Abstract
Morphology and biomechanics are linked by causal morphogenesis (‘Wolff's law’) and the interplay of mutations and selection (Darwin's ‘survival of the fittest’). Thus shape-based selective pressures can be determined. In both cases we need to know which biomechanical factors lead to skeletal adaptation, and which ones exert selective pressures on body shape. Each bone must be able to sustain the greatest regularly occurring loads. Smaller loads are unlikely to lead to adaptation of morphology. The highest loads occur primarily in posture and locomotion, simply because of the effect of body weight (or its multiple). In the skull, however, it is biting and chewing that result in the greatest loads. Body shape adapted for an arboreal lifestyle also smooths the way towards bipedality. Hindlimb dominance, length of the limbs in relation to the axial skeleton, grasping hands and feet, mass distribution (especially of the limb segments), thoracic shape, rib curvatures, and the position of the centre of gravity are the adaptations to arboreality that also pre-adapt for bipedality. Five divergent locomotor/morphological types have evolved from this base: arm-swinging in gibbons, forelimb-dominated slow climbing in orang-utans, quadrupedalism/climbing in the African apes, an unknown mix of climbing and bipedal walking in australopithecines, and the remarkably endurant bipedal walking of humans. All other apes are also facultative bipeds, but it is the biomechanical characteristics of bipedalism in orang-utans, the most arboreal great ape, which is closest to that in humans. If not evolutionary accident, what selective factor can explain why two forms adopted bipedality? Most authors tend to connect bipedal locomotion with some aspect of progressively increasing distance between trees because of climatic changes. More precise factors, in accordance with biomechanical requirements, include stone-throwing, thermoregulation or wading in shallow water. Once bipedality has been acquired, development of typical human morphology can readily be explained as adaptations for energy saving over long distances. A paper in this volume shows that load-carrying ability was enhanced from australopithecines to Homo ergaster (early African H. erectus), supporting an earlier proposition that load-carrying was an essential factor in human evolution.
Key words: biomechanics of arboreality, biomechanics of walking, human evolution, selective pressure, trunk shape
Introduction
As Foley (2000) observed, bipedality is the outstanding characteristic of our own evolutionary lineage, and the most obvious adaptive distinction from our closest living relatives. The fossil record clearly indicates that bipedality appeared before the (species-specific) expansion of the brain. My focus is thus an old but nevertheless still fascinating question: why did our ancestors become bipeds and not quadrupeds?
Any progress of our knowledge about human evolution must ultimately be linked to the fossil record, and fossils contain primarily information about morphology. In turn, morphological traits must be closely connected to biomechanics, in particular by causal morphogenesis (‘Wolff's law’) and by the interplay of mutations and selection (Darwin's ‘survival of the fittest’). In principle, both linkages allow the identification of selective pressures on the basis of shape. In both cases, the main challenge is clearly to identify which biomechanical factors lead to the observed form of the skeletal elements under consideration, and which factors exert selective pressures on body shape. These mechanisms are complementary, rather than alternatives.
The shape of specific skeletal elements can be explained by analysis of the mechanical stresses that the elements must sustain in life, under normal function. Good examples are the curvature of the phalanges and metapodials (metacarpals and metatarsals) in tree-climbing primates (Preuschoft, 1969, 1971, 1973; Susman et al. 1984; Susman, 1988), the arrangement of trabeculae in the tarsal elements (Preuschoft, 1969, 1973), the epiphyseal sutures (Preuschoft & Tardieu, 1996), the positions of the joint surfaces and the cross-sectional arrangement of compact bone (Pauwels, 1958; Amtmann, 1979) in long bones. All these traits are, or at least can be, acquired during the lifetime of the individual. The development of shape follows processes described by Pauwels (1958) and Kummer (1972) as applications of Wolff's law. It is self-evident that to exclude failure in everyday situations, each skeletal element must be able to sustain the greatest of the regularly occurring loads. In the postcranial skeleton, these highest loads occur primarily in positional behaviour (i.e. posture and locomotion), simply because of the action of body weight (or, in accelerations, a multiple of it). In the skull, however, biting and chewing lead to the greatest loads.
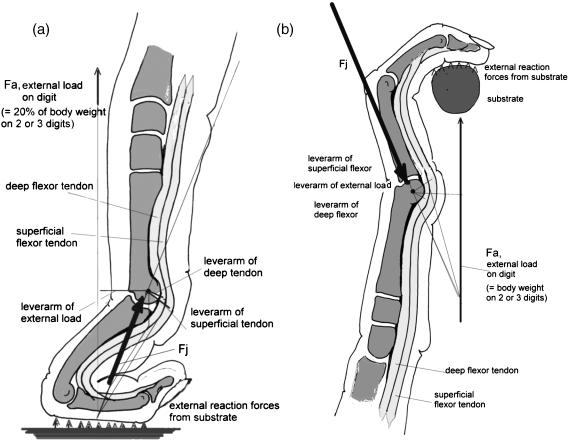
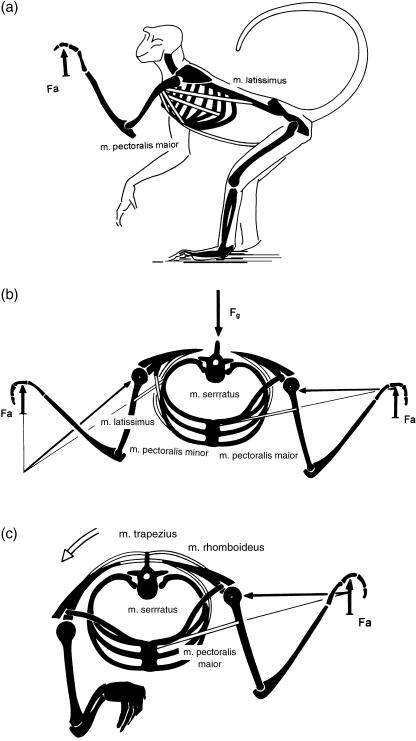
Smaller loads are less likely to lead to morphological traits that tolerate this special loading, i.e. to ‘adapted’ shape. As examples we may consider the relative lack of success of attempts to find ‘adaptations’ to knuckle walking (e.g. Richmond & Strait, 2000; Richmond, 2001) or to tool-making. Concerning the former, Kimura et al. (1979) and Kimura (1995) showed that only some 20% of total body weight is carried on the forelimbs (Fig. 1a), whereas in hanging by one arm about three times body weight must be sustained by the metacarpophalangeal joints (Fig. 1b). Similarly, in tool-making or tool-using, forces in the phalanges and metacarpals are rarely comparable in magnitude with those that occur in locomotion. Nevertheless, the interplay of mutation and selection cannot be ruled out completely as the origin of morphological traits of bones.
Fig. 1.
Hand of a chimpanzee, loaded in knuckle-walking (a). The external load Fe is about 20% of body weight. (b) Loaded in suspended posture. The external load Fe is proportional to total body weight. In arm-swinging, it may readily increase to twice body weight, because of centrifugal force. The forces acting on the metacarpal are indicated in both cases and drawn to the same scale.
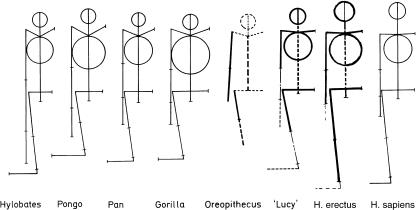
The explanation of shape via ‘functional adaptation’, or ‘causal morphogenesis’, fails however in all cases in which relative lengths or segment mass distribution are the focus of interest. However, the most obvious morphological differences between humans and our closest biological relatives are indeed mainly in body proportions (e.g. Schultz, 1956; Jouffroy & Lessertisseur, 1979; Jungers, 1985) (see Fig. 2).
Fig. 2.
Proportions of several hominoids redrawn after Schultz (1956). From left to right: Hylobates, Pongo, Pan, Gorilla, Oreopithecus, Australopithecus afarensis AL 288-1 ‘Lucy’, Homo ergaster (early African H. erectus), H. sapiens.
Therefore, identification of biomechanical reasons for the evolution of overall body shape presents a major challenge. To solve the problem, we have first of all to search for mechanical conditions in which a given set of traits (such as the typical length of the hindlimbs in humans, or the length and gracility of the autopodia in climbers) is likely to offer an advantage over the opposite set of traits (e.g. shortness of the hindlimbs in apes and robusticity of the autopodia in some non-arboreal primates). Such mechanical conditions can often be found in the kinetics of such species-specific locomotion (e.g. Alexander, 1984a,b, 2004). As a second step, we must attempt to explain all combinations of traits that are realized, and as a third, to describe the advantages quantitatively, that is in the form: combination X contains under these clearly defined conditions an advantage of Y per cent over combination Z. Only then can we readily consider why the morphological features under consideration have been realized. However, there is no evidence to suggest that the proportions of an animal can, in a similar way, ‘adapt’ during its lifetime to mechanical conditions.
If we need to understand the morphology of a genus, to reconstruct its mode of locomotion and to relate this to its environment – as for example in the case of a newly discovered fossil – it is very effective to begin with body proportions. Analysis of morphological detail, based on a causal morphology approach, can be performed later, and may often serve only to help verify conclusions made on the basis of body proportions. This sequence of analysis, however, is often impossible.
The basic theoretical assumption concerning evolution that underpins this procedure is that viable mutations occur by chance. Changes that lead to increase of limb length in bipeds, for example, will permit greater speed and/or lower energy expenditure (Mochon & McMahon, 1980, 1981; Preuschoft & Witte, 1991, 1993; Witte et al. 1991) and hence improve the efficiency of endurant walking. Reduction of the forelimbs, at the same time, would reduce body weight. Thus individuals carrying these mutations could expand further into open country than could conspecifics lacking elongated hindlimbs, but retaining stronger as well as longer forelimbs. The latter would benefit, on the other hand, by requiring to spend less force in climbing on tree-trunks, and a longer reach when foraging in the canopy. Thus, in general form: animals do not adapt to an existing ecological niche but find not yet existing niches for which their morphology is suited. In this case, it can be seen that the term ‘adaptation’ is somewhat misleading, because it implies that a species continues to occupy a pre-existing niche.
The aim of this study is to not to discuss species or individual specializations, but simply the general features that apply to all, or at least the majority of individuals within the taxa concerned. For this study I therefore employed relatively simple theoretical mechanics described in detail in texts such as Lehmann (1972/1974) and use as my data ubiquitous information on skeletal shape and proportions of chimpanzees (P. paniscus and P. troglodytes), orang-utans (Pongo pygmaeus), western lowland gorillas (G. gorilla), gibbons and siamangs (genus Hylobates) and modern humans (H. sapiens). Among australopithecines, the best preserved postcranial individuals: AL 288-1, Olduvai Hominid 8, Olduvai Hominid 64 and Sts 14 yield most information; for H. erectus the juvenile skeleton KNM-WT 15000. At this level of analysis, Neanderthalers appear very similar postcranially to H. sapiens, and are subsumed under this species.
Biomechanical characteristics of arboreal locomotion and their morphological indicators
During the last two decades, numerous features have been identified as related to the mechanical conditions of arboreality, but they have only recently been assembled into a coherent whole in meeting volumes, including Strasser et al. (1998) for the 1995 Davis meeting on primate locomotion and Okada & Preuschoft (2002) for the 1998 Kyoto meeting on locomotor adaptations for arboreality, which both show to what extent body form suited for arboreality pre-adapts for the acquisition of bipedality.
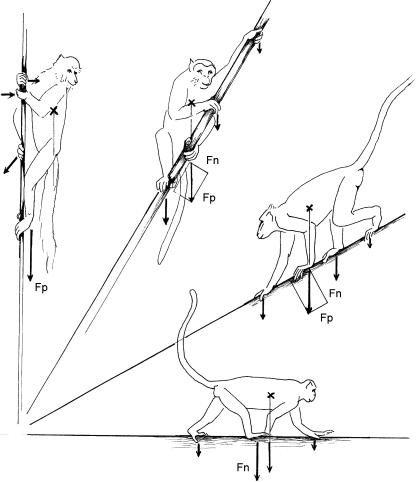
To begin with, we need to identify the basic mechanical conditions of arboreal climbing in trees. Studies including Morbeck (1975), Rose (1979), Nieschalk (1991), Hoeschen et al. (1995) and Arms et al. (2002) show that most primates prefer more or less horizontal arboreal substrates, and on these perform gaits nearly identical to the usual terrestrial gaits. Most common is the walk, in which primates prefer the diagonal footfall sequence (Vilensky, 1983; Preuschoft et al. 1996). At higher speeds, primates avoid the trot, and prefer the gallop rather than the bound or half-bound. The biomechanics of arboreal gaits thus differ little from those of terrestrial locomotion (Preuschoft, 2002). However, the more inclined the substrate, the more load is carried by that pair of limbs positioned closer to the ground (Fig. 3). On near-vertical substrates, almost the total body weight may come to be borne by the lower limbs, and the upper pair of limbs exert only small, mostly tensile forces to prevent the body swinging away from the substrate. [See Nakano (2002) for an experimental analysis of this situation.]
Fig. 3.
Distribution of body weight on fore- and hindlimbs on inclined substrates. Note that the pair of limbs closer to the ground receives a greater share of the substrate reaction force.
As long as animals maintain a body posture in which the head is upwards and the tail is hanging down, the greater share of body weight will consistently act on the hindlimbs. A division of labour between the fore- and hindlimbs becomes possible: the hindlimbs carry (and accelerate) body mass; the forelimbs only balance, and may thus be freed for other tasks, such as investigating and manipulating objects, food intake and social behaviour. Structural reinforcement of the hindlimbs is to be expected, and can indeed be observed in comparisons between more terrestrial primates and closely related, but more arboreal, primates (Kimura, 1985, 1992, 1995, 2002). This result is in accordance with the observation made by Kimura et al. (1979), and confirmed by Demes et al. (1994), that primates carry a larger part of their body weight on their hindlimbs; by contrast, cursorial, primarily ground-living mammals carry more weight on their forelimbs. The overall pattern of body form in primates, namely the combination of a short trunk and neck with very long limbs, also contributes to this peculiarity of the primates. Elongation of all extremities offers a long reaching distance per se, which permits bridging of gaps in the discontinuous arboreal environment, and facilitates feeding within a three-dimensionally large envelope from a single, safe position. The length of the limbs, in combination with a short neck (a short neck is tolerable because contacts with the environment are mediated mainly by the hands, not by the mouth), leads, in walking, to placement of the feet below the body centre of gravity; hence the finding of Kimura et al. (1979) concerning the high share of body weight borne on the hindlimbs.
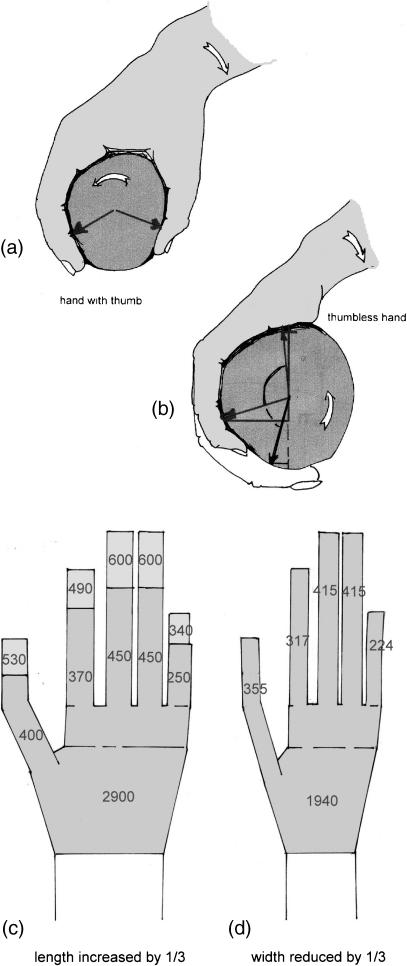
In terrestrial locomotion, the relatively small horizontal force components that occur are usually balanced by friction. Friction becomes greater if the compression between body and ground is increased. On strongly inclined substrates, however, the weight force has a large component parallel to the substrate surface, so reducing compression (Cartmill, 1974, 1985). Because friction is proportional to the pressure exerted, and weight force in a climber may act parallel to the substrate, it is essential to exert pressure independent from body weight. This difficulty can be overcome by prehensile hands and feet, in most cases by a forceps-like grasp between the lateral digits and the hallux or pollex (Cartmill, 1974, 1985). In some species, however, a brace is provided against the pressure of the fingers by the soft tissues over the heads of the metapodials (thenar or hypothenar, Fig. 4). Long hands and feet are obviously advantageous for a tree-living animal, because they allow grasping of thick branches, or grasp of multiple smaller supports among the foliage to make use of their summed strength. Unfortunately, length will also enlarge the contact area, and thus reduce the compressive force, between the substrate and the autopodium (hand or foot). A very narrow shape can compensate by reducing the contact area and so increase the pressure between autopodium and substrate (Preuschoft et al. 1996).
Fig. 4.
Grasping with digits, pressing a grasped object against an abutment. In the feet of primates, this is commonly the hallux; in the hands, the pollex (a) may be replaced by (b) the soft tissue over the heads of the metacarpals, or the thenar plus hypothenar eminences. Increased length of the autopodia (hands and feet), which increases the span of the grip, nevertheless reduces the contact pressure (c). Reduced width of the hand (d) leads to higher pressure over the contact area. Because friction depends on the contact pressure, this allows the transmission of greater rotating moments.
Grasping hands and feet also permit the transmission of tensile forces between the animal's body and the substrate (Fig. 1b). This can alternatively be accomplished by claws (Cartmill, 1974, 1985). Prehensility in addition allows the transmission of rotating moments (Fig. 4). The dermatoglyphic ridges increase the friction resistance (according to the experiments of Buck & Bär, 1993) by some 20%, but the compliance of the palmar and plantar surfaces also enables a tight fit between the autopodium and the substrate.
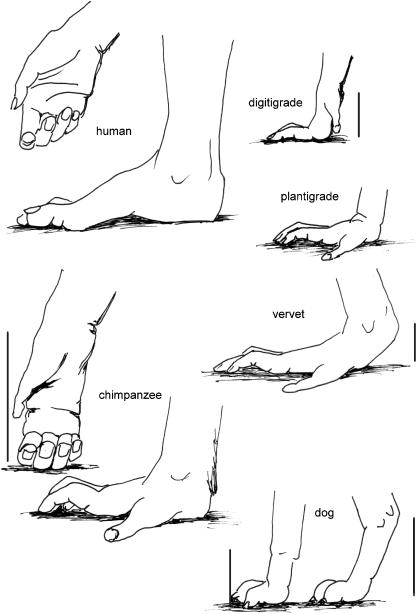
The pressure exerted by the fingers and toes is created by the flexor muscles, the largest of which have their origins on the zeugopodia [forearm, and lower leg (crus or calf)]. These muscles, however, influence the posture of the wrist and ankle joints. Independence of the grip is secured by the short intrinsic muscles of the hand and the foot. Because of the length of the digits, these muscles must be very strong – and thus heavy. The resulting increase in weight has consequences for the animal: studies by Günther (see Preuschoft et al. 1998) found the autopodia as well as the zeugopodia of the primates to be twice as heavy as in other, non-arboreal mammals (Table 1, based on Preuschoft et al. 1998). This makes high-frequency gaits in primates, especially the trot, very energy-consuming. Consequently, primates use the trot rarely (Vilensky, 1983, 1989; D’Août et al. 2004; my personal observation), except during early childhood (Nakano, 1996). In addition, the use of the palmar and plantar surfaces for securing the grip precludes elevation of the wrist and ankle joints from the substrate (see Fig. 5). Elsewhere, such elevation of the metacarpus, tarsus or metatarsus can contribute to elongation of the functional length of the (inverted) limb-pendulum (see Preuschoft & Günther, 1994; Preuschoft et al. 1994).
Table 1.
Mass distribution of extremities in primates and non-primates. Values in percentage of body weight are summarized from Preuschoft et al. (1998). ‘Prosimians’ includes Tarsius
| Upper arm | Forearm | Hand | Thigh | Lower leg | Foot | |
|---|---|---|---|---|---|---|
| Non-primates | 1.8 | 1.1 | 0.4 | 4.6 | 1.9 | 0.7 |
| Prosimians | 2.0 | 1.7 | 0.8 | 6.7 | 2.8 | 1.6 |
| Simians | 3.0 | 2.4 | 0.8 | 6.5 | 2.7 | 1.3 |
Fig. 5.
Schematic drawings of fore- and hindlimbs to show the effect of elevation of carpus or heel, respectively, from the ground for elongation of the functionally important limb length (= inverted limb pendulum). The examples are plantigrade humans, semiplantigrade vervets, digitigrade vervets, chimpanzee, dog hind- and forelimbs. The vertical bars indicate the elongation of the inverted limb pendulum.
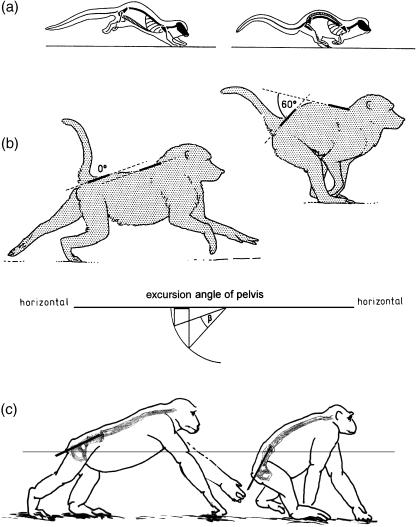
For rapid progression, primates, even small Callithricidae, normally use the true gallop (Morbeck, 1979; Rose, 1979; Rosenberger & Stafford, 1994; Voges, 1999; Schmidt & Fischer, 2000; Arms et al. 2002; Witte et al. 2002; Vilensky et al. 1994). The continuous footfall sequence in the gallop (compared with the bound or half-bound) reduces the risk of inducing vibration of the substrate, or even substrate failure. In all ‘springing’ asymmetrical gaits (the bound, half-bound and gallop) flexion and extension of a long and mobile lumbar spine play a prominent role (Fig. 6a,b), at least in small mammals (Fischer, 1994; Fischer & Lehmann, 1998; Witte et al. 2002). A long, slender trunk, and a mobile and also rather long lumbar spine are thus both advantageous for such locomotion. An elongated pelvis, with a long ‘iliac neck’ (Berge, 1993) increases the excursions of the hip joint and so contributes to stride length: for example, Fischer (1994) found in the hyrax that displacements of the hip joint account for 40% of the stride length of the hindlimbs. Although monkeys may not achieve a similar contribution, trunk and pelvic flexion is evident in their gallop (Fig. 6).
Fig. 6.
Trunk flexion in springing gaits of quadrupedal primates. Drawn after film recordings. The vertebral columns and pelvic outlines are tentative. (a): Cheirogaleus, (b): baboon, (c): chimpanzee.
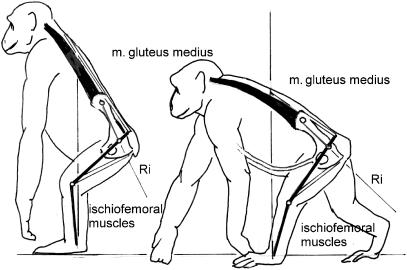
Such flexions also seem to be of importance in chimpanzees (Fig. 6c) but not in gorillas, orang-utans and gibbons. Rather, most apes, especially great apes, show a pronounced tendency to shorten their trunks, reducing the lumbar spine to as little as three vertebrae (Schultz, 1961). Nevertheless the great apes retain their long ‘iliac necks’. The ilium is not necessarily thereby exposed to great bending moments, nor the sacroiliac joint to great torques, because the hip joint resultants pass along the longitudinal axis of the ilium from the hip to the sacroiliac joint (Fig. 7).
Fig. 7.
Chimpanzee in the usual semi-upright posture. The tensile forces along the dorsal contour are provided by the erector spinae and the gluteus medius. A rough estimate of the hip resultant (Ri) shows that it passes close to the sacro-iliac joint, which therefore is not exposed to high torques. The same holds true for the chimpanzee in quadrupedal posture.
A short trunk will obviously tend to facilitate upright posture, in sitting as much as in standing. In the apes, the width of the iliac blade gives a large area of origin for the very large and strong gluteus medius. Its contraction (to keep the hip joint in equilibrium) contributes tensile force along the dorsal contour, exactly where the bending moments will assume their greatest values (Figs 7,8). Bending moments within the trunk occur in vertical climbing (see below) and these may be more important in great apes other than humans than the occasional assumption of erect posture. These bending moments are also reduced by shortening of the trunk (Preuschoft, 1978). A long ilium gives the gluteus medius and minimus (the so-called ‘scansorius’, see Preuschoft, 1961) great fibre length, and hence they can exert force over a large range of hip postures, which may be vital during climbing.
Fig. 8.
Plot of the torques (bending moments) evoked by the weight of head, neck, forelimbs and trunk at the lumbo-sacral joint. All these parts are represented by a cylinder. Horizontal axis: increased erection of trunk axis, inclination angle from pronograde to orthograde. Vertical axis: ratio between length/diameter increasing from 10 : 3 (thick-bellied gorilla) to 10 : 1.5 (slender human).
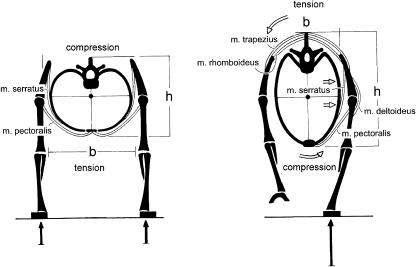
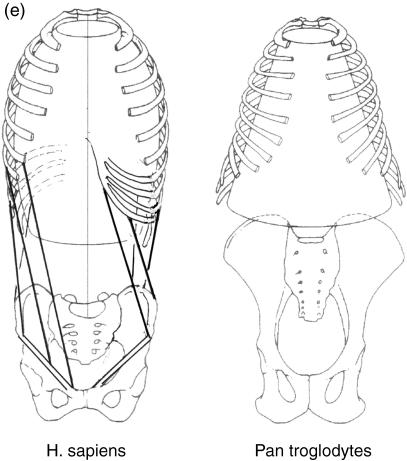
A further characteristic of the apes, which distinguishes them from purely terrestrial mammals, is their wide thorax with curved, rather than flat, ribs (Mollison, 1911). This feature was recently interpreted by Preuschoft et al. (2003) by a comparison between cursorial mammals (such as the horse) and a primate. In the cursor the trunk is invariably suspended between the extended forelimbs, or by a single forelimb (Fig. 9). In this situation, bending as well as rotatory moments act on the cross-section, and these will be minimized by a narrow thorax with low curvature of the ribs. In the shoulder joint only small medially directed forces occur, if any. In a discontinuous arboreal environment, however, the hands must be placed at the level of the head (Fig. 10a), or the arms abducted laterally (Fig. 10b,c). Both positions require powerful contractions of the latissimus dorsi and pectorals to keep the shoulder joint in equilibrium (Fig. 10b,c). As a consequence, medially directed force components occur, are taken over in part by the clavicle (missing in the cursorial mammals), and in part by a scapular blade rotated on the cross-section, so that the glenoid fossa faces laterally (Schmidt et al. 2002). The scapula is supported, against joint reaction forces, by the serratus anterior. This muscle, as well as pectoralis major, takes its origin from the ribcage. The ribs can only resist the extremely large bending moments applied to them if their long sections are more or less parallel to the muscle forces. Thus the ribs must be strongly curved, leading to a wide and shallow thorax. In turn, because of the rather dorsal position of the centre of gravity of the trunk, the assumption of upright posture is facilitated by the resulting truncal shallowness (Fig. 11).
Fig. 9.
Schematic cross-section through the shoulder region of a cursorial mammal in (left) two-limb support and (right) one-limb support. Skeleton: black, muscles: double lines. The narrower the trunk, the smaller the moments. The joint forces in the shoulder joint lie in a parasagittal plane. A clavicle is superfluous.
Fig. 10.
Shoulder region of a climber, in side view (a), and in cranial view (b,c). The joint resultant in the shoulder joint has a large component in the medial direction. To counterbalance this component, the scapula must offer a glenoid surface that faces laterally. The muscles pectoralis, latissimus and serratus exert high bending moments on the ribs. A pronounced curvature of the ribs gives them the necessary strength to sustain these bending moments. In addition, a clavicle is of advantage to prevent giving way of the scapula.
Fig. 11.
Deep trunk (left) compared with a shallow trunk (right) in upright posture.
All the above, from hindlimb strength through pelvic and thoracic shape, make acquisition of bipedality only a short step away. We may thus state that the trunk shape of an arboreal primate is ‘pre-adaptive’ for upright posture.
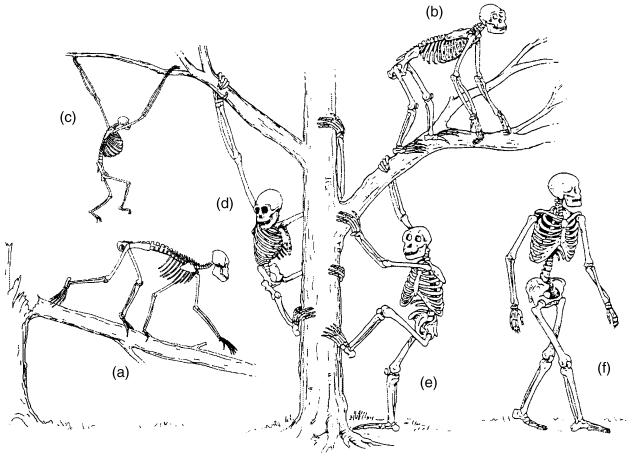
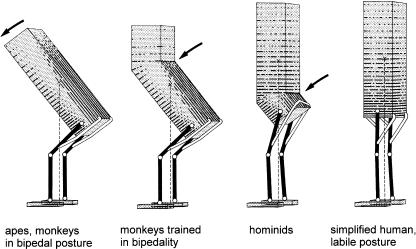
On this basis, however, five divergent ‘Baumuster’ (pairings of locomotor mode and form, Fig. 12) have evolved: arm-swinging in gibbons; forelimb-dominated slow climbing in orang-utans, and probably in Oreopithecus; quadrupedalism with climbing in the African apes; a largely enigmatic combination of climbing and bipedal walking in australopithecines; and remarkably endurant bipedal walking in humans. As only two taxa among these five ‘Baumuster’ are, or were, habitually bipedal, permanently upright body posture does not seem to be a necessary consequence of these ‘adaptations’. Non-human apes also quite often stand and walk bipedally; in spite of the fluent bipedal movements of chimpanzees (Thorpe et al. 2003) and gibbons, it is the biomechanical characteristics of bipedal walking in orang-utans, the most arboreal among the large apes, which is closest to that of humans (Crompton et al. 2003). In the fossil Oreopithecus, bipedality seems to have become habitual (Moya-Sola & Köhler, 1996).
Fig. 12.
Six ‘Baumuster’ (specializations), realized among hominoids: (a) the quadrupedal arborealist Proconsul; (b) chimpanzee, as representative of the African apes, generalist, adapted climber and quadrupedal walker with bipedal potential; (c) gibbon, highly specialized brachiator; (d) orang-utan and the similar Oreopithecus, slow climbers in trees, on the ground or on rigid, horizontal branches, bipedalists who use their arms for support; (e) Australopithecus, bipedal ground-dweller with high potential for climbing in trees; (f) Homo, highly specialized terrestrial bipedalist, not afraid of the third dimension, but not really gifted in using it.
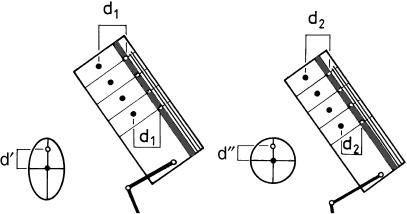
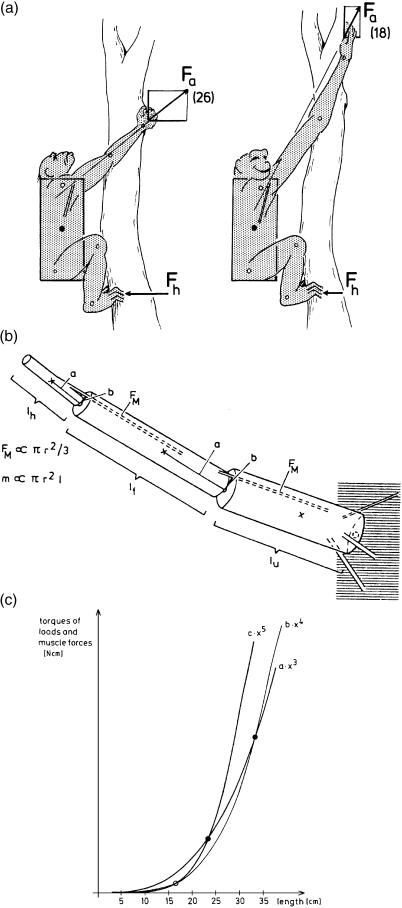
At this point it is tempting to follow the evolutionary pathways leading to the largely arboreal apes. Their major morphological characteristics are a short, bulky trunk, short hindlimbs and long forelimbs. According to Jungers (1985), the forelimbs scale positively with body mass in all primates, the regression line being the same in all forms, including the Hominoidea. This has recently been confirmed by A. Gallagher (personal communication). It seems as if trunk length scales negatively with body mass. Nevertheless, the ratio of arm length to trunk length is as remarkable as the shortness of the hindlimbs. Shortness of the limbs implies relative safety given the likely frequency of instability: Preuschoft (1990) collected data from the literature, which show that a quarter to a third of skeletons in museum collections show traces of (healed) fractures of long bones, but the shorter the limb, the smaller the bending moments when balance is lost. This simple reason may account for the shortness of the hindlimbs. In reference to the forelimbs, climbing tree-trunks is more important in larger than in smaller animals. Apart from the evident advantage of long reach, the suspension of body mass from the forelimb may be accomplished using less force – and hence less energy – if the forelimbs are long (Fig. 13a,b). Length is limited by increased mass of the shoulder muscles necessary to control a long forelimb, because the mass moments of inertia that must be overcome are fifth powers of linear dimensions (Fig. 13c). However, it should be noted that elongation and strengthening of the forelimbs is by no means essential for vertical clinging and climbing. Long and powerful hindlimbs can also support body weight with low expenditure of energy – as can be seen from the lemurids, indriids and tarsiers (see Preuschoft et al. 1992) although these are to a great extent leapers. Humans, when climbing on vertical tree-trunks, also utilize the hindlimb for support (Preuschoft & Witte, 1993).
Fig. 13.
(a) Energy-saving effect of long forelimbs. The longer the arms in vertical clinging or climbing, the lower the forces acting on the forelimbs. (b) Length increase of the arms is limited by the disproportional increase of arm mass in comparison with muscle strength. (c) Increase of mass is a third power function of linear dimensions; increase of load moments of rotation a fourth power function; and increase of mass moments of inertia, which must be overcome in rapid movements, a fifth power function of linear dimensions. Muscle strength on the opposite side is a second power function.
Once a long, powerful forelimb is acquired, it opens new possibilities of locomotion in the outer canopy: arm-swinging locomotion in the (small-sized) gibbon, and forelimb-dominated climbing in the (larger) orang-utan (Fig. 12c,d). In both, the great tensile strength of small branches along their long axis is recruited, and the consequences of their limited bending strength across this axis are equally avoided, maintaining all the time orthograde (upright), head-up body posture. Both forelimb length and body size are, however, limited by mechanical conditions (Preuschoft & Demes, 1984, 1985, as shown in Fig. 13).
Biomechanical characteristics of bipedality and their morphological indicators
Once bipedal posture and locomotion has been assumed by an early human ancestor, the eventual development of the typical morphological configuration seen in Homo may be explained simply in terms of enhancements that reduce the cost of walking below that in the apes or in Australopithecus, so that very long distances can be covered at considerable speed while consuming the minimum energy.
This holds true for the structure of the foot, especially the metatarsus and other details of the limb skeleton (Preuschoft, 1971; Stern & Susman, 1983), for length of the hindlimbs, the distribution of muscle mass on the hindlimbs and the loss of prehensility of the feet, which results from reduction of flexor muscle mass. These traits and their mechanical relevance have been analysed in earlier studies (Preuschoft & Witte, 1991, 1993; Witte et al. 1991; Preuschoft et al. 1992). Here I concentrate rather on the shape of the trunk, including pelvis, thorax and shoulder.
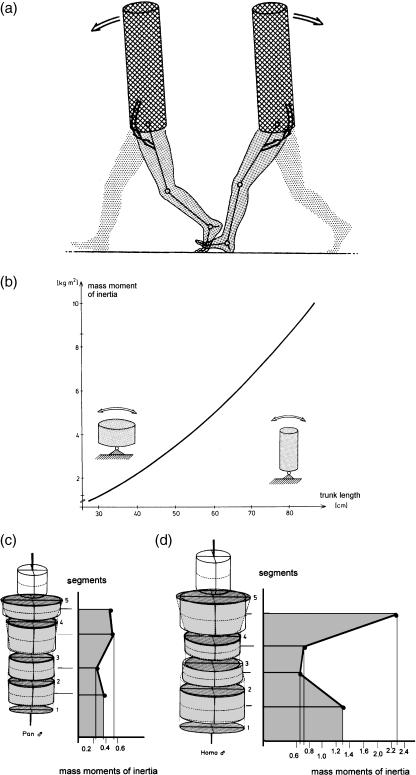
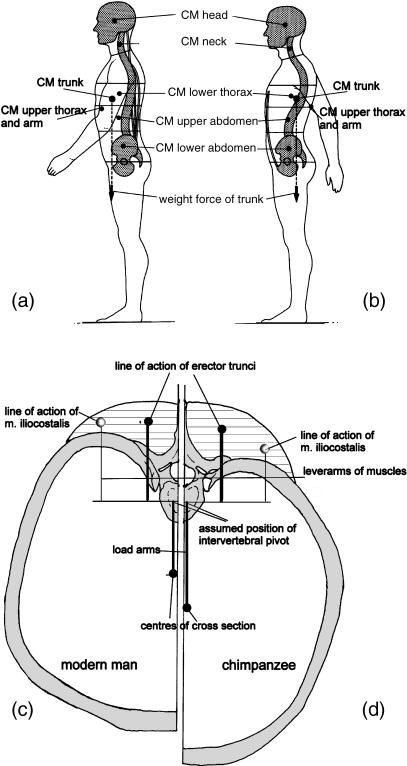
The specific elongation of the trunk seen in humans (in contrast to its shortness in the other Hominoidea; Figs 8,14a,b) offers the mass moment of inertia necessary to stabilize the trunk and head against the movements of heavy and long hindlimbs (Witte et al. 1991). A tendency for the trunk to rotate about its vertical axis during walking is resisted by the pattern of distribution of body mass about its cross-section (Fig. 14c,d; see also Witte et al. 2004). This distribution explains the greater pelvic and shoulder breadth in Homo compared with other apes. Elastic stretching and recoil of the oblique muscles of the abdomen recover energy during steady-state walking, while forming a waist between the pelvis and thorax (Fig. 14e; see also Witte et al. 2004). A sagittal orientation of the most ventral part of the iliac crest (Fig. 15i) gives the abdominal obliques a long lever arm and keeps the stresses, exerted by the muscles attached to the ilium lateral to the iliac pillar, low. The attachment of hip muscles on the large outer surface of the pelvis concentrates considerable muscle mass around the joint without increasing the mass moment of inertia of the lower limb (Witte et al. 1991).
Fig. 14.
The specific elongation of the trunk (a) seen in humans, in contrast to its shortness in the other Hominoidea (b), offers the mass moment of inertia necessary to stabilize the trunk and head against the movements of heavy and long hindlimbs. A tendency for the trunk to rotate about its vertical axis during walking is resisted by the pattern of distribution of body mass about its cross-section (c,d). This distribution explains the greater pelvic and shoulder breadth in Homo compared with other apes. Elastic stretching and recoil of the oblique muscles of the abdomen (e) recover energy during steady-state walking, while forming a waist between the pelvis and thorax.
Fig. 15.
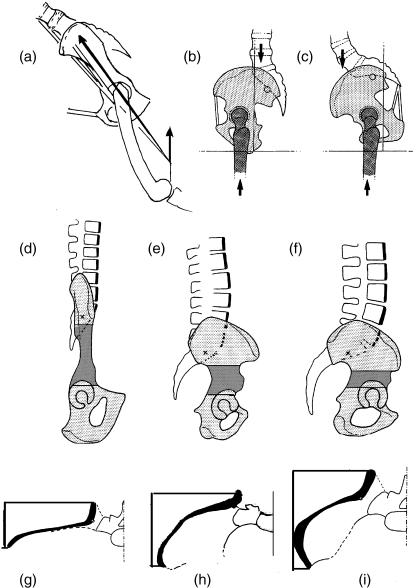
Pelvic shapes in side view (top and middle) and cranial view (bottom). (a) Chimpanzee in a position different from those shown in Fig. 7; the resultant of all forces acting on the hip joint, Ri, also passes through the iliosacral joint, relieving the ilium from bending. In contrast, the iliac neck in humans (b,c) is usually exposed to bending moments. The iliac neck (hatched) therefore can be long in the chimpanzee (d) and must be short in Homo (f). The iliac crest (black, g–i) indicates low resistance to bending in the chimpanzee (g) and high resistance to bending in Homo (i). Australopithecus (e,h) assumes an intermediate position.
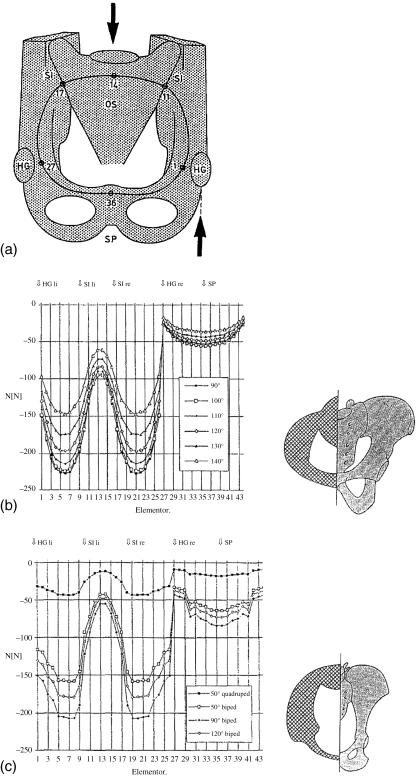
The mechanics of the hip joint, femoral neck and pelvis may be understood following the approach proposed by Pauwels (1958): consider the pelvis from in front, and reduced to a simple ring. The strength necessary for the whole pelvic girdle can be calculated, as performed by Lenz (1993), who substituted for these requirements an ‘adapted’ shape, assuming that all the bone is of equal strength. The shapes he thus obtained were in reasonable agreement with the distribution of bone in the pelvis of a chimpanzee or a human, respectively (Fig. 16). The shortcomings of this approach are that both the outline of the pelvic girdle and its spatial orientation relative to the vertical have to be assumed as a priori conditions, when they are in fact what we are trying to determine.
Fig. 16.
Stresses in the pelvis according to Lenz (1993). The pelvic entrance (a) is taken as a ring, loaded by body weight applied to the sacrum and supported by the hindlimbs at the hip joints. The stresses are shown as negative values for several postures (b,c); the distribution of bone material allows the stresses to be kept at the same level along the entire pelvic ring. The calculation leads to a distribution of material that roughly corresponds to the arrangement of bone in a human (b) or a chimpanzee (c).
Another approach might therefore be more rewarding. Looking at an orthograde trunk in side view, bending along its length must occur in semi-upright postures (Fig. 17). Tensile force must be produced by dorsal muscles, mainly the erector spinae, and this requires expenditure of energy. If part of the trunk is fully upright, bending moments will largely be confined to the section caudal to the lordotic flexion. In the craniad section, compressive stresses should prevail.
Fig. 17.
Schematic variation of trunk shapes to minimize the bending moments along the trunk axis. The dorsal muscles have to exert tensile forces to keep the mobile spine in its position. Left, in semi-upright posture, muscle activity is necessary along the entire trunk; second left, if a lordotic flexion is assumed, the muscle activity can be confined to the part caudal to the flexion; second right, if the flexion is shifted caudally, muscle force can be saved, but this is only possible if the pelvis is short (as in australopithecines); or right, if the pelvis itself is involved in the lordotic curvature (as in Homo). The inclined position of the long axis of the pelvis, from iliac crest to the ischial tuberosity, allows the musculature to remain without great functional change.
Pronounced lordosis of the trunk can be observed in bipedally trained Japanese macaques (Preuschoft et al. 1988), in which the long iliac ‘neck’ prevents dorsal (i.e. rearwards) shifting of the lordosis. The further caudally it is placed, the less muscular energy is wasted. Obviously, shortening of the iliac ‘neck’ would be a considerable advantage for a biped, and this feature can be seen both in Homo and Australopithecus when compared with Pan (Fig. 15). In addition, the lordotic flexion in the trunk may even be shifted into the pelvis, forming the sciatic notch typical of Homo, not pronounced in Australopithecus and completely missing in non-human apes (Fig. 15d–f). We may describe this configuration by saying that the lordotic curvature in Homo is displaced caudally into the iliac ‘neck’. The lordosis of the lumbar spine in adult humans is largely confined to intervertebral joint L5/S1, forming the lumbo-sacral angle of about 135° (Preuschoft, 1999). The human lumbar spine, which commonly possesses five vertebrae, is rather long for a hominoid: this is in accordance with the ability of a long trunk to provide large mass moments of inertia. The upright posture of the cervical, thoracic and lumbar spine keeps bending moments low.
However, the arrangement we have just described should lead to very large bending stresses within the pelvis, especially within the ilia. Experiments on living subjects by Witte et al. (2004) have shown that such bending moments do in fact occur (Fig. 15b–c). It is well known that the pelvis of other apes differs from that in humans in the orientation of the iliac blades: frontal in the apes, but ventrally curved in humans (Fig. 15g–i). This change, in combination with the long sagittal diameter of the iliac ‘neck’, gives the ilium its required bending strength (Fig. 15d–f). The arrangement of the trabeculae of the pelvis is in accordance with stress-coat experiments I performed some time ago, which also suggest marked bending of the human iliac ‘neck’.
In all traits mentioned here, Australopithecus assumes a position intermediate between those of chimpanzees and those of humans (Fig. 15). This statement, however, is a qualitative one that explains neither how the earliest hominids moved nor how the evolution of human locomotion was initiated. Only the short length and the ventral tilt of the iliac blade, in combination with the long medio-lateral diameter of the ‘neck’, makes the pelvis of Australopithecus well suited for upright posture. Whether its clear divergence from the pelvis of Homo is a matter of size or of special locomotor features requires further investigation. The best preserved fossil vertebral column (STS 14) was described by Robinson (1972). It is reported to have had as many as six lumbar vertebrae, which is unusually long for a hominoid.
A short lumbar spine makes sense (as do other traits of the human and australopithecine pelves) only when a species ceases moving on all fours. In this respect, members of genus Homo, and other apes, seem to represent functionally divergent evolutionary lines.
A further important factor influencing trunk shape was mentioned above: the curvature of the ribs. This is primarily a necessity of climbing, because curved ribs resist the medially directed force components that occur in elevated and abducted arm postures better than do flat ribs. In humans, the vertebral column is located dorsal to the segment centre of gravity (Fig. 18a,b), so that moderate bending moments persist, which must be counterbalanced by muscle tone. In the upper lumbar and thoracic sections of the trunk, the vertebral column of upright humans is ‘sunk’ into the thorax, so that the centres of gravity of the segments are very close to the columnar support provided by vertebral bodies (Fig. 18c), the load arms being short and the force arms long.
Fig. 18.
Because of the eccentric position of the vertebral column, even when the upper lumbar and thoracic segments are fully upright, a moderate torque remains at the intervertebral joints. In the human trunk, the vertebral column is displaced ventrally (compare a and b) to give the body weight components short (and the muscles long) lever arms. (c,d) Comparison of the position of the load and lever arms in relation to the vertebral column in cross-sections at the level of a thoracic vertebra in (c) a human and (d) a chimpanzee.
The human waist (Fig. 14e) favours rotations of the upper half of the trunk, comprising the thorax and shoulder, on the lower, namely the pelvis. These rotations are controlled by the oblique muscles of the anterolateral trunk wall and by multifidus. Witte et al. (1997, 2004) present strong arguments that elastic stretching and recoil of muscles contributes to the fore-swing of the hindlimbs, thus saving energy. The lateral contour of the abdomen, i.e. the waist, is formed by superimposing the shortest possible connections between the thoracic wall and the iliac crest (Witte et al. 2004). The wide shoulder, which discriminates humans from other apes, is produced in a very simple way by placing the shoulder more caudally on the thorax, so that the clavicle is swung about its sternal attachment and its length pushes the shoulder laterally. In quadrupedal posture, this arrangement entails the disadvantage that the distance between the supports of the forelimb, at the shoulder joint, are removed from the centre of gravity (Fig. 9), so that large rotational moments must be compensated for in one-limb support. It is not known whether australopithecines had a shoulder position like that of humans or like that of other apes. An indirect clue, according to Schmid (1983, 1991) may be provided by the funnel-shaped thorax present in australopithecines such as AL 288-1 (‘Lucy’). Neither a narrowing of the lower aperture of the thorax (Fig. 14e) nor a curvature of the iliac crests in transverse section– bringing the anterior iliac spine to point forward – is present (Fig. 15i). Both traits seem to indicate a lack of the torsional spring mechanism mentioned above. In australopithecines, this mechanism may not have existed: if a waist was indeed absent, width of the shoulders would carry no disadvantage.
Conclusions
We may draw from all the above the conclusion that a large number of highly characteristic traits of all hominoids (including humans) are functionally related to arboreal quadrupedalism, and that divergencies between humans and other apes are related primarily to endurant bipedal walking. We may now return to our original question: why upright? In this context, reference is often made to the ‘knuckle-walking’ concept. This term should never be employed to signify a vague ‘stage’ of evolution, but used only in its original sense, as introduced by Tuttle (1967) as a descriptor of the characteristic locomotion of the African apes. A shortness of the digital flexors, which compels the animal to support its body on the dorsal surfaces of the middle phalanges, makes sense only as a consequence of (or adaptation to) habitual climbing. So ‘knuckle-walking’ is no more than the consequence of shortness of the digital flexors in a large mammal adapted primarily to climbing (Preuschoft, 1973, 1976) together with the need for roll-off of the hand in quadrupedal walking. In interpretations of human evolution, however, ‘knuckle-walking’ is essentially a recourse to the old idea, proposed by Keith (1923), of gradual elevation through the stages: quadrupedal – elongated forelimbs – brachiating – upright. In the first part of this paper, I have shown that the ‘adaptations’ of the known hominoids cannot be arranged along such a simple sequence.
If an evolutionary accident in the form of a chance mutation is not held responsible, what selective factor can explain why two hominoid forms (Australopithecus and Oreopithecus) adopted bipedality? Most authors tend to connect bipedal locomotion with some aspect of progressively increasing distance between trees, because of climatic changes. The scenario at least seems to correspond with palaeoclimatic and palaeoenvironmental data (Pickford, 2002): at the time at which bipedal hominids evolved, the climate in East Africa became drier than before, and vast grasslands with gallery forests appeared. Nevertheless, as a proximate cause for the evolution of bipedality this coincidence is not completely satisfying, given our knowledge of chimpanzee behaviour in open landscapes such as Gombe (Goodall, 1968). In addition, the morphological characteristics, in the same environment, of the largely terrestrial baboons, patas and vervet monkeys make them more efficient quadrupedal cursors than their strictly arboreal relatives (Kimura, 1985, 1995, 2002). Other proximate factors, which have derived some support, include control of body temperature (Wheeler, 1985, 1991a,b), throwing stones (Calvin, 1985; Fifer, 1987; Dunsworth et al. 2003) and wading in shallow water (Niemitz, 2000, 2002). The first of these proposals is not in contradiction with biomechanical considerations, the last two in perfect accordance (Preuschoft, 2003).
For effective throwing, elongation of the arms allows a long acceleration time [comparable with the situation in leapers as analysed by Peters & Preuschoft (1984), Günther (1989) as well as Preuschoft et al. (1998) for the projectile], and yields high centrifugal speed along the trajectory of the hand. A limiting factor for arm elongation, however, is the force required to move the arm. Arm elongation is limited because the proportion of arm mass involved in throwing must not exceed a limit, where the mass of the body parts not involved in accelerating the projectile can counterbalance the impulse exerted by the active shoulder girdle muscles to their attachments. Some aspects of a biomechanical analysis of these problems are treated in Fifer (1987), Dunsworth et al. (2003) and Kirschmann (1999), but a complete analysis is lacking, as is a comparison with sports performances such as javelin or discus throwing. At present, claims reach further than do facts.
Niemitz's (2000, 2002) reasoning in his ‘amphibischer Generalisten-Theorie’ is more ecological and behavioural than biomechanical. He begins with a generalised quadruped that was spending as much time on the ground as on trees. This animal is assumed to prefer to exploit the animal protein resources such as the invertebrates living in shallow water along river banks and on the seashore. In wading, upright posture increases loads on the hindlimbs and therefore gives a better contact with the ground than does quadrupedal posture, which is more subject to the effects of buoyancy. Further factors include water resistance acting against the limbs during fore-swing (two limbs receive less water resistance than do four) and the more mundane matter of avoiding becoming wet.
There is a connection, not always realized, between stone-throwing, the popular theory of tool-making (for which this paper provides no support), the proven influence of load carrying, wading and the exploitation of food resources in shallow water: the hands of the assumed protobiped are always occupied by tasks other than locomotion. This of course favours the hindlimb-dominated locomotion, as illustrated by Nakatsukasa's (2004) observations on an armless Japanese monkey.
Although the step from an arboreal lifestyle towards bipedality was undoubtedly not a big one (and see Crompton et al. 2003), no single reason for an ancestral hominoid to become a specialized biped is both generally accepted and appears so convincing that it excludes all other ideas. It is worthwhile for us to continue the search.
Once bipedality has been acquired, however, development of the typical human morphology can readily be explained as adaptations for energy saving over long distances. Studies by the Liverpool group (Crompton et al. 2003; Wang & Crompton, 2003; Wang et al. 2003; Sellers et al. 2004) show that the energetic efficiency of overall body shapes may even be quantified. Further, and contributing to our question ‘why upright?, Wang (1999), Wang et al. (2002) and Wang & Crompton (2004) found that the ability to carry large loads is enhanced from Australopithecus to Homo erectus and possibly on to Homo sapiens, according to an earlier proposal that carrying loads in various contexts was an essential factor in human evolution.
Acknowledgments
Drs R. H. Crompton and M. M. Günther have induced me to review the results we have obtained in many years of work, and to add new arguments. In addition, they have carefully checked and edited the text. Many thanks.
References
- Alexander R McN%. Walking and running. Am. Sci. 1984a;72:348–354. [Google Scholar]
- Alexander R McN%. Human walking and running. J. Biol. Education. 1984b;18:135–140. [Google Scholar]
- Alexander R McN%. Bipedal animals, and their differences from people. J. Anat. 2004;204:321–330. doi: 10.1111/j.0021-8782.2004.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amtmann E. Biomechanical interpretation of form and structure of bones: role of genetics and function in growth and remodeling. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behaviour and Morphology: Dynamic Interactions in Primates. New York:: G Fischer.; 1979. pp. 347–366. [Google Scholar]
- Arms A, Voges D, Fischer MS, Preuschoft H. Arboreal locomotion in small New-World monkeys. Z. Morph. Anthropol. 2002;83:243–263. [PubMed] [Google Scholar]
- Berge C. L’évolution de la Hanche et Du Pelvis Des HominidésBipédie, Parturition, Croissance, Allometrie. Paris: Editions du CNRS; 1993. [Google Scholar]
- Buck C, Bär H. Investigations on the biomechanical significance of dermatoglyphic ridges. In: Preuschoft H, Chivers DJ, editors. Hands of Primaftes. Vienna: Springer-Verlag; 1993. pp. 285–308. [Google Scholar]
- Calvin WH. A stone's throw and its launch window: timing precision and and its implications for language and hominid brains. J. Theor. Biol. 1985;104:121–135. doi: 10.1016/0022-5193(83)90405-8. [DOI] [PubMed] [Google Scholar]
- Cartmill M. Pads and claws in arboreal locomotion. In: Jenkins FA, editor. Primate Locomotion. New York: Academic Press; 1974. pp. 45–83. [Google Scholar]
- Cartmill M. Climbing. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge, MA: Harvard University Press; 1985. pp. 73–88. [Google Scholar]
- Crompton RH, Li Y, Thorpe SK, Wang WJ, Savage R, Payne R, et al. The biomechanical evolution of erect bipedality. Cour. Forsch.-Inst. Senckenberg. 2003;243:115–126. [Google Scholar]
- D’Août K, Vereecke E, Schoonaert K, De Clercq D, Van Elsacker L, Aerts P. Locomotion in bonobos (Pan paniscus): differences and similarities between bipedal and quadrupedal terrestrial walking, and a comparison with other locomotor modes. J. Anat. 2004;204:353–361. doi: 10.1111/j.0021-8782.2004.00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demes B, Larsen SG, Stern JT, Biknevicius AR, Schmitt D. The kinetics of primate quadrupedalism: hindlimb drive reconsidered. J. Hum. Evol. 1994;26:353–374. [Google Scholar]
- Dunsworth HJ, Challis H, Walker A. Throwing and bipedalism: a new look at an old idea. Cour. Forsch.-Inst. Senckenberg. 2003;243:105–110. [Google Scholar]
- Fifer FC. The adoption of bipedalism by the hominids: a new hypothesis. Hum. Evol. 1987;2:135–147. [Google Scholar]
- Fischer MS. Crouched posture and high fulcrum, a principle in the locomotion of small mammals. The example of the rock hyrax (Procavia capensis) (Mammalia, Hyracoidea) J. Hum. Evol. 1994;26:501–524. [Google Scholar]
- Fischer MS, Lehmann R. Application of cineradiography for the metric and kinematic study of in-phase gaits during locomotion of the pika (Ochotona rufescens, Mammalia, Lagomorpha) Zoology. 1998;101:148–173. [Google Scholar]
- Foley R. Menschen Vor Homo SapiensWie und warum unsere Art sich durchsetzte Reihe SpeciesÜberarbeitete Übersetzung aus dem Englischen (1995): Humans before Humanity. Berlin: J. Thorbecke Verlag; 2000. [Google Scholar]
- Goodall J. The behaviour of free-living chimpanzees in the Gombe Stream Reserve. Anim Behav. Monogr. 1968;1:161–311. [Google Scholar]
- Günther MM. PhD thesis. Free University of Berlin; 1989. Funktionsmorphologische Untersuchungen zum Sprungverhalten an mehreren Halbaffenarten (Galago moholi, Galago (Otolemur) garnetti, Lemur catta) [Google Scholar]
- Hoeschen G, Preuschoft H, Schulze H. Substratpräferenzen bei Schlankloris (Loris tardigradus, Halbaffen) in Menschenobhut. Zool. Garten, N. F. 1995;65:258–266. [Google Scholar]
- Jouffroy FK, Lessertisseur J. Relationships between limb morphology and locomotor adaptation among prosimians: an osteometric study. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behaviour and Morphology: Dynamic Interactions in Primates. New York: G. Fischer.; 1979. pp. 143–182. [Google Scholar]
- Jungers WL. Body size and limb proportions in primates. In: Jungers WL, editor. Size and Scaling in Primate Biology. New York: Plenum Press; 1985. pp. 345–382. [Google Scholar]
- Keith A. Man's posture: its evolution and disorders. Br. Med. J. 1923;1:451–454. 499–502, 545–548, 587–590, 624–626, 669–672. doi: 10.1136/bmj.1.3246.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura T, Okada M, Ishida H. Kinesiological characteristics of primate walking: its significance in human walking. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behavior, Morphology: Dynamic Interactions in Primates. New York: G. Fischer.; 1979. pp. 297–312. [Google Scholar]
- Kimura T. Bipedal and quadrupedal walking of primates: comparative dynamics. In: Kondo S, editor. Primate Morphophysiology, Locomotor Analysis and Human Bipedalism. Tokyo: University of Tokyo Press; 1985. pp. 81–104. [Google Scholar]
- Kimura T. Hindlimb dominance during primate high-speed locomotion. Primates. 1992;33:465–476. [Google Scholar]
- Kimura T. Long bone characteristics of primates. Z. Morph. Anthropol. 1995;80:265–280. [Google Scholar]
- Kimura T. Primate limb bones and locomotor types in the arboreal environment. In: Okada M, Preuschoft H, editors. Arboreal Locomotor Adaptation in Primates and its Relevance to Human Evolution. Vol. 83. Z. Morph.: Anthropol; 2002. pp. 201–219. [PubMed] [Google Scholar]
- Kirschmann E. Das Zeitalter der Werfer. Eine Neue Sicht Des Menschen. Hannover: Kirschmann.; 1999. [Google Scholar]
- Kummer B. Biomechanics of bone. In: Fung YC, Perrone N, Anlicker M, editors. Biomechanics: its Foundations and Objectives. Englewood Cliffs, NJ: Prentice Hall; 1972. pp. 237–271. [Google Scholar]
- Lehmann T. Elemente der MechanikI: Einführung III Kinetik. Braunschweig: Vieweg.; 19721974. [Google Scholar]
- Lenz S. MD thesis. Ruhr-University, Bochum; 1993. Zur Biomechanik Des Beckens: Eine Vergleichend-Funktionsmorphologische Studie. [Google Scholar]
- Mochon S, McMahon TA. Ballistic walking. J. Biomech. 1980;13:49–57. doi: 10.1016/0021-9290(80)90007-x. [DOI] [PubMed] [Google Scholar]
- Mochon S, McMahon TA. Ballistic walking: an improved model. Mathemat. Biosci. 1981;52:241–260. [Google Scholar]
- Mollison T. Die Korperproportionen der Primaten. Morph. Jb. 1911;42:79–304. [Google Scholar]
- Morbeck ME. Positional behavior of Colobus guereza: a preliminary quantitative analysis. In: Kondo S, Kawai M, Ehara A, Kawamura S, editors. Symposium Vth CongrInternational PrimatolSoc1974. Tokyo: Japan Science Press; 1975. pp. 331–343. [Google Scholar]
- Morbeck ME. Forelimb use and positional adaptation in Colobus guereza: integration of behavioral, ecological and antomical data. In: Morbeck ME, Preuschoft H, Gomberg N, editors. EnvironmentBehaviour and Morphology: Dynamic Interactions in Primates. New York: G Fischer.; 1979. pp. 95–118. [Google Scholar]
- Moya-Sola S, Köhler M. Body posture of Oreopithecus bambolii. C. R. Acad. Sci. Paris. 1996;324:141–148. [Google Scholar]
- Nakano Y. Footfall patterns in early development of the quadrupedal walking of Japanese macaques. Folia Primatol. 1996;66:113–125. doi: 10.1159/000157189. [DOI] [PubMed] [Google Scholar]
- Nakano Y. The effects of substratum inclination on locomotor patterns in primates. In: Okada M, Preuschoft H, editors. Arboreal Locomotor Adaptations in Primates and its Relevance to Human Evolution. Vol. 83. Z. Morph.: Anthropol; 2002. pp. 189–199. [PubMed] [Google Scholar]
- Nakatsukasa M. Acquisition of bipedalism: the Miocene hominoid record and modern analogues for bipedal protohominids. J. Anat. 2004;204:000–000. doi: 10.1111/j.0021-8782.2004.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemitz C. A theory on the evolution of human bipedalism: die Amphibische Generalistentheorie. In: Zeller U, editor. Origin and Evolutionary Transformations of Mammals: Using Biological Signals in Understanding Earth History. Berlin: Natural History Museum, Humboldt-Universität.; 2000. pp. 46–48. [Google Scholar]
- Niemitz C. A theory on the evolution of the habitual orthograde human bipedalism. The Amphibische Generalistentheorie. Anthropol. Anzeiger. 2002;60:3–66. [PubMed] [Google Scholar]
- Nieschalk U. PhD thesis. Ruhr-University, Bochum; 1991. Fortbewegung und Funktionsmorphologie von. Loris Tardigradus und anderen kleinen Halbaffen in Anpassung an unterschiedliche Habitate. [Google Scholar]
- Okada M, Preuschoft H. Introduction of the guest editors. Z. Morph. Anthropol. 2002;83:145–148. [Google Scholar]
- Pauwels F. Die Bedeutung der Bauprinzipien des Stütz- und Bewegungsapparates für die Beanspruchung der Röhrenknochen. Z. Anat. Entw. Gesch. 1958;114:129–166. [PubMed] [Google Scholar]
- Peters A, Preuschoft H. External biomechanics of leaping in Tarsius and its morphological and kinematic consequences. In: Niemitz C, editor. Biology of Tarsiers. Stuttgart: Fischer.; 1984. pp. 227–255. [Google Scholar]
- Pickford M. Paleoenvironments and hominoid evolution. In: Okada M, Preuschoft H, editors. Arboreal Locomotor Adaptation in Primates and its Relevance to Human Evolution. Vol. 83. Z. Morph.: Anthropol; 2002. pp. 337–348. [Google Scholar]
- Preuschoft H. Muskeln und Gelenke der Hinterextremitaet des Gorilla. Morph. Jb. 1961;101:432–540. [Google Scholar]
- Preuschoft H. Statische Untersuchungen am Fuß der Primaten. I. Phalangen und Metatarsalia. Z. Anat. Entw. Gesch. 1969;129:285–345. [Google Scholar]
- Preuschoft H. Body posture and mode of locomotion in Early Pleistocene Hominids. Folia Primatol. 1971;14:209–240. doi: 10.1159/000155351. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. Functional anatomy of the upper extremity. In: Bourne GH, editor. Arboreal Locomotor Adaptation in Primates and its Relevance to Human EvolutionThe Chimpanzee. Vol. 6. Basel: Karger; 1973. pp. 34–120. [Google Scholar]
- Preuschoft H. Aufs. u. Reden D. Senckenberg. Naturf.Ges, Evoluierende Systeme I + II. Frankfurt: Kramer Verlag; 1976. Funktionelle Anpassung evoluierender Systeme. – 5. Arbeitsgespräch zu Phylogenetik und Systematik, Lochmühle/Spessart; pp. 98–117. [Google Scholar]
- Preuschoft H. Recent results concerning the biomechanics of man's acquisition of bipedality. In: Chivers DJ, editor. Proceedings of the 6th CongrInternational PrimatSoc. London: Academic Press; 1978. pp. 435–458. [Google Scholar]
- Preuschoft H, Demes B. Biomechanics of brachiation. In: Preuschoft H, Brockelman WY, Chivers DJ, Creel N, editors. The Lesser ApesEvolutionary and Behavioral Biology. Edinburgh: Edinburgh University Press; 1984. pp. 96–118. [Google Scholar]
- Preuschoft H, Demes B. Influence of size and proportions on biomechanics of brachiation. In: Jungers WL, editor. Size and Scaling in Primate Biology. New York: Plenum Press; 1985. pp. 383–398. [Google Scholar]
- Preuschoft H, Hayama S, Günther MM. Curvature of the lumbar spine as a consequence of mechanical necessities in Japanese macaques trained for bipedalism. Folia Primatol. 1988;50:42–58. doi: 10.1159/000156333. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. Gravity in primates and its relation to body shape and locomotion. In: Jouffroy FK, Stack MH, Niemitz C, editors. Gravity, Posture and Locomotion in Primates. Firenze: II Sedicesimo; 1990. pp. 109–127. [Google Scholar]
- Preuschoft H, Witte H. Biomechanical reasons for the evolution of hominid body shape. In: Senut B, Pickford M, editors. Origine(s) de la Bipédie Chez les Hominidés. Paris: Editions du CNRS; 1991. pp. 59–77. [Google Scholar]
- Preuschoft H, Witte H, Demes B. Biomechanical factors that influence overall body shape of large apes and humans. In: Matano S, Tuttle RH, Ishida H, Goodman M, editors. Topics in Primatology, Evolutionary Biology, Reproductive Endocrinology and Virology. Vol. 3. Tokyo: University of Tokyo Press; 1992. pp. 259–289. [Google Scholar]
- Preuschoft H, Witte H. Die Körpergestalt des Menschen als Ergebnis biomechanischer Erfordernisse. In: Voland E, editor. Evolution und AnpassungWarum die Vergangenheit die Gegenwart Erklärt. Stuttgart: S. Hirzel Verlag; 1993. pp. 43–74. [Google Scholar]
- Preuschoft H, Witte H, Christian H, Recknagel S. Körpergestalt und Lokomotion bei großen Säugetieren. Verh. Dt. Ges. Zool. 1994;87:147–163. [Google Scholar]
- Preuschoft H, Tardieu C. Biomechanical reasons for the divergent morphology of the knee joint and the distal epiphyseal suture in hominoids. Folia Primatol. 1996;66:82–92. doi: 10.1159/000157187. [DOI] [PubMed] [Google Scholar]
- Preuschoft H, Witte H, Christian A, Fischer MS. Size influences on primates locomotion and body shape, with special emphasis on the locomotion of ‘small mammals’. Folia Primatol. 1996;66:93–112. doi: 10.1159/000157188. [DOI] [PubMed] [Google Scholar]
- Preuschoft H, Günther MM, Christian A. Size dependence in prosimian locomotion and its implications for the distribution of body mass. Folia Primatol. 1998;69:60–81. doi: 10.1159/000052699. [DOI] [PubMed] [Google Scholar]
- Preuschoft H. Zur Evolution der menschlichen Becken- und Rumpfform. In: Biedermann N, editor. Manual-Therapie bei Kindern. Stuttgart: Enke.; 1999. pp. 77–88. [Google Scholar]
- Preuschoft H. What does ‘arboreal locomotion’ mean exactly? and what are the relationships between ‘climbing’, environment and morphology? In: Okada M, Preuschoft H, editors. Arboreal Locomotor Adaptation in Primates and its Relevance to Human Evolution. Vol. 89. Z. Morph.: Anthropol; 2002. pp. 171–188. [PubMed] [Google Scholar]
- Preuschoft H. Warum wurden die Vormenschen zu Zweibeinern? Zbl. Geol. Paläont. 2003;II(1/2):1–20. [Google Scholar]
- Preuschoft H, Schmidt M, Hayama S, Okada M. The influence of three-dimensional movements of the forelimb on the shape of the thorax and its importance for erect body posture. Cour. Forsch.-Inst. Senckenberg. 2003;243:9–24. [Google Scholar]
- Richmond BG, Strait DS. Evidence that humans evolved from a knuckle-walking ancestor. Nature. 2000;404:382–384. doi: 10.1038/35006045. [DOI] [PubMed] [Google Scholar]
- Richmond BG. Origin of human bipedalism: the knuckle-walking hypothesis revisited. Yearb. Phys. Anthropol. 2001;44:70–105. doi: 10.1002/ajpa.10019.abs. [DOI] [PubMed] [Google Scholar]
- Robinson JT. Early Hominid Posture and Locomotion. Chicago: University of Chicago Press.; 1972. [Google Scholar]
- Rose M. Positional behavior of natural populations: Some quantitative results of a field study of Colobus guereza and Cercopithecus aethiops. In: Morbeck ME, Preuschoft H, Gomberg N, editors. Environment, Behavior, and Morphology: Dynamic Interaction in Primates. New York: G Fischer.; 1979. pp. 75–94. [Google Scholar]
- Rosenberger AL, Stafford BJ. Locomotion in captive Leontocebus and Callimico: a multimedia study. Am. J. Phys. Anthropol. 1994;94:379–394. doi: 10.1002/ajpa.1330940307. [DOI] [PubMed] [Google Scholar]
- Schmid P. Eine Rekonstruktion des Skelettes von A.L. 288–1 (Hadar) und deren Konsequenzen. Folia Primatol. 1983;40:283–306. doi: 10.1159/000156111. [DOI] [PubMed] [Google Scholar]
- Schmid P. The trunk of the australopithecines. In: Coppens Y, Senut B, editors. Origine(s) des la Bipédie Chez les Hominidés. Paris: Editions du CNRS.; 1991. pp. 225–234. [Google Scholar]
- Schmidt M, Fischer MS. Cineradiographic study of forelimb movements during quadrupedal walking in the Brown Lemur (Eulemur fulvus, Primates: Lemuridae) Am. J. Phys. Anthropol. 2000;111:245–262. doi: 10.1002/(SICI)1096-8644(200002)111:2<245::AID-AJPA9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Voges D, Fischer MS. Shoulder movement during quadrupedal locomotion in arboreal primates. Z. Morph. Anthropol. 2002;83:235–242. [PubMed] [Google Scholar]
- Schultz AH. Post embryonic age changes. In: Hofer H, Schultz AH, Starck D, editors. Primatologia, Handbuch der Primatenkunde I. Basel: S. Karger; 1956. pp. 887–964. [Google Scholar]
- Schultz AH. Vertebral column and thorax. In: Hofer H, Schultz AH, Starck D, editors. Primatologia, Handbuch der Primatenkunde, IV. Basel: S. Karger; 1961. pp. 5/1–5/66. [Google Scholar]
- Sellers WI, Dennis LA, Wang WJ, Crompton RH. Evaluating alternative gait strategies using evolutionary robotics. J. Anat. 2004;204:343–351. doi: 10.1111/j.0021-8782.2004.00294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern JT, Susman RL. The locomotor anatomy of Australopithecus afarensis. Am. J. Phys. Anthropol. 1983;60:279–317. doi: 10.1002/ajpa.1330600302. [DOI] [PubMed] [Google Scholar]
- Strasser E, Fleagle J, Rosenberger A, McHenry H. Primate Locomotion: Recent Advances. New York: Plenum Press; 1998. [Google Scholar]
- Susman RL, Stern JT, Jr, Jungers WL. Arboreality and bipedality in the Hadar hominids. Folia Primatol. 1984;43:113–156. doi: 10.1159/000156176. [DOI] [PubMed] [Google Scholar]
- Susman RL. New postcranial remains from Swartkrans and their bearing on the fuctional morphology and behaviour of Paranthropus robustus. In: Grine FE, editor. Evolutionary History of the Robust Austrlopithecines. New York: Aldine de Gruyter.; 1988. pp. 149–172. [Google Scholar]
- Thorpe SKS, Crompton RH, Chamberlain AT. Bipedal behaviour in captive Chimpanzees, Pan troglodytes. In: Harcourt CS, Sherwood B, editors. New Perspectives in Primate Evolution and Behaviour. Location: Westbury Publishing; 2003. pp. 235–252. [Google Scholar]
- Tuttle RH. Knuckle-walking and the evolution of hominoid hands. Am. J. Phys. Anthropol. 1967;26:171–206. [Google Scholar]
- Vilensky JA. Gait characteristics of two macaques with emphasis on relationship with speed. Am. J. Phys. Anthropol. 1983;61:255–265. doi: 10.1002/ajpa.1330610215. [DOI] [PubMed] [Google Scholar]
- Vilensky JA. Primate quadrupedalism: how and why does it differ from that of typical quadrupeds? Brain Behav. Evol. 1989;34:357–364. doi: 10.1159/000116522. [DOI] [PubMed] [Google Scholar]
- Vilensky JA, Moore AM, Libii JN. Squirrel monkey locomotion on an inclined treadmill: implications for the evolution of gaits. J. Hum. Evol. 1994;26:375–386. [Google Scholar]
- Voges D. PhD thesis. University of Jena; 1999. Roentgenkinematographische Untersuchungen der Lokomotion von Saguinus oedipus (Linné, 1758) (Callithrichidae, Primates) [Google Scholar]
- Wang WJ. PhD Thesis. University of Liverpool; 1999. The mechanics of bipedalism in relation to load-carrying. Biomechanical optima in hominind evolution. [Google Scholar]
- Wang WJ, Crompton RH, Li Y, Günther MM. Optimum ratio of upper to lower limb lengths under the assumptions of frequency coordination and hand-carrying of a load. J. Biomech. 2002;36:249–252. doi: 10.1016/s0021-9290(02)00315-9. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Crompton RH. Size and power required for motion with implications for the evolution of early hominids. J. Biomech. 2003;36:1237–1246. doi: 10.1016/s0021-9290(03)00165-9. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Crompton RH, Li Y, Günther MM. Energy transformation during erect and bent-hip-bent-knee walking by humans with implications for the evolution of bipedalism. J. Hum. Evol. 2003;44:563–580. doi: 10.1016/s0047-2484(03)00045-9. [DOI] [PubMed] [Google Scholar]
- Wang WJ, Crompton RH. The role of load-carrying in the evolution of modern body proportions. J. Anat. 2004;204:417–430. doi: 10.1111/j.0021-8782.2004.00295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler PE. The loss of functional body hair in man: the influence of thermal environment, body form and bipedality. J. Hum.Evol. 1985;14:23–28. [Google Scholar]
- Wheeler PE. The thermoregulatory advantages of hominid bipedalism in open equatorial environments: the contribution of increased convective heat loss and cutaneous evaporative cooling. J. Hum. Evol. 1991a;21:117–136. [Google Scholar]
- Wheeler PE. Body hair reduction and tract orientation in man: Hydrodynamics or thermoregulatory aerodynamics? In: Roede M, Wind J, Patrick J, Reynolds V, editors. The Aquatic Ape, Fact or Fiction. London: Souvenir Press; 1991b. pp. 221–236. [Google Scholar]
- Witte H, Preuschoft H, Recknagel S. Human body proportions explained on the basis of biomechanical principles. Z. Morph. Anthropol. 1991;78:407–423. [PubMed] [Google Scholar]
- Witte H, Recknagel S, Lesch C, Rao JG, Preuschoft H. Is elastic energy storage of quantitative relevance for the functional morphology of the human locomotor apparatus? Acta Anat. 1997;158:106–111. doi: 10.1159/000147919. [DOI] [PubMed] [Google Scholar]
- Witte H, Preuschoft H, Fischer M. The importance of the evolutionary heritage of locomotion on flat ground in small mammals for the development of arboreality. Z. Morph. Anthropol. 2002;83:221–233. [PubMed] [Google Scholar]
- Witte H, Hoffmann H, Hackert R, Schilling C, Fischer M, Preuschoft H. Biomimetic robotics should be based on functional morphology. J. Anat. 2004;204:331–342. doi: 10.1111/j.0021-8782.2004.00297.x. [DOI] [PMC free article] [PubMed] [Google Scholar]