Abstract
A detailed study of the ontogenesis of deer stomach has not been undertaken to date, and our aim was to sequence several histological phenomena that occur during the ontogenesis of one of the gastric compartments, the rumen. Histomorphometric and immunohistochemical analyses were carried out on 50 embryos and fetuses of deer from the initial stages of prenatal life until birth. For the purposes of testing, the animals were divided into five experimental groups: group I, 1.4–3.6 cm crown–rump length, 30–60 days, 1–25% of gestation; group II, 4.5–7.2 cm crown–rump length, 67–90 days, 25–35% of gestation; group III, 8–19 cm crown–rump length, 97–135 days, 35–50% of gestation; group IV, 21–33 cm crown–rump length, 142–191 days, 45–70% of gestation; and group V, 36–40 cm crown–rump length, 205–235 days, 75–100% of gestation. The rumen of the primitive gastric tube was observed at approximately 60 days. At 67 days the rumen consisted of three layers: internal or mucosal, middle or muscular, and external or serosal layer. The stratification of the epithelial layer was accompanied by changes in its structure with the appearance of ruminal pillars and papillae. The outline of the ruminal papillae began to appear at 142 days of prenatal development as evaginations of the basal zone toward the ruminal lumen, pulling with it in its configuration the stratum basale, the lamina propria and the submucosa. From the pluripotential blastemic tissue at 60 days we witnessed the histodifferentiation of the primitive tunica muscularis, composed of two layers of myoblasts with a defined arrangement. It was also from the pluripotential blastemic tissue, at 97 days, that the lamina propria and the submucosa were differentiated. The serosa showed continuity in growth as well as differentiation, already detected in the undifferentiated outline phase. The tegumentary mucosa of deer rumen was shown without secretory capacity in the initial embryonic phases; neutral mucopolysaccharides appeared from 67 days. The presence of neuroendocrine cells (non-neuronal enolase) in the ruminal wall of deer during development was not detected until 97 days. The glial cells were detected at 142 days for glial fibrillary acidic protein and at 67 days for vimentin. The immunodetection of neuropeptides vasointestinal peptide and neuropeptide Y progressively increased with gestation period, starting from 97 days. In terms of the structure of the rumen of the primitive gastric tube, our observations revealed that the deer is less precocious than small and large domestic ruminants. Thus its secretory capacity, detected by the presence of neutral mucopolysaccharides, and its neuroendocrine nature, determined by the presence of positive non-neuronal enolase cells, were evident in more advanced stages of prenatal development than those detected in the sheep, goat and cow.
Keywords: immunohistochemistry, prenatal development, red deer, rumen
Introduction
Red deer in the Iberian Peninsula are a species that need to be conserved for two principal reasons: as a species to be hunted, and for its role in the economic activity of the region. Red deer provide a wealth of genetic material, and its autochthonous nature is necessary for the maintenance of the ecosystem. Red deer meat also has an increasingly important part in the food industry.
The hunting of red deer constitutes one of the main economic uses of Mediterranean forests and ‘dehesas’ (open oak forests) in the south-west of the Iberian Peninsula. The management of its population has undergone a notable evolution in recent years (Carranza, 1999). The traditional system of intensive farming methods is gradually being replaced by a mixed system of intensive/semi-intensive methods, in which new management methods require intervention in many areas, food being the area of most concern here.
Food supplements, on the one hand, give rise to ecological and behavioural changes in the species, because the addition of food in winter can result in a large increase in the number of animals that an area can support. Moreover, the period of shortage in the southernmost areas coincides with the rutting season (Carranza, 1999). On the other hand, added to the undesirable ecological effects from the spatial concentration of food are the changes that result from such supplements on the histophysiology of the red deer stomach.
The ruminal complex, in its function as a fermentation chamber, plays a fundamental role in the breakdown of cellulose (the food base of ruminants) thanks to the mass anaerobic fermentation that occurs, caused by the synergy between the amount of bacteria and protozoa that are present in this compartment. The morphological adaptation of the ruminal mucosa to its degradative function of food ingestion leads to the formation of a tegumentary mucosa. This structure of the rumen in postnatal life originates in prenatal development, because once it has become separate from the primitive gastric tube, its mucosa undergoes histological modifications as the animals ages, leading to the definitive formation found in postnatal stages and in perinatal stages.
The prenatal development of the stomach of ruminants has not been studied in detail. Our research group focused on the ontogenetic approach taken in the stomach of sheep (Franco et al. 1992, 1993a,b, c; Regodón et al. 1996). In previous studies of prenatal development of the sheep rumen (Franco et al. 1992), the entire ontogenesis was found to involve a gradual adaptation of the ruminal mucosa to the profound changes that it has to go through in its structural formation in order to become adapted to the food modifications that occur as a consequence of birth. The postnatal development of the rumen is thus a continuation of the differentiation and formation in its structure that is initiated in prenatal life. An ontogenetic study of deer stomach has not been undertaken, however, and our aim was to sequence some histological phenomena that occur during the ontogenesis of one of the gastric compartments – the rumen. Immunohistochemical analysis was performed to detect neuroendocrine cell markers (non-neuronal enolase), glial cell markers (glial fibrillary acidic protein, GFAP, and vimentin, VIM), and markers of peptidergic innervation (neuropeptide Y, NPY, and vasointestinal peptide, VIP). VIP and NPY are used as neuropeptide markers because they are widely distributed throughout the mammalian peripheral nervous system, and may be found in the neurons of the gastrointestinal tract. The study has been carried out in an extensive deer-farming system, without food supplements, with the objective of establishing a morphological database of prenatal development of the rumen without the possible influences that arise from these supplements. The study was also intended to serve as a reference database for future studies looking at these influences.
Materials and methods
Animals
Deer embryos and fetuses (n = 25) from the initial prenatal stages until birth were studied. The specimens were divided into five groups of five animals each, with reference to the most relevant histomorphogenic characteristics (Table 1). To obtain embryos and fetuses at various stages of development, a total of 125 Caesarian sections on the same number of females were performed. The females were taken from ten hunting grounds from extensive and non-enclosed estates from the Sierra of San Pedro (north-east of the Province of Cáceres, Spain).
Table 1.
Neuropeptides present in the rumen of red deer during prenatal development
| Group I CRL (cm) (1.4–3.6) | Group II CRL (cm) (4.5–7.2) | Group III CRL (cm) (8–19) | Group IV CRL (cm) (21–33) | Group V CRL (cm) (36–40) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | |
| NSE | – | – | – | – | – | – | – | – | – | + | + | – | – | ++ | ++ | – | – | ++ | ++ | – |
| GFAP | – | – | – | – | – | – | – | – | – | – | – | – | – | ++ | ++ | ++ | – | ++ | ++ | ++ |
| VIM | – | – | – | – | – | + | + | + | + | + | + | + | – | ++ | ++ | ++ | – | ++ | ++ | ++ |
| VIP | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – |
| NPY | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | ++ | – | – | – |
–, non immunoreactivity; +, low immunoreactivity; ++, moderate immunoreactivity; +++, high immunoreactivity. E = epithelium, LP-S = lamina propia-submucosa, T = tunica muscularis, S = serosa.
Sampling and processing
Once the ruminal compartment was separated, it was analysed by visual and stereomicroscopic inspection. The colouring and the consistency of the ruminal mucosa were determined, as well as the morphology of the ruminal papillae.
Square specimens measuring 1.5 × 0.5 cm were taken from the medial region of the dorsal sac and from the cranial sac of the rumen of each animal. Tissues for histological study were fixed in 4% buffered formaldehyde, processed by standard paraffin-embedding methods, and 5-µm-thick sections were cut and treated with haematoxylin–eosin (H-E); periodic acid-Schiff (pH 7.2) and PAS-alcian blue (pH 7.2) for specific differentiation of neutral and acid mucopolysaccharides; Masson's Trichrome, Van Gieson and Reticuline of Gomori.
Morphometric analysis
Specimens for morphometric analysis were embedded in paraffin, stained with H-E, and viewed through a microscope (Optiphot, Nikon Inc, Tokyo, Japan) equipped with a video camera. The image was reflected on to the screen of a semi-automatic image analyser (Vid IV, Rego and Cía, Madrid, Spain). Variables studied were height of various tissue strata (epithelium, lamina propria and submucosa, tunica muscularis and serosa) and total wall thickness. Eight specimens were selected for each group, and 50 measurements were made for each tissue stratum.
The results are shown as the average ± standard error. The data were analysed using analysis of the variance. In the cases where the anova was significant, a post hoc (Tukey) analysis was carried out in order to study the significant differences among the distinct groups. A value of P ≤ 0.05 was considered significant.
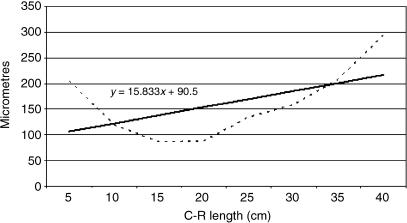
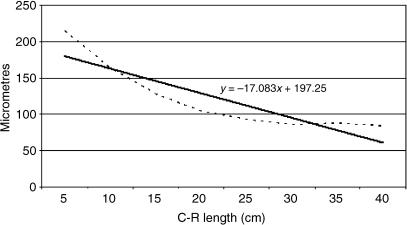
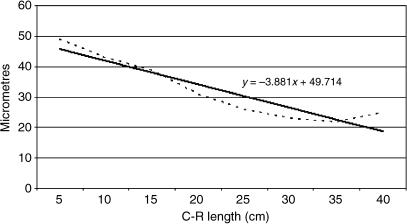
Tissue growth models were created, using a personal computer and statistics program (Statgraphics V 2.1 (1986)). In Figs 25–29 below, the graphs represent the averages of the real growth values next to the adjusted line of regression. The goodness of fit of this adjustment was measured using the rate of determination, r2. In all cases, embryo body length (crown–rump length, CRL, in centimetres) was used as the independent variable; the thickness of each tissue stratum served as the dependent variable.
Fig. 25.
Mathematical model of rumen growth (tunica muscularis).
Immunocytochemical analysis
ExtrAvidin peroxidase staining (EAS) was performed on deparaffinized sections from the dorsal and ventral sac of the rumen to detect the neuroendocrine cells markers [non-neuronal enolase (NNE)]; glial cell markers (GFAP and VIM) and markers of peptidergic innervation (NPY and VIP). Tissue was deparaffinized, hydrated and treated sequentially with 15% hydrogen peroxide for 30 min in order to block endogenous peroxidase activity. Non-specific tissue binding sites were blocked by incubation in 1% normal goat serum for 30 min. Samples were incubated with the following dilution in phosphate-buffered saline (PBS) of primary antisera: 1: 200 monoclonal anti-human NNE (Sigma/Aldrich Química, Madrid, Spain, no. S5768); 1: 400 monoclonal antihuman GFAP (Sigma/Aldrich Química, Madrid, Spain, no. G-3893); 1: 20 monoclonal anti-human VIM (Sigma/Aldrich Química, no. V-5255); 1: 200 monoclonal anti-human NPY (Sigma/Aldrich Química, no. N9528); and 1: 20 monoclonal anti-human VIP (Sigma/Aldrich Química, no. V3508) for 3 h at 20 °C. Biotinylated goat anti-mouse IgG (1: 200 dilution) (Sigma/Aldrich Quimica, no. B7151) was then added to the sections for 30 min. Sections were finally incubated with diluted (1: 50) ExtrAvidin horseradish peroxidase (Sigma/Aldrich Quimica, no. E2886) for 1 h. After diaminobenzidine reaction, nuclear counterstaining with Mayer's haematoxylin was applied. Finally, the sections were mounted with Entellan (Merck 7961).
The specificity of the staining reaction was determined in control experiments. These comprised either substitution of the primary antibody by PBS or normal mouse serum 1: 100, or omission of both primary and secondary antibodies; in addition, prior absorption of the primary antibody (overnight preincubation of the primary antisera with the repective peptide, 50–100 µm). Next, the antibody/peptide mixture was applied to sections in the identical manner and at the same concentration of the primary antibody.
Results
Macroscopic findings
From 60 days of gestation and until the perinatal stages, the deer rumen displayed a smooth surface, on which the incipient papillae that had started to rise emerged. The ruminal surface displayed a non-keratinized appearance, of soft consistency and with an absence of descamative phenomena. As prenatal development progressed, the papillae rose above the ruminal surface, reaching a considerable length at birth. The morphology of these papillae was foliated and at stages close to birth they displayed a greater keratinization and considerable descamative phenomena.
Ruminal histomorphogenesis
Group I (1.4–3.6 cm CRL, 30–60 days, 1–25% of gestation)
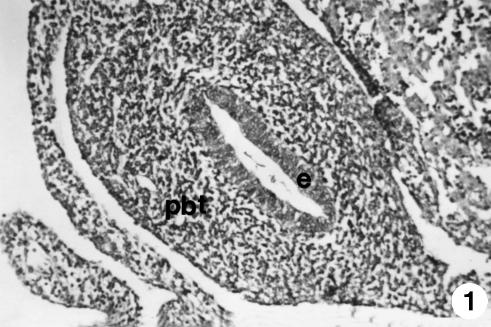
At 30 days of prenatal development, the primitive outline of the gastric tube showed a thin cavity surrounded by a thick wall (250 ± 10 µm) (Fig. 1). The wall was composed of two layers: an internal epithelial layer (50 ± 6 µm), adjacent to the tube, and an external layer, of pluripotential blastemic tissue. The epithelium was cylindrically stratified and was not ciliated. The thickness of pluripotential blastemic tissue was 183 ± 10 µm. It was composed of cells of irregular morphology and arrangement, immersed in abundant ground substance. A considerable number of blood capillaries could be seen in the thickness of the pluripotential blastemic tissue.
Fig. 1.
Photomicrograph of a section of the undifferentiated stomach at 1.4 cm CRL. The wall was composed of two layers: epithelium (e) and pluripontetial blastemic tissue (pbt). H-E, ×180.
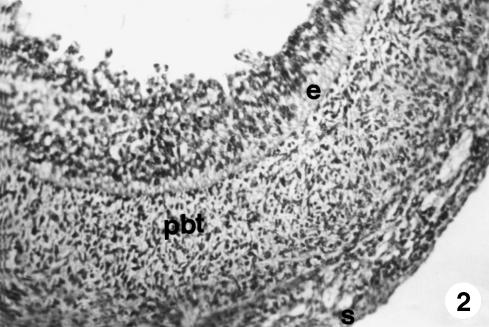
At 60 days of gestation (Fig. 2), the outline of the ruminal compartment appears. Its wall (313 ± 16 µm) was composed of three well-defined layers. The internal epithelial layer showed identical morphological characteristics to those described above but was of greater thickness (62 ± 5 µm). In the middle layer or pluripotential blastemic tissue, of a similar thickness (195 ± 10 µm), longitudinally arranged morphologically fusiform myoblastic cells appeared, the ontogenetic base of the future tunica muscularis, subdividing it into two zones: a highly cellular internal zone and a further external one, in which ground substance predominated, and which would later form the subserosa. The final layer was the external layer or serosa (56 ± 3 µm), formed by a mesothelium of flat cells and a lax connective tissue of cellular support. The thickness of the walls was 358 ± 18 µm.
Fig. 2.
Photomicrograph of a section of the ruminal wall at 3.6 cm CRL. Three layers are visible: epithelium (e), pluripotential blastemic tissue (pbt) and serosa (s). H-E, ×250.
Group II (4.5–7.2 cm CRL, 67–90 days, 25–35% of gestation)
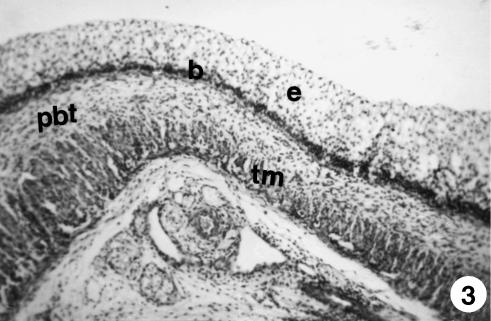
We witnessed an evident thickening of the stratified epithelial layer (105 ± 9 µm) that covers the ruminal lumen, attributable to an increase in the number of its layers (Fig. 3). In the basal zone of the epithelium we witnessed nuclear condensation with a visible layer of two or three cells that formed the stratum basale. Papilliform projections in the epithelial layer toward the ruminal lumen form the outline of the pillars (Fig. 3) that will later divide the rumen into its different sacs.
Fig. 3.
Photomicrograph of a section of the ruminal pillar at 4.5 cm CRL. Epithelium (e), basal membrane (b), pluripotential blastemic tissue (pbt) and tunica muscularis (tm) were observed. H-E, ×250.
In the extremely vascularized pluripotential blastemic tissue (205 ± 16 µm), signs of cellular differentiation were evident. There was a first layer, adjacent to the serosa, formed by myoblastic elements arranged in circular formations to the ruminal lumen, and a second layer of less differentiated cells, arranged longitudinally (Fig. 3). The outline of the tunica muscularis showed slight swelling as it entered the inner zone of the pillar parenchyma, thus participating in the formation and growth of the pillars.
The serosa (48 ± 5 µm) was extremely vascularized and formed by an epithelium and subserosa (Fig. 3). The thickness of the walls was 460 ± 28 µm.
Group III (8–19 cm CRL, 97–135 days, 35–50% of gestation)
At this stage of development (Figs 4–7), the ruminal wall comprised four layers: mucosa (with epithelial layer and lamina propria), submucosa, tunica muscularis and serosa.
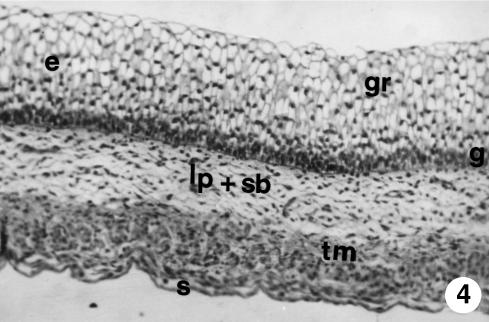
Fig. 4.
Photomicrograph of a section of the ruminal wall at 97 days. The epithelium (e) is stratified in two zones: a basal zone or stratum germinativum (g) and another apical zone or stratum granulosum (gr); pluripotential blastemic tissue subdivided into lamina propria and submucosa (lp + sb); tunica muscularis (tm) and serosa (s). H-E, ×250.
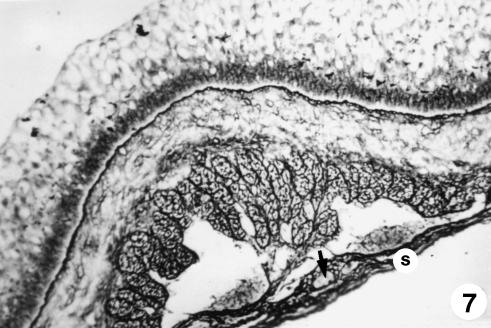
Fig. 7.
Photomicrograph of a section of the ruminal wall at 135 days. Serosa (s) with mesothelium in a subserosa rich in reticulin fibres and highly vascularized (arrow). Reticuline of Gomori, ×350.
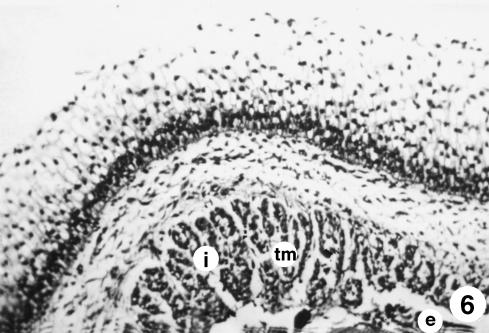
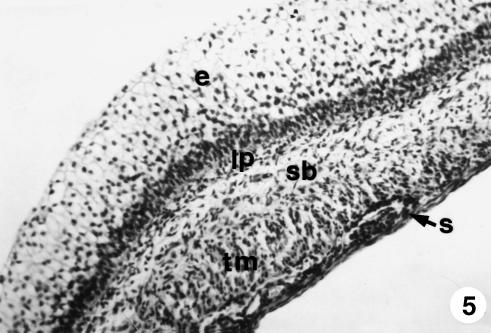
The stratified epithelial layer continued its growth (250 ± 20 µm). It displayed two clearly defined zones: a basal zone or stratum germinativum, formed by 3–5 layers of morphologically oval cells, with a large central nucleus taking up most of the cell and a basophilic cytoplasm; and another external zone of 10–12 layers of polyhedral cells, with small nucleus and light-staining cytoplasm that we will term the stratum granulosum (Figs 4–6). Adjacent to the epithelial layer, two layers developing from the pluripotential blastemic tissue – the lamina propria and submucosa – were differentiated. The lamina propria accompanied the raising of the basal layer of the epithelium (Figs 4 and 5) and was composed of morphologically stellate cells surrounded by a small quantity of ground substance. The submucosa, adjacent to the tunica muscularis, contained fewer cell elements and a greater quantity of ground substance. Both layers together had a thickness of 101 ± 8 µm. The tunica muscularis (76 ± 11 µm) was structured in an internal circular bundle and another longitudinal external bundle (Figs 6 and 7). The serosa (33 ± 6 µm) showed a mesothelium in a subserosa rich in ground substance, reticulin fibres, vascularization (Fig. 7), nerve fibres and nerve cell bodies. Finally, the ruminal pillars showed a thin epithelium germinativum, as in the lamina propria and the submucosa. The muscular layer that was detected in the pillars was thicker, forming the muscular body of the pillar (Fig. 5). The thickness of the walls was 725 ± 48 µm.
Fig. 6.
Photomicrograph of a section of the ruminal wall at 135 days. Substantial development of the tunica muscularis (tm), with two bundles: internal circular bundle (i) and an external longitudinal bundle (e). H-E, ×350.
Fig. 5.
Photomicrograph of a section of the ruminal wall at 13 cm CRL. It was formed by four layers: mucosa, with epithelial layer (e) and lamina propria (lp); submucosa (sb); tunica muscularis (tm); and serosa (s). H-E, ×250.
Group IV (21–33 cm CRL, 142–191 days, 45–70% of gestation)
In this stage of development (Figs 8–11), the epithelium increased considerably in thickness with respect to the previous stages (430 ± 31 µm) and showed greater stratification (Fig. 10). The stratum germinativum, in the basal zone, was formed by extremely darkly stained cells. The stratum granulosum, in the apical zone, was formed by polyhedral vesiculiform cells of clear cytoplasm and nuclei polarized toward one of the membranes. The appearance of intercellular bridges, directly above the stratum basale, represented the morphological expression of the lucidum–spinosum stratum. The stratum corneum also appeared, with morphologically flat elongated cells in contact with the lumen. The outline of the ruminal papillae began to appear as evaginations of the basal zone toward the ruminal lumen, pulling with it in its formation the stratum basale, the lamina propria and the submucosa (Figs 9–11). The lamina propria and submucosa together had a thickness of 86 ± 7 µm (Figs 8–10). The tunica muscularis was extremely thick (186 ± 14 µm) (Fig. 12), especially in the internal bundle. Blood vessels, nerve fibres and nerve cell bodies could be observed in the intermuscular and perimuscular connective tissue. Finally, the serosa (23 ± 4 µm) did not have characteristics different from those of the previous stage. The thickness of the walls was 725 ± 48 µm.
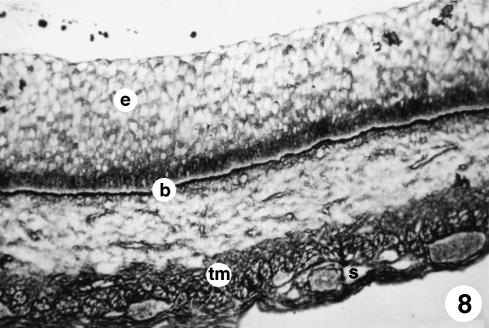
Fig. 8.
Photomicrograph of a section of the ruminal wall at 142 days. Visible basal membrane (b) separating a highly stratified epithelium (e) from a tunica muscularis (tm) and a highly vascularised serosa (s). Reticuline of Gomori, ×250.
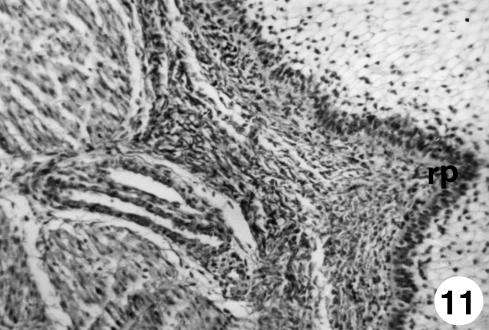
Fig. 11.
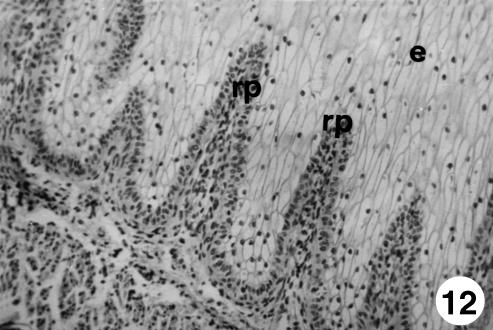
Photomicrograph of a section of the ruminal wall at 191 days.Outline of ruminal papillae (rp).Van Gieson,×350.
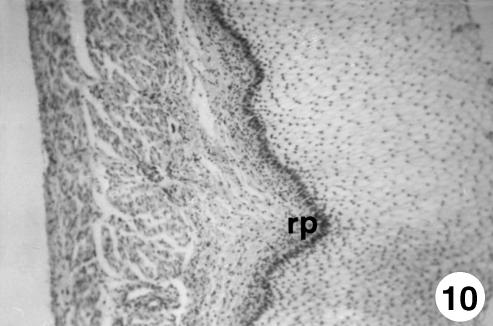
Fig. 10.
Photomicrograph of a section of the ruminal wall at 30 cm CRL. Outline of ruminal papillae (rp). H-E, ×250.
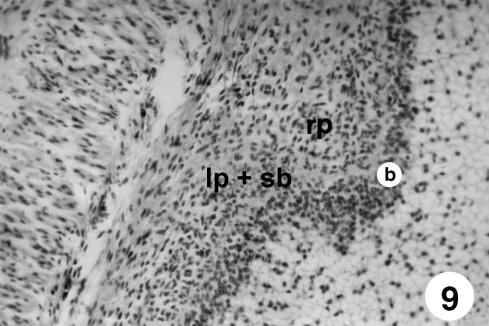
Fig. 9.
Photomicrograph of a section of the ruminal wall at 142 days. Outline of ruminal papillae (rp) as evagination of the basal lamina toward the ruminal lumen, pulling with it in its formation the stratum basale (b), lamina propria and submucosa (lp + sb). H-E, ×250.
Fig. 12.
Photomicrograph of a section of the ruminal wall at 205 days. Ruminal papillae (rp) reaching the third apical of the epithelium (e). H-E, ×250.
Group V (36–40 cm CRL, 205–235 days, 75–100% of gestation)
The thickest epithelial layer (550 ± 36 µm) was composed of a barely stratified epithelium formed by: stratum basale or germinativum of small cells arranged in a line; stratum granulosum; lucidum–spinosum stratum, in which the presence of intercellular bridges and stratum corneum in contact with the ruminal lumen was apparent. Growth of the ruminal papillae was considerable; at 205 days they reached the highest third of the epithelium (Fig. 12), and at 207 days were positioned close to the epithelial surface. Some papillae, on being transversely cut, showed a cylindrical form (Fig. 13). The papillary body appeared delimited by the stratum basale and displayed a highly cellular connective tissue with a small quantity of collagen and reticulin that formed the framework of the papilla (Figs 12 and 14). The submucosa, less cellular than the lamina propria (Fig. 12), showed a lax connective tissue that was a continuation of the lamina propria without clear delimitation. A large number of blood vessels and isolated nerve fibres could be observed. Both layers together displayed a thickness of 182 ± 8 µm. The tunica muscularis (192 ± 12 µm) was structured in two layers of smooth muscular tissue: an internal circular layer and another longitudinally arranged external layer (Fig. 14). The extremely thin serosa, as in the previous stage (25 ± 3 µm), appeared covered with a mesothelium in an extremely lax subserosa, with a small quantity of collagenous and elastie fibers (Fig. 15). Variable quantities of fat, blood vessels and nerve tissue could be observed at some points. The thickness of the walls was 949 ± 52 µm.
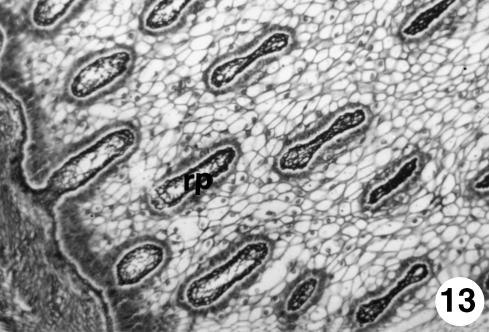
Fig. 13.
Photomicrograph of a section of the ruminal wall at 40 cm CRL. Cylindrical form of the ruminal papillae (rp) on being transversely cut. RG, ×350.
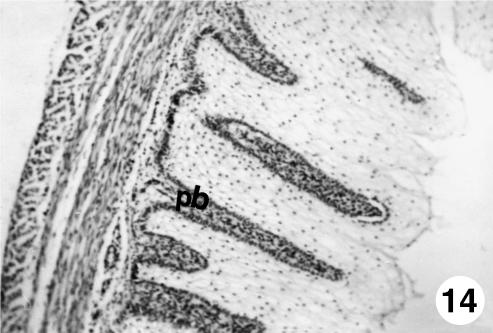
Fig. 14.
Photomicrograph of a section of the ruminal wall at 40 cm CRL. Papillary body (pb) delimited by a basal stratum, formed by a highly cellular connective tissue. H-E, ×250.
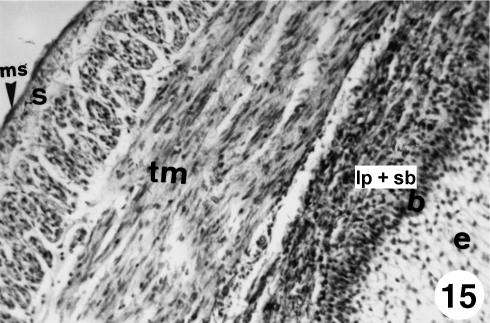
Fig. 15.
Photomicrograph of a section of the ruminal wall at 40 cm CRL. At birth a tunica muscularis (tm) with well-defined bundles and a thin serosa covered by a mesothelium (ms) in a lax subserosa (s) were visible. Epithelium (e), stratum basale (b) and lamina propria and submucosa (lp + sb) were also obseved. VG, ×250.
Histochemical behaviour of the epithelium
Neutral mucopolysaccharides appeared at 67 days and gradually decreased throughout the period of prenatal development until birth. They were found around the tegumentary mucosa in the deeper layers of the epithelium, without affecting the stratum corneum. Acid mucopolysaccharides, mucins and mucoid compounds were not found during development.
Immunohistochemical observation
Table 1 shows the neuropeptides present in the rumen of of red deer during prenatal development. Immunohistochemical findings in the rumen of the five groups of red deer studied are summarized in Figs 16–22.
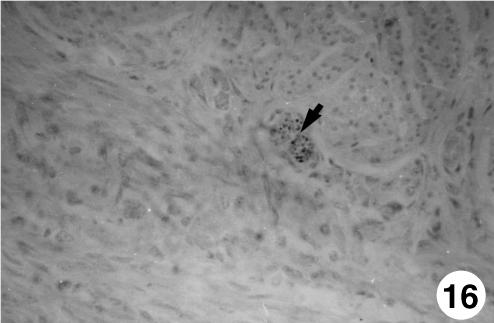
Fig. 16.
Photomicrograph of a section of the ruminal wall at 97 days. Presence of neuroendocrine cells (arrow, NNE) in myenteric plexus. EAS, ×250.
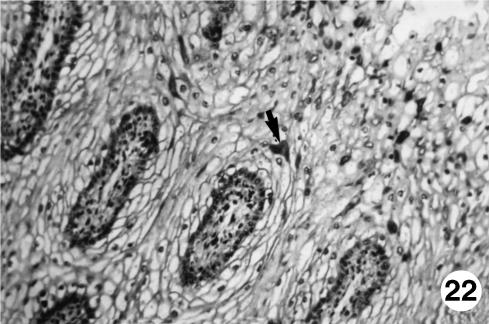
Fig. 22.
Photomicrograph of a section of the ruminal wall at 40 cm CRL.Positive immunodetection of NPY (arrow)in the epithelial thickness of fetuses at term.EAS,×250.
The immunodetection of NNE was positive from 97 days of gestation in the lamina propria and the submucosa. This rise was progressive until 142 days of prenatal development, remaining until birth. A positive immunoreaction for GFAP was observed at 142 days of prenatal life in the lamina propria, tunica muscularis and serosa and was prolonged until birth. The VIM antigen was detected in an identical location as that of GFAP, although in previous stages of prenatal development (67 days of gestation). Immunopositivity for VIP and NPY was only detected in the epithelial layer and in perinatal states.
Histomorphometric observations
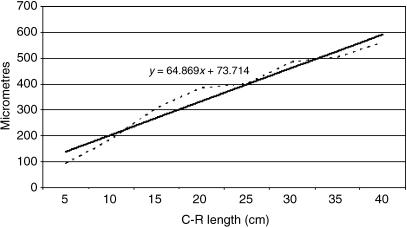
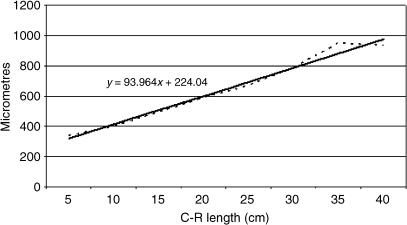
Table 2 shows the tissue layer thickness in the rumen of red deer during prenatal development. Each tissue stratum was fitted to mathematical growth models (Figs 23–27), using the corresponding growth equation.
Table 2.
Morphometrical and statistical findings of the tissue layer thickness in the rumen of red deer during prenatal development (µm)
| Group I | Group II | Group III | Group IV | Group V | |
|---|---|---|---|---|---|
| Epithelium | 62 ± 5 | 105 ± 9 | 250 ± 20 | 430 ± 31 | 550 ± 36 |
| Lp + Sb | pbt | pbt | 101 ± 8 | 86 ± 7 | 182 ± 8 |
| Tm | 195 ± 10* | 205 ± 16* | 76 ± 11 | 186 ± 14 | 192 ± 12 |
| Serosa | 56 ± 3 | 48 ± 5 | 33 ± 6 | 23 ± 4 | 25 ± 3 |
| Wall | 313 ± 16 | 358 ± 18 | 460 ± 28 | 725 ± 48 | 949 ± 52 |
Lp + Sb = lamina propia and submucosa.
Tm = tunica muscularis; pbt = pluripotential blastemic tissue.
The pluripotential blastic tissue of groups I and II, which will later give rise to the lamina propria and submucosa, were not statistically compared owing to the fact that one structure will give rise to various others.
Fig. 23.
Mathematical model of rumen growth (epithelium).
Fig. 27.
Mathematical model of ruminal wall growth.
A factorial anova indicated that the mean growth value of group I epithelium was significantly lower than in groups II–V (F = 14.68; Tukey test: P = 0.003). By contrast, the mean growth value of serosa of group I was significantly higher than in groups II–V (F = 8.40; Tukey test: P = 0.002) and the same for wall (F = 9.50; Tukey test P = 0.001). As indicated by main factor analysis in factorial anova, the lamina propria and submucosa of group III was significantly different from these layers in groups IV and V (F = 6.80; P = 0.002) and the tunica muscularis of group III was also significantly different from this tunica in groups IV and V (F = 15.70; P = 0.0002).
The epithelial layer experienced rapid growth until 174 days of prenatal development; from 174 until 200 days of gestation there is a period of stabilization. Subsequently, growth of the epithelial layer again became rapid until birth. The lamina propria and submucosa experienced a rapid regression until 174 days of gestation; following this a phase of stabilization that lasts until the perinatal states was detected. The tunica muscular demonstrated regression until 135 days of gestation followed by progressive growth until birth. The serosa showed regression in its development until 200 days of gestation; from 200 days of prenatal development a slight increase in growth until birth was witnessed. The integral ruminal wall demonstrated gradual and progressive growth with age and perinatal stabilization.
Discussion
The appearance of the rumen of the primitive gastric tube was observed at approximately 60 days of gestation. In comparison with other ruminants, prenatal development of red deer rumen was later than in sheep, at approximately 33 days (Franco et al. 1992) and at 34 days (Del Rio Ortega, 1973) (22% of gestation), although authors such as Fath El-Bab et al. (1983) established a period of development later still, and placed it at about 97 days (65% of gestation). In goat, Molinari & Jorquera (1988) described ruminal development at 36 days (24% of gestation). In cow, Vivo et al. (1990) reported it very early at 1.7 cm CRL, equivalent to a 30-day period (11%) of gestation.
At 67 days the rumen appeared to be formed by three layers: an internal or mucosal layer, a middle or muscular layer, and an external or serosal layer. In terms of the mucosa, the transition from an epithelium of an ‘embryonic type’ (Warner, 1958) toward a stratified epithelium with uniform distribution of germinativum and granulosum strata was evident. In this sense, our results differ from those of Panchamukhi & Srivastava (1979) in buffalo and Vivo et al. (1990) in cow, as these authors showed the epithelial stratification without a concrete disposition of strata. The stratum corneum appeared at about 142 days. There is no general agreement among previous researchers regarding the appearance of the stratum corneum. Thus, although we concur with Franco et al. (1992), who placed it in sheep at 138 days (92% of gestastion), in this same species Wardrop (1961) and Del Rio Ortega (1973) described it at birth and at 57 days (38% of gestation), respectively. In goat, Molinari & Jorquera (1988) described it at 104 days (70% of gestation). In buffalo, Tiwari & Jamdar (1970) and Panchamukhi & Srivastava (1979) described the appearance of the stratum corneum at 102 days (32% of gestation). In cow it is described in the developmental stages close to birth (Warner, 1958; Asari et al. 1981; Vivo et al. 1990). The appearance of the lucidum–spinosum stratum at around 142 days has only been described by Osman & Berg (1981) in buffalo in advanced stages of development. This stratification of the epithelial layer was accompanied by modifications in its structure, with the appearance of the ruminal pillars and papillae. The presence of papilliform projections toward the lumen that formed the outline of the pillars was timed at around 67 days. In this sense we agree with the findings of Del Rio Ortega (1973) and Franco et al. (1992) in sheep, and with Vivo et al. (1990) in cow, who described their appearance at 39 (26% of gestation), 42 (28% of gestation) and 44 days (16% of gestation), respectively. All parietal layers take part in its formation, although the serosa takes part to a lesser extent. The outline of the ruminal papillae started to appear at 142 days, as evaginations of the basal zone toward the ruminal lumen, pulling with it in its formation the stratum basale, the lamina propria and the submucosa. Opinions differ with regard to the timing of its appearance; thus in sheep Del Rio Ortega (1973), Fath El-Bab et al. (1983) and Franco et al. (1992) put it at 64 (43% of gestation), 103 (69% of gestation) and 61 days (41% of gestation), respectively. In goat, Molinari & Jorquera (1988) place it at around 136 days (91% of gestation). Arias & Cabrera Valencia (1978), Amasaki & Daygo (1987) and Vivo et al. (1990), in cow, describe it in stages close to birth. In buffalo, Osman & Berg (1981) reference it at 74 days of prenatal development (23% of gestation).
At 60 days, from the pluripotential blastemic tissue we witnessed the histodifferentiation of the primitive tunica muscularis composed of two layers of myoblasts thought to be of defined arrangement. Del Rio Ortega (1973) and Franco et al. (1992), in sheep, and Vivo et al. (1990), in cow, placed it earlier at 33 days (22% and 12% of gestation, respectively). However, no agreement has been formed since Fath El-Bab et al. (1983), also in sheep, placed it later, at 50 days (33% of gestation). In deer, this differentiation reached its maximum expression at 205 days (85% of gestation). Vivo et al. (1990), in cow, and Franco et al. (1992), in sheep, described this phenomenon at an earlier stage, at 83 days (30% and 55% of gestation, respectively), coinciding with the largest growth and development of the pillars. For this reason and in agreement with these authors, we believe that although the epithelial layer takes part in the formation of the pillars of the rumen, it is the development of the tunica muscularis that is responsible to a greater extent for this formation in the construction of the body of the pillar.
It is also from the pluripotential blastemic tissue, at 97 days, that the lamina propria and submucosa are differentiated. In sheep, Wardrop (1961) and Franco et al. (1992) placed it at 53 days of prenatal development (35% of gestation), and in cow Vivo et al. (1990) placed it at 50 days (18% of gestation).
During embryonic development in red deer, the connective tissue underwent regression in its growth due primarily to the expansion of the structures between which, internally, the epithelial layer and, externally, the tunica muscularis are situated. This has been previously been reported by Franco et al. (1992) during the prenatal life of sheep.
The serosa, meanwhile, showed continuity, in growth as well as in differentiation, as already detected in the undifferentiated outline phase. At 97 days an intense vascularization was apparent. This has been described in sheep, at 53 days (35% of gestation), by Wardrop (1961) and Franco et al. (1992).
Results indicate that the tegumentary mucosa of the deer rumen lacked secretory capacity during initial embryonic stages; from 67 days neutral mucopolysaccharides were detected. In sheep, Franco et al. (1992) placed this appearance at 89 days (60% of gestation). Mechanical protection of the first products of embryonic metabolism against aggressions is a function located in the stratum corneum. By contrast, in the same way as pointed out by Franco et al. (1992) in sheep, in deer there existed a gradually increasing relationship between neutral mucopolysaccharide content in the deeper epithelial layers and the gradual adaptation of the ruminal mucosa in its function as a chemical protector in postnatal life; it thus acts as a buffer system in the neutralization of the acid compounds produced during ruminal fermentation, and of the acid compounds incorporated in amniotic fluid, given that the ingestion of this is a normal process during gestation.
The presence of neuroendocrine cells in the ruminal mucosa of deer was not detected until 97 days. These cells were located in the lamina propria and submucosa, close to the epithelium, although not detected within it. Similar opinions were reported by Kitamura et al. (1986) in cow, and Groenewald (1994) in sheep. The glial cells were detected at 142 days for GFAP and at 67 days for VIM, demonstrating that VIM is a marker of more primitive glial cells, a fact reported in the prenatal development of the sheep pineal gland (Franco et al. 1997). The localization of the glial cells in the ruminal mucosa of deer during development involved the lamina propria, submucosa, tunica muscularis and serosa. Finally, the immunodetection of the neuropeptides VIP and NPY occurred in stages close to birth. The former were more abundant and always in the intercellular spaces of the epithelial layer, although on a few occasions we observed them around the vascular structures. None of the authors consulted have detected the presence of these neuropeptides in the epithelial layer, although they have been detected distributed around other tissue strata in the stomach of pigs (Timmermans et al. 1990); in the rumen and omasum of sheep (Vergara-Esteras et al. 1990; Groenewald, 1994; Yamamoto et al. 1994); and in the stomach of cow (Kitamura et al. 1986, 1993).
In the comparison with cow and sheep, deer were shown as being less precocious with regard to structural division, in their capacity to secrete neutral mucopolysaccharides, and in their neuroendocrine nature, as determined by the presence of positive NNE cells.
Fig. 17.
Photomicrograph of a section of the ruminal wall at 191 days.Presence of neuroendocrine cells (arrow,NNE)in myenteric plexus.EAS,×250.
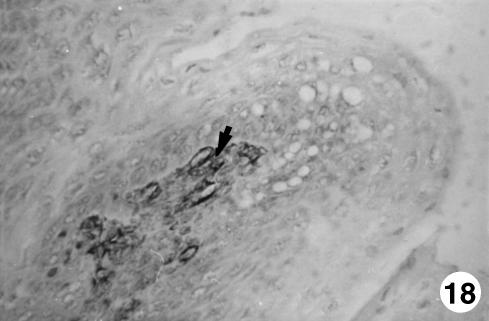
Fig. 18.
Photomicrograph of a section of the ruminal wall at 40 cm CRL.Presence of GFAP-positive cells (arrow)in the interior of the papillary body.EAS,×350.
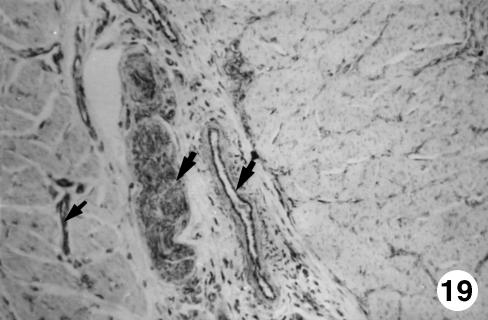
Fig. 19.
Photomicrograph of a section of the ruminal wall at 40 cm CRL.Presence of GFAP-positive cells (arrows)in the intervascular connective tissue,with perivascular disposition and in the interior of the myenteric plexus.EAS,×350.
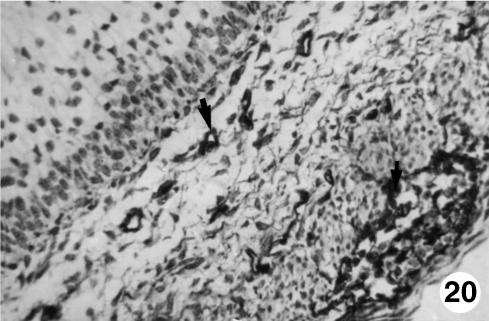
Fig. 20.
Photomicrograph of a section of the ruminal wall at 97 days.Slight presence of VIM-positive cells (arrows)occupying the lamina propria of the submucosa and in the interior of the myenteric plexus.EAS,×350.
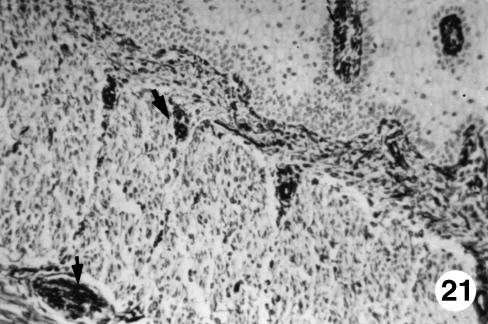
Fig. 21.
Photomicrograph of a section of the ruminal wall at 40 cm CRL.Abundant presence of VIM-positive cells (arrows)in the interior of the papillary body,intervascular connective tissue and in the myenteric plexus.EAS,×250.
Fig. 24.
Mathematical model of rumen growth (lamina propria and submucosa).
Fig. 26.
Mathematical model of rumen growth (serosa).
References
- Amasaki H, Daygo M. Prenatal development of subepitelial vasculature related to appearance of ruminal papillae in the cow rumen. Anat. Anzeiger. 1987;164:139–147. [PubMed] [Google Scholar]
- Arias JLR, Cabrera Valencia A. Observations on the histological development of the rumen papillae of cow. Morphological changes due to age. Anat. Histol. Embryol. 1978;7:140–151. doi: 10.1111/j.1439-0264.1978.tb00664.x. [DOI] [PubMed] [Google Scholar]
- Asari M, Fukaya K, Kano Y, Eguichi Y. Development of the fetus and neonatal bovine stomach. Anat. Histol. Embryol. 1981;10:264–274. doi: 10.1111/j.1439-0264.1981.tb00524.x. [DOI] [PubMed] [Google Scholar]
- Carranza J. Aplicaciones de la etología al manejo de las poblaciones de ciervo en el suroeste de la Península Ibérica: producción y conservación. Etología. 1999;7:5–18. [Google Scholar]
- Del Rio Ortega S. Desarrollo prenatal del estómago de la oveja. 1973. Doctoral thesis Facultad de Veterinaria, Zaragoza. [Google Scholar]
- Fath El-Bab MR, Schwart R, Ali AMA. Micromorphological studies on the stomach of sheep during prenatal life. Anat. Histol. Embryol. 1983;12:139–153. doi: 10.1111/j.1439-0264.1983.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Franco A, Regodon S, Robina A, Redondo E. Histomorphometric analysis of the rumen of the sheep during development. Am. J. Vet. Res. 1992;53:1209–1217. [PubMed] [Google Scholar]
- Franco A, Robina A, Regodon S, Vivo JM, Masot AJ, Redondo E. Histomorphometric analysis of the omasum of sheep during development. Am. J. Vet. Res. 1993a;54:1221–1229. [PubMed] [Google Scholar]
- Franco A, Robina A, Guillen MT, Mayoral AI, Redondo E. Histomorphometric analysis of the abomasum of sheep during development. Anat. Anzeiger. 1993b;175:119–125. doi: 10.1016/s0940-9602(11)80164-0. [DOI] [PubMed] [Google Scholar]
- Franco A, Robina A, Regodon S, Vivo JM, Masot AJ, Redondo E. Histomorphometric analysis of the reticulum of the sheep during development. Histol. Histopathol. 1993c;8:547–556. [PubMed] [Google Scholar]
- Franco A, Regodon S, Masot AJ, Redondo E. A combined immunohistochemical and electron microscopic study of the second cell type in the developing sheep pineal gland. J. Pineal Res. 1997;22:130–136. doi: 10.1111/j.1600-079x.1997.tb00314.x. [DOI] [PubMed] [Google Scholar]
- Groenewald HB. Neuropeptides in the myenteric ganglia and nerve fibres of the forestomach and abomasum of grey, white and black karakul lambs. Onderstepoort J. Vet. Res. 1994;61:207–213. [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase-and peptide-containing nerves in the reticulorumen and the reticular groove of the cattle. J. Compar. Neurol. 1986;248:223–234. doi: 10.1002/cne.902480205. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamamoto Y, Yamashita T. Substance P-immunoreactive neurons of the bovine forestomach mucosa: their presumptive role in a sensory mechanism. Arch. Histol. Cytol. 1993;56:399–410. doi: 10.1679/aohc.56.399. [DOI] [PubMed] [Google Scholar]
- Molinari E, Jorquera B. Intrauterine development stages of the gastric compartments of the goat Capra hircus. Anat. Histol. Embryol. 1988;17:121–137. doi: 10.1111/j.1439-0264.1988.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Osman AHR, Berg R. Studies on the histogenesis of the tunica mucosa of the stomach of the egyptian water buffalo (Bos bubbalis) 1. Histogenesis of the ruminal mucosa. Anat. Anzeiger. 1981;149:232–240. [PubMed] [Google Scholar]
- Panchamukhi BG, Srivastava HC. Histogenesis of the rumen of the buffalo (Bubalus bubalis) stomach. Anat. Histol. Embryol. 1979;8:97–105. doi: 10.1111/j.1439-0264.1979.tb00682.x. [DOI] [PubMed] [Google Scholar]
- Regodón S, Franco A, Masot AJ, Redondo E. Estudio ontogénico comparativo de la lámina epitelial de los compartimentos gástricos no glandulares en ovinos merinos. Anat. Histol. Embryol. 1996;25:233–241. doi: 10.1111/j.1439-0264.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Timmermans JP, Scheuermann DW, Stach W, Adriansen D, De Groot-Lasseel MHA. Distinct distribution of CGRP-, enkephalin-, galanin-, neuromedin U-, neuropeptide Y-, somastotatin-, substance P-, VIP- and serotonin-containing neurons in the two submucosal ganglionic neural netwoks of the porcine small intestine. Cell Tissue Res. 1990;260:367–379. doi: 10.1007/BF00318639. [DOI] [PubMed] [Google Scholar]
- Tiwari GP, Jamdar NM. Studies of the gross and histological structure and development on the forestomach of indian water buffalo calf in early postnatal life with reference to normal feeding. 2. Reticulum. Indian J. Anim. Sci. 1970;57:335–340. [Google Scholar]
- Vergara-Esteras P, Harrison FA, Brown D. The localization of somatostatin-like immunoreactivity in the alimentary tract of the sheep with observations of the effect of an infection with the parasite Haemonchus contortus. Exp. Physiol. 1990;75:779–789. doi: 10.1113/expphysiol.1990.sp003460. [DOI] [PubMed] [Google Scholar]
- Vivo JM, Robina A, Regodon S, Guillen MT, Franco A, Mayoral AI. Histogenetic evolution of bovine gastric compartments during prenatal period. Histol. Histopathol. 1990;5:461–476. [PubMed] [Google Scholar]
- Wardrop JD. Some preliminary observations on the histological development on the forestomach of the lamb. I. Histological changes due to age in the period from 46 days of foetal life to 77 days of posnatal life. J. Agric. Sci. 1961;57:335–341. [Google Scholar]
- Warner ED. The organogenesis and early histogenesis of the bovine stomach. Am. J. Anat. 1958;102:33–63. doi: 10.1002/aja.1001020103. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Kitamura N, Yamada J, Yamashita T. Immunohistochemical study of the distributions of the peptide and catechol-containing nerves in the omasum of the seep. Acta Anat. 1994;149:104–110. doi: 10.1159/000147564. [DOI] [PubMed] [Google Scholar]