Abstract
Deletion studies in transgenic mice indicate that the potassium inward rectifying channel Kir4.1 is crucial for oligodendrocyte differentiation and has a special role in regulation of extracellular potassium (K+), a major function of astrocytes. However, there are conflicting reports on whether Kir4.1 is expressed by white matter astrocytes and oligodendrocytes, raising doubts over its functions. Here, we have examined Kir4.1 expression in astrocytes and oligodendrocytes of the rat optic nerve, a typical central nervous system white matter tract. Single and double immunofluorescence labelling was performed on frozen sections from optic nerves aged postnatal day (P)5, 10, 15, 20 and adult, using anti-Kir4.1 antibodies and the glia-specific antibodies glial fibrillary acidic protein (GFAP, astrocytes), carbonic anhydrase II (CAII, oligodendrocyte somata and processes) and myelin basic protein (MBP, oligodendrocyte myelin sheaths). The results demonstrate Kir4.1 expression in rows of glial cells as early as P5, and this pattern persisted throughout development and into adulthood, consistent with early expression of Kir4.1 on developing oligodendrocytes. Clear co-expression of Kir4.1 and GFAP is first evident at P10 and increases to adult levels by P15 and P20, which correlates with the development of K+ regulation between P15 and P20. Astrocyte expression of Kir4.1 is localized to perivascular end-feet and fine processes within the fascicles of myelinated axons, consistent with a role in K+ spatial buffering between nodes of Ranvier and blood vessels. By contrast, Kir4.1 is concentrated in the cell bodies of oligodendrocytes, and there is no apparent co-expression with MBP+ myelin sheaths, suggesting oligodendroglial Kir4.1 channels are not involved in K+ regulation. The results support roles for Kir4.1 in both oligodendrocyte differentiation and K+ regulation by astrocytes.
Keywords: development, glia, immunohistochemistry, inward rectifying potassium channel, myelin
Introduction
Astrocytes and oligodendrocytes comprise the main glial cell types in the central nervous system (CNS). Oligodendrocytes are the myelin-forming cells of the CNS, whereas astrocytes have been assigned many functions, a key one being regulation of extracellular potassium ([K+]o) (Orkand et al. 1966; Newman et al. 1984). Neuronal activity causes an increase in [K+]o, which if uncorrected would result in the depolarization of neuronal membranes and the disruption of axonal conduction and synaptic transmission (Ransom & Orkand, 1996). Hertz (1965) first suggested that excess K+ may be cleared from the extracellular space by astrocytes, by a process later described as ‘spatial buffering’ (Orkand et al. 1966) or ‘siphoning’ (Newman et al. 1984; Newman, 1995). Uptake of extracellular K+ by glia occurs through a combination of active uptake, co-transport and through inward rectifying potassium channels (Kirs) (reviewed by Walz, 2000). Kirs are important for setting the resting membrane potential (RMP) of glia close to the equilibrium potential for K+, which provides an electrochemical gradient for driving K+ into glia without the expenditure of energy (Newman, 1995).
Kir4.1 has been proposed to be a specific glial Kir subunit (Takumi et al. 1995), suggesting it may have a special function in glial K+ regulation (reviewed by Horio, 2001; Neusch et al. 2003). Recent studies on Kir4.1 knockout mice have demonstrated a clear role for Kir4.1 in K+ siphoning in retinal Müller cells (Kofuji et al. 2000, 2002). In addition, deletion of Kir4.1 in mice leads to CNS hypomyelination (Neusch et al. 2001), indicating that the Kir4.1 channel subunit is crucial for normal oligodendrocyte maturation or myelination. However, immunohistochemical studies indicate that Kir4.1 expression may be restricted to subpopulations of astrocytes, in the deep cerebellar nuclei and hippocampus, but not astrocytes of white matter (Poopalasundaram et al. 2000; Higashi et al. 2001) or the retina (Kofuji et al. 2002). This is not consistent with a primary role for Kir4.1 in K+ regulation, which is one of the few established functions of astrocytes. Furthermore, the study of Higashi et al. (2001) did not detect expression of Kir4.1 by oligodendrocytes, which is incompatible with deletion studies showing Kir4.1 is crucial for oligodendrocyte differentiation (Neusch et al. 2001), and is in contradiction to the findings of Poopalasundaram et al. (2000) who found greater expression of Kir4.1 in oligodendrocytes than in astrocytes. A major aim of the present study therefore was to clarify whether astrocytes and oligodendrocytes express Kir4.1 in the rat optic nerve, a typical CNS white matter tract.
The concepts of spatial buffering and siphoning are that K+ is taken up into the glial syncytium at the site of neuronal activity and released at a site distal to the active neurons (Orkand et al. 1966; Newman et al. 1984). In the optic nerve, therefore, K+ regulation occurs between nodes of Ranvier, the sites of action potential propagation, and blood vessels. Furthermore, K+ regulatory mechanisms and nodes of Ranvier are not established in young nerves and develop after postnatal day (P)15 (Connors et al. 1982; Butt & Ransom, 1993; Rasband et al. 1999). Oligodendrocyte differentiation, by contrast, commences earlier, between P5 and P10 (Butt & Ransom, 1993). If Kir4.1 channels are important in K+ regulation and oligodendrocyte development, they should be expressed in the right place at the right time. To test this, we have examined the expression of Kir4.1 in the developing rat optic nerve, using the anti-Kir4.1 antibody developed by Wilkin and colleagues (Poopalasundaram et al. 2000). The present study shows Kir4.1 expression in both astrocytes and oligodendrocytes in the rat optic nerve, and that the spatiotemporal pattern of expression is consistent with separate roles in astroglial K+ regulation and oligodendrocyte differentiation.
Materials and methods
Animals and tissue
Wistar rats of both sexes were used, aged at P5, 10, 15, 20 and adult. Rats were humanely killed according to Home Office guidelines, either by lethal overdose of anaesthetic (Lethobarb, J.M. Loverage plc, Southampton, UK) administered by intraperitoneal injection, or by anaesthetic overdose followed by intracardiac perfusion of paraformaldehyde in phosphate-buffered saline (PBS).
Antibodies
As previously described (Poopalasundaram et al. 2000), the polyclonal rabbit anti-Kir4.1 antibody was raised against the C-terminal region of rat Kir4.1 using a synthetic peptide corresponding to amino acid residues at position 350–368; (Cys)-G-D-P-E-K-L-K-L-E-E-S-L-R-E-Q-A-E-K-E where the terminal cysteine was coupled to Imject® maleimide-activated keyhole limpet haemocyanin. The antibody was then affinity purified through a Kir4.1 peptide–Sepharose column. Antibody specificity was confirmed using Western blotting and immunolabelling of the rat cerebellum, which were as reported in our previous study (Poopalasundaram et al. 2000), and which were blocked by pre-incubation with the cognate peptide used to raise the antibody.
Rabbit anti-Kir4.1 antibody was used at a dilution of 1: 100. Other primary antibodies used were: mouse anti-glial fibrillary acidic protein (GFAP) antibody (1: 250; Sigma, Poole, Dorset, UK); mouse anti-myelin basic protein (MBP) antibody (1: 10; Serotec, Oxford, UK); and rabbit anti-carbonic anhydrase II (CAII) antiserum (1: 1500; prepared by Dr Norman Gregson, King's College London, UK). Secondary antibodies used were biotinylated goat anti-rabbit IgG (1: 400; Vector Laboratories, Peterborough, UK) and extravidin-conjugated FITC (1: 100; Sigma) (both for Kir 4.1), TRITC-conjugated anti-mouse IgG (GFAP and MBP) or TRITC-conjugated anti-rabbit IgG (CAII) (both 1: 100; Sigma). In addition, triple labelling used the blue Alexa Fluor® 350 goat anti-rabbit IgG (1: 500; Molecular Probes from Cambridge Bioscience).
Fixation protocols
Optimal preparation for Kir4.1 immunolabelling was in fresh frozen tissue post-fixed in methanol (Poopalasundaram et al. 2000). Freshly dissected optic nerves were slowly frozen by lowering into isopentane (BDH; 99% assay) at −40 °C. Optic nerves were mounted in OCT and sectioned at 10–20 µm. Sections were dried for 1 h on to slides coated with gelatin chrome alum and fixed for 2 min in methanol at −20 °C. Sections were then washed in PBS prior to immunolabelling. This protocol was used throughout, with the exception of CAII labelling, which required paraformaldehyde fixation (Butt & Kirvell, 1996). Perfusion fixed nerves were dissected free and post-fixed in 4% paraformaldehyde for 1 h, followed by cryprotection in a 30% sucrose solution overnight at 4 °C, prior to freezing and sectioning as above, but without fixation in methanol.
Immunohistochemistry
Sections were washed three times in PBS for 10 min; all subsequent washes were done in this manner unless otherwise stated. Antibodies were diluted in PBS containing 1% bovine serum albumin (BSA/PBS). Sections were blocked in 1.5% normal goat serum (NGS) for 1 h at room temperature before sections were incubated overnight at 4 °C with rabbit anti-Kir4.1 antibody (1: 100). Sections were washed in PBS and then incubated for 1 h at room temperature with biotinylated goat anti-rabbit IgG (1: 400; Vector Laboratories). Following PBS washes, Kir4.1 labelling was visualized using extravidin-conjugated FITC (used at a 1: 100 dilution) for 1 h at room temperature. In negative controls, the rabbit anti-Kir4.1 antibody was pre-incubated with the immunizing peptide for 30 min prior to incubation with optic nerve sections. In the case of single immunolabelling, tissues were then washed in PBS and mounted in Citifluor anti-fade reagent. For double immunolabelling, sections were incubated a second time in 1.5% NGS after labelling for Kir4.1 and prior to incubation in the second primary antibody, either rabbit anti-CAII, mouse anti-GFAP or mouse anti-MBP, overnight at 4 °C. In the case of CAII double immunolabelling, sections were fixed with 4% paraformaldehyde for 10 min and then washed in PBS (six times, 10-min washes), prior to incubation in the anti-CAII antiserum. In all cases, sections were washed in PBS and visualized using the appropriate TRITC-conjugated anti-mouse IgG (GFAP and MBP) or TRITC-conjugated anti-rabbit IgG (CAII). Triple immunofluorescence labelling was also performed, using Alexa Fluor® 350 goat anti-rabbit IgG (blue) to visualize CAII- and TRITC-conjugated anti-mouse IgG for MBP labelling. Finally, sections were mounted in Citifluor and viewed using an Olympus Provis Fluorescence Microscope and images captured using a Zeiss Axiophot digital camera and software.
Results
A number of controls were used to test the specificity of the anti-Kir4.1 antibody. The Kir4.1 immunolabelling of glial profiles (Fig. 1A) was blocked by pre-incubation of sections with the immunizing peptide (Fig. 1B), and there was no immunofluorescence in the absence of the secondary antibody (not illustrated). In addition, immunolabelling for Kir4.1 was the same in single labelled sections (Fig. 1) and when double labelled with GFAP (Figs 2 and 3), CAII (Fig. 4) or MBP (Fig. 5), indicating there was no cross-reaction between antibodies.
Fig. 1.
Developmental changes in Kir4.1 expression in the rat optic nerve. Anti-Kir4.1 antibodies immunolabelling of glia (A) is blocked when the antibody is pre-incubated with the immunizing peptide (B). Kir4.1 immunolabelling is concentrated within the somata of glia from P5 (A,C), through P10 (D), P15 (E,F), P20 (G) and into adulthood (H). Kir4.1-immunopositive glia are arrayed in rows along the length of the nerve (A,F,G,H) and in groups of two or more cells at the chiasm (C,D,E). The density of Kir4.1-immunoexpressing cells appears to increase between P5 (C) and P10 (D), and to decrease between P20 (G) and adult (H). Throughout development, Kir4.1-immunopositive glia are prominent between the immunonegative axon bundles. Scale bars: A,B,D,H = 50 µm; C = 25 µm; E = 100 µm; F,G = 30 µm.
Fig. 2.
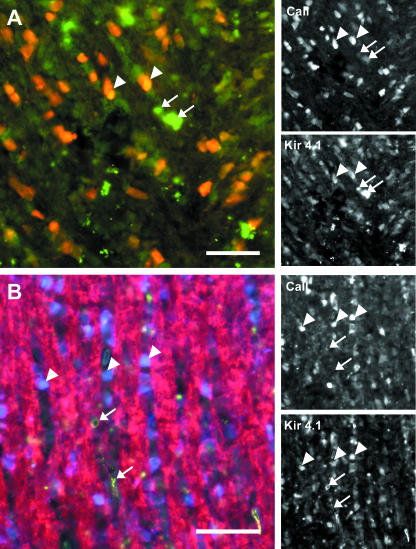
Developmental expression of Kir4.1 in astrocytes. Double immunofluorescence labelling for Kir4.1 (green) and GFAP (red), at P10 (A), P15 (B) and P20 (C,D). (A) In the P10 nerve, there was little co-labelling for GFAP and Kir4.1 (curved arrow), and GFAP+/Kir4.1+ astrocytes were rarely observed (arrow); co-expression appears yellow–orange. The majority of Kir4.1+ cells did not express GFAP (arrowhead). (B) In the P15 nerve, there was extensive co-labelling for GFAP and Kir4.1 (arrows), together with numerous GFAP−/Kir4.1+ profiles (arrowheads). (C) A GFAP+/Kir4.1+ astrocyte (arrow) within a row of GFAP−/Kir4.1+ presumptive oligodendrocytes (arrowhead) in the P20 nerve. (D) Stellate astrocytes at the optic chiasm, indicating most if not all astrocytes express Kir4.1. Scale bars: 30 µm (B,C); 50 µm (A,D).
Fig. 3.
Expression of Kir4.1 by astroglial perivascular and intrafascicular processes. Double immunofluorescence labelling for Kir4.1 (A,B, green) and GFAP (A,B red) or MBP (C, red) in sections of P20 optic nerve. (A) Kir4.1 immunolabelling is localized to perivascular end-feet of GFAP+ astrocytes (arrows), surrounding a capillary profile (BV); co-expression appears yellow–orange. (B) Kir4.1+/GFAP+ processes form multiple associations with GFAP−/Kir4.1+ cell bodies (arrowheads) and are evident within the fascicles of immunonegative axons (arrows). (C) Double immunofluorescence labelling for Kir4.1 and MBP illustrates Kir4.1+ processes running parallel and apposed to myelinated axons (arrows). Scale bars: 40 µm (A); 30 µm (B,C).
Fig. 4.
Expression of Kir4.1 by oligodendrocyte somata. Double (A) and triple (B) immunofluorescence labelling for Kir4, CAII and MBP, in sections of adult rat optic nerve. (A) CAII+ oligodendrocytes (red) are co-labelled with anti-Kir4.1 antibodies (green); co-expression appears yellow–orange (arrowheads). There was not perfect co-expression, and there are numerous Kir4.1+/CAII− cells (arrows), consistent with astroglial labelling demonstrated above. (B) There was co-expression of Kir4.1 (green) in the cell bodies of most if not all CAII+ oligodendrocytes (blue), but there was no evident co-labelling with MBP (red). Kir4.1+/CAII−/MBP− profiles and processes are also evident (arrows). Scale bars: 50 µm (A); 60 µm (B).
Fig. 5.
Lack of expression of Kir4.1 in myelin. Double immunofluorescence labelling of Kir4.1 (green) and MBP (red) in P15 (A) and P20 (B) nerves. There was no co-expression of Kir4.1 and MBP, and rows of interfascicular Kir4.1+/MBP− glial profiles (arrowheads) are clear between the Kir4.1−/MBP+ myelinated axon bundles. Scale bars: 100 µm.
Developmental expression of Kir4.1
Ontogenic changes in Kir4.1 expression were first analysed by single immunofluorescence labelling in optic nerve sections from P5, P10, P15, P20 and adult rats (n = 3–5 for each age group) (Fig. 1). At P5, Kir4.1 immunolabelling is concentrated within the somata of glia, which are arranged in rows along the length of the nerve (Fig. 1A) and in groups of two or more cells at the chiasm, where retinal ganglion cell axons decussate (Fig. 1C). This distribution of cells is characteristic of optic nerve oligodendrocytes, which are small rounded cells sited in rows of four or more cells, interspersed with larger solitary polygonal astrocytes (Butt & Kirvell, 1996). The frequency of Kir4.1-expressing cells is increased in the P10 nerve (Fig. 1D), and by P15 the interfascicular rows of Kir4.1-immunopositive glia are prominent between the immunonegative axon bundles (Fig. 1E). In the P15 nerve (Fig. 1F) and at P20 (Fig. 1G), there is labelling of fine processes within axon fascicles, in addition to labelling of glial rows. This was also evident in adult nerves (Fig. 1H), but there was an apparent decrease in the frequency of Kir4.1-labelled cells, consistent with the growth of the immunonegative fascicles of myelinated axons.
Expression of Kir4.1 by optic nerve astrocytes
In P5 or P10 nerves, Kir4.1+/GFAP+ astrocytes were rarely observed (arrow in Fig. 2A) within rows of Kir4.1+/GFAP− presumptive oligodendrocytes (arrowhead, Fig. 2A), but overall there was little apparent co-localization of GFAP and Kir4.1 at these ages (curved arrow, Fig. 2A). By P15 (Fig. 2B) and P20 (Fig. 2C,D), there was clear and extensive co-expression of Kir4.1 and GFAP, which persisted in adults. After P15, Kir4.1 immunolabelling was present on both the cell bodies and the processes of GFAP+ astrocytes (some indicated by arrows in Fig. 2B–D), along the length of the nerve (Fig. 2B), and at the chiasm (Fig. 2D), where astrocytes have a distinctive stellate morphology. There was not perfect co-localization of Kir4.1 and GFAP, and solitary astrocytes are sited within rows of three or more Kir4.1+/GFAP− cells (Fig. 2C, some indicated by arrowheads), shown below to be CAII+ oligodendrocytes (Fig. 4). The majority of GFAP+ processes appear to be Kir4.1+, although not all processes are labelled to the same intensity (Fig. 3). Kir4.1 immunolabelling appeared to be localized to processes terminating on blood vessels (Fig. 3A). In addition, Kir4.1+/GFAP+ processes (Fig. 3B, some indicated by arrows) are evident within the fascicles of Kir4.1−/MBP+ myelinated axons (Fig. 3C, some indicated by arrows) and between the rows of Kir4.1+/GFAP− glia (Fig. 3B, some indicated by arrowheads).
Expression of Kir4.1 by optic nerve oligodendrocytes
In optic nerves from P5 and P10 rats, the majority of Kir4.1+ cells observed in rows are presumed to be immature oligodendrocytes, because this is where oligodendrocytes are sited in adults (Fig. 4) and there was little evidence of co-expression of Kir4.1 with GFAP at these ages (Fig. 2A). However, this could not be confirmed because immature oligodendrocytes did not express CAII or MBP. Nonetheless, double immunofluorescence labelling with CAII, which is specific for differentiated oligodendrocytes in the optic nerve (Butt & Kirvell, 1996), confirmed that rows of oligodendrocytes express Kir4.1 in P15, P20 and adult nerves (Fig. 4, adult illustrated). It is noteworthy that throughout development Kir4.1 immunolabelling was greatest in oligodendrocyte somata. It appeared that all CAII+ oligodendrocytes co-expressed Kir4.1 (some indicated by arrowheads in Fig. 4A,B), but there were numerous Kir4.1+/CAII− cells (some indicated by arrows in Fig. 4A), and processes within myelinated axon bundles (some indicated by arrows in Fig. 4B); these are presumed to be astrocytes (see Fig. 3B,C). Double immunolabelling for Kir4.1 and MBP clearly illustrates the rows of Kir4.1-expressing glia between the fascicles of myelinated axons in the optic nerve chiasm at P15 (Fig. 5A) and P20 (Fig. 5B), and there was no apparent co-expression of Kir4.1 and MBP at any age studied.
Discussion
Deletion studies in mice have indicated a role for Kir4.1 in the regulation of extracellular K+ in the retina (Kofuji et al. 2000, 2002). In addition, a study in the Kir4.1 knockout mouse indicated the Kir4.1 channel subunit is essential for oligodendrocyte maturation and myelination (Neusch et al. 2001). The results of the present study demonstrate that astrocytes and oligodendrocytes express Kir4.1 in the rat optic nerve, a typical CNS white matter tract. We show that Kir4.1 expression in GFAP-positive astrocytes develops between P10 and P15, and provide evidence that CAII-negative oligodendrocytes express Kir4.1 as early as P5, which correlate, respectively, with the development of astroglial K+ regulatory mechanisms and oligodendrocyte differentiation (Connors et al. 1982; Butt & Ransom, 1993). To be directly involved in K+ uptake, expression of Kir4.1 should be present at nodes of Ranvier, the sites of action potential propagation. This is possibly the case in astrocytes but not in oligodendrocytes. Astroglial Kir4.1 channels are present on processes within myelinated axon bundles, which may subserve nodes of Ranvier (Butt et al. 1994), as well as on perivascular end-feet, which is consistent with a role in K+ spatial buffering or siphoning between these two sites. By contrast, oligodendrocytes did not appear to express Kir4.1 on paranodal myelin sheaths, but did on their cell bodies, which is not consistent with a role in K+ regulation. The results indicate that in the optic nerve Kir4.1 is in the right place at the right time to play a key role in K+ regulation by astrocytes and an unresolved role in oligodendrocyte differentiation.
Optic nerve astrocytes and oligodendrocytes express the Kir4.1 channel subunit
The pattern of Kir4.1 immunolabelling in optic nerves at all ages, and co-expression with CAII, MBP and GFAP after P15, indicates that both white matter oligodendrocytes and astrocytes express Kir4.1. Previous studies on Kir4.1 expression have been conflicting. Poopalasundaram et al. (2000) showed prominent labelling of grey matter astrocytes and oligodendrocytes of the rat cerebellum or cortex, but not in white matter astrocytes. In direct contrast, Higashi et al. (2001) found Kir4.1 immunolabelling in only a small fraction of astrocytes and none in oligodendrocytes. A further study by Li et al. (2001) localized Kir4.1 channel subunit expression on astrocytes in mouse cortical white matter, but again not on oligodendrocytes. It is difficult to reconcile the latter two reports with a study in Kir4.1 knockout mice, which shows that the Kir4.1 channel subunit is essential for oligodendrocyte development (Neusch et al. 2001). The present study demonstrates strong Kir4.1 expression in oligodendrocytes, which is consistent with the results of Poopalasundaram et al. (2000) and Neusch et al. (2001). The expression of Kir4.1 by optic nerve astrocytes demonstrated here contrasts with white matter astrocytes of the cerebellum or cortex (Poopalasundaram et al. 2000), which may indicate that astrocytes are heterogeneous with respect to Kir4.1 expression.
Differences between the findings of our study and that of Higashi et al. (2001) may be explained by differences in antibodies and fixation protocols. The anti-Kir4.1 antibody used in the present study recognizes the same peptide sequence as Poopalasundaram et al. (2000), using amino acid residues 350–368, whereas that of Higashi et al. (2001) was produced using a shorter sequence, from amino acid residues 366–378. In addition, the latter study used a fixation protocol of paraformaldehyde perfusion followed by a lengthy 48-h post-fixation in the same fixative (Higashi et al. 2001). In our experience, Kir4.1 immunolabelling is poor in paraformaldehyde-fixed tissue, and the optimal protocol is to use fresh frozen tissue with a brief methanol fixation following sectioning. Using methanol fixation, we found the same pattern of immunolabelling for Kir4.1 in the cerebellum as previously reported (Poopalasundaram et al. 2000), whereas labelling of oligodendrocytes and astrocytes in the cerebellum and optic nerve was compromised in paraformaldehyde-fixed tissue. Furthermore, the anti-Kir4.1 antibody labelling of optic nerve glia was blocked by pre-incubation of sections with the immunizing peptide, and Western blot analysis confirmed the antibody recognized a band at 42–45 kDa in P5, P10, P15, P20 and adult optic nerves and brain, and that this was also blocked by pre-incubation with the immunizing peptide. We are confident therefore that the Kir4.1 immunolabelling of astrocytes and oligodendrocytes in the optic nerve is a true representation of expression of the Kir4.1 potassium channel subunit.
Developmental changes in glial Kir4.1 expression
Expression of Kir4.1 was detected from P5, and expression appeared to increase between P10 and P15. There was little evident co-expression of Kir4.1 with GFAP In P5–P10 nerves, and the strongest labelling was in rows of glia. Strong astrocyte labelling for Kir4.1 was first observed in P15–P20 nerves, and expression was primarily on perivascular astroglial processes. There was an apparent decrease in the extent of Kir4.1 immunolabelling between P20 and adult, due to the growth of the myelinated axon fascicles driving apart the rows of Kir4.1+ glia. Nonetheless, Kir4.1 expression remained prominent on both astrocytes and the cell bodies of oligodendrocytes in the adult nerve, whereas there was no evident co-localization of Kir4.1 and MBP at any age studied. It is concluded that the CAII−/GFAP−/Kir4.1+ cells sited in rows in P5 nerves are immature oligodendrocytes, because oligodendrocytes but not astrocytes are located in rows of three or more cells in older nerves. The optic nerve at this age contains mainly immature CAII− oligodendrocytes, GFAP+ astrocytes and NG2+ cells (Butt & Ransom, 1993; Butt & Kirvell, 1996; Greenwood & Butt, 2003). It is not excluded that NG2+ cells express Kir4.1, but immunolabelling for NG2 and Kir4.1 requires markedly different fixation protocols and has not yet been successful. Nonetheless, it is reasonable to conclude that GFAP+ astrocytes do not express Kir4.1 before P10, and that the majority of Kir4.1+ cells sited in rows in the P5 nerve are immature oligodendrocytes. This indicates that Kir4.1 expression is spatiotemporally different in oligodendrocytes and astrocytes. Oligodendrocyte expression appears to increase between P5 and P10, the major period of oligodendrocyte maturation and the commencement of axon myelination in the optic nerve (Butt & Ransom, 1993). Astrocyte expression of Kir4.1 develops after P10 and correlates with their development of a more negative RMP and maturation of K+ regulatory mechanisms (Connors et al. 1982; Butt & Jennings, 1994). The developmental up-regulation of Kir4.1 in oligodendrocytes and then astrocytes is consistent with electrophysiological studies showing a relative shift from outwardly rectifying K+ channels to Kir during glial development (Barres et al. 1990; Berger et al. 1991; Bordey & Sontheimer, 1997; MacFarlane & Sontheimer, 2000).
Astrocyte Kir4.1 channel subunits are expressed in the right place at the right time to be important in K+ regulation
The expression of Kir4.1 on optic nerve astrocytes is consistent with a role for Kir 4.1 in the regulation of [K+]o. A single action potential causes a transient increase in [K+]o of approximately 1 mm (Frankenhauser & Hodgkin, 1956) and, if uncorrected, the K+ accumulation from repetitive action potentials would result in axonal depolarization and changes in excitability. One of the few recognized functions of astrocytes is to take up K+ at the site of activity, and redistribute it to areas of low K+ concentration, such as at blood vessels (Orkand et al. 1966; Newman et al. 1984; Newman, 1995). Optic nerve astrocytes extend perinodal processes to both nodes of Ranvier, the sites of action potential propagation, and blood vessels (Butt et al. 1994). The present study shows high Kir4.1 expression in perivascular end-feet and on processes within myelinated fascicles, some of which are likely to be perinodal processes. However, Kir4.1 was not concentrated at nodes of Ranvier, as it was at blood vessels. There is evidence that localization of Kir4.1 within glial cell membranes depends on laminin, which in the CNS is almost entirely restricted to the basement membranes of blood vessels (Ishii et al. 1997; Horio, 2001). The surface expression of Kir4.1 at nodes of Ranvier may therefore be prevented by the lack of laminin. This is equivalent to Kir4.1 expression in retinal Müller cells, in which strongest expression of Kir4.1 is in end-foot membranes facing the vitreous body and surrounding retinal blood vessels, whereas Kir2.1 is expressed at the site of neuronal activity (Kofuji et al. 2002). In Müller cells, K+ siphoning involves co-operation between Kir, with the strongly rectifying Kir2.1 being expressed where K+ enters the glial cell, and the weakly rectifying Kir4.1 being expressed where K+ leaves the cell (Kofuji et al. 2002). Kir2.1 has been shown to be concentrated in perinodal processes of Schwann cells (Mi et al. 1996), and it is possible that optic nerve astrocytes function in a similar way, whereby K+ released at nodes enters the glial synctium through perinodal Kir2.1 to be dispersed to blood vessels via Kir4. The amount of K+ released at nodes is directly related to axonal activity, and its redistribution and release by astroglial perivascular end-feet would provide a mechanism for coupling cerebral blood flow to neuronal activity (Newman et al. 1984). In addition, Kir4.1 is co-localized with AQP4 in Müller glia (Nagelhus et al. 1999), and may together play a role in regulating the extracellular volume, during neuronal activity and in oedema formation following blood–brain barrier disruption.
What is the function of Kir4.1 in oligodendrocytes?
Kir4.1 appears to be expressed on immature oligodendrocytes as early as P5, and there is subsequently a marked increase in the Kir4.1 expression at P10 and P15. The function of Kir in oligodendrocytes may be to set the RMP, which occurs during neonatal differentiation by the up-regulation of Kir (Barres et al. 1990; Berger et al. 1991; Knutson et al. 1997). Deletion of Kir4.1 blocks the differentiation of oligodendrocytes, which are chronically depolarized (Neusch et al. 2001). Oligodendroglial K+ channels and RMP determine proliferation and development of oligodendrocytes (Gallo et al. 1996; Knutson et al. 1997) and inactivation of Kir4.1 results in apoptosis at an early stage of differentiation (Neusch et al. 2001). The results of the present study support the proposition that Kir4.1 is the major K+ channel subunit in oligodendrocytes (Neusch et al. 2001), and therefore is the most important channel setting the oligodendrocyte RMP and controlling oligodendrocyte development. Deletion of Kir4.1 in knock-out mice resulted in hypomyelination (Neusch et al. 2001), and the persistent expression of Kir4.1 in mature oligodendrocytes (present study) indicates a possible role for Kir4.1 in maintaining oligodendrocyte and myelin integrity.
In conclusion, our findings place Kir4.1 in the right place at the right time for a key role in oligodendrocyte development and the redistribution of extracellular potassium following neuronal activity.
Acknowledgments
This research was upported by the BBSRC. K.G. received an Anatomical Society of Great Britain & Ireland PhD studentship. A.S.K. received a Wellcome Trust summer vacation scholarship. We would like to thank Dr Patrick Foran at Imperial College London for assistance in preparing the anti-Kir4.1 antibody.
References
- Barres BA, Koroshetz WJ, Swartz KJ, Chun LL, Corey DP. Ion channel expression by white matter glia: the O-2A glial progenitor cell. Neuron. 1990;4:507–524. doi: 10.1016/0896-6273(90)90109-s. [DOI] [PubMed] [Google Scholar]
- Berger T, Schnitzer J, Kettenmann H. Developmental changes in the membrane current pattern, K+ buffer capacity, and morphology of glial cells in the corpus callosum slice. J. Neurosci. 1991;11:3008–3024. doi: 10.1523/JNEUROSCI.11-10-03008.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey A, Sontheimer H. Postnatal development of ionic currents in rat hippocampal astrocytes in situ. J. Neurophysiol. 1997;78:461–477. doi: 10.1152/jn.1997.78.1.461. [DOI] [PubMed] [Google Scholar]
- Butt AM, Ransom BR. Morphology of astrocytes and oligodendrocytes during development in the intact rat optic nerve. J. Comp. Neurol. 1993;338:141–158. doi: 10.1002/cne.903380110. [DOI] [PubMed] [Google Scholar]
- Butt AM, Colquhoun K, Tutton M, Berry M. Three-dimensional morphology of astrocytes and oligodendrocytes in the intact mouse optic nerve. J. Neurocytol. 1994a;23:469–485. doi: 10.1007/BF01184071. [DOI] [PubMed] [Google Scholar]
- Butt AM, Duncan A, Berry M. Astrocyte associations with nodes of Ranvier: ultrastructural analysis of HRP-filled astrocytes in the mouse optic nerve. J. Neurocytol. 1994;23:486–499. doi: 10.1007/BF01184072. [DOI] [PubMed] [Google Scholar]
- Butt AM, Jennings J. The astrocyte response to gamma-aminobutyric acid attenuates with age in the rat optic nerve. Proc. R. Soc. Lond. B. Biol. Sci. 1994;258:9–15. doi: 10.1098/rspb.1994.0134. [DOI] [PubMed] [Google Scholar]
- Butt AM, Kirvell S. Glial cells in transected optic nerves of immature rats. II. An immunohistochemical study. J. Neurocytol. 1996;25:381–392. doi: 10.1007/BF02284809. [DOI] [PubMed] [Google Scholar]
- Connors BW, Ransom BR, Kunis DM, Gutnick MJ. Activity-dependent K+ accumulation in the developing rat optic nerve. Science. 1982;216:1341–1343. doi: 10.1126/science.7079771. [DOI] [PubMed] [Google Scholar]
- Frankenhauser B, Hodgkin AL. The after-effects of impulses in the giant nerve fibres of Loligo. J. Physiol. 1956;131:341–376. doi: 10.1113/jphysiol.1956.sp005467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo V, Zhou JM, McBain CJ, Wright P, Knutson PL, Armstrong RC. Oligodendrocyte progenitor cell proliferation and lineage progression are regulated by glutamate receptor-mediated K+ channel block. J. Neurosci. 1996;16:2659–2670. doi: 10.1523/JNEUROSCI.16-08-02659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood K, Butt AM. Evidence that NG2 glia are not conventional oligodendrocyte progenitors and do not depend on axons for their survival. MCN. 2003;23:544–558. doi: 10.1016/s1044-7431(03)00176-3. [DOI] [PubMed] [Google Scholar]
- Hertz L. Possible role of neuroglia: a potassium-mediated neuronal–neuroglial–neuronal impulse transmission system. Nature. 1965;206:1091–1094. doi: 10.1038/2061091a0. [DOI] [PubMed] [Google Scholar]
- Higashi K, Fujita A, Inanobe A, Tanemoto M, Doi K, Kubo T, Kurachi Y. An inwardly rectifying K+ channel, Kir4.1, expressed in astrocytes surrounds synapses and blood vessels in brain. Am. J. Physiol. Cell Physiol. 2001;281:C922–931. doi: 10.1152/ajpcell.2001.281.3.C922. [DOI] [PubMed] [Google Scholar]
- Horio Y. Potassium channels of glial cells: distribution and function. Jpn. J. Pharmacol. 2001;87:1–6. doi: 10.1254/jjp.87.1. [DOI] [PubMed] [Google Scholar]
- Ishii M, Horio Y, Tada Y, Hibino H, Inanobe A, Ito M, et al. Expression and clustered distribution of an inwardly rectifying potassium channel, KAB-2/Kir4.1, on mammalian retinal Müller cell membrane: their regulation by insulin and laminin signals. J. Neurosci. 1997;17:7725–7735. doi: 10.1523/JNEUROSCI.17-20-07725.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson P, Ghiani CA, Zhou J-M, Gallo V, McBain CJ. K+ channel expression and cell proliferation are regulated by intracellular sodium and membrane depolarization in oligodendrocyte progenitor cells. J. Neurosci. 1997;17:2669–2682. doi: 10.1523/JNEUROSCI.17-08-02669.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Ceelen P, Zahs KR, Surbeck LW, Lester HA, Newman EA. Genetic inactivation of an inwardly rectifying potassium channel (Kir4.1 subunit) in mice: phenotypic impact in retina. J. Neurosci. 2000;20:5733–5740. doi: 10.1523/JNEUROSCI.20-15-05733.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P, Biedermann B, Siddharthan V, Raap M, Iandiev I, Milenkovic I, et al. Kir potassium channel subunit expression in retinal glial cells: implications for spatial potassium buffering. Glia. 2002;39:292–303. doi: 10.1002/glia.10112. [DOI] [PubMed] [Google Scholar]
- Li L, Head V, Timpe LC. Identification of an inward rectifier potassium channel gene expressed in mouse cortical astrocytes. Glia. 2001;33:57–71. doi: 10.1002/1098-1136(20010101)33:1<57::aid-glia1006>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Mi H, Deerinck TJ, Jones M, Ellisman MH, Schwarz TL. Inwardly rectifying K+ channels that may participate in K+ buffering are localized in microvilli of Schwann cells. J. Neurosci. 1996;16:2421–2429. doi: 10.1523/JNEUROSCI.16-08-02421.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, et al. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Müller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Neusch C, Rozengurt N, Jacobs RE, Lester HA, Kofuji P. Kir4.1 potassium channel subunit is crucial for oligodendrocyte development and in vivo myelination. J. Neurosci. 2001;21:5429–5438. doi: 10.1523/JNEUROSCI.21-15-05429.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neusch C, Weishaupt JH, Bahr M. Kir channels in the CNS: emerging new roles and implications for neurological diseases. Cell Tissue Res. 2003;311:131–138. doi: 10.1007/s00441-002-0669-x. [DOI] [PubMed] [Google Scholar]
- Newman E, Frambach D, Odette L. Control of extracellular potassium levels by retinal glial cell K+ siphoning. Science. 1984;225:1174–1175. doi: 10.1126/science.6474173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman EA. Glial cell regulation of extracellular potassium. In: Ketterman H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 1995. pp. 717–731. [Google Scholar]
- Orkand RK, Nicholls JG, Kuffler SW. The effect of nerve impulses on the membrane potential of glial cells in the central nervous system of amphibia. J. Neurophysiol. 1966;29:788–806. doi: 10.1152/jn.1966.29.4.788. [DOI] [PubMed] [Google Scholar]
- Poopalasundaram S, Knott C, Shamotienko OG, Foran PG, Dolly JO, Ghiani CA, et al. Glial heterogeneity in expression of the inwardly rectifying K+ channel, Kir4.1, in adult rat CNS. Glia. 2000;30:362–372. doi: 10.1002/(sici)1098-1136(200006)30:4<362::aid-glia50>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Orkand RK. Glial–neuronal interactions in non-synaptic areas of the brain: studies in the optic nerve. TINS. 1996;19:352–358. doi: 10.1016/0166-2236(96)10045-x. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Peles E, Trimmer JS, Levinson SR, Lux SE, Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi T, Ishii T, Horio Y, Morishige K-I, Takahashi N, Yamada M, et al. A novel ATP-dependant inward rectifier potassium channel expressed predominantly in glial cells. J. Biol. Chem. 1995;270:16339–16346. doi: 10.1074/jbc.270.27.16339. [DOI] [PubMed] [Google Scholar]
- Walz W. Role of astrocytes in the clearance of excess extracellular potassium. Neurochem. Int. 2000;36:291–300. doi: 10.1016/s0197-0186(99)00137-0. [DOI] [PubMed] [Google Scholar]