Abstract
The rumen and reticulum of sheep serve as a fermentation chamber. Both compartments exhibit specific motility patterns. With developmental changes, the size of the reticulorumen dramatically increases when newborn lambs mature to adult sheep. This makes it possible to investigate the intrinsic innervation of the reticuloruminal muscles in lambs by taking the entire reticulum and rumen into account. The aim of the study was to analyse the projections and neurochemistry of myenteric neurons in the rumen and reticulum, which project to the inner or outer muscle layer, respectively. Therefore, we applied retrograde tracing with the fluorescent dye 1,1′-didodecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (Dil) and subsequent immunohistochemical detection of choline acetyltransferase (ChAT), substance P (SP) and vasoactive intestinal peptide (VIP). In both compartments innervation of both the inner and the outer muscle layer consisted mainly of cholinergic neurons (65–70%). The majority of them co-localized SP. The non-cholinergic neurons projecting to the muscle expressed immunoreactivity for VIP. Polarized innervation of the muscle layers was found neither in the rumen nor in the reticulum. Consequently, intrinsic innervation patterns for the smooth muscle layers in the rumen and reticulum differ from all gastrointestinal regions examined thus far.
Keywords: choline acetyltransferase, Dil tracing, forestomach, substance P, vasoactive intestinal peptide
Introduction
The rumen and reticulum are compartments within the ruminant forestomach that mainly take part in mixing and fermentation of the ingesta. Although the term ‘reticulorumen’ implies that they are functionally a unit, they exhibit specific motility patterns that are unique to each compartment. Motility of the rumen consists mostly of mixing of the ingesta, whereas the reticulum additionally participates in regurgitation of forestomach contents (Ruckebusch, 1989).
Reticuloruminal motility is at least partly driven by myenteric neurons of the enteric nervous system (Gregory, 1982; Pfannkuche et al. 2002b). Enteric neurons are thought to control motility events by the release of specific combinations of neurotransmitters. For this purpose, a direct innervation of the smooth muscle layers by projections of enteric neurons is necessary. In other parts of the gastrointestinal tract, intrinsic innervation of the muscle layers is organized in a highly conserved manner, especially in the circular muscle layer (Brookes, 2001). Innervation patterns consist of ascending cholinergic and descending nitrergic neurons, representing the anatomical basis for the generation of the peristaltic reflex (Brookes, 2001). It is noteworthy that, in the rumen of the adult sheep, such strictly polarized projections cannot be found (Pfannkuche et al. 2002b). Nevertheless, one limiting factor for the analysis of intrinsic neuronal pathways in the forestomach is the filling capacity of this organ. In contrast to the gastrointestinal tract of small laboratory mammals like guinea-pigs, only a very small area of the forestomach can be studied with retrograde tracing techniques. Consequently, in relation to the size of the forestomach, only short-projecting neurons can be taken into account. The reticulorumen of the newborn lamb is smaller in size and is comparable with the guinea-pig stomach. This provides the advantage that projections over a large portion of the reticulorumen can be studied. It has also been found that, apart from differences in their proportion, the myenteric plexus subpopulations in the reticulorumen of the adult sheep and the suckling lamb are comparable in their neurochemical coding (Pfannkuche et al. 2003a). Therefore, we set out to identify the myenteric neurons projecting to the inner or outer muscle layer of the rumen and the reticulum by use of the retrograde tracer 1,1′-didodecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate (Dil). Dil-labelled neurons were further analysed by immunohistochemical detection of cholineacetyl-transferase (ChAT), substance P (SP) and vasoactive intestinal peptide (VIP).
Materials and methods
Tissue preparation
Specimens were taken from male and female newborn Merino lambs (7–10 days old). The experiments were performed in accordance with legislation covering the protection of animals. The lambs were killed by captive bold and subsequent exsanguination at the faculty slaughterhouse. The forestomach was removed, both sides of the ventral ruminal sac and of the reticulum were dissected and contents were rinsed off by washes in ice-cold Krebs–Ringer solution of the following composition: 117 mm NaCl, 4.7 mm KCl, 1.2 mm MgCl2, 1.2 mm NaH2PO4, 25 mm NaHCO3, 2.5 mm CaCl2, 11.5 mm glucose and 1 μm nifedipine, pH 7.4, gassed with 5% CO2/95% O2. The same solution was used for transportation of the tissues.
Retrograde tracing
For retrograde tracing, mucosal and submucosal layers were dissected from muscularis propria. The latter was placed into sterile Krebs–Ringer solution and washed six times by changing the solution every 10 min. After the washings, the tissue was flatly pinned and maximally streched at the bottom of a Sylgard-covered Petri dish. Tissues were used to label neurons projecting to the inner muscle layer or to the outer muscle layer. The inner and outer muscle layers of the reticulorumen are comparable with the circular and longitudinal muscle layers in other gastrointestinal regions, respectively. Specimens used to label neurons projecting to the inner muscle layer were pinned inner muscle layer side up. For labelling neurons projecting to the outer muscle layer, specimens were pinned outer muscle layer side up.
The retrograde tracer Dil (Molecular Probes, Eugene, OR, USA) was used to identify neurons projecting to the inner or outer muscle layer. Dil was evaporated on to small glass beads (200 μm in diameter). One glass bead was placed on to the inner muscle layer or on to the outer muscle layer in the centre of each tissue. After the Dil application, the tissue was rinsed three times for 10 min each with sterile Krebs solution and cultured for 5 days in sterile culture medium (Dulbecco's modified Eagle's nutrient medium F12, supplemented with 10% heat-inactivated fetal calf serum, 100 IU mL−1 penicillin, 100 μg mL−1 streptomycin, 1.24 μg mL−1 amphotericin B, 20 μg mL−1 gentamicin, 2.1 mg mL−1 NaHCO3 and 1 μm nifedipine, pH 7.4; all chemicals were from Sigma, Deisenhofen, Germany) at 37 °C and 5% CO2. During the culture period, tissues were continuously agitated using a rocking tray. Culture medium was changed once daily. For the last 16 h of the culture period, the culture medium was supplemented with 40 μm colchicine.
After organotypic culture the specimens were fixed for 24 h at 4 °C in 0.1 m phosphate buffer containing 4% paraformaldehyde and 15% picric acid. They were washed in 0.1 m phosphate buffer and stored in phosphate-buffered saline (PBS, 0.1 m) containing 0.1% NaN3.
Immunohistochemistry
To characterize the Dil-labelled neurons of the myenteric plexus, whole mount preparations were obtained by removing inner and outer muscle layers. Unlike in small mammals such as guinea-pigs, it is possible and also necessary to remove both muscle layers even in preparations of the newborn lambs. Otherwise, remaining muscles would make examination of the myenteric plexus impossible.
For immunohistochemical characterization of the Dil-labelled neurons, preparations were permeabilized in buffered (0.5 m NaHCO3/NaCO3, pH 8.6, containing 0.1% NaN3) glycerol solution of ascending concentrations (30 min in 50%, 30 min in 80%, 180 min in 100% glycerol). The permeabilized preparations were washed thoroughly with PBS (4 × 15 min) and afterwards pre-incubated in a solution of PBS containing 0.1% NaN3 and 4% horse serum for 1 h at room temperature. The primary antibodies were diluted in the same solution. The tissues were incubated for 72 h at room temperature in the solution containing primary antibodies. The following antisera were used in the respective concentrations: rabbit anti-choline acetyltransferase (ChAT, 1 : 1000, P3YEB; Schemann et al. 1993), rat anti-substance P (SP, 1 : 1000, 10-S015, Fitzgerald, Concord, MA, USA) and guinea-pig anti-vasoactive intestinal polypeptide (VIP, 1 : 1000, GHC7161, Peninsula, San Carlos, CA, USA).
After three further washes in PBS, the specimens were incubated for 48 h in buffer solution containing the secondary antibodies. Affinity-purified secondary anti-rabbit, anti-rat and anti-guinea-pig raised in donkeys conjugated to Carbocyanin (Cy2), Biotin or Indodicarbocyanin (Cy5, all purchased from Dianova, Hamburg, Germany) were used. The final dilutions of secondary antibodies were 1 : 200 (Cy2 conjugates), 1 : 500 (Cy5 conjugates) and 1 : 50 (Biotin conjugates). Biotin-conjugated secondary antibodies were visualized using streptavidin conjugated with Aminomethylcoumarin-acetat (AMCA) at a dilution of 1 : 50. Finally, the specimens were washed in PBS, ‘whole mounts’ were mounted on poly-l-lysine-coated slides and coverslipped with a solution of NaHCO3/Na2CO3 (0.5 m, pH 7.0) containing 0.1 NaN3 and 80% glycerol.
The preparations were examined with an epifluorescence microscope (IX50, Olympus, Japan). Appropriate filters were used to visualize the Dil and the fluorophores separately (Pfannkuche et al. 1998). Pictures were acquired with a black-and-white video camera (Mod. 4910, Cohu, San Diego, CA, USA) connected to a personal computer and controlled by Scion image software (Scion Corp., Frederick, MD, USA). Frame integration and contrast enhancement were employed for image processing.
Computer-assisted mapping of Dil-labelled neurons
The localization of Dil-labelled neurons was analysed by using a computer-assisted stage mapping system (Märzhäuser and Kassen, Wetzlar, Germany). The Dil application site was defined as the origin of the coordinate system (x = 0, y = 0) and the y-axis was orientated in parallel to the course of the inner muscle layer beneath the application site.
The entire tissue was examined for Dil-labelled neurons. Their location relative to the Dil application site (x and y coordinates) and their neurochemical code was recorded for each tissue. Using the x- and y-coordinates of the retrogradely labelled cell bodies, direction of axonal projection was determined for each neuron. According to studies from the guinea-pig (Brookes et al. 1998) Dil-labelled neurons were defined as ascending or descending neurons with respect to their localization relative to the course of the inner muscle layer (y-axis). Dil-labelled cells located orally to the y-axis (x-value < 0) had axons projecting aborally towards the application sites and were considered as descending neurons. Consequently, somata located aborally to the application site (x-value > 0) had oral axonal projections and were classified as ascending neurons.
Statistics
The results obtained from each preparation were normalized by calculating percentages relative to all Dil-labelled neurons or to the number of neurons in a given subpopulation (neurochemical coding and/or axonal projection). In addition, the median value of the projection length was calculated for each preparation. Results are expressed as mean ± standard deviation (SD; n = number of preparations, N = number of animals).
To determine differences in the proportions of subpopulations within one localization (rumen or reticulum) and application (inner or outer muscle layer) a one-way anova with subsequent multiple comparisons (Student–Newman–Keuls test) was used. Differences between the projection length in the direction of the inner or outer muscle layer were analysed using a t-test.
Statistical tests were performed using SigmaStat 2.0 (SPSS Science, Chicago, IL, USA). Differences were considered as statistically significant at values of P < 0.05.
Results
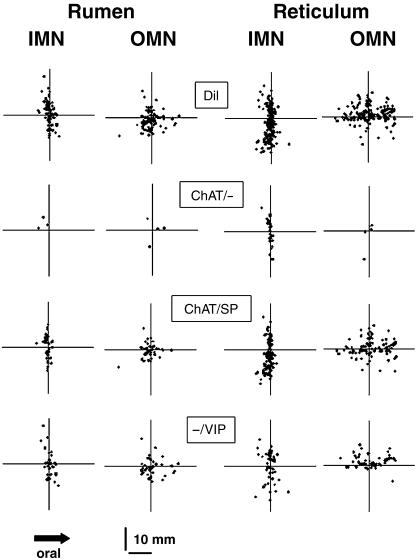
Application of Dil on to the inner or outer muscle layer of rumen and reticulum resulted in labelling of myenteric neurons. The number of Dil-labelled neurons did not differ between rumen and reticulum or between inner and outer muscle layers. It was 30 ± 22 (n = 4; N = 4) for the ruminal inner muscle layer, 31 ± 9 (n = 4; N = 4) for the ruminal outer muscle layer, 99 ± 59 (n = 3; N = 3) for the reticular inner muscle layer and 62 ± 53 (n = 3; N = 3) neurons for the reticular outer muscle layer. Neurons projecting to the inner muscle layer are further referred to as inner muscle neurons (IMN), and neurons retrogradely labelled from the outer muscle layer as outer muscle neurons (OMN). According to previous studies (Pfannkuche et al. 2002b), Dil-labelled neurons were divided in two groups. Neurons that have their soma located oral from the application site with anally projecting axons were defined as ‘descending’ neurons. Consequently, neurons located anal to the application site were defined as ‘ascending’ neurons. Neither in the rumen nor in the reticulum was a projection preference in the ascending or descending direction found for IMN or OMN (Fig. 1).
Fig. 1.
Computer-assisted map of the different subpopulations of Dil-labelled neurons innervating the circular and longitudinal muscle of rumen and reticulum. In each graph the respective data from three or four original preparations stained for ChAT, SP and VIP are shown. Application of Dil resulted in a total labelling of 107 IMN and 108 OMN in the rumen, and 268 IMN and 163 OMN in the reticulum. The Dil-labelled neurons were subdivided into neurons immunoreactive for ChAT/– (rumen: three IMN, five OMN; reticulum: 39 IMN, six OMN), ChAT/SP (rumen: 66 IMN, 55 OMN; reticulum: 177 IMN, 96 OMN) or –/VIP (rumen: 38 IMN, 48 OMN; reticulum: 52 IMN, 61 OMN).
Projection length
The average projection length did not differ between OMN and IMN in the rumen and reticulum. It was 4.0 ± 1.9 mm (range 0.61–16.9 mm) for ruminal IMN, 3.6 ± 1.0 (range 0.66–15.9 mm) for ruminal OMN, 4.6 ± 0.7 (range 0.18–15.69 mm) for reticular IMN and 5.9 ± 1.2 (range 0.97–14.81 mm) for reticular OMN. To compare the circumferential and longitudinal displacement of Dil-labelled neurons projecting to the different targets, the standard deviation of the y- and x-projections was used, respectively. For IMN in the reticulum, the standard deviations for the circumferential spread (3.4 ± 0.3 mm) were significantly greater than for the longitudinal spread (1.2 ± 0.7 mm). For reticular OMN a significantly greater spread in the longitudinal (3.6 ± 0.5 mm) than in the circumferential (2.6 ± 0.4 mm) direction was found. In the rumen, a tendency (P = 0.052) for a pronounced spread in the circumferential direction (2.1 ± 1.0 mm) compared to the longitudinal direction (0.7 ± 0.4 mm) was found for IMN. OMN in the rumen did not show any difference for their spread in the circumferential (2.1 ± 0.6 mm) or longitudinal (2.4 ± 1.1 mm) direction.
Neurochemical coding of Dil-labelled neurons
In all specimens examined, Dil-labelled neurons were divided into two subpopulations by their immunoreactivity for ChAT. For the reticular outer and inner muscle layer and for the ruminal inner muscle layer, ChAT-positive neurons were more numerous than ChAT-negative neurons (Table 1). For the ruminal outer muscle layer only a tendency for a cholinergic dominance could be found. Neither cholinergic nor non-cholinergic neurons expressed a projection preference in ascending or descending direction (Fig. 1).
Table 1.
Neurochemical code of neurons projecting to the inner or outer muscle layer within the rumen and reticulum of the lamb. Within rows no significant differences between the subpopulations could be obtained
| Rumen | Reticulum | |||
|---|---|---|---|---|
| CMN | LMN | CMN | LMN | |
| ChAT positive (%) | 65.5 ± 16.9* | 65.3 ± 21.7 | 81.6 ± 11.6* | 70.2 ± 14.5* |
| ChAT negative (%) | 34.5 ± 16.9 | 34.7 ± 21.7 | 18.4 ± 11.6 | 29.8 ± 14.5 |
| ChAT/SP (%) | 63.4 ± 15.4A | 60.7 ± 23.1A | 65.6 ± 5.4A | 62.7 ± 12.7A |
| ChAT/– (%) | 2.1 ± 2.4B | 4.6 ± 4.0B | 16.0 ± 12.3B | 7.5 ± 6.5B |
| –/VIP (%) | 34.5 ± 16.9C | 34.7 ± 21.7A | 18.4 ± 11.6B | 29.8 ± 14.5B |
Significant difference between the proportion of ChAT-positive and ChAT-negative neurons. t-test; P < 0.05.
A, B and C indicate significant differences between the different neurochemical subpopulations within one column. One-way anova; P < 0.05.
ChAT-positive and ChAT-negative Dil-labelled neurons were further characterized by demonstration of the neuropeptides SP and VIP. In this way, three different subpopulations innervating the smooth muscle layers of the rumen and the reticulum could be distinguished: neurons immunoreactive only for ChAT (ChAT/–), for ChAT and SP (ChAT/SP) or only for VIP (–/VIP) (Figs 1–3). Localizations (i.e. rumen and reticulum) and target tissues (i.e. inner and outer muscle layer) were not related to a specific neurochemical code (Fig. 1, Table 1).
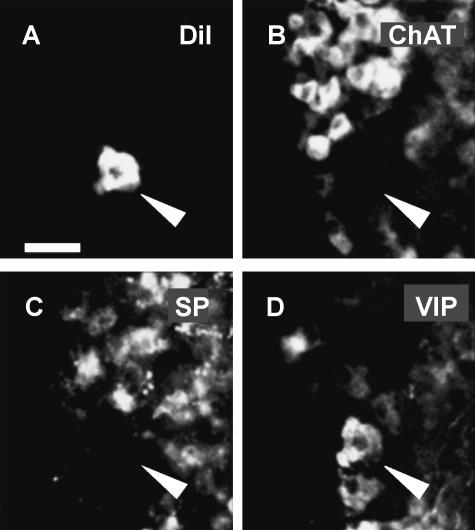
Fig. 3.
Fig. 3 Dil-labelled neuron (indicated by an arrowhead in panel A) projecting to the inner muscle layer of the reticulum. The neuron did not express immunoreactivity for ChAT (B) or SP (C) but did for VIP (D). Therefore, it is encoded –/VIP. Scale bar=50 μm.
Discussion
This study revealed projections of myenteric neurons to the circular and longitudinal muscle layer in the rumen and reticulum of lambs. Neither ruminal nor reticular muscle motor neurons had any polarized projection of ascending cholinergic and descending non-cholinergic pathways. This is consistent with findings from the rumen of adult sheep where again no polarized projections of enteric muscle motor neurons were detected (Pfannkuche et al. 2002b).
Studies in the newborn lamb have the advantage that because of the size of the forestomach, in contrast to the adult sheep, a complete half from the ventral rumen sac and from the reticulum could be taken for analysis. Nevertheless, general projection patterns such as the distribution of Dil-labelled neurons around the application site and the lack of a polarized innervation seem to be comparable between the newborn and the adult sheep where only a relatively small area of the forestomach may be studied (Pfannkuche et al. 2002b).
In our study we found a striking relationship between the orientation of the muscle layers and the distribution of Dil-labelled motor neurons around the Dil application site. The most likely explanation for this phenomenon is the observation that axons of motor neurons run for long distances parallel to the muscle bundles once they leave the plane of the plexus and enter the muscle layer. A distribution of IMN along the course of the inner muscle layer has been demonstrated in various gastrointestinal regions (Pfannkuche et al. 1998, 2002b; Michel et al. 2000; Neunlist et al. 2001). A distribution of OMN along the outer muscles layer was recently found in the guinea-pig gastric corpus and proximal colon (Michel et al. 2000; Neunlist et al. 2001) but interestingly not in the bovine abomasum (Pfannkuche et al. 2002a). Dil-labelled neurons in the reticulorumen were either ChAT positive or VIP positive. It has been reported recently that reticuloruminal myenteric neurons in the lamb are immunoreactive for either ChAT or for NOS (Pfannkuche et al. 2003a, b). Therefore, we assumed that ChAT-negative and VIP-positive Dil-labelled neurons expressed the code NOS/VIP. This neurochemical code for putative inhibitory neurons projecting to the forestomach muscle can also be detected in the rumen of adult sheep (Pfannkuche et al. 2002b) and various gastrointestinal tissues from animals with simple forms of stomach (Brookes, 2001). An inhibitory function of these neurons is supported by the fact that nitric oxide and VIP induce a relaxation of the smooth muscle layers in the forestomach (Denac et al. 1987; Schneider & Eades, 1998). The majority of the Dil-labelled neurons expressed the code ChAT/SP, which indicates a function as excitatory motor neurons to the smooth muscle layers, as has been proposed also in the stomach of animals with simple forms of stomach (Pfannkuche et al. 1998) and intestine (Brookes, 2001). Acetylcholine and SP cause a contractile response of the forestomach muscle layers (Vassileva et al. 1978; Veenendaal et al. 1982; Wong & McLeay, 1988).
Our interpretation of the projection preference of ChAT/SP-positive and NOS/VIP-positive neurons to the muscle, as well as their varicose appearance in the muscle layers, is that these cells probably function as muscle motor neurons. However, it cannot be ruled out that some of these neurons serve as intrinsic afferent neurons, in particular because a proportion of ChAT/SP immunoreactive myenteric neurons in the guinea-pig intestine appear to have a sensory function (Furness et al. 1998).
The results of the present study, together with our previous data from adult sheep (Pfannkuche et al. 2002b), suggest age-associated differences in the proportion of ChAT/SP or NOS/VIP IMN. The proportions changed from 63.4% ChAT/SP and 34.5% NOS/VIP expressing IMN in the suckling lamb to 80.3% ChAT/SP and 19.7% NOS/VIP immunoreactive IMN in the adult sheep. For OMN, no age-related difference in the neurochemical code can be found. It is important to emphasize that the decrease in the population of NOS/VIP immunoreactive IMN and the parallel increase in ChAT/SP immunoreactive IMN in adult sheep is not due to an age-associated overall change in neurochemical coding within the ruminal myenteric plexus (Pfannkuche et al. 2003a). In fact, the proportion of NOS/VIP immunoreactive neurons increased with age, and size of the ChAT/SP population remains stable (Pfannkuche et al. 2003a). This clearly indicated that the age-associated changes in the proportion of the two particular cell populations are related to their function, i.e. they only occur in nerve cells that projected to the inner muscle.
Besides the two populations encoding ChAT/SP or NOS/VIP, a third population exclusively immunoreactive for ChAT was identified. In the rumen of adult sheep, by contrast, no projections of purely cholinergic neurons to the muscle layers have been found (Pfannkuche et al. 2002b). The population of purely cholinergic neurons decreased from the newborn to the adult sheep (Pfannkuche et al. 2003a). Nevertheless, also in the present study, only a small proportion of myenteric neurons with the code ChAT/– projected to the muscle layers in the rumen. The intrinsic innervation patterns between rumen and reticulum did not substantially differ in the newborn sheep. This is in agreement with the fact that rumen and reticulum work as one functional unit (Ruckebusch, 1989). The intrinsic innervation by myenteric neurons might mainly control local contractions as well as tonus of the forestomach wall (Gregory, 1982; Ruckebusch, 1989). Specific motility patterns of the rumen and the reticulum that can be observed during primary and secondary cycles are almost exclusively driven by the parasympathetic nervous system (Ruckebusch, 1989).
In conclusion, in this study we found intrinsic innervation patterns that seem to be comparable between rumen and reticulum of the newborn lamb, but not to any other examined region within the gastrointstinal tract, indicating a specific intrinsic control of the ovine forestomach motility.
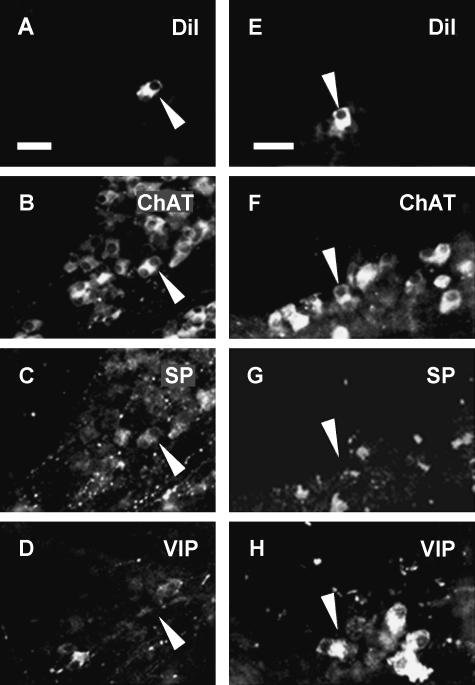
Fig. 2.
Neurochemical code of one IMN (A–D) and one OMN (E–H) in the reticulum. The Dil-labelled neuron shown in panel A by an arrowhead expressed immunoreactivity for ChAT (B) and SP (C) but not for VIP (D) and is therefore encoded ChAT/SP. The Dil-filled neuron demonstraded in panel E expresses only immunoreactivity for ChAT (F) but not for SP (G) nor for VIP (H). Consequently is has the code ChAT/–. Scale bars=50 μm.
Acknowledgments
The skilful technical assistance of Petra Philipp is gratefully acknowledged. This study was sponsored by a grant from the Deutsche Forschungsgemeinschaft and Akademie für Tiergesundheit (AfT), grant numbers PF 403/1-1 and 1-2.
References
- Brookes SJ, Hennig G, Schemann M. Identification of motor neurons to the circular muscle of the guinea pig gastric corpus. J. Comp. Neurol. 1998;397:268–280. doi: 10.1002/(sici)1096-9861(19980727)397:2<268::aid-cne8>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Brookes SJ. Retrograde tracing of enteric neuronal pathways. Neurogastroenterol. Motil. 2001;13:1–18. doi: 10.1046/j.1365-2982.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- Denac M, Marti J, Scharrer E. Relaxation of ruminal smooth muscle by vasoactive intestinal polypeptide (VIP) Zentralbl. Veterinarmed[a] 1987;34:317–320. doi: 10.1111/j.1439-0442.1987.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Furness JB, Kunze WA, Bertrand PP, Clerc N, Bornstein JC. Intrinsic primary afferent neurones of the intestine. Prog. Neurobiol. 1998;54:1–18. doi: 10.1016/s0301-0082(97)00051-8. [DOI] [PubMed] [Google Scholar]
- Gregory PC. Forestomach motility in the chronically vagotomized sheep. J. Physiol. 1982;328:431–447. doi: 10.1113/jphysiol.1982.sp014275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel K, Reiche D, Schemann M. Projections and neurochemical coding of motor neurones to the circular and longitudinal muscle of the guinea pig gastric corpus. Pflugers Arch. 2000;440:393–408. doi: 10.1007/s004240000299. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Michel K, Aube AC, Galmiche JP, Schemann M. Projections of excitatory and inhibitory motor neurones to the circular and longitudinal muscle of the guinea pig colon. Cell Tissue Res. 2001;305:325–330. doi: 10.1007/s004410100387. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Reiche D, Sann H, Schemann M. Different subpopulations of cholinergic and nitrergic myenteric neurones project to mucosa and circular muscle of the guinea-pig gastric fundus. Cell Tissue Res. 1998;292:463–475. doi: 10.1007/s004410051075. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Reiche D, Hoppe S, Schemann M. Cholinergic and noncholinergic innervation of the smooth muscle layers in the bovine abomasum. Anat. Rec. 2002a;267:70–77. doi: 10.1002/ar.10087. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Schemann M, Gäbel G. Ruminal muscle of sheep is innervated by non-polarized pathways of cholinergic and nitrergic myenteric neurones. Cell Tissue Res. 2002b;309:347–354. doi: 10.1007/s00441-002-0554-7. [DOI] [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Aschenbach JR, Gäbel G. Age-associated plasticity in the intrinsic innervation of the ovine rumen. J. Anat. 2003a;203:277–282. doi: 10.1046/j.1469-7580.2003.00218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Gäbel G. Reticular grove and reticulum are innervated by myenteric neurons with different neurochemical codes. Anat. Rec. 2003b;274A:917–922. doi: 10.1002/ar.a.10104. [DOI] [PubMed] [Google Scholar]
- Ruckebusch Y. Gastrointestinal motor functions in ruminants. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology The Gastrointestinal System. Vol. 1. New York: Oxford University Press; 1989. pp. 1225–1283. [Google Scholar]
- Schemann M, Sann H, Schaaf C, Mader M. Identification of cholinergic neurons in enteric nervous system by antibodies against choline acetyltransferase. Am. J. Physiol. 1993;265:G1005–G1009. doi: 10.1152/ajpgi.1993.265.5.G1005. [DOI] [PubMed] [Google Scholar]
- Schneider DA, Eades SC. Antagonist of nitric oxide synthesis inhibits nerve-mediated relaxation of isolated strips of rumen and reticulum. J. Dairy Sci. 1998;81:2588–2594. doi: 10.3168/jds.S0022-0302(98)75816-3. [DOI] [PubMed] [Google Scholar]
- Vassileva P, Stoyanov I, Loukanov Y. Neurotransmitted responses of smooth-muscle strips of complex sheep stomach after electrical field stimulation. Acta Physiol. Pharmacol. Bulg. 1978;4:11–18. [PubMed] [Google Scholar]
- Veenendaal GH, Woutersen-van Nijnanten FM, Van Miert AS. Responses of goat ruminal musculature to substance P in vitro and in vivo. Vet. Res.Commun. 1982;5:363–367. doi: 10.1007/BF02215006. [DOI] [PubMed] [Google Scholar]
- Wong MH, McLeay LM. In vitro spontaneous motility of gastric smooth muscles of the sheep. Q. J. Exp. Physiol. 1988;73:521–531. doi: 10.1113/expphysiol.1988.sp003172. [DOI] [PubMed] [Google Scholar]