Abstract
In this study, we investigated the profile of integrin expression in human and porcine intervertebral disc tissue. Differences in extracellular matrix composition between anulus fibrosus (AF) and nucleus pulposus (NP) regions of the disc, as well as differences in cellular responses to environmental stimuli, suggest a role for integrins in presenting matrix signals that may mediate these responses. Human disc tissue and porcine AF and NP tissue were stained with antibodies to alpha integrin subunits 1–6, V and IIb, and beta integrin subunits 1–6 and graded for evidence of positive staining on a scale from 0 (no staining) to 3 (high incidence of staining). Human tissue expressed α and β integrin subunits shown to be present in articular cartilage, including α1, α5 and αV. Porcine AF tissue expressed similar integrin subunits to human disc, with both expressing α1, α5, β1, β3 and β5 subunits, whereas porcine NP tissue expressed higher levels of α6, β1 and β4 than AF tissue. The expressed subunits are known to interact with proteins including collagens, fibronectin and laminin; however, additional studies will be required to characterize the interactions of the integrin subunits with specific matrix constituents, as well as their specific involvement in regulating environmental stimuli.
Keywords: anulus fibrosus, cell–matrix interaction, human, nucleus pulposus, porcine
Introduction
Integrins are a class of cell adhesion molecules that regulate interactions between a cell and its surrounding matrix. One alpha (α) and one beta (β) subunit associate non-covalently to form each functional integrin. To date, eight β and 18 α subunits are known to exist in humans, which are known to form only 24 distinct heterodimers. Integrins participate in signalling events in many cell types (Hynes, 2002), controlling gene expression (Hynes, 1992; Danen & Yamada, 2001; Takahashi et al. 2003), cell cycle (Gumbiner, 1996; Segat et al. 2002; Watt, 2002) and apoptosis (Bates et al. 1995; Matter & Ruoslahti, 2001; Lewis et al. 2002; Miranti & Brugge, 2002). Cells of cartilaginous tissues exist in an avascular and alymphatic environment, where they are surrounded by considerable amounts of extracellular matrix that provides important signals to mediate cell differentiation, survival and metabolism (Loeser, 2002). Loeser and co-workers have documented the presence of many α and β integrin subunits in articular cartilage, and demonstrated their importance in presenting matrix signals to chondrocytes from relevant proteins, including collagens and fibronectin (Loeser, 1993). Human articular chondrocytes may use α1β1, α2β1 and α10β1 integrins to interact with type II collagen, and α1β1 and α2β1 may also mediate adhesion to type VI collagen and matrilin-1 (Camper et al. 1998; Loeser et al. 2000). The α5β1 subunit is used ubiquitously by many cell types, including chondrocytes, to interact with fibronectin, along with the αvβ3 integrin, which may also be used for this interaction. Studies in chondrocytes have demonstrated the importance of matrix signals from fibronectin in regulating collagenase expression and in promoting cell survival (Forsyth et al. 2002; Loeser et al. 2003). Furthermore, studies have shown an up-regulation in integrin expression in situ in osteoarthritic cartilage compared with normal cartilage (Loeser et al. 1995), suggesting a role for integrins in regulating disease states in cartilage. Still other studies have shown that integrin expression changes as articular cartilage matures from fetal cartilage to adult cartilage, indicating a role for integrins in chondrocyte proliferation and differentiation (Holmvall et al. 1995; Salter et al. 2001). Little is known of integrin expression patterns in cartilaginous tissues other than articular cartilage, however, nor their functional role in mediating cell metabolism or cell cycle.
The intervertebral disc is composed of two distinct cartilaginous tissues, the anulus fibrosus (AF) and the nucleus pulposus (NP). The AF is considered to be fibrocartilaginous, and to consist primarily of layers, or lamellae, composed of highly orientated fibres of types I and II collagen (Schollmeier et al. 2000). The NP contains a higher content of type II collagen that is randomly orientated, with a much higher concentration and polydispersity of proteoglycans. Cells of the AF regions have been shown to orientate along the predominant collagen fibre directions of the lamella, and to form elongated processes that suggest a strong physical interaction with the extracellular matrix (Errington et al. 1998; Bruehlmann et al. 2002; Baer et al. 2003). Cells of the innermost AF and NP regions appear more rounded and may accumulate significant pericellular matrix regions containing fibronectin, type VI and II collagens, and proteoglycans (Roberts et al. 1991, 1994). It is noteworthy that cells of the AF and NP regions respond differently to environmental stimuli, with evidence that AF fibrochondrocytic cells change their gene expression in response to physical stimuli such as compression or osmotic pressure, whereas cells of the NP do not respond in a similar manner to these same stimuli (Lotz et al. 2001; Chen et al. 2002, 2003; MacLean et al. 2003; Boyd et al. 2004). Differences in the composition of extracellular matrix between AF and NP regions, as well as the evident differences in cellular responses to environmental stimuli, suggest a role for integrins in presenting matrix signals that mediate cellular responses in the intervertebral disc.
The objective of this study was to characterize the profile of integrin subunit expression in human and porcine tissues of the intervertebral disc. A subgroup of integrins were selected for study because of their known interactions with proteins expressed in fibrocartilage and hyaline cartilage, including types I, II (α1β1, α2β1), VI (α1β1) and XI collagens (α3, α6), and fibronectin (α3, α4, α5, αV). Results of this study reveal similarities in integrin expression between mature human and porcine AF tissues, as well as differences between porcine AF and NP tissues that may be key to understanding the extracellular matrix interactions that regulate their differential biological behaviours.
Materials and methods
Tissue isolation and sample preparation
Intervertebral disc tissues were obtained from three human patients (ages 42–50) undergoing surgery for transforaminal lumbar interbody fusion. One disc sample, largely encompassing the inner and outer anulus fibrosus, was obtained from lumbar level L4–5 or L5–S1 of each patient. All samples showed morphological evidence of moderate degeneration. Tissue specimens were washed in phosphate-buffered saline (PBS), equilibrated in 30% sucrose dissolved in PBS and then flash frozen in liquid nitrogen for cryosectioning. In the mature human disc, there is little demarcation between discrete zones of the fibrocartilaginous AF and NP tissues; thus, we undertook a study of skeletally immature porcine tissues in order to examine zonal differences in integrin expression. Intervertebral discs (n = 2 for each of three animals) were obtained from the lumbar region of skeletally immature porcine spines (3 months old, Nahunta Pork Outlet, Raleigh, NC, USA) and dissected into regions containing the AF and NP tissues. Tissue samples were immediately flash frozen in liquid nitrogen for cryosectioning.
Immunohistochemistry
Anti-human anti-integrin antibodies were purchased from Chemicon (Temecula, CA, USA) except anti-β1, which was purchased from Coulter Corporation (Miami, FL, USA). Seven-micrometre-thick sections for immunohistochemistry were fixed in 100% acetone. Sections were then washed in water, followed by PBS, before being incubated with 10% normal non-immune goat serum (Zymed, South San Francisco, CA, USA) for 2 h. Sections were then incubated with one of the following anti-integrin primary antibodies: rabbit polyclonal anti-α1 (AB1934), mouse monoclonal anti-α2 (MAB1950Z), anti-α3 (MAB1952Z), anti-α4 (MAB16983Z), rabbit polyclonal anti-α5 (AB1928), mouse monoclonal anti-α6 (CD149f), rat monoclonal anti-αV (MAB1378), mouse monoclonal anti-αIIb (MAB1990), mouse monoclonal anti-β1 (4B4), anti-β2 (MAB1414), rabbit polyclonal anti-β3 (AB1932), anti-β4 (AB1922), anti-β5 (AB1926), or mouse monoclonal anti-β6 (MAB2076Z). Antibodies against the α1, α5 and β3 integrin subunits are known to cross-react with porcine tissues, whereas all other antibodies cross-reacted with porcine NP, AF or porcine meniscus tissue in preliminary screening. Control sections were incubated with an equal volume of non-immune goat serum. Following overnight incubation at 4 °C, sections were washed three times in PBS and then incubated with appropriate fluorescent secondary antibodies (Alexa Fluor® 488, Molecular Probes, Eugene, OR, USA) for 1 h at room temperature. Sections were then mounted with GVA mounting medium (Zymed) and immediately imaged on an inverted fluorescence microscope (Zeiss Axiovert S100, Thornwood, NY, USA).
Analysis
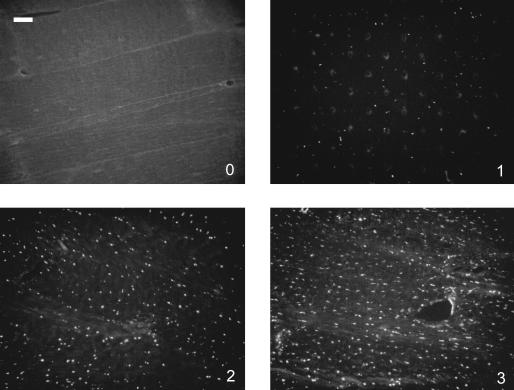
Each human disc, or porcine AF or NP, was treated as one sample. Two sections from each sample were analysed. From each section, five representative images were taken for each integrin subunit. NP cells from young discs were observed to exist in large clusters, often with membrane–membrane contacts, as has been previously described (Trout et al. 1982; Baer et al. 2003; Hunter et al. 2003), making it difficult to determine cell boundaries, where integrin staining was expected to be seen. These observations were consistent with the pattern of integrin staining observed in the NP, which often followed the morphology of the entire cell cluster, rather then individual cells. Although it may have been possible to ascertain individual cell staining in the AF sections, the staining pattern observed in the NP made it difficult to ascertain staining for individual cells in this region. Because it was our aim to compare levels of integrin expression between AF and NP tissues, all sections were graded semi-quantitatively on a scale according to intensity of staining, as 0 (no staining), 1 (low incidence of staining), 2 (moderate incidence of staining) or 3 (high incidence of staining) (Fig. 1). Some background staining was observed in human and porcine AF sections due to collagen autofluorescence; however, images containing background staining were not graded based on background fluorescence, but rather on the number and intensity of punctate staining, as would be expected to be associated with integrins. A mean score was calculated for each integrin subunit based on all scores from all samples. Scores are reported as mean score ± standard deviation.
Fig. 1.
Images of porcine AF sections stained for integrin subunits demonstrating the grading scale: (A) no staining, (B) low incidence of staining, (C) moderate incidence of staining, (D) high incidence of staining. Scale bar = 100 µm.
Results
Mean scores and standard deviations for each integrin subunit for each tissue are given in Table 1. The most highly expressed integrins in human tissue sections (moderate to high incidence of staining) were α5 and αV (Table 1a), and β1, β3, β5 and β6 subunits (Table 1b). A consistent incidence of staining was also observed for α1, β1, β3 and β6 subunits (low to moderate staining). A similar staining profile was observed in porcine AF tissue sections, except for the absence of αv and β6 subunits in the porcine tissue, and a higher incidence of staining for α1, α6 and β4 subunits. The expression of subunits differed for the porcine NP tissue as compared with either human or porcine AF tissues, with a higher incidence of α6, β1 and β4 subunits. Notably, there was a moderate to high incidence of staining for the α6 subunit in the NP tissue, expressed at low levels or completely absent in human or porcine AF tissues (Table 1a).
Table 1.
Mean incidence of staining for (a) alpha subunits and (b) beta subunits in human disc, porcine NP and porcine AF tissues. Data are presented as mean ± SD
| (a) | α1 | α2 | α3 | α4 | α5 | α6 | αV | αIIb |
|---|---|---|---|---|---|---|---|---|
| Human disc | 0.8 ± 1.0 † | 0 ± 0 * | 0 ± 0 * | 0 ± 0* | 1.7 ± 0.6 ‡ | 0 ± 0 * | 1.7 ± 0.5 ‡ | 0 ± 0 * |
| Porcine AF | 2.5 ± 0.7 ‡ | 0 ± 0 * | 0.2 ± 0.4 * | 0.1 ± 0.3 * | 2.1 ± 0.7 ‡ | 0.5 ± 0.7 † | 0.0 ± 0.2 * | 0.2 ± 0.4 * |
| Porcine NP | 2.6 ± 0.7 ‡ | 0.0 ± 0.6 * | 0.0 ± 1.0 * | 0.0 ± 0.2 * | 1.5 ± 0.6 ‡ | 2.2 ± 0.6 ‡ | 0.2 ± 0.4 * | 0.3 ± 0.5 * |
| (b) | β1 | β2 | β3 | β4 | β5 | β6 |
|---|---|---|---|---|---|---|
| Human disc | 1.2 ± 0.8 † | 0.2 ± 0.4 * | 1.5 ± 0.6 † | 0.3 ± 0.5 * | 3.0 ± 0.0 ‡ | 0.5 ± 0.6 * |
| Porcine AF | 1.2 ± 0.4 † | 0 ± 0 * | 1.8 ± 0.6 ‡ | 1.3 ± 0.7 † | 2.8 ± 0.4 ‡ | 0 ± 0 * |
| Porcine NP | 2.3 ± 0.9 ‡ | 0.5 ± 0.8 * | 1.7 ± 0.5 ‡ | 1.7 ± 0.5 ‡ | 2.6 ± 0.5 ‡ | 0 ± 0 * |
No to low incidence of staining (0–0.5);
low to moderate incidence of staining (0.5–1.5);
moderate to high incidence of staining (≥1.5).
Discussion
This study presents the first available data for the integrin expression profile of cells of the intervertebral disc. Although the data presented here are obtained from a small number of human discs (n = 3), tissue was examined from multiple regions (n = 5) and sections (n = 6). In addition, there was evidence of little or no variability in integrin expression among the tissue sections. Results for the porcine tissue samples, for which we had a larger sample number, showed no more or less variable findings. Because of this low variability and the descriptive nature of this study, data were deemed sufficient for the analyses presented.
The integrin expression profile of human disc tissues had some similarities to that documented for cartilage from normal human femoral heads (Ostergaard et al. 1998; Table 2). Similarities in integrin subunit expression between these two tissues may relate to similarities in the composition of their extracellular matrices. For example, subunits for the dimer α1β1 are expressed by both tissues, which is most commonly recognized as the type II collagen receptor for chondrocytes; however, α1β1 can also mediate adhesion to type VI collagen and to matrilin-1. Both tissues also express subunits for α5β1, which is the primary fibronectin receptor used by chondrocytes; however, αv, which has been shown to be present in human articular cartilage, was also expressed by human AF and may mediate interactions with fibronectin. Both tissues lacked expression of the α2 subunit in situ; however, it has recently been reported by that α2 is expressed by isolated articular chondrocytes and may play a role in adhesion to type II or VI collagen (Forsyth et al. 2002). Whereas chondrocytes have been shown to express α2, this expression has not been shown at the tissue level, and was also not detectable in human disc tissue in this study.
Table 2.
Mean incidence of staining for alpha and beta subunits in human disc and human articular cartilage
| α1 | α2 | α3 | α4 | α5 | α6 | αV | β1 | β2 | β3 | β4 | β5 | β6 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human disc | ++ | – | – | – | ++ | – | ++ | ++ | – | ++ | + | +++ | ++ |
| Articular cartilage* | +++ | – | – | – | ++ | – | +++ | ++ | – | – | – | ++† | – |
No staining (–), low incidence of staining (+), moderate incidence of staining (++), high incidence of staining (+++).
Data taken from Ostergaard et al. (1998); high Incidence of staining here represents those sections with a median score of 3–4 in Ostergaard et al.
Data for αvβ5.
Some differences are apparent between the human AF tissues studied here and the results for articular cartilage reported previously. Human AF tissue exhibited a higher incidence of staining for the β3, β4 and β6 subunits than human articular cartilage and a slightly lower incidence of staining for the αV subunit than articular cartilage.
Importantly, porcine AF tissue showed a similar staining profile to human AF tissues, with only a few notable exceptions. Incidences of staining for α5, β1, β3 and β5 subunits were similar for both human and porcine AF tissues; however, human tissue expressed α1 and β4 at lower levels than porcine AF tissue. Notably, the human AF tissue stained for αv and β6 subunits whereas the porcine AF did not, and the porcine AF tissue stained for the α6 subunit whereas the human tissue did not. Neither tissue expressed α2, α3, α4, αIIb or β2. Similarities between the human surgical specimens and porcine AF tissues were surprising owing to the significant differences in age between these specimens. Similarities in occurrence of the integrin subunits between human and porcine tissues do not imply similarities in function, however, as both integrin specificity and intensity have been found to differ with age and degeneration in human articular cartilage (Loeser et al. 1995). Nevertheless, these similarities reflect the origin of the human specimens from the fibrocartilaginous region of the intervertebral disc.
Differences in integrin expression profiles were also observed between AF and NP tissues in the skeletally immature disc tissues of the pig. Of particular interest is the consistent and moderate to high incidence of staining for the α6, β1 and β4 subunits in NP tissues, as compared with a low incidence of staining for α6, β1 and β4 subunits in the AF. The α6β1 and α6β4 integrins are classical laminin receptors, of which α6β1 is present in cartilage and may play a role in mediating chondrocyte interactions with laminin (Durr et al. 1996). The α6 subunit exists in two isoforms, α6a and α6b, and expression of both isoforms is required for mesenchymal cells to differentiate down the chondrogenic pathway, and express types II and X collagen (Segat et al. 2002). Thus it is possible that NP tissues from young porcine spines may be undergoing chondrogenesis, with the possibility that the expression of α6 may change with maturity. It is also noteworthy that NP cells may be notochordal and thus derived from the laminin-rich ectoderm where expression of α6 would be expected. Nevertheless, because of the dramatic differences in staining for this subunit in immature disc tissue, expression of the α6 integrin may have an important role in contributing to differences seen in the biological responses to environmental stimuli between the NP and AF.
Although this study presents a profile of integrin subunit expression in tissues of the intervertebral disc, neither the functional state of the integrins nor their formation of heterodimers was studied. Both α and β subunits contribute to ligand specificity, so that substituting for either subunit, along with effects of alternative splicing, will affect the nature of the interaction between the integrin heterodimer and its ligand. For this reason, together with the fact that integrins can bind to more than one ligand and that ligands can recognize more than one integrin, the diversity and complexity of integrin–ligand interactions and downstream signalling requires knowledge far beyond the expression of individual integrin subunits (Hynes, 1992). Once functionality is confirmed, additional studies will be important for assessing how functional integrin expression contributes to zonal variations in the interverterbal disc cell response to environmental stimuli.
Acknowledgments
This work was supported by NIH (1R01AR47442) and an NSF Graduate Student Fellowship. We would also like to thank Dr Richard F. Loeser for many helpful discussions.
References
- Baer AE, Laursen TA, Guilak F, Setton LA. The micromechanical environment of intervertebral disc cells determined by a finite deformation, anisotropic, and biphasic finite element model. J. Biomech. Eng. 2003;125:1–11. doi: 10.1115/1.1532790. [DOI] [PubMed] [Google Scholar]
- Bates RC, Lincz LF, Burns GF. Involvement of integrins in cell survival. Cancer Metastasis Rev. 1995;14:191–203. doi: 10.1007/BF00690291. [DOI] [PubMed] [Google Scholar]
- Boyd LM, Chen J, Setton LA. Conditioned medium differentially regulates matrix protein gene expression in cells of the intervertebral disc. Spine in press. 2004 doi: 10.1097/01.brs.0000142747.90488.1d. [DOI] [PubMed] [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J. Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper L, Hellman U, Lundergren-Akerlund E. Isolation, cloning, and sequence analysis of the integrin subunit alpha10, a beta1-associated collagen binding integrin expressed on chondrocytes. J. Biol. Chem. 1998;273:20383–20389. doi: 10.1074/jbc.273.32.20383. [DOI] [PubMed] [Google Scholar]
- Chen J, Baer AE, Paik PY, Yan W, Setton LA. Matrix protein gene expression in intervertebral disc cells subjected to altered osmolarity. Biochem. Biophys. Res. Commun. 2002;293:932–938. doi: 10.1016/S0006-291X(02)00314-5. [DOI] [PubMed] [Google Scholar]
- Chen J, Wei Y, Setton LA. Compression-induced changes in proteoglycan gene expression vary with zone of cell origin in the intervertebral disc. Trans. ORS. 2003;28:1132. [Google Scholar]
- Danen EHJ, Yamada KM. Fibronectin, integrins, and growth control. J. Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- Durr J, Lammi P, Goodman SL, Aigner T, von der Mark K. Identification and immunolocalization of laminin in cartilage. Exp. Cell Res. 1996;222:225–233. doi: 10.1006/excr.1996.0028. [DOI] [PubMed] [Google Scholar]
- Errington RJ, Puustjarvi K, White IR, Roberts S, Urban JP. Characterisation of cytoplasm-filled processes in cells of the intervertebral disc. J. Anat. 1998;192:369–378. doi: 10.1046/j.1469-7580.1998.19230369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth CB, Pulai J, Loeser RF. Fibronectin fragments and blocking antibodies to alpha2beta1 and alpha5beta1 integrins stimulate mitogen-activated protein kinase signaling and increase collagenase 3 (matrix metalloproteinase 13) production by human articular chondrocytes. Arthritis Rheum. 2002;46:2368–2376. doi: 10.1002/art.10502. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Holmvall H, Camper L, Johansson S, Kimura JH, Lundgren-Akerlund E. Chondrocyte and chondrosarcoma cell integrins with affinity for collagen type ii and their respose to mechanical stress. Exp. Cell Res. 1995;221:496–503. doi: 10.1006/excr.1995.1401. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J. Anat. 2003;202:279–291. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Lewis JM, Truong TN, Schwartz MA. Integrins regulate the apoptotic response to DNA damage through modulation of p53. Proc. Natl Acad. Sci. USA. 2002;99:3627–3632. doi: 10.1073/pnas.062698499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeser RF. Integrin-mediated attachment of articular chondrocytes to extracellular matrix proteins. Arthritis Rheum. 1993;36:1103–1110. doi: 10.1002/art.1780360811. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Carlson CS, McGee MP. Expression of b1 integrins by cultured articular chondrocytes and in osteoarthritic cartilage. Exp. Cell Res. 1995;217:248–257. doi: 10.1006/excr.1995.1084. [DOI] [PubMed] [Google Scholar]
- Loeser RF, Sadiev S, Tan L, Goldring MB. Integrin expression by primary and immortalized human chondrocytes: evidence of a differential role for a1b1 and a2b2 integrins in mediating chondrocyted adhesion to types ii and vi collagen. Osteoarthritis Cartilage. 2000;8:96–105. doi: 10.1053/joca.1999.0277. [DOI] [PubMed] [Google Scholar]
- Loeser RF. Integrins and cell signaling in chondrocytes. Biorheology. 2002;39:119–124. [PubMed] [Google Scholar]
- Loeser RF, Forsyth CB, Samarel AM, Im HJ. Fibronectin fragment activation of proline-rich tyrosine kinase pyk2 mediates integrin signals regulating collagenase-3 expression by human chondrocytes through a protein kinase c-dependent pathway. J. Biol. Chem. 2003;278:24577–24585. doi: 10.1074/jbc.M304530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotz JC, Hsieh AH, Walsh AL, Palmer EI, Chin JR. Mechanobiology of the intervertebral disc. Biochem. Soc. Trans. 2001;30:853–858. doi: 10.1042/bst0300853. [DOI] [PubMed] [Google Scholar]
- MacLean JJ, Lee CR, Ito K, Alini M, Iatridis J. Loading history and configuration affect intervertebral disc cell response to cyclic compression. Trans. ORS. 2003;28:1146–000. [Google Scholar]
- Matter ML, Ruoslahti E. A signaling pathway from the alpha5beta1 and alpha (v) beta3 integrins that elevates bcl-2 transcription. J. Biol. Chem. 2001;276:27757–27763. doi: 10.1074/jbc.M102014200. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Q. Nat. Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Ostergaard K, Salter DM, Petersen J, Bendtzen K, Hvolris J, Andersen CB. Expression of α and β subunits of the integrin superfamily in articular cartilage from macroscopically normal and osteoarthritic human femoral heads. Ann. Rheumatic Dis. 1998;57:303–308. doi: 10.1136/ard.57.5.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Ayad S, Menage PJ. Immunolocalisation of type vi collagen in the intervertebral disc. Ann. Rheum. Dis. 1991;50:787–791. doi: 10.1136/ard.50.11.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts S, Caterson B, Evans H, Eisenstein SM. Proteoglycan components of the intervertebral disc and cartilage endplate: an immunolocalization study of animal and human tissues. Histochem. J. 1994;26:402–411. doi: 10.1007/BF00160052. [DOI] [PubMed] [Google Scholar]
- Salter DM, Millward-Sadler SJ, Nuki G, Wright MO. Integrin-interleukin-4 mechanotransduction pathways in human chondrocytes. Clin. Orthop. 2001;39:49–60. doi: 10.1097/00003086-200110001-00006. [DOI] [PubMed] [Google Scholar]
- Schollmeier G, Lahr-Eigen R, Lewandrowski KU. Observations on fiber-forming collagens in the anulus fibrosus. Spine. 2000;25:2736–2741. doi: 10.1097/00007632-200011010-00004. [DOI] [PubMed] [Google Scholar]
- Segat D, Riccardo C, Di Marco E, Strangio A, Cancedda R, Franzi AT, et al. Integrins alpha 6a beta 1 and alpha 6b beta 1 promote different stages of chondrogenic cell differentiation. J. Biol. Chem. 2002;277:31612–31622. doi: 10.1074/jbc.M203471200. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Onodera K, Sasano Y, Mizoguchi I, Bae J-W, Mitani H, et al. Effect of stretching on gene expression of beta-1 integrin and focal adhesion kinase and on chondrogenesis through cell–extracellular matrix interactions. Eur. J. Cell Biol. 2003;82:182–192. doi: 10.1078/0171-9335-00307. [DOI] [PubMed] [Google Scholar]
- Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral-disk. 2. Cells of the nucleus pulposus. Anat. Rec. 1982;204:307–314. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- Watt FM. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 2002;21:3919–3926. doi: 10.1093/emboj/cdf399. [DOI] [PMC free article] [PubMed] [Google Scholar]