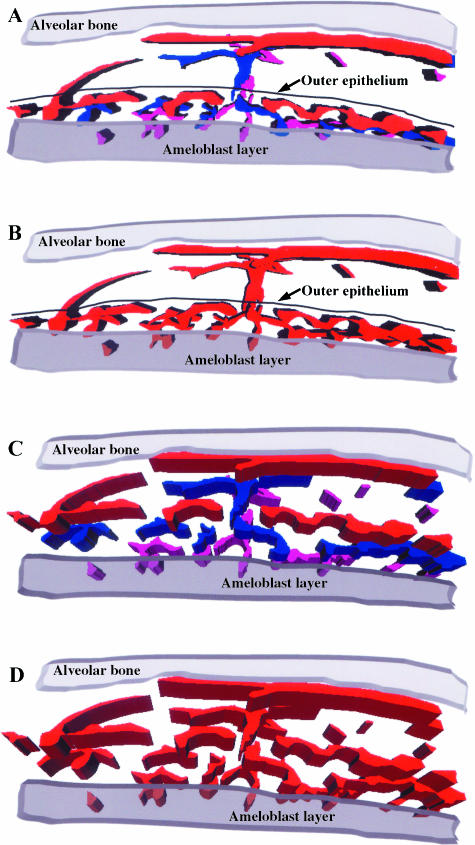
The three serial sections (20 μm each) of the tooth germ lateral region used for the 3D reconstruction are shown in Fig. 3 (A–C). These three consecutive sections exhibiting the blue Indian-ink-filled blood vessels of the enamel organ and dental follicle were outlined for computer reconstruction. Structures used as fiducial points for the three serial sections are indicated by the numbers 1, 2, 3 and 4 for each section. Although enamel is not shown, it is possible to observe polarized secretory ameloblasts and the overlaying involuting stellate reticulum. In Fig. 4(A) the Indian-ink-filled blood vessels of each serial section used for the reconstruction are displayed in three different colours, whereas in Fig. 4(B) the vessels of the three serial sections are shown in the same colour. Fig. 4(C) is the same image as in Fig. 4(A) but tilted by about 30° along the x-axis (horizontal axis). In Fig. 4(D) the image shown in Fig. 4(B) was tilted by about 30° along the y-axis (vertical axis) and the x-axis.
Fig. 3.
Light micrographs of three serial sections (20 μm thick each) of the same lateral region of the enamel organ of a molar tooth germ from a 5-day-old rat injected with blue Indian ink. Blood vessels in the dental follicle (DF) and in the enamel organ (EO) are filled with blue Indian ink. The enamel organ (EO) shows polarized ameloblasts (AM) at the stage of enamel formation and involuting stellate reticulum. These consecutive sections were outlined for a 3D computer reconstruction of the blood vessels (V), as shown in Fig. 4. Structures used as fiducial points for alignment of the three serial sections are indicated by the numbers 1, 2, 3 and 4. AB, alveolar bone; OE, outer enamel epithelium. H&E. Scale bar = 40 μm.
Fig. 4.
A 3D computer reconstruction of the Indian-ink-filled blood vessels observed in the three serial sections shown in Fig. 3(A–C). (A) The blood vessels for each section are shown in different colours, i.e. red for A, blue for B and pink for C. (B) The blood vessels from the three sections are shown in the same colour to demonstrate their continuity and their 3D arrangement. (C,D) The images of the 3D reconstruction as in panels A and B have been tilted clockwise around the x-axis to demonstrate the interconnecting arrangement of the vessels in the region of the stratum intermedium.
Our results allowed a 3D visualization of the distribution, arrangement and relationships of the capillaries within the enamel organ. Our findings have shown that the vessels are not distributed randomly but are in fact arranged in three distinct regions: (1) penetrating and leaving capillaries in relation to the outer enamel epithelium; (2) capillaries crossing and branching inside the stellate reticulum; and (3) capillaries branching and anastomosing profusely within the stratum intermedium, thereby forming an extensive capillary plexus intimately associated with the cells of the stratum intermedium. It should be noted, however, that there are variations in the degree of penetration of the vessels into different regions of the enamel organ as well as in different species (Adams, 1962; Tasumi, 1967; Okada et al. 1990).
In addition, our results confirmed that vessels from the dental follicle penetrate the enamel organ and cross the stellate reticulum towards the stratum intermedium and subsequently return to the dental follicle (Bernick, 1966; Tasumi, 1967; Bonnaud, 1984; Sasaki & Garant, 1986). Our images, however, did not allow us to identify which vessels were entering or leaving the enamel organ. Although previous reports have described that blood vessels are located next to the stratum intermedium layer (Decker, 1967; Sasaki & Garant, 1986; Yoshida et al. 1989), we have shown the existence of a complex network of anastomosing capillaries between the cells of the stratum intermedium.
The function of the intra-epithelial capillaries remains largely unknown. It has previously been suggested that the capillaries were responsible for the transport of inorganic salts, mainly calcium ions, required for calcification of the enamel (Bernick, 1960; Adams, 1962; Bonnaud, 1984; Sasaki et al. 1984). However, recent evidence has shown that cytokines produced by stellate reticulum cells are transported from the enamel organ to the dental follicle (Wise & Lin, 1994, 1995). Moreover, the importance of stratum intermedium in odontogenesis has been recently emphasized by the demonstration that it produces the signalling and morphogenetic factor –Sonic hedgehog (Koyama et al. 2001). Thus, the existence of a conspicuous capillary plexus closely intermingled with cells of the stratum intermedium, as shown in our results, suggests that some molecules produced by cells of the stratum intermedium could be released into the capillary plexus and thereafter carried to the dental follicle. However, further studies are required to clarify the exact function of the vascular plexus between the cells of the stratum intermedium.
Acknowledgments
We thank Mrs Gisela S. Scarpati for her technical assistance. This research was supported by CNPq (Brazil).
References
- Adams D. The blood supply to the enamel organ of the rodent incisor. Arch. Oral Biol. 1962;7:279–286. doi: 10.1016/0003-9969(62)90018-3. [DOI] [PubMed] [Google Scholar]
- Baratella L, Arana-Chavez VE, Katchburian E. Apoptosis in the early involuting stellate reticulum of rat molar tooth germs. Anat. Embryol. 1999;200:49–54. doi: 10.1007/s004290050258. [DOI] [PubMed] [Google Scholar]
- Bernick S. Vascular supply to the developing teeth of rats. Anat. Rec. 1960;137:141–151. doi: 10.1002/ar.1091370210. [DOI] [PubMed] [Google Scholar]
- Bernick S. Vascular and nerve supply to the molar teeth of guinea pigs. J. Dent. Res. 1966;45:249–260. doi: 10.1177/00220345660450020601. [DOI] [PubMed] [Google Scholar]
- Bonnaud A. Modifications cellulaires au niveau de l’épithélium adamantin externe au cours de la vascularisation de l’organe de l’émail chez le rat. Biol. Buccale. 1984;12:225–237. [PubMed] [Google Scholar]
- Decker JD. The development of a vascular supply to the rat molar enamel organ. An electron microscopic study. Arch. Oral Biol. 1967;12:453–458. doi: 10.1016/0003-9969(67)90020-9. [DOI] [PubMed] [Google Scholar]
- El-Agroudi MA, Selliseth NJ, Selvig KA. Microvascular system of the rat incisor enamel organ. A scanning electron microscopic study of vascular corrosion casts. Eur. J. Oral Sci. 1998;106:1013–1021. doi: 10.1046/j.0909-8836.1998.eos106606.x. [DOI] [PubMed] [Google Scholar]
- Fiala JC, Harris KM. Computer-based alignment and reconstruction of serial sections. Microsc. Anal. 2002;52:5–7. [Google Scholar]
- Garant PR, Gillespie R. The presence of fenestrated capillaries in the papillary layer of the enamel organ. Anat. Rec. 1969;163:71–80. doi: 10.1002/ar.1091630109. [DOI] [PubMed] [Google Scholar]
- Koyama E, Wu C, Shimo T, Iwamoto M, Ohmori T, Kurisu K, et al. Development of stratum intermedium and its role as a Sonic hedgehog-signaling structure during odontogenesis. Dev. Dyn. 2001;222:178–191. doi: 10.1002/dvdy.1186. [DOI] [PubMed] [Google Scholar]
- Okada S, Ohta Y, Nishimura K, Matsushita J-I, Nakamura M. Microvascular architecture of the enamel organ of the upper major incisor in the rabbit. Okajimas Folia Anat. Jpn. 1990;67:231–242. doi: 10.2535/ofaj1936.67.4_231. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Tominaga H, Higashi S. Microvascular architecture of the enamel organ in the rat-incisor maturation zone. Scanning and transmission electron microscopic studies. Acta. Anat. 1984;118:205–213. doi: 10.1159/000145846. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Garant PR. An ultrastructural study of the papillary layer and its vascular bed in the kitten enamel organ. Anat. Rec. 1986;214:353–364. doi: 10.1002/ar.1092140404. [DOI] [PubMed] [Google Scholar]
- Scott JH, Symons NBB. Introduction to Dental Anatomy. 9. New York: Churchill Livingstone; 1982. [Google Scholar]
- Tasumi M. The vascular system in the developing molar teeth of the rat. Bull. Tokyo Med. Dent. University. 1967;14:123–139. [PubMed] [Google Scholar]
- Ten Cate AR. Oral Histology: Development, Structure, and Function. 5. St Louis: Mosby-Year Book, Inc.; 1998. [Google Scholar]
- Wise GE, Lin F. Regulation and localization of colony-stimulating factor-1 mRNA in cultured rat dental follicle cells. Arch. Oral Biol. 1994;39:621–627. doi: 10.1016/0003-9969(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Wise GE, Lin F. The molecular biology of initiation of tooth eruption. J. Dent. Res. 1995;74:303–306. doi: 10.1177/00220345950740010301. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Ohshima H, Kobayashi S. Vascularization of the enamel organ in developing molar teeth of rats – scanning electron microscope study of corrosion casts. Okajimas Folia Anat. Jpn. 1989;66:99–112. doi: 10.2535/ofaj1936.66.2-3_99. [DOI] [PubMed] [Google Scholar]