Abstract
When applied prior to excitotoxic lesions, ciliary neurotrophic factor (CNTF) has been shown to be neuroprotective. However, data concerning the endogenous CNTF content of the intact rat striatum are rare and have not until now been available for the quinolinic acid (QA)-lesioned striatum. Therefore, we investigated the CNTF content in the QA-lesioned rat striatum for at least 1 month using immunohistochemistry and Western blot analysis. In lesioned striata a neuronal loss was observed by Nissl staining and by a reduction of NeuN-immunoreactive cells, whereas increased glial fibrillary acidic protein immunoreactivity showed a gliotic reaction. With CNTF immunohistochemistry we found that in the QA-lesioned striatum CNTF was increased over time, whereas it was not detectable in intact and sham-lesioned striata. CNTF-immunoreactive cells had the morphology of protoplasmatic astrocytes. Furthermore, quantitative Western blotting demonstrated that the content of CNTF protein from striatal lysates containing 1 mg of whole protein 1 month after QA lesioning (2.76 ± 1.71 ng) was significantly increased (P < 0.05, U-test) compared with sham-lesioned hemispheres (0.68 ± 0.25 ng) and intact controls (0.55 ± 0.25 ng). We conclude that CNTF content is correlated with glial scar formation and suggest that our results may be of relevance to cell grafting strategies for the treatment of Huntington's disease.
Keywords: excitotoxic lesion, gliosis, Huntington's disease, neurodegeneration, neurotrophin
Introduction
Huntington's disease (HD) is an inherited autosomal dominant neurodegenerative disorder primarily characterized by the loss of neostriatal GABA-ergic projection neurons leading to impaired motor behaviour and, in later stages, to cognitive and psychiatric dysfunctions, and finally to death (Reiner et al. 1988). HD is not curable and currently there is no satisfactory therapy; therefore, extensive research for the treatment of HD is currently being performed using various animal models (Beal et al. 1986; DiFiglia, 1990; Menalled & Chesselet, 2002). Excitatory amino acids, for example quinolinic acid (QA), can be used to induce lesions of the caudate putamen (CPu) in the rat or the corpus striatum of non-human primates, similar to the neural cell loss of HD (DiFiglia, 1990). This excitotoxic lesion model is well established and can be used to investigate various strategies for the treatment of HD, for example the application of neurotrophic factors (Hefti, 1994; Alberch et al. 2002) or cell replacement therapies (Björklund & Lindvall, 2000). One of the most promising candidates of potent neurotrophins for the treatment of HD is the ciliary neurotrophic factor (CNTF), which is a member of the alpha-helical cytokine superfamily (Richardson, 1994; Sendtner et al. 1994). Its receptor, CNTF-receptor alpha, is widely distributed in neurons of the intact adult central nervous system, including the CPu (Ip et al. 1993). In previous studies, CNTF yielded neuroprotective effects when applied prior to an excitotoxic QA lesion by direct infusion into the brain (Anderson et al. 1996), when implanted cells genetically engineered to overexpress CNTF were used as intraparenchymal biological pumps (Emerich et al. 1996, 1997a,b; Weinelt et al. 2003) or through in vivo gene transfer that led to an increase of CNTF in the CPu (De Almeida et al. 2001; Regulier et al. 2002). Furthermore, a clinical phase I trial using intrathecal infusion of CNTF according to the protocol from Bachoud-Levi et al. (2000) has recently been finished with encouraging results (M. Peschanski, unpubl. observ.).
Other cytokine derivates and neurotrophins were also able to induce neuroprotection in the rat QA lesion model, for example glial cell line-derived neurotrophic factor (GDNF) (Perez-Navarro et al. 1999; Alberch et al. 2002), brain-derived neurotrophic factor (BDNF) (Alberch et al. 2002) or nerve growth factor (NGF) (Schumacher et al. 1991; Emerich et al. 1994; Menei et al. 2000). Meanwhile, it was observed that the mRNA-expression for GDNF and its receptor GFRalpha1 increased after a QA lesion (Bresjanac & Antauer, 2000; Marco et al. 2002). The same effect was described for mRNA levels of NGF (Canals et al. 1998) and BDNF (Checa et al. 2000), respectively.
Nevertheless, comparable data concerning the expression of CNTF mRNA or protein in the CPu after a QA lesion or other excitotoxic lesions of the CPu have not been available until now, and data about the CNTF protein content in the intact CPu are scarce and contradictory (De Almeida et al. 2001; Regulier et al. 2002). Thus, we investigated the time course of the expression pattern of CNTF protein in the CPu after an unilateral QA lesion using immunohistochemistry and, in addition, quantitative Western blotting to determine the CNTF protein contents in protein lysates from the CPu of intact control, sham-lesioned and QA-lesioned animals 29 days after surgery.
Materials and methods
Animals
Adult male Wistar rats aged 3–5 months were housed at 22 ± 2 °C under a 12-h light/dark cycle, with free access to standard food and water. Housing was in groups of two individuals per cage. All animal care and handling were conducted in compliance with the regulations and licensing of the local authorities.
Excitotoxic lesions
Stereotaxic surgery was conducted on animals (n = 17) under anaesthesia with fentanyl (0.25 mg kg−1, Curamed, Karlsruhe, Germany) and dehydrobenzperidol (5 mg kg−1, Janssen-Cilag, Neuss, Germany) using a stereotaxic frame (Kopf, Tujunga, CA, USA). Lesions were made in the left CPu by injection of 2 × 0.5 μL of 0.09 m QA (Sigma) dissolved in 0.1 m phosphate-buffered saline (PBS; titrated with 1 m NaOH to pH 7.4) delivered over 4 min each via a 26-gauge 5-μL Hamilton syringe (Hamilton, Bonaduz, Switzerland). The coordinates with reference to bregma were (1) A = +1.2, L = 2.8, V = −5.5, and (2) A = +0.0, L = 3.6, V = −5.5 (Paxinos & Watson, 1992). Furthermore, the animals received two sham injections consisting of 0.5 μL 0.1 m PBS into the contralateral CPu (respective coordinates).
Assessment of successful lesions
Apomorphine-induced rotation provides a sensitive and rapid behavioural correlate of unilateral striatal damage (Norman et al. 1988; Björklund et al. 1994). Seven, 14, 21 and 28 days after QA lesion, animals were tested for apomorphine-induced rotation (1.0 mg kg−1 in isotonic saline, Teclapharm, Lüneburg, Germany, injected subcutaneously) using a self-constructed automated rotometry device. Whole body turns (rotations) towards the lesioned side measured over 30 min were defined as complete 360° ipsilateral turns and are reported as net differences between the two directions per minute.
Immunohistochemistry
Preparation of the lesioned animals for immunohistochemistry and histology were performed 7 (n = 3), 14 (n = 3) and 29 days (n = 7) after QA lesion. Intact animals (n = 5) served as controls. After anaesthesia with ether, the rats were perfused transcardially with ice-cold 0.9% sodium chloride (50 mL), followed by 400 mL of 3.7% paraformaldehyde (dissolved in 0.1 m PBS, pH 7.4). Brains were immediately removed from the skull, post-fixed for 4 h, and transferred into PBS (pH 7.4) containing 20% sucrose overnight at 4 °C. The cryoprotected brains were frozen in isopentane (−50 °C) and stored at −80 °C until further processing.
Immunohistochemistry was performed as described previously for the staining of brain tissue sections without the use of detergents (Haas et al. 2000), because, in our hands, the various antibodies used to detect CNTF protein in this study only showed a specific staining when the use of detergents was omitted. In brief, 25-μm-thick coronal cryostat sections containing the region of the CPu, or as positive controls sections containing the optical nerve or the olfactory bulb (Stöckli et al. 1991; Dobrea et al. 1992; Kirsch et al. 1998), were stored free floating overnight in PBS containing 30% sucrose at 4 °C. Cryoprotected brain sections were pretreated for immunohistochemical staining by successive freeze/thaw cycles (five times) in hyperosmotic medium and liquid nitrogen, to make intracellular epitopes accessible for antibodies. The sections were then washed twice in 0.1 m TRIS buffer (pH 7.4).
For fluorescence microscopy, free-floating sections were incubated for 4 h at room temperature in 0.1 m TRIS containing 3% bovine serum albumine (BSA, Sigma) and 5% normal goat or normal horse serum (according to the secondary antibodies), followed by an incubation overnight at 4 °C with primary antibodies (dissolved in 0.1 m TRIS, 1% BSA) against rat CNTF (goat polyclonal, 1 : 250, R & D Systems; or chicken polyclonal, 1 : 300, Promega), glial fibrillary acidic protein (GFAP, mouse monoclonal, 1 : 400, Sigma) or neuronal nuclei antigen (NeuN, mouse monoclonal, 1 : 1000, Chemicon). Tissue sections were then washed three times in 0.1 m TRIS and incubated overnight at 4 °C with biotinylated secondary antibodies against chicken IgY (donkey polyclonal, 1 : 200, Dianova, Hamburg, Germany), against goat IgG (donkey polyclonal, 1 : 200, Dianova) or with Cy2-conjugated anti-mouse IgG (goat polyclonal, 1 : 400, Dianova). After three rinses in 0.1 m TRIS, sections stained for GFAP and NeuN were mounted on to gelatine-coated glass slides and embedded in anti-fading fluorescence mounting medium, whereas sections for CNTF visualization were further incubated for 2 h in ABC-complex (1 : 500 for solutions A and B, Vector Laboratories) followed by three rinses in 0.1 m TRIS, for 2 h at room temperature in Fluorescein–Avidin D (1 : 250, Vector Laboratories) and finally rinses in 0.1 m TRIS (3 × 5 min). Specimens were also mounted on gelatine-coated glass slides and embedded in anti-fading fluorescence mounting medium.
For light microscopy freeze/thawed sections were, after two rinses in 0.1 m TRIS, incubated for 20 min in 3% H2O2 to quench endogenous peroxidases. The sections were then rinsed three times in 0.1 m TRIS and incubated for 1 h in 0.1 m TRIS containing 3% BSA and 5% normal horse serum, followed by an incubation overnight at 4 °C with the primary antibodies against rat CNTF (goat polyclonal, 1 : 250, R & D Systems) dissolved in 0.1 m TRIS containing 1% BSA. After three washes in 0.1 m TRIS, sections were incubated overnight at 4 °C with biotinylated secondary antibodies directed against goat IgG (donkey polyclonal, 1 : 200, Dianova) followed by three washes in 0.1 m TRIS, then for 2 h in the ABC-Complex (1 : 500 for solutions A and B, Vector Laboratories), and after three rinses in 0.1 m TRIS the final detection with the chromophore DAB (Sigma) for 10 min at room temperature (5 mg DAB dissolved in 100 mL 0.1 m TRIS containing 1 μL of 30% H2O2) was performed. Sections were mounted on to gelatine-coated glass slides, dehydrated in graded alcohol concentrations and coverslipped with Depex mounting medium (Serva).
Nissl and myelin staining
For histology, parallel sections were mounted on to gelatine-coated glass slides and stained for Nissl substance with 0.1% Cresyl violet acetate (Sigma) or, for myelin, using a silver impregnation slightly modified from Zilles (1985).
For myelin staining, the sections were fixed in a 0.1 m neutral phosphate-buffered 3.7% paraformaldehyde solution for 45 min and subsequently immersed in a solution of 67% pyridine (Merck) and 33% acetic anhydride (Roth, Karlsruhe, Germany) for 30 min. Following three rinses in distilled water, the sections were transferred into an ammonium silver nitrate solution (0.1% ammonium nitrate, Sigma; 0.1% silver nitrate, VEB Feinchemie, Sebnitz, Germany; 3 mm sodium hydroxide, Sigma) for 30 min in the dark and subsequently rinsed three times in 1% acetic acid. Thereafter, sections were immersed in a ‘physical developer’ (0.3 m sodium bicarbonate, Merck; 0.1% ammonium nitrate; 0.1% silver nitrate; 0.5% tungstosilicic acid, Sigma; 0.08% Agfacolour bleach fixer, Agfa). Development of the sections was stopped after 20 min by immersion in 0.5% acetic acid for 2 × 5 min and subsequent rinsing in running tap water for 10 min. Next, staining was fixed in a photographic fixer (Acidofix, Agfa) for 10 min. After an additional rinse in running tap water for 10 min and a short rinse in distilled water, the sections were dehydrated through ethanol to xylene and coverslipped with Depex mounting medium (Serva).
Analysis and documentation of tissue sections
Brain sections were analysed with a Leitz Aristoplan microscope (Wetzlar, Germany) using the FITC filter unit for immunofluorescence inspections. When documenting the immunofluorescence of the sham-lesioned control hemisphere and the contralateral QA-lesioned side the film exposure time was fixed to demonstrate the differences of emitted light intensities as indicators for treatment-dependent protein expression patterns.
Western blot analysis
Four weeks after QA lesion four animals were killed by pentobarbital overdose (80 mg kg−1, Serva) and transcardially perfused with 4 °C isotonic saline (50 mL). The brains were removed from the skull and tissue blocks containing the QA-lesioned and the sham-lesioned CPu were excised. Tissue blocks from the CPu of intact animals served as intact controls (n = 4). Comparable amounts of fresh tissue blocks (50 mg of tissue per 1000 μL sample buffer) were boiled for 5 min in SDS (sodium dodecyl sulphate) sample buffer (Laemmli, 1970). SDS polyacrylamide gel electrophoresis (SDS-PAGE) was performed with ready-to-use criterion mini gels (Biorad, Munich, Germany) consisting of 4–20% polyacrylamide. A 30-μL SDS sample buffer from the various CPu was loaded per lane. Measurements of whole protein contents in these lysates were performed according to the method described by Dieckmann-Schuppert & Schnittler (1997). Additionally, recombinant rat CNTF (R & D Systems) was also boiled for 5 min in SDS sample buffer and various concentrations were loaded, serving as a positive control and furthermore to evaluate the CNTF protein content in tissue lysates. After electrophoresis, proteins were transferred on to nitrocellulose membranes (Amersham, Freiburg, Germany). Membranes were blocked for 1 h at room temperature in 0.1 m PBS (pH 7.4), 0.1% Tween 20 (PBS-T, Sigma) and 1% BSA (Sigma). Primary antibodies directed against β-actin, the product of a housekeeping gene (mouse monoclonal, 1 : 3000, Sigma) and rat CNTF (goat polyclonal, 1 : 1000, R & D Systems) were simultaneously performed overnight at 4 °C. Membranes were washed in PBS-T (4 × 15 min) and incubated for 1 h at room temperature with secondary antibodies conjugated with horseradish peroxidase (anti-mouse, 1 : 5000 and anti-goat, 1 : 10 000, both Vector Laboratories). After washing the membranes in PBS-T (4 × 15 min), the peroxidase activity was visualized on X-ray films with an enhanced chemiluminescence kit (Amersham). Exposure time for films was 2 min.
Quantification of Western blot dots
X-ray films were digitized by a high-resolution transparent flat bed scanner (Duoscan, Agfa) at a lateral resolution of 800 ppi. Hence, these parameters were used for all blots. To determine the intensity of six different known CNTF concentrations, these ‘standards’ were measured by an interactive image analytical procedure. Each dot of CNTF resembling 0.05, 0.1, 0.2, 0.3, 0.4 or 0.5 ng lane−1 was demarcated five times interactively to reduce variability of measurements. The individual background of each X-ray film was subtracted in order to preserve comparability. The evaluation was realized by a script for the image analysis system KS400 (Zeiss Vision). We observed no stronger differences by evaluating the images by a densitometric (transformed grey-level values) (Oberholzer et al. 1996) or an intensitometric (original grey-level values) approach. Blots were performed in quadruplicate. Using the means, a first-order exponential model fitting was performed to obtain the standard curve. The resulting equation was used for calculating the CNTF content within the CPu lysates.
The dots of the three groups (intact control n = 4, sham-lesioned n = 4, QA-lesioned n = 4) were quantified for CNTF and the housekeeping protein β-actin (internal standard). Each dot was demarcated and quantified three times to reduce measurement variability. Data are expressed as mean ± SD. The means of the three groups were compared by the U-test.
Results
Apomorphine-induced rotations
We used apomorphine-induced rotations to evaluate the degree of unilateral excitotoxic lesion of the CPu of rats, because they provide a sensitive and behavioural correlate of striatal damage. One week after lesion the mean numbers of apomorphine-induced rotations were 3.8 ± 1.1 rotations min−1 and increased up to 5.2 ± 1.1 rotations min−1 2 weeks after the QA lesion, then reaching a plateau and remaining constant with 4.6 ± 0.6 and 5 ± 1.1 rotations min−1, 3 or 4 weeks post lesion, respectively, which is in the reported range of successfully lesioned animals (Norman et al. 1988; Björklund et al. 1994; Weinelt et al. 2003). This behavioural analysis suggests that 2 weeks after lesion the loss of striatal neurons and the decrease of dopamine receptors in the lesioned CPu was completely achieved.
Morphological changes in the QA-lesioned CPu
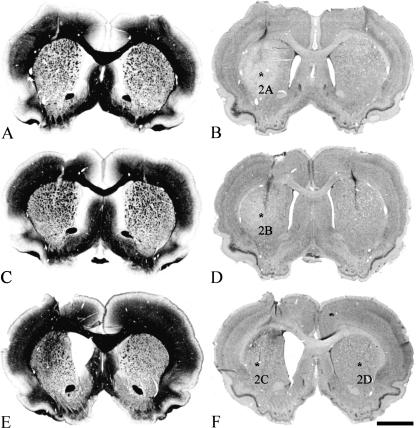
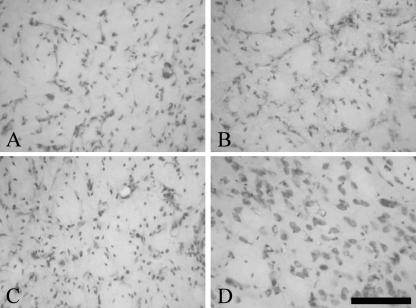
Excitatory amino acid lesions of the CPu mimic some of the neuropathological features that occur during HD (DiFiglia, 1990). We used myelin and Nissl stainings to demonstrate gross morphological changes in the lesioned CPu (Fig. 1). A nearly complete loss of striatal neurons over time and partial replacement by glial cells was observed in the Nissl stainings (Fig. 2) and was also verified with immunohistochemistry against the glial marker GFAP and the neuronal marker NeuN (Fig. 3). The various fibre bundles that pass through the CPu seemed to be unaffected by the lesioning. However, over time they become closer to each other as a result of shrinkage of striatal neuronal tissue (Fig. 1A,C,E). Concomitantly with this reduction of tissue we observed an ongoing enlargement of the lateral ventricle in the lesioned hemisphere (Fig. 1). The contralateral sham-lesioned hemisphere did not show these morphological changes and did not differ from those of intact animals (Figs 1 and 2D). Only in the vicinity of the needle tract was a glial scar formation typical for this kind of stab wound observed (Fig. 1D).
Fig. 1.
Histological evaluation of successful QA lesions of the CPu. Frontal sections through the injection site 7 days (A,B), 14 days (C,D) and 29 days (E,F) after a unilateral QA lesion (left hemisphere) are illustrated. Myelin stainings are shown in the left column (A,C,E) whereas Nissl stainings are shown to the right (B,D,F). The decreasing tissue volume of the CPu and the enlargement of the lateral ventricle over time after lesion, compared with the sham-lesioned side, are clearly visible. Asterisks (B,D,F) indicate the corresponding regions shown in Fig. 2. Scale bars, 500 μm (A–F).
Fig. 2.
Neuronal loss and increasing gliosis after QA lesion. High magnification of Nissl stainings from CPu 7 days (A), 14 days (B) and 29 days (C) after QA lesion showing the loss of neurons and the increase of glial cells over time, whereas in the contralateral sham-lesioned hemisphere 29 days after surgery (D) the amount of glial cells and neurons cannot be distinguished from intact animals. Scale bars, 100 μm (A–D).
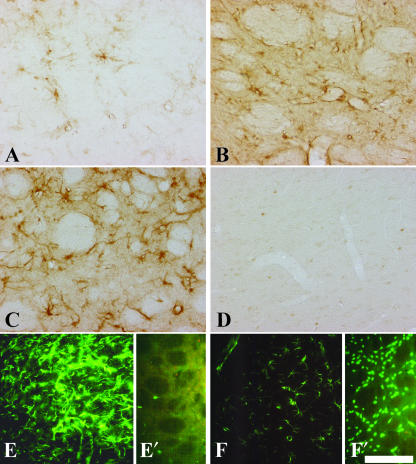
Fig. 3.
Immunohistochemical detection of CNTF, GFAP and NeuN in the QA-lesioned and sham-lesioned contralateral CPu. Increasing staining intensity for CNTF 7 days (A), 14 days (B) and 29 days (C) after a QA lesion is shown. The sham-lesioned CPu (D) does not show any immunoreactivity for CNTF. In the QA-lesioned CPu 29 days post-lesion (E), GFAP immunoreactivity is strongly increased compared with sham-lesioned CPu (F). Twenty-nine days after QA lesion the loss of neurons is clearly detectable by immunostaining against the neuronal nuclei antigen in the lesioned CPu (E′), compared with the sham-lesioned (F′) CPu with many NeuN-immunoreactive cells. Scale bars, 100 μm (A–D), 175 μm (E,F).
CNTF expression in QA-lesioned CPu documented with immunohistochemistry
We used two different primary antibodies directed against CNTF, and various detection systems, including immunofluorescence or final visualization with the chromogen DAB (Fig. 3A–D); we always obtained similar results for the pattern of CNTF immunostaining. According to several groups (Stöckli et al. 1991; Dobrea et al. 1992; Kirsch et al. 1998), positive controls showed a strong CNTF immunoreactivity within cells of the the olfactory bulb, contributing to the glial limiting membrane, and also in cells of the optic nerve having close contact with the glial limiting membrane or blood vessels (not shown). Each negative control performed by exclusion of the primary antibodies showed a complete negative staining (not shown), as was the case for stainings of the CPu from intact control rats. In contrast to the negative stainings in the CPu of intact control animals, the CPu of QA-lesioned animals led to a weak staining for CNTF with single CNTF-containing cells 1 week after surgery (Fig. 3A). Two weeks after lesion, the intensity of CNTF labelling and, furthermore, the number of CNTF-immunoreactive cells increased (Fig. 3B). Twenty-nine days after the QA lesion the CNTF labelling was further intensified (Fig. 3C). The cells that contained CNTF (Fig. 3A–C) showed a similar morphology and distribution in the CPu as the GFAP-immunoreactive protoplasmatic astrocytes with large, swollen cell bodies (Fig. 3E). In the contralateral hemisphere receiving PBS injections, CNTF was only detectable directly along the needle tracts, as described previously (Asada et al. 1995). However, in the remaining parts of the sham-lesioned CPu there was no detectable CNTF labelling (Fig. 3D) and only a sparse to moderate GFAP labelling (Fig. 3F), comparable with stainings from intact controls (not shown). In the CPu, 29 days after lesion, where the CNTF and GFAP expression was high (Fig. 3C,E), only single NeuN-positive cells could be found (Fig. 3E′), whereas in the contralateral PBS-injected hemisphere many NeuN–immunoreactive nerve cells were visible (Fig. 3F′).
Measurement of CNTF protein content
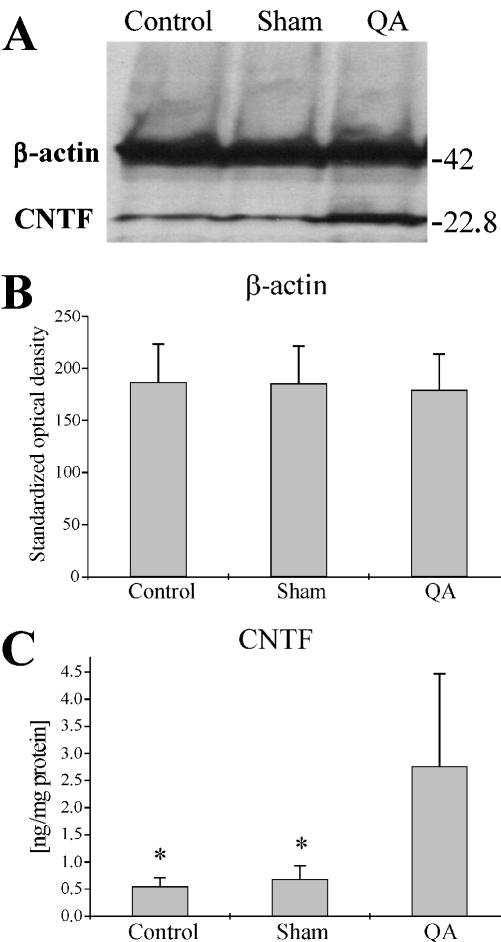
Using quantitative Western blotting we determined the CNTF content in the excised CPu from intact controls and sham-lesioned and QA-lesioned animals 29 days after surgery (Fig. 4). Because there were no significant intergroup differences of the β-actin content (Fig. 4B) no corrections of the calculated CNTF-dot intensities had to be performed. In lysates containing 1 mg of whole protein we found a significantly greater content of CNTF in the CPu of the QA-lesioned hemispheres (2.76 ± 1.71 ng, n = 4) in comparison with both the sham-lesioned hemispheres (0.68 ± 0.25 ng, n = 4) and the intact controls (0.55 ± 0.16 ng, n = 4) at P < 0.05 (Fig. 4C). No significant difference in CNTF-content was observed between the intact control and the sham-lesioned group.
Fig. 4.
CNTF content in intact control and sham-lesioned and QA-lesioned CPu 29 days post-surgery determined with Western blotting. A typical result from a Western blot (A) with tissue lysates from intact control, sham-lesioned or QA-lesioned CPu incubated simultaneously against CNTF (22.8 kDa) and β-actin (42 kDa). When measuring the standardized optical density for the dots of β-actin in the various groups, no significant differences were detectable (B). Measurements of the CNTF dots (C), however, revealed a significantly higher CNTF content in QA-lesioned CPu as compared with both sham-lesioned and intact control CPu (*P < 0.05, U-test). In B and C the error bars show standard deviations.
Discussion
CNTF is one of the most potent factors with neurotrophic effects when applied prior to excitotoxic lesions of the CPu in rats (Beal et al. 1986; Emerich et al. 1994, 1997a; Weinelt et al. 2003) or the corpus striatum of non-human primates (Emerich et al. 1997b). Its potential as a treatment for human patients suffering from HD when applied intrathecally has been furthermore investigated in a clinical phase I study (Bachoud-Levi et al. 2000; M. Peschanski, unpubl. observ.). However, data concerning the endogenous contents of CNTF mRNA or CNTF protein in the intact rat CPu were rare (Stöckli et al. 1991; De Almeida et al. 2001; Regulier et al. 2002) and data regarding endogenous CNTF content in the CPu that has been lesioned by excitotoxic agents were not available. Therefore, it seems important to obtain precise information regarding the endogenous CNTF protein content after a unilateral QA lesion in the CPu, up to 29 days post-lesion, a commonly accepted time-scale for this model (Beal et al. 1986; Emerich et al. 1994, 1997a, b; Regulier et al. 2002; Weinelt et al. 2003). The results of the time-dependent changes in CNTF content were closely correlated with the morphological changes, as demonstrated by immunohistochemical visualization of CNTF, using two different primary antibodies that were detected with similar results by immunofluorescence or by the ABC-method with the chromophore DAB. Both primary antibodies against CNTF led to similar results and we verified their specificity by negative and positive controls. The latter was realized by incubating a specimen of the optic nerve and the olfactory bulb of the rat that are known to present high levels of CNTF protein in astrocytes (Stöckli et al. 1991; Dobrea et al. 1992; Kirsch et al. 1998). In the QA-lesioned CPu, CNTF was also high, being specifically labelled in cells having the morphology of protoplasmatic astrocytes, whereas we did not observe these staining patterns in the sham-lesioned hemisphere. In the latter, we detected a weak CNTF immunoreactivity in the vicinity of the needle tract only. The high amount of regional CNTF expression due to such a stab wound has been described earlier by in situ hybridization (Asada et al. 1995) and also indicates the specificity of the antibodies presently used. Moreover, in the Western blot experiments the specificity of one of the primary antibodies was shown by use of recombinant rat CNTF and the specific labelling in the brain protein lysates from the various animals was shown with a single band at the same molecular weight as recombinant rat CNTF.
In Stöckli et al. (1991), where absolute contents of CNTF mRNA were not listed, the highest CNTF mRNA content in adult rats was found in the sciatic nerve, whereas for the central nervous system the CNTF mRNA content in the striatum was defined as low, in comparison with the optic nerve and olfactory bulb, which both contained about 20% of the CNTF mRNA of the sciatic nerve. In that study no specific data were given concerning CNTF protein content in the adult or developing rat striatum. In more recent studies of De Almeida et al. (2001) and Regulier et al. (2002), an enzyme-linked immunosorbent assay (ELISA) was applied to determine the CNTF protein content in the rat striatum without or after lentiviral CNTF delivery prior to an excitotoxic lesion using QA. In both studies the CNTF protein content of the control striata (n = 3, De Almeida et al. 2001; n = 2, Regulier et al. 2002) was below the detection level. Nevertheless, in the first study the CNTF protein content subsequent to lentiviral transfection of the striatum was about 0.94 ± 0.33 ng mg−1 protein and in the second study it was between 13.1 ± 0.8 ng mg−1 and 15.5 ± 4.7 ng mg−1 protein depending on the ratio of the injected vectors, which was 1 : 1 or 1 : 5 for a cocktail containing TRE-CNTF and PGK-tTA lentiviruses. Furthermore, in both studies the treatment of the animals with the lentiviral constructs led to neuroprotection of striatal tissue and also to a decrease of apomorphine-induced rotations. The CNTF protein content we determined, using Western blots, in the intact CPu (0.55 ± 0.16 ng mg−1 protein) or in the sham-lesioned CPu (0.68 ± 0.25 ng mg−1 protein) seemed to be in the range of undetectable levels for CNTF as described by De Almeida et al. (2001) and Regulier et al. (2002) for their control tissues using an ELISA. In our experimental set-up we observed 1 month after a QA lesion a CNTF protein content of 2.76 ± 1.71 ng mg−1 protein in the CPu, which is clearly elevated compared with intact controls, but is below the described values for the lentiviral transfected striata in the study of Regulier et al. (2002).
The fact that a dramatic time-dependent local increase of CNTF, detectable with immunohistochemistry, occurred only between 14 days and 29 days after QA lesion could surely explain why a neuroprotective effect of the endogenous CNTF protein could not be observed in our lesion model, as a result of the late up-regulation at a time point when the striatal neurons already had undergone cell death (Beal et al. 1986; DiFiglia, 1990). The role of the endogenous CNTF protein expression at this late time point is possibly linked to astrogliosis and formation of a glial scar. There is recent evidence that CNTF induces astrocytic differentiation or gliosis (Lee et al. 2000). This effect of CNTF has been shown in vitro in differentiating neural stem cells (Sun et al. 2001), in O-2A-immunoreactive progenitor cells (Hughes et al. 1988), for radial glia cells in cortical slice cultures (Hasling et al. 2003) and for differentiating mouse embryonic stem cells (Barberi et al. 2003). A study investigating the brain from mice overexpressing CNTF showed an increase of GFAP immunoreactivity and hypertrophy in astrocytes, compared with their wild-type littermates (Winter et al. 1995). Stereotaxic injection of CNTF in the brain of wild-type mice also led to a gliotic reaction with an increase of GFAP immunoreactivity 48 h after injection, whereas the heat-inactivated CNTF protein did not lead to such an effect (Winter et al. 1995) and comparable results have also been obtained using rats (Clatterbuck et al. 1996). In another study performed by Kahn et al. (1995), infusion of CNTF into the neonate rat brain also induced an up-regulation of GFAP in the brain of the recipients. Adenovirus-mediated gene transfer leading to CNTF overexpression, for example in the striatum, led also to an increase of GFAP immunoreactivity and to an hypertrophied phenotype in astrocytes (Lisovoski et al. 1997).
Our results deliver important information for cell replacement strategies, including neural stem cells, that are actually discussed for the treatment for HD (Björklund & Lindvall, 2000). Petersen et al. (1999) investigated the influence of CNTF on graft survival of cells, derived from the lateral ganglionic eminence from embryonic 14-day-old rats and transplanted into the CPu of rats that received a QA lesion into the CPu 14 days prior to transplantation. Interestingly, they found that cell suspensions, containing about 0.6 ng of CNTF per injection (3 μL of suspension in toto), showed no better survival of neural cells than cells from control grafts without added CNTF. Comparing these results with our study, the explanation for the failure of a significant protection of the grafted cells could be the high amounts of endogenous CNTF, which could exert maximal survival-enhancing effects on the grafted cells. Unfortunately, Petersen et al. exclusively used markers for neurons and did not investigate the expression of glial markers in their study. The lateral ganglionic eminence of E14 rats contains neural stem cells that have the capacity to undergo differentiation in several cell types of the central nervous system (Cattaneo & Conti, 1998; Zhou & Chiang, 1998). It would be of interest to see if there was a difference of differentiation into several cell lineages, e.g. GFAP-positive astrocytes, between neural stem cells grafted in the QA-lesioned vs. the intact hemisphere as a result of the high amounts of endogenous CNTF expressed after this excitotoxic insult. In addition, in other transplantation experiments it has been shown that nestin-positive temperature-sensitive immortalized neural stem cells grafted into the striatum of rats 2 weeks prior to an excitotoxic lesion with ibotenic acid differentiated preferentially into GFAP-positive cells (Lundberg & Björklund, 1996). Because no data are available regarding the CNTF content in the brain of HD patients, one can only speculate what endogenous CNTF could exert on the grafted cells in patients suffering from HD and, furthermore, that exogenously infused CNTF could perhaps induce an unintentional glial differentiation of the grafted fetal progenitor/stem cells. Astrogliosis, mainly in the basal ganglia, is one of the hallmarks of HD (Selkoe et al. 1982; Leigh et al. 1985). Therefore, a study referring to the question of the expression of various neurotrophic factors in HD would be surely of great interest, because it is known that GDNF, and also CNTF, are depleted in the substantia nigra from patients with Parkinson's disease (Chauhan et al. 2001).
In conclusion, our study provides results showing that in the QA lesion model, which reproduces various neuroanatomical changes occurring during HD, there is a significant increase of CNTF protein over time in the lesioned CPu and that the CNTF protein is expressed in cells having the morphology of protoplasmatic astrocytes. The up-regulation of CNTF seems to be correlated with glial scar formation in the lesioned CPu. If and how astrocytic CNTF expression may prevent neuronal cell death and induce neuronal or glial differentiation in vivo, especially of stem cell grafts, need to be investigated in future studies to bring us a step forward to reducing progression of neurodegenerative diseases.
Acknowledgments
We thank Professor G. Nikkhah (Abteilung Stereotaktische Neurochirurgie, Universitätsklinikum Freiburg) for providing us with the plans for the self-constructed rotometry device and Dr Ing. H. Janik (Institut für Arbeitsmedizin, Universität Rostock) and A. Stave (Institut für Physiologie, Universität Rostock) for the construction of this device. We also thank Heidemarie Waldmann (Institut für Anatomie, Universität Rostock) for technical assistance.
References
- Alberch J, Perez-Navarro E, Canals JM. Neuroptotection by neurotrophins and GDNF family members in the excitotoxic model of Huntington's disease. Brain Res. Bull. 2002;57:817–822. doi: 10.1016/s0361-9230(01)00775-4. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Anderson KD, Panayotatos N, Corcoran TL, Lindsay RM, Wiegand SJ. Ciliary neurotrophic factor protects striatal output neurons in an animal model of Huntington disease. Proc. Natl Acad. Sci. USA. 1996;93:7346–7351. doi: 10.1073/pnas.93.14.7346. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada H, Ip NY, Pan L, Razack N, Parfitt MM, Plunkett RJ. Time course of ciliary neurotrophic factor mRNA expression is coincident with the presence of protoplasmatic astrocytes in traumatized rat striatum. J. Neurosci Res. 1995;40:22–30. doi: 10.1002/jnr.490400104. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Bachoud-Levi AC, Deglon N, Nguyen JP, Bloch J, Bourdet C, Winkel L, et al. Neuroprotective gene therapy for Huntington's disease using a polymer encapsulated BHK cell line engineered to secrete human CNTF. Hum. Gene Ther. 2000;11:1723–1729. doi: 10.1089/10430340050111377. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Barberi T, Klivenyi P, Calingasan NY, Lee H, Kawamata H, Loonam K, et al. Neural subtype specification of fertilization and nuclear transfer embryonic stem cells and application in parkinsonian mice. Nature Biotechnol. 2003;21:1200–1207. doi: 10.1038/nbt870. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Beal MF, Kowall NW, Ellison DW, Mazurek MF, Swartz DW, Martin JB. Replication of the neurochemical characteristics of Huntington's disease by quinolinic acid. Nature. 1986;321:168–171. doi: 10.1038/321168a0. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Björklund A, Campbell K, Sirinathsinghji DJ, Fricker RA, Dunnett SB. Functional capacity of striatal transplants in rat Huntington model. In: Dunnett SB, Björklund A, editors. Functional Neural Transplantation. New York: Raven Press; 1994. pp. 157–195. 10.1111/j.0021-8782.2004.00279.x. [Google Scholar]
- Björklund A, Lindvall O. Cell replacement therapies for central nervous system disorders. Nature Neurosci. 2000;3:537–544. doi: 10.1038/75705. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Bresjanac M, Antauer G. Reactive astrocytes of the quinolinic acid-lesioned rat striatum express GFRalpha1 as well as GDNF in vivo. Exp. Neurol. 2000;164:53–59. doi: 10.1006/exnr.2000.7416. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Canals JM, Marco S, Checa N, Michels A, Perez-Navarro E, Arenas E, et al. Differential regulation of the expression of nerve growth factor, brain-derived neurotrophic factor, and neurotrophin-3 after excitotoxicity in a rat model of Huntington's disease. Neurobiol. Dis. 1998;5:357–364. doi: 10.1006/nbdi.1998.0211. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Cattaneo E, Conti L. Generation and characterization of embryonic striatal conditionally immortalized ST14A cells. J. Neurosci Res. 1998;53:223–234. doi: 10.1002/(SICI)1097-4547(19980715)53:2<223::AID-JNR11>3.0.CO;2-7. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Chauhan NB, Siegel GJ, Lee JM. Depletion of glial cell line-derived neurotrophic factor in substantia nigra neurons of Parkinson's disease brain. J. Chem. Neuroanat. 2001;21:277–288. doi: 10.1016/s0891-0618(01)00115-6. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Checa N, Canals JM, Alberch J. Developmental regulation of BDNF and NT-3 expression by quinolinic acid in the striatum and its main connections. Exp. Neurol. 2000;165:118–124. doi: 10.1006/exnr.2000.7451. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Clatterbuck RE, Price DL, Koliatsos VE. Ciliary neurotrophic factor stimulates the expression of glial fibrillary acidic protein by brain astrocytes in vivo. J. Comp. Neurol. 1996;369:543–551. doi: 10.1002/(SICI)1096-9861(19960610)369:4<543::AID-CNE5>3.0.CO;2-4. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- De Almeida LP, Zala D, Aebischer P, Deglon N. Neuroprotective effect of a CNTF-expressing lentiviral vector in the quinolinic acid rat model of Huntington's disease. Neurobiol. Dis. 2001;8:433–446. doi: 10.1006/nbdi.2001.0388. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Dieckmann-Schuppert A, Schnittler HJ. A simple assay for quantification of protein in tissue sections, cell cultures, and cell homogenates, and of protein immobilized on solid surfaces. Cell Tissue Res. 1997;288:119–126. doi: 10.1007/s004410050799. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- DiFiglia M. Excitotoxic injury of the neostriatum: a model for Huntington's disease. Trends Neurosci. 1990;13:286–289. doi: 10.1016/0166-2236(90)90111-m. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Dobrea GM, Unnerstall JR, Rao MS. The expression of CNTF message and immunoreactivity in the central and peripheral nervous system of the rat. Dev. Brain Res. 1992;66:209–219. doi: 10.1016/0165-3806(92)90082-8. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Hammang JP, Baetge EE, Winn SR. Implantation of polymer-encapsulated human nerve growth factor-secreting fibroblasts attenuates the behavioral and neuropathological consequences of quinolinic acid injections into rodent striatum. Exp. Neurol. 1994;130:141–150. doi: 10.1006/exnr.1994.1193. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Lindner MD, Winn SR, Chen EY, Frydel BR, Kordower JH. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington's disease. J. Neurosci. 1996;16:1568–1581. doi: 10.1523/JNEUROSCI.16-16-05168.1996. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich DF, Cain CK, Greco C, Saydoff JA, Hu ZY, Liu H, et al. Cellular delivery of human CNTF prevents motor and cognitive dysfunction in a rodent model of Huntington's disease. Cell Transplant. 1997a;6:249–266. doi: 10.1177/096368979700600308. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Emerich DF, Winn SR, Hantraye PM, Peschanski M, Chen EY, Chu Y, et al. Protective effects of encapsulated cells producing neurotrophic factor CNTF in a monkey model of Huntington's disease. Nature. 1997b;386:395–399. doi: 10.1038/386395a0. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Haas SJP, Bauer P, Rolfs A, Wree A. Immunocytochemical characterization of in vitro PKH26-labelled and intracerebrally transplanted neonatal cells. Acta Histochem. 2000;102:273–280. doi: 10.1078/S0065-1281(04)70035-5. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Hasling TA, Gierdalski M, Jablonska B, Juliano SL. A radialization factor in normal cortical plate restores disorganized radial glia and disrupted migration in a model of cortical displasia. Eur. J. Neurosci. 2003;17:467–480. doi: 10.1046/j.1460-9568.2003.02468.x. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Hefti F. Neurotrophic factor therapy for nervous system degenerative diseases. J. Neurobiol. 1994;25:1418–1435. doi: 10.1002/neu.480251109. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Hughes SM, Lillien LE, Raff MC, Rohrer H, Sendtner M. Ciliary neurotrophic factor induces type-2 astrocyte differentiation in culture. Nature. 1988;335:70–72. doi: 10.1038/335070a0. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Ip NY, McClain J, Barrezueta NX, Aldrich TH, Pan L, Li Y, et al. The alpha component of the CNTF receptor is required for signaling and defined potential CNTF targets in the adult and during development. Neuron. 1993;10:89–102. doi: 10.1016/0896-6273(93)90245-m. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Kahn MA, Ellison JA, Speight GJ, de Vellis J. CNTF regulation of astrogliosis and the activation of microglia in the developing rat central nervous system. Brain Res. 1995;685:55–67. doi: 10.1016/0006-8993(95)00411-i. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Kirsch M, Schneider T, Lee MY, Hofmann HD. Lesion-induced changes in the expression of ciliary neurotrophic factor and its receptor in rat optic nerve. Glia. 1998;23:239–248. 10.1111/j.0021-8782.2004.00279.x. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Lee JC, Mayer-Proschel M, Rao MS. Gliogenesis in the central nervous system. Glia. 2000;30:105–121. doi: 10.1002/(sici)1098-1136(200004)30:2<105::aid-glia1>3.0.co;2-h. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Leigh RJ, Parhad IM, Clark AW, Buettner-Ennever JA, Folstein SE. Brainstem findings in Huntington's disease. Possible mechanisms for slow vertical saccades. J. Neurol. Sci. 1985;71:247–256. doi: 10.1016/0022-510x(85)90063-2. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Lisovoski F, Akli S, Peltekian E, Vigne E, Haase G, Perricaudet M, et al. Phenotypic alteration of astrocytes induced by ciliary neurotrophic factor in the intact adult brain, as revealed by adenovirus-mediated gene transfer. J. Neurosci. 1997;17:7228–7236. doi: 10.1523/JNEUROSCI.17-19-07228.1997. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg C, Björklund A. Host regulation of glial markers in intrastriatal grafts of conditionally immortalized neural stem cell lines. Neuroreport. 1996;7:847–852. doi: 10.1097/00001756-199603220-00002. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Marco S, Canudas AM, Canals JM, Gavalda N, Perez-Navarro E, Alberch J. Excitatory amino acids differentially regulate the expression of GDNF, neurturin, and their receptors in the adult rat striatum. Exp. Neurol. 2002;174:243–252. doi: 10.1006/exnr.2001.7859. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Menalled LB, Chesselet MF. Mouse models of Huntington's disease. Trends Pharmacol. Sci. 2002;23:32–39. doi: 10.1016/s0165-6147(00)01884-8. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Menei P, Pean JM, Nerriere-Daguin V, Jollivet C, Brachet P, Benoit JP. Intracerebral implantation of NGF-releasing biodegradable microspheres protects striatum against excitotoxic damage. Exp. Neurol. 2000;161:259–272. doi: 10.1006/exnr.1999.7253. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Norman AB, Calderon SF, Giordano M, Sanberg PR. Striatal tissue transplants attenuate apomorphine-induced rotational behavior in rats with unilateral kainic acid lesions. Neuropharmacology. 1988;27:333–336. doi: 10.1016/0028-3908(88)90053-6. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Oberholzer M, Östreicher M, Christen H, Brühlmann M. Methods in quantitative image analysis. Histochem. Cell Biol. 1996;105:333–355. doi: 10.1007/BF01463655. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1992. [Google Scholar]
- Perez-Navarro E, Arenas E, Marco S, Alberch J. Intrastriatal grafting of a GDNF-producing cell line protects striatonigral neurons from quinolinic acid excitotoxicity in vivo. Eur. J. Neurosci. 1999;11:241–249. doi: 10.1046/j.1460-9568.1999.00433.x. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Petersen A, Emgard M, Brundin P. Impact of a preceding striatal excitotoxic lesion and treatment with ciliary neurotrophic factor on striatal graft survival. Brain Res. Bull. 1999;50:275–281. doi: 10.1016/s0361-9230(99)00202-6. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Regulier E, De Almeida LP, Sommer B, Aebischer P, Deglon N. Dose-dependent neuroprotective effect of ciliary neurotrophic factor delivered via tetracycline-regulated lentiviral vectors in the quinolinic acid rat model of Huntington's disease. Hum. Gene Ther. 2002;13:1981–1990. doi: 10.1089/10430340260355383. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Reiner A, Albin RL, Anderson KD, D’Amato CJ, Penney JB, Young AB. Differential loss of striatal projection neurons in Huntington's disease. Proc. Natl Acad. Sci. USA. 1988;85:5733–5737. doi: 10.1073/pnas.85.15.5733. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM. Ciliary neurotrophic factor: a review. Pharmacol. Ther. 1994;63:187–198. doi: 10.1016/0163-7258(94)90045-0. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Schumacher JM, Short MP, Hyman BT, Breakefield XO, Isacson O. Intracerebral implantation of nerve growth factor-producing fibroblasts protects striatum against neurotoxic levels of excitatory amino acids. Neuroscience. 1991;45:561–570. doi: 10.1016/0306-4522(91)90271-o. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ, Salazar FJ, Abraham C, Kosik KS. Huntington's disease: changes in striatal proteins reflect astrocytic gliosis. Brain Res. 1982;245:117–125. doi: 10.1016/0006-8993(82)90344-4. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Sendtner M, Carroll P, Holtmann B, Hughes RA, Thoenen H. Ciliary neurotrophic factor. J. Neurobiol. 1994;25:1436–1453. doi: 10.1002/neu.480251110. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Stöckli KA, Lillien LE, Näher-Noe M, Breitfeld G, Hughes RA, Raff MC, et al. Regional distribution, developmental changes, and cellular localization of CNTF-mRNA and protein in the rat brain. J. Cell Biol. 1991;115:447–459. doi: 10.1083/jcb.115.2.447. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga A, Hua X, et al. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Weinelt S, Peters S, Bauer P, Mix E, Haas SJP, Dittmann A, et al. Ciliary neurotrophic factor overexpression in neural progenitor cells (ST14A) increases proliferation, metabolic activity, and resistance to stress during differentiation. J. Neurosci Res. 2003;71:228–236. doi: 10.1002/jnr.10477. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Winter CG, Saotome Y, Levison SW, Hirsh D. A role for ciliary neurotrophic factor as an inducer of reactive gliosis, the glial response to central nervous system injury. Proc. Natl Acad. Sci. USA. 1995;92:5865–5869. doi: 10.1073/pnas.92.13.5865. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou FC, Chiang YH. Longt-term nonpassaged EGF-responisve neural precursor cells are stem cells. Wound Repair Regen. 1998;6:337–348. doi: 10.1046/j.1524-475x.1998.60409.x. 10.1111/j.0021-8782.2004.00279.x. [DOI] [PubMed] [Google Scholar]
- Zilles K. The Cortex of the RatA Stereotaxic Atlas. Berlin: Springer; 1985. [Google Scholar]