Abstract
The porcine infrapatellar fat pad is a structure composed of adipocytes and adipose connective tissues. Limited information is available concerning its chemical composition. Samples of the fat pad collected from young hogs were dissected into two portions: a relatively hard core of the pad with cushioning properties (inner tissue), and a soft adipose tissue surrounding the core (outer tissue). The inner tissue contained less moisture and nitrogen than did the outer tissue. The yield of dry-delipidated tissue was also lower in the inner tissue, indicating a higher content of lipid in this tissue. Fatty acid analysis showed that the proportions of C18: 1, C16: 1 and C18: 2n-6 are higher, and the proportion of C16: 0 is lower in the inner than in the outer tissue. Collagen is the major protein, with relatively small amounts of glycosaminoglycans in both tissues. The content of hyaluronic acid relative to sulphated galactosaminoglycan was lower in the inner than in the outer tissue. The electrophoresis pattern of sulphated galactosaminoglycan was also different between the two tissues. These results suggest that chemical composition varies between adipose tissues with different biomechanical function.
Keywords: adipose connective tissue, hyaluronic acid, sulphated galactosaminoglycan
Introduction
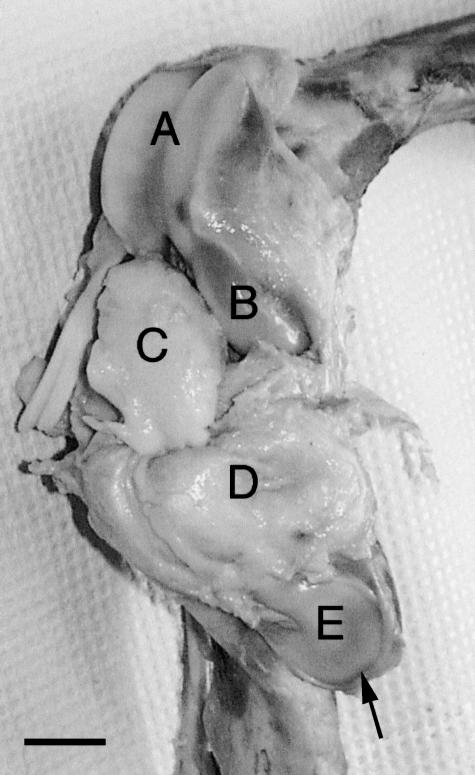
The infrapatellar fat pad found in the human knee (Gray, 1985) or swine stifle joint is composed of adipocytes and adipose connective tissues containing collagen and glycosaminoglycans. Its proximal end is attached to the distal end of the patella, and its distal end is attached to the anterior portions of the medial and lateral menisci. The surface of the infrapatellar fat pad is covered with synovial membrane and articulates with the trochleal cartilage of the distal femur. The fat pad may have an important function as a shock absorber. The chemical composition and structure of cartilage macromolecules in the knee joint have been studied by many researchers, but there is very limited information available concerning the chemical composition of the infrapatellar fat pad found in the same joint. Such information may be important to researchers studying the physiological function of knee joint tissues. The infrapatellar fat pad can be divided into two portions, inner and outer tissues (Fig. 1). The inner tissue is a relatively hard pillow-like adipose tissue that has cushioning properties, as detected by touching with a blunt probe, whereas the outer tissue is a soft adipose tissue surrounding the inner tissue (T. Nakano, unpublished observations). The inner tissue may be subjected to a compressive load, whereas the outer tissue may remain unloaded or be subjected to a tensional load. Previous studies of adipose tissues have demonstrated site-specific variations in the collagen concentration (Pond & Mattacks, 1989; Pond et al. 1993) and fatty acid composition (Calder et al. 1992) among adipose tissues of different anatomical origin. It has also been reported that chemical composition varies between connective tissues with different biomechanical functions. For example, in the bovine temporomandibular joint disc, the distribution of glycosaminoglycan differs between inner and outer tissues subjected to compressive and tensional forces, respectively (Nakano & Scott, 1996). Glycosaminoglycan composition is also different among the zones of the stifle meniscus with different compressive loads (Nakano et al. 1997a).
Fig. 1.
Right stifle joint tissues of swine showing articular cartilages of trochlea (A), medial femoral condyle (B) and patella (E), and the inner tissue (C) separated from the outer tissue (D). The inner and outer tissues show the ventral and dorsal surfaces, respectively. An arrow shows the proximal end of the patella. Scale bar = 2 cm.
The extracellular matrix of adipose connective tissue is composed of collagen fibres embedded in an amorphous ground substance containing glycosaminoglycans. Glycosaminoglycans are straight-chain acidic polysaccharides composed of repeating disaccharide units of hexosamine and uronic acid (or galactose in keratan sulphate). With the exception of a non-sulphated glycosaminoglycan, hyaluronic acid (which exists as a free polymer), all glycosaminoglycans are sulphated and covalently attached to core proteins to form proteoglycans (Nakano et al. 2002). This study was undertaken to examine whether there is any difference in chemical composition between the inner and outer tissues from the infrapatellar fat pad of young pigs.
Materials and methods
Infrapatellar fat pads were obtained from young (5–6 months old) hogs at a local abattoir. All fat pad samples collected appeared normal. They were dissected free from synovial membrane and patellar ligament, and separated into inner and outer tissues. Each tissue was cut into small pieces (approximately 0.5 cm in diameter), and thoroughly mixed for chemical analysis. A total of eight replicates for inner or outer tissues, each derived from a different animal, were prepared. A portion of each tissue was studied for its moisture and nitrogen contents. The remaining portion was treated with chloroform/methanol (2 : 1) (Folch et al. 1957), and the lipid extracted with this solvent was assayed for fatty acid composition. The delipidated tissues obtained were dried at 80 °C for 1 h and subjected to chemical analysis and glycosaminoglycan studies.
The moisture content was estimated from the loss of weight of each sample by heating at 110 °C overnight. The nitrogen content was determined using the Kjeldahl method (AOAC, 1998). The content of hydroxyproline (reflecting that of collagen) was determined by the method of Stegemann & Stalder (1967), and the uronic acid content was measured using the diphenyl reaction (Blumenkrantz & Asboe-Hansen, 1973). With this method, the colour yields of glucuronic acid and iduronic acid are similar. Sialic acid (a carbohydrate moiety of glycoprotein) was analysed by the thiobarbituric acid reaction (Warren, 1959) after hydrolysis of samples in 0.1 n sulphuric acid at 80 °C for 1 h. The chromophore formed was extracted using 1-propanol (Nakano et al. 1994) instead of cychlohexanone as used by Warren (1959). Fatty acid composition was determined by gas liquid chromatography (Sunwoo et al. 1995). Analytical data obtained with eight replicates were expressed as mean ± standard deviation (SD). Analysis of variance was used to detect a significant difference between means. Computations were made by using a linear model for paired comparison (SAS Institute, 1990).
For glycosaminoglycan studies, dry-delipidated samples of inner or outer tissue were pooled, and a portion of pooled sample was digested with twice crystallized papain (20 units mg−1 protein, Sigma-Aldrich Canada Ltd) at an enzyme to sample ratio of 1 : 250 in 0.1 m sodium acetate buffer, pH 5.5 (Harab & Mourão, 1989) containing 0.005 m disodium EDTA, 0.005 m cystein hydrochloride and 0.02% (wt/vol) sodium azide at 60 °C overnight (Scott, 1960). After proteolysis, cold trichloroacetic acid was added to the digest to a final concentration of 7% (wt/vol) and the mixture was left at 4 °C for 2 h. After removal of the precipitated protein by centrifugation, the supernatant was transferred to a dialysis tube (molecular weight cutoff 6–8 kDa) previously boiled in deionized water for 10 min, and dialysed in running tap water for 24 h and for another 24 h in deionized water at 4 °C. The non-dialysable product obtained, referred to as papain digest, was freeze-dried and examined using cellulose acetate electrophoresis, enzymatic digestion and gel chromatography.
Cellulose acetate electrophoresis of papain digest was carried out using 0.1 m pyridine/1.2 m acetic acid, pH 3.5 (Habuchi et al. 1973). The proportion of hyaluronic acid in total glycosaminoglycan was determined densitometrically by measuring peak areas for hyaluronic acid and sulphated glycosaminoglycan from electrophoretograms stained with Alcian blue. Values for hyaluronic acid were corrected for the low staining intensity (0.56) of the non-sulphated glycosaminoglycan relative to that of a 1 : 1 mixture of chondroitin sulphate and dermatan sulphate as 1.0 (Nakano & Scott, 1996). The tissue content of hyaluronic acid was calculated from the total uronic acid content and the proportion of hyaluronic acid in total glycosaminoglycan. The tissue content of sulphated glycosaminoglycan was calculated from the difference between the total uronic acid content and the content of uronic acid in hyaluronic acid. Gel chromatography of papain digest was performed using a 1.0 × 110-cm column of Sephacryl S-300 (Pharmacia Biotech Inc.) equilibrated and eluted with 0.5 m sodium acetate, pH 5.6, containing 0.02% (wt/vol) sodium azide.
Digestions with Streptomyces hyaluronidase and chondroitinase-ABC were carried out as described previously (Nakano & Scott, 1996). The digests were examined by cellulose acetate electrophoresis or a turbidimetric assay with cetyltrimethylammonium bromide (DiFerrante, 1956). In the latter, the enzyme suscestibility of glycosaminoglycan was estimated by determining the percentage decrease of turbidity upon digestion with enzyme.
Results
Palpation confirmed that the infrapatellar fat pad comprises a relatively hard core with cushioning properties, whereas the outer tissue is a soft adipose tissue. Analysis of fresh tissues showed that both moisture content and nitrogen content were lower (P < 0.05) in the inner than in the outer tissue (Table 1). This was consistent with the larger (P < 0.05) dry-delipidated tissue weight in the latter (Table 1), reflecting a higher lipid concentration in the inner tissue. Analysis of dry-delipidated tissues demonstrated that there is no difference (P > 0.05) in nitrogen, hydroxyproline, sialic acid and uronic acid concentrations between the inner and outer tissues (Table 1).
Table 1. Analysis of the infrapatellar fat pad tissues.
| Inner tissue | Outer tissue | |
|---|---|---|
| Fresh sample1 | ||
| Moisture | 13.0 ± 1.9 | 26.6 ± 6.7* |
| Nitrogen | 4.5 ± 1.4 | 7.3 ± 0.4* |
| Dry-delipidated tissue | 5.5 ± 0.2 | 9.0 ± 0.9* |
| Dry-delipidated sample2 | ||
| Nitrogen | 15.1 ± 0.9 | 15.4 ± 0.3 |
| Hydroxyproline | 95.0 ± 0.7 | 93.4 ± 5.2 |
| Uronic acid | 2.2 ± 0.1 | 2.2 ± 0.2 |
| Sialic acid | 1.9 ± 0.3 | 2.1 ± 0.0 |
Values are expressed as percentage of wet weight and presented as mean ± SD obtained from eight replicates.
Values are expressed as μg/mg dry weight and presented as mean ± SD obtained from eight replicates.
Value in the outer tissue is significantly (P < 0.05) different from that in the inner tissue.
Fatty acid analysis of the infrapatellar fat pad tissues (Table 2) showed that C18: 1 is the predominant fatty acid (average 48%), followed by C16: 0 (24%) and C18: 0 (11%). The proportions of C18: 1, C16: 1 and C18: 2n-6 were higher (P < 0.05) in the inner than in the outer tissue, whereas the proportion of C16: 0 was higher (P < 0.05) in the outer tissue. The proportion of saturated fatty acid was higher (P < 0.05), whereas the proportion of monounsaturated fatty acid and the unsaturation index value were lower (P < 0.05), in the outer tissue. The proportions of the remaining fatty acids were similar (P > 0.05) between the inner and outer tissues.
Table 2. Fatty acid composition (wt% of total fatty acid) of infrapatellar fat pad 1.
| Inner tissue | Outer tissue | |
|---|---|---|
| C16: 0 | 23.45 ± 1.04 | 25.10 ± 0.99* |
| C16: 1 | 4.32 ± 0.57 | 3.33 ± 0.40* |
| C18: 0 | 9.30 ± 1.86 | 11.83 ± 1.41 |
| C18: 1 | 48.46 ± 1.07 | 47.20 ± 1.61* |
| C18: 2n-6 | 8.50 ± 1.08 | 6.61 ± 1.18* |
| SAFA | 34.95 ± 2.78 | 39.10 ± 2.03* |
| MUFA | 54.31 ± 1.52 | 51.86 ± 2.00* |
| PUFA | 9.04 ± 1.96 | 10.74 ± 1.51 |
| Unsaturation index | 78.05 ± 4.97 | 70.34 ± 3.93* |
Minor fatty acids accounting for less than 2% of total fatty acid were not included. Values are presented as mean ± SD obtained from eight replicates.
Value in the outer tissue is significantly (P < 0.05) different from that in the inner tissue.
SAFA: saturated fatty acids; MUFA: monounsaturated fatty acids;
PUFA: polyunsaturated fatty acids; Unsaturation index is the sum of [number of double bonds for each fatty acid ×% of each fatty acid].
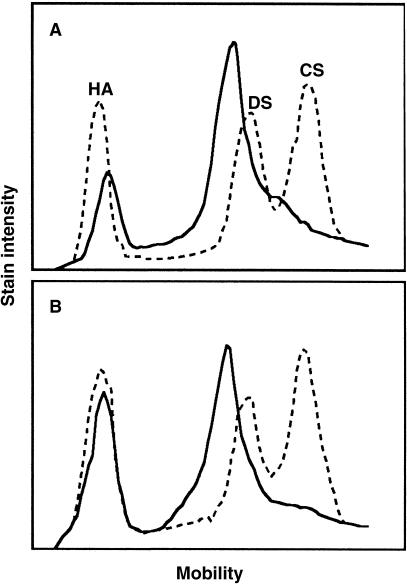
Cellulose acetate electrophoresis of papain digests, accounting for 1.7 and 2.3% of dry-delipidated inner and outer tissues, respectively, showed two major bands in both tissues (Fig. 2). The slow-moving band had a mobility similar to that of hyaluronic acid, and was highly susceptible to Streptomyces hyaluronidase, an enzyme specific to hyaluronic acid (Ohya & Kaneko, 1970) (data not shown). Therefore, the component in the band was identified as hyaluronic acid. The fast-moving band had mobility close to but slightly slower than that of dermatan sulphate. In addition, the inner tissue showed a relatively minor band between dermatan sulphate and chondroitin sulphate bands. Such a band was not seen in the outer tissue (Fig. 2). All these bands from either tissue were highly susceptible to chondroitinase-ABC (data not shown), suggesting the presence of galactosaminoglycans. The densitometrically determined proportion of hyaluronic acid in the total glycosaminoglycan was lower in the inner (31%) than in the outer (51%) tissue. The tissue contents of hyaluronic acid and sulphated galactosaminoglycan expressed as μg uronic acid per mg dry-delipidated tissue were 0.7 and 1.5, respectively, in the inner tissue. In the outer tissue, the content of uronic acid (1.1 μg mg−1) was similar between the two glycosaminoglycans.
Fig. 2.
Cellulose acetate electrophoresis of papain digests from the inner (A) and outer (B) tissues. (—) Papain digest; (…) standard glycosaminoglycans, including hyaluronic acid (HA), dermatan sulphate (DS) and chondroitin sulphate (CS).
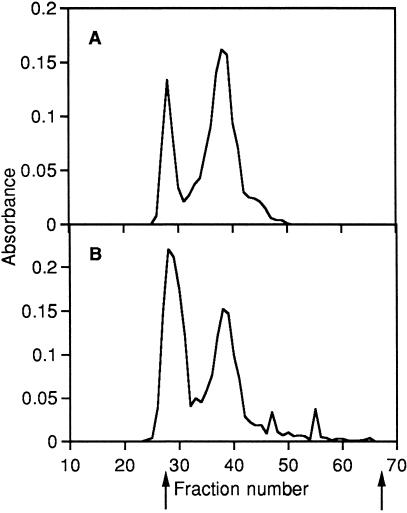
Gel chromatography of papain digest on Sephacryl S-300 (Fig. 3) gave two major uronic acid peaks. This is consistent with the results obtained with cellulose acetate electrophoresis. The first peak (Kav 0.07) seen in fractions 25–31 was highly susceptible to Streptomyces hyaluronidase (susceptibility 98% as measured by the fall of turbidity, see Methods), and the second peak (Kav 0.26) in fractions 35–42 was susceptible to chondroitinase-ABC (susceptibility 93%). Therefore, the first peak contained hyaluronic acid, and the second peak galactosaminoglycan. The proportion of hyaluronic acid in total glycosaminoglycan estimated from the peak area was higher in the outer (45%) than in the inner tissue (26%), as seen with cellulose acetate electrophoresis (see above).
Fig. 3.
Chromatography of papain digests from the inner (A) and outer (B) tissues on Sephacryl S-300. Fractions (1.4 mL) collected were monitored for uronic acid content by the diphenyl reaction (absorbance at 520 nm).
Discussion
The present results suggest that collagen is the major protein of the porcine fat pad connective tissue, in that type I collagen is probably the major protein providing the tissue with tensile strength. The content of collagen is similar between the inner and outer tissues, averaging 68% of the dry-delipidated tissue [calculated using a factor of 7.25 (Nakano et al. 1996) to convert from the hydroxyproline content]. However, because of the difference in the dry-delipidated tissue content between the two tissues (Table 1), the collagen content in the fresh tissue is calculated to be 1.6 times lower in the inner tissue (3.8%) than in the outer tissue (6.1%), as measured from the nitrogen content (Table 1). The value for the inner tissue is comparable with the collagen content (mean ± SD = 3.9 ± 1.7%) calculated from the hydroxyproline content (0.54 ± 0.24% found in the groin adipose tissues from animals; Pond & Mattacks, 1989). The collagen content for the outer tissue is close to the upper range of reported values. The content of glycosaminoglycan in the dry-delipidated fat pad tissue can be estimated to be 0.7% using a factor of 3 (Nakano et al. 1996) to convert from uronic acid content. No information was available on the glycosaminoglycan content in the adipose tissue.
The collagen content in the dry-delipidated fat pad tissue is apparently lower than that (72%) found in the skeletal muscle epimysium from pigs of similar age (Nakano et al. 1996). By contrast, the glycosaminoglycan content (see above) is two-fold higher than that (0.35%) found in the porcine epimysium (Nakano et al. 1996), indicating a higher collagen to glycosaminoglycan ratio (reflecting the amount of collagen fibre relative to amorphous ground substance) in the epimysium compared with the fat pad. The muscle connective tissue, epimysium, may need a framework with a higher proportion of collagen than the adipose connective tissue, which helps transfer force generated during muscle contraction.
It has been demonstrated that the bovine (Nakano et al. 1997a) and porcine (T. Nakano, unpublished observations) adipose connective tissues covering the longissimus dorsi muscle contain decorin, a galactosaminoglycan containing proteoglycan. The galactosaminoglycan found in the fat pad (Figs 2 and 3) is probably derived from decorin. This will be confirmed immunohistochemically with the anti-decorin monoclonal antibody used in the previous study (Nakano et al. 1997b). Decorin is a low-molecular-weight (∼100 kDa) leucine-rich proteoglycan containing a single galactosaminoglycan chain. Because decorin can bind to type I collagen fibrils and transforming growth factor-β (TGF-β) through its protein core (Kresse et al. 1994), it has been suggested that decorin has roles in regulating collagen fibrillogenesis and neutralizing the effect of TGF-β (Kresse et al. 1994). It would be interesting to know whether decorin actively contributes to the formation of adipose tissues by controlling the activity of TGF-β, which stimulates collagen and proteoglycan synthesis. Very little is known regarding whether the adipose connective tissue contains galactosaminoglycan bearing proteoglycans, versican and biglycan, which are found in fibrous connective tssues (Nakano et al. 2002).
The qualitative and quantitative differences observed between the inner and outer tissues in this study appear to reflect the difference in their functions. The inner tissue, which contains less water and more fat than does the outer tissue, may need less hyaluronic acid, a glycosaminoglycan with high water-holding capacity. The inner tissue, which is probably subjected to a compressive load, appears to need more sulphated galactosaminoglycan, which has a higher electrophoretic mobility than dermatan sulphate. A similar situation was reported in the bovine temporomandibular joint disc, in which the concentration of chondroitin sulphate galactosaminoglycan (but not dermatan sulphate proteoglycan) is higher in inner (presumably compressed) than in outer (presumably non-compressed) tissue (Nakano et al. 1996).
The fatty acid composition of porcine fat pad determined in this study is in general similar to that of pig fat reported elsewhere (Lawrie, 1974), showing C18: 1, C16: 0 and C18: 0 accounting for 46, 28 and 13% of the total fatty acid, respectively. No satisfactory explanation is available concerning the difference seen in the proportions of fatty acids between the inner and outer tissues. Site-specific differences in fatty acid composition have been reported in human adiopose tissues (Calder et al. 1992). Pond et al. (1992) analysed fat depots from polar bears and reported differences in fatty acid composition between inner (dorsal) and outer (ventral) regions of the superficial adipose tissue. Differences in metabolic activity between adipose tissues may be an important causative factor for variation of fatty acid composition. The present study provides a basis for more detailed investigation of the structure and composition of proteoglycans together with causes of variation of fatty acid composition in the porcine infrapatellar fat pad.
Acknowledgments
We gratefully acknowledge the financial support from the Alberta Agricultural Research Institute and the Natural Sciences and Engineering Research Council of Canada.
References
- AOAC. Official Methods of Analysis of AOAC International. 16. Gaithersburg, MD: Association of Official Analytical Chemists; 1998. [Google Scholar]
- Blumenkrantz N, Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal. Biochem. 1973;54:484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Calder PC, Harvey DJ, Pond CM, Newsholme EA. Site-specific difference in the fatty acid composition of human adipose tissue. Lipids. 1992;27:716–720. doi: 10.1007/BF02536031. [DOI] [PubMed] [Google Scholar]
- DiFerrante N. Turbidimetric measurement of acid mucopolysaccharides and hyaluronidase activity. J. Biol. Chem. 1956;220:303–306. [PubMed] [Google Scholar]
- Folch J, Lees M, Sloan-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–507. [PubMed] [Google Scholar]
- Gray H. Anatomy of the Human Body. Philadelphia: Lea & Febiger; 1985. pp. 404–405. [Google Scholar]
- Habuchi H, Yamagata T, Iwata H, Suzuki S. The occurrence of a wide variety of dermatan sulfate-chondroitin sulfate copolymers in fibrous cartilage. J. Biol. Chem. 1973;248:6019–6028. [PubMed] [Google Scholar]
- Harab RC, Mourão PAS. Increase of chondroitin 4-sulfate concentration in the endochondral ossification cartilage of normal dogs. Biochim. Biophys. Acta. 1989;992:237–240. doi: 10.1016/0304-4165(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Kresse H, Hausser H, Schönherr E. Small proteoglycans. In: Jollès P, editor. Proteoglycans. Basel: Birkhäuser-Verlag; 1994. pp. 73–100. [Google Scholar]
- Lawrie R. Meat Science. 2. Oxford: Pergamon Press; 1974. [Google Scholar]
- Nakano K, Nakano T, Ahn DU, Sim JS. Sialic acid contents in chicken eggs and tissues. Can. J. Anim. Sci. 1994;74:601–606. [Google Scholar]
- Nakano T, Scott PG. Changes in the chemical composition of the bovine temporomandibular joint disc with age. Arch. Oral. Biol. 1996;41:845–853. doi: 10.1016/s0003-9969(96)00040-4. [DOI] [PubMed] [Google Scholar]
- Nakano T, Sunwoo HH, Li X, Price MA, Sim JS. Study of sulfated glycosaminoglycans from porcine skeletal muscle epimysium including analysis of iduronosyl and glucuronosyl residues in galactosaminoglycan fractions. J. Agric. Food. Chem. 1996;44:1424–1434. [Google Scholar]
- Nakano T, Dodd CM, Scott PG. Glycosaminoglycans and proteoglycans from different zones of the porcine knee meniscus. J. Orthop. Res. 1997a;15:213–220. doi: 10.1002/jor.1100150209. [DOI] [PubMed] [Google Scholar]
- Nakano T, Li X, Sunwoo HH, Sim JS. Immunohistochemical localization of proteoglycans in bovineskeletal muscle and adipose connective tissues. Can. J. Anim. Sci. 1997b;77:169–172. [Google Scholar]
- Nakano T, Dixon WT, Ozimek L. Proteoglycans (glycosaminoglycans/mucopolysaccharides) In: Vandamme EJ, De Baets S, Steinbüchel A, editors. Biopolymers Polysaccharides II. Polysaccharides from Eukaryotes. Vol. 6. Weinheim: Wiley-VCH-Verlag GmbH; 2002. pp. 575–604. [Google Scholar]
- Ohya T, Kaneko Y. Novel hyaluronidase from Streptomyces. Biochim. Biophys. Acta. 1970;198:607–609. doi: 10.1016/0005-2744(70)90139-7. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA. Biochemical correlates of the structural allometry and site-specific properties of mammalian adipose tissue. Comp. Biochem. Physiol. 1989;92A:455–463. doi: 10.1016/0300-9629(89)90591-4. [DOI] [PubMed] [Google Scholar]
- Pond CM, Mattacks CA, Colby RH, Ramsay MA. The anatomy, chemical composition, and metabolism of adipose tissue in wild polar bears (Ursus maritimus) Can. J. Zool. 1992;70:326–341. [Google Scholar]
- Pond CM, Mattacks CA, Calder PC, Evans J. Site-specific properties of human adipose depots homologous to those of other mammals. Comp. Biochem. Physiol. 1993;104A:819–824. doi: 10.1016/0300-9629(93)90160-6. [DOI] [PubMed] [Google Scholar]
- SAS Institute. SAS User's Guide. Cary, NC: SAS Institute Inc; 1990. [Google Scholar]
- Scott JE. Aliphatic ammonium salts in the assay of acidic polysaccharides from tissues. Meth. Biochem. Anal. 1960;8:145–197. doi: 10.1002/9780470110249.ch4. [DOI] [PubMed] [Google Scholar]
- Stegemann H, Stalder K. Determination of hydroxyproline. Clin. Chim. Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- Sunwoo HH, Nakano T, Hudson RJ, Sim JS. Chemical composition of antlers from wapiti (Cervus elaphus) J. Agric. Food Chem. 1995;43:2846–2849. [Google Scholar]
- Warren L. The thiobarbituric acid assay of sialic acids. J. Biol. Chem. 1959;234:1971–1975. [PubMed] [Google Scholar]