Abstract
This study set out to determine the pattern of development and distribution of the interstitial cells of Cajal (ICC) in the intestinal tract of the equine fetus and neonate. Intestinal tissue samples from 12 naturally aborted equine fetuses and three euthanized neonates were collected and fixed in formalin prior to applying standard immunohistochemical labelling techniques targeting the c-Kit protein of the ICC. At 6 months of gestation, a network of ICC was present in the myenteric plexus region of both the small and the large intestine. ICC were also present within the circular muscle layer. In the large intestine, a proximal to distal gradient of distribution was evident, with few ICC observed in the more distal parts of the large intestine in the younger fetuses compared with the near-term animals. A transmural gradient of distribution was also evident within the large intestine, with the most luminal part of the muscularis externa being the last area to be colonized by ICC. This region did not appear fully developed until the early neonatal period. An increased density of ICC was noted throughout the large intestine in the regions of the taenial bands in all animals. This study is the first to describe ICC development and distribution in the equine fetus and neonate.
Keywords: c-Kit, foal, horse, immunohistochemistry, intestinal smooth muscle
Introduction
The interstitial cells of Cajal (ICC) generate pacemaker activity throughout the gastrointestinal tract by initiating the slow wave activity that is integral to the coordination of gastrointestinal motility (Thuneberg, 1982; Hara et al. 1986; Ward et al. 1991; Sanders, 1996). In addition, ICC are thought to be involved in mediating neurotransmission and facilitating active propagation of electrical events (Thuneberg, 1982; Daniel & Posey-Daniel, 1984; Sanders et al. 1990; Daniel et al. 1998; Wang et al. 1999, 2000). It has been proposed that this wide range of functions is carried out by separate classes of ICC (Sanders, 1996). Recently, ICC have also been identified in the horse (Hudson et al. 1999).
As no previous work has been undertaken to investigate the normal development of ICC in the horse, all current knowledge is based on comparative studies primarily in the mouse, rat and chicken (Torihashi et al. 1995, 1997; Lecoin et al. 1996; Young et al. 1996; Kluppel et al. 1998). There have also been a number of human clinical studies demonstrating that ICC, or precursors of ICC, start to colonize the intestinal tract at an early stage of fetal development (Kenny et al. 1998a, b,1999; Wester et al. 1999). Generally, this seems to occur about one-third to half way through gestation, with some variation between species (Lecoin et al. 1996; Young et al. 1996; Torihashi et al. 1997; Kenny et al. 1999; Wester et al. 1999).
ICC express the proto-oncogene c-kit, which encodes c-Kit, a receptor tyrosine kinase (Ward et al. 1994; Huizinga et al. 1995; Torihashi et al. 1995). The c-Kit protein can be used as a marker for these cells using standard immunohistochemical labelling techniques (Sanders, 1996). Prior to the use of c-Kit immunohistochemistry, developmental studies of ICC were difficult to perform because definitive identification required ultrastructural analysis (Faussone-Pellegrini, 1984, 1987; Faussone-Pellegrini et al. 1985). Furthermore, immature ICC possess few of the ultrastructural features associated with mature ICC, making ultrastructural identification difficult (Faussone-Pellegrini et al. 1996). The use of c-Kit as a marker for ICC not only enabled easier identification of the cells but also allowed earlier detection during their development (Lecoin et al. 1996; Young et al. 1996; Torihashi et al. 1997).
In support of a pacemaker-generating function of the ICC, it has been shown that the onset of electrical rhythmicity and slow wave activity occurs after the development of ICC networks, although there appears to be anatomical and interspecies variation regarding the exact timing of slow wave onset (Torihashi et al. 1997; Ward et al. 1997). An oral to anal temporal gradient in the functional development of ICC in the mouse has been reported in one study (Ward et al. 1997).
Other studies have demonstrated that concomitant with abnormal ICC morphology, numbers or function, there is a corresponding lack of normal electrical patterns in the intestinal tract, providing further evidence for the role of ICC as pacemaker cells (Ward et al. 1994; Huizinga et al. 1995; Torihashi et al. 1995). The abnormal development of ICC has been implicated in a number of motility disorders in the human neonate, including hypertrophic pyloric stenosis (Langer et al. 1995), anorectal malformations (Kenny et al. 1998a) and transient neonatal intestinal pseudo-obstruction (Kenny et al. 1998b; Yamataka et al. 1998).
There are several well-recognized gastrointestinal disturbances observed in the equine neonate, including meconium impaction (Palmer, 1985), intestinal malformations and intussusceptions (Greet, 1992). Other disturbances include abnormal motility patterns and ileus typically observed in premature and dysmature foals (Wilson & Cudd, 1990) as well as in foals with hypoxic ischaemic insults in the immediate peripartum period (Vaala, 1994).
The purpose of this study was to determine the pattern of development and distribution of ICC in the normal equine fetus and neonate.
Materials and methods
Animals, sampling and processing
Surplus intestinal tissue samples from 22 naturally aborted equine fetuses were collected during routine abortion investigations. Two fetuses were examined as part of a post-mortem investigation associated with the death of the mare. The ages of the fetuses ranged from 6 to 11 months of gestation. A wide range in gestational length has been described in Thoroughbreds (320–360 days, mean 340 days) and ponies (315–350 days, mean 333 days) (Rossdale et al. 1991; Rossdale, 1993). Two fetuses included in this study were aborted from pony mares, and the remaining animals were all Thoroughbreds. As there was sometimes a delay before the fetus was presented for post-mortem examination, ten of the 22 fetuses had to be excluded from the study owing to the degree of tissue autolysis (see below).
In addition, surplus intestinal tissue samples were collected from three euthanized foals aged 4, 7 and 8 weeks postpartum, following the routine post-mortem investigations of these animals. One foal had contracted Tyzzer's disease (a bacterial hepatitis), one had an intestinal volvulus of the proximal small intestine (at a site distant to the collected tissue), and the third foal was euthanized due to severe orthopaedic disease.
Samples of intestine were collected from all animals at anatomically defined sites. From the small intestine, this was ileum (at a position level with the midpoint of the ileocaecal fold); from the large intestine, this included caecal base, pelvic flexure apex (located at the junction between the left ventral colon and left dorsal colon), and the distal third of the small colon (equivalent to the descending colon in humans).
Following collection, all samples were immediately placed in 10% phosphate-buffered formalin and fixed for at least 24 h. Tissue processing consisted of rinsing the tissue samples in running tap water for 1 h prior to soaking in graded sucrose solutions (10 and 30% sucrose in phosphate-buffered saline, PBS), in order to cryoprotect the samples. The samples were frozen rapidly in isopentane precooled in liquid nitrogen and subsequently cut at thicknesses of 10 µm and 20 µm. All sections were mounted on Tespa-coated (3-aminopropyltriethoxysilane) (Sigma, Poole, UK) slides and allowed to air-dry overnight. After washing the sections in PBS, sections were incubated for 30 min in 0.3% hydrogen peroxide in methanol to quench endogenous peroxidase activity. Sections were then incubated for 1 h in 1% goat serum (Vector Laboratories, Burlingame, CA, USA) to block non-specific antibody binding. After subsequent washes with PBS, the sections were incubated overnight at 4 °C in humid chambers in a rabbit-raised polyclonal antiserum to c-Kit (Ab-1) (Oncogene Research Products, Cambridge, MA, USA) at a concentration of 1 µg mL−1. Further washing in PBS was followed by a 1-h incubation with biotin-conjugated goat anti-rabbit immunoglobulin (Vector Laboratories) at a dilution of 1 : 200. Immunoreactivity was revealed using the avidin–biotin method (ABC) (Vectastain Elite ABC Kit, Vector Laboratories) using a diaminobenzidine substrate (DAB) (BDH Laboratory Supplies, Poole, UK). Sections were dehydrated in ethanol, cleared in xylene and then mounted in Depex (Merck, Glasgow, UK).
For all batches processed, both negative and positive controls were included. In negative controls, the primary antibody was replaced with normal rabbit serum and resulted in complete absence of immunolabelling. Tissue samples from a normal control animal from a previous study that had been shown to have an abundance of c-Kit immunoreactivity were used as positive controls.
ICC were differentiated from round c-Kit-immunoreactive mast cells in the submucosa by their morphology (e.g. presence of cellular processes in ICC), or by staining parallel sections with toluidine blue to demonstrate mast cell metachromasia (Galli et al. 1993; Hudson et al. 1999).
In addition to immunohistochemical labelling, all processed tissues were stained with haematoxylin and eosin in order to assess tissue integrity. This was carried out to help clarify whether reduced or absent c-Kit immunoreactivity was a primary finding or secondary to a degree of tissue autolysis. Only samples with good tissue integrity (and not worse than a mild degree of mucosal degradation) were included in order to ensure that immunohistochemical grading was reliable. Ten fetuses were rejected from the study on this basis. For analysis, the 12 fetuses and three foals were grouped together in different age groups as described in Table 1.
Table 1.
Age groups of fetuses and foals
| Fetal/foal age | F6–F7 | F8–F9 | F10–F11 | Full term | 4–8 weeks postpartum |
|---|---|---|---|---|---|
| No. of animals | 2 | 4 | 4 | 2 | 3 |
Key: F6–7, 6th and 7th month of fetal development, etc.
Results
In the small intestine (ileum) of all the fetuses examined, c-Kit-immunoreactive ICC were identified in the myenteric plexus region displaying a mixture of bipolar- and stellate-shaped morphologies. Individual ICC could be identified in the youngest fetuses but, in contrast, in the older fetuses and foals, the increasingly dense ICC network that developed surrounding the myenteric ganglia made it more difficult to identify individual cells (Figs 1 and 2). There was a limited and inconsistent degree of branching of ICC from the myenteric plexus region into the longitudinal muscle layer in the fetuses. However, this branching became more prominent and consistent in the neonates (Fig. 2).
Fig. 1.
c-Kit immunoreactivity in the ileum of the small intestine in three fetuses, at 6 months (A), 7 months (B) and 10 months (C) of gestation. Individual c-Kit-immunoreactive ICC can be observed in both the myenteric plexus region and the circular muscle layer (arrows). LM, longitudinal muscle layer; CM, circular muscle layer; MG, myenteric ganglia.
Fig. 2.
c-Kit immunoreactivity in the ileum of the small intestine in a 2-month-old foal. A dense network of ICC surrounds the myenteric ganglia with branching of ICC from the myenteric plexus region into the longitudinal muscle layer (arrows). LM, longitudinal muscle layer; CM, circular muscle layer.
Interstitial cells were also observed in the small intestinal circular muscular layer (Figs 1 and 2) in all samples. In the youngest fetus (6 months of gestation), few cells were observed within this muscle layer compared with the older fetus and neonate (Figs 1 and 2). The majority of ICC in the circular muscle of the ileum were bipolar in shape, although in the older fetus there appeared to be a slight increase in the proportion of stellate-shaped cells. The orientation of the long axis of ICC was parallel to the circular muscle fibres.
In the large intestine (caecum, pelvic flexure and small colon), both bipolar- and stellate-shaped c-Kit-immunoreactive ICC were present in the myenteric plexus region, with no predominance of either morphology. The ICC did not form the dense networks observed in the small intestine. The distribution and density of ICC in this region did not appear to change significantly during fetal development or indeed into the neonatal period (see Fig. 6).
Fig. 6.
Schematic illustration of the proximal to distal developmental distributions of ICC in the myenteric plexus region and the circular muscle layer of the equine large intestine. The age-related changes in the transmural distribution of ICC within the circular muscle layer are also illustrated.
Bipolar- and stellate-shaped ICC were also observed in the circular muscle layer of the muscularis externa of the large intestine in all samples (Fig. 3). However, in contrast to the ileum, there was no clear predominance of either morphological type. A proximal to distal gradient of ICC distribution was detected, with more ICC present in the proximal large intestine than in the distal large intestine. This gradient was most evident in the youngest fetuses (6–8 months of gestation) but became less distinct as the fetuses developed (Fig. 4). A summary of this proximal to distal gradient of distribution is illustrated later in Fig. 6.
Fig. 3.
Bipolar-shaped (b) and stellate-shaped (s) c-Kit-immunoreactive ICC in the large intestine of a fetus at 6 months of gestation. The ICC shown are located in the circular smooth muscle layer of the pelvic flexure and are typical of those seen elsewhere in the small and large intestines of the horse.
Fig. 4.
c-Kit immunoreactivity in the large intestine of the fetus.
(A) Caecal base at 7 months of gestation; (B) distal small colon at 7 months of gestation (same fetus as shown in A); (C) distal small colon at 10 months of gestation. OCM, outer region of circular muscle layer; ICM, inner region of circular muscle layer.
In addition, a transmural gradient of ICC distribution was present in the circular muscle layer of the large intestine. In the youngest fetuses, the majority of ICC in the most proximal part of the large intestine (caecum) were seen in the outer (more serosal) two-thirds of this muscle layer, with the innermost region having a lower ICC density (Fig. 4A). However, at the same stage of gestation, in the most caudal part of the large intestine, the distal small colon, very few ICC were observed in any part of the circular muscle layer (Fig. 4B). In the developing fetus, the transmural distribution observed in the pelvic flexure (a site approximately midway between the caecum and distal small colon) was more limited than in the caecum but more extensive than in the distal small colon. In late gestation, an area of low ICC density at the innermost part of the circular muscle layer was still evident in the most caudal part of the large intestine (Fig. 4C). The transmural gradient of ICC distribution was no longer apparent in the neonatal animals (Fig. 5). A schematic representation of the transmural gradient is shown in Fig. 6.
Fig. 5.
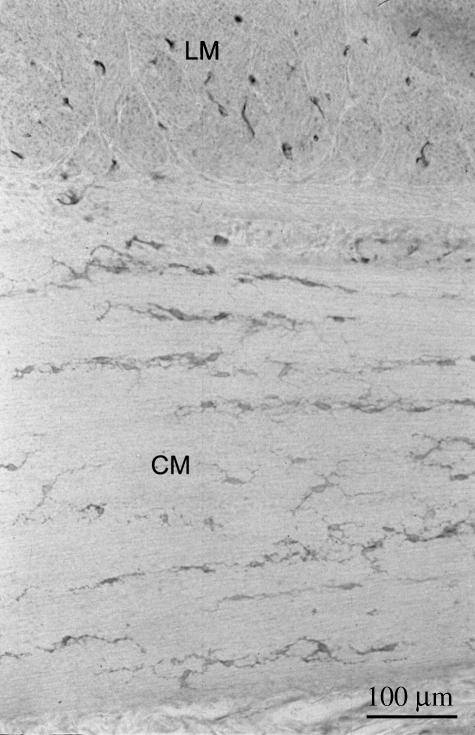
c-Kit immunoreactivity in the distal small colon of a 2-month-old foal. The transmural gradient of ICC distribution seen in the developing fetus is no longer apparent and there is now a uniform distribution of ICC throughout the circular muscle layer. LM, longitudinal muscle layer; CM, circular muscle layer.
In the areas where taenial bands were located, ICC density was consistently higher, both in the myenteric plexus region and in the circular muscle layer than in areas away from the taenial band (Fig. 7). This was found to be the case in both the developing fetus and the foals, irrespective of age and anatomical location.
Fig. 7.
c-Kit-immunoreactivity in the distal small colon of a fetus at 7 months of gestation. (A) Taenial band region. Numerous ICC can be observed in the circular muscle layer (arrows) (B) away from the taenial band in the same animal. Note the lower ICC density in this region compared with A. LM/T, longitudinal muscle layer/taenial band; LM, longitudinal muscle; CM, circular muscle layer; MG, myenteric ganglia.
Discussion
This is the first study to describe ICC development and distribution in the equine fetus and neonate. Developmental studies in other species have demonstrated that ICC start to colonize the intestinal tract about one-third to half way through gestation (Lecoin et al. 1996; Young et al. 1996; Torihashi et al. 1997; Kenny et al. 1999; Wester et al. 1999) and it seems reasonable to speculate that a similar time-scale of development also occurs in the equine fetus. This would suggest that the initial colonization of the equine intestinal tract would commence around the 4th to 6th month of gestation. However, considering the wide range of normal gestational lengths in this species (Rossdale et al. 1991; Rossdale, 1993), there may also be some individual variation in the time of initial ICC colonization. Unfortunately, equine fetuses under 6 months of gestation were not available for this study, principally because many abortions at this stage of pregnancy occur undetected.
At full term, ICC distribution and density in the small intestine appeared to be well developed. The cells were detectable not only in dense networks in the myenteric plexus region but also in the circular muscle layer of the muscularis externa. This distribution differs somewhat from other species in which ICC in the circular muscle layer were only sparsely, if at all, detectable until the neonatal period (Faussone-Pellegrini, 1984; Torihashi et al. 1995, 1997; Young et al. 1996; Ward et al. 1997; Kenny et al. 1999; Wester et al. 1999).
It is thought that ICC located around the myenteric plexus in the small intestine are involved in generating pacemaker activity in this region, whereas those located in the circular muscle layer primarily mediate neurotransmission (Sanders, 1996). Assuming this also applies to the horse, the findings in the current study suggest that, in the equine fetus, development of ICC classes of both the pacemaker-generating as well as the neurotransmission-mediating functions may be relatively advanced at the time of birth compared with other mammalian species. This apparent maturity is further supported by the observation that there did not appear to be significant changes in ICC density and distribution in the small intestine in the equine neonate compared with that of the full-term fetus. However, it is recognized that classification of the different types of ICC in the horse needs further investigation. For example, to investigate possible differences in function of the two morphological categories of ICC, it may be necessary to conduct both electrophysiological and electron microscopical studies (Rumessen et al. 1993; Liu et al. 1998).
The maturity of small intestinal ICC networks at full term differed somewhat from that of the large intestine. In the latter, a proximal to distal gradient of ICC distribution was evident, with the most distal part of the large intestine showing some evidence of ongoing development into the neonatal period. This type of developmental gradient has also been recognized in previous studies in other species (Faussone-Pellegrini et al. 1996; Ward et al. 1997; Kenny et al. 1998a). Ward et al. (1997) demonstrated that this developmental pattern also mirrored the onset of electrical rhythmicity in the murine gastrointestinal tract, providing further evidence that some of these cells generate pacemaker activity.
At full term, in the most distal part of the large intestine, there was incomplete ICC colonization of the innermost aspect of the circular muscle layer. Faussone-Pellegrini (1987) demonstrated in an ultrastructural study that the innermost aspect of the circular muscle layer in the mouse colon was the least differentiated layer at the time of birth when fibroblast-like cells still differentiated into smooth muscle cells. Lecoin et al. (1996) subsequently demonstrated that ICC were mesenchymal in origin and hence share a common developmental origin with smooth muscle cells and fibroblasts. Indeed, this close relationship was further demonstrated when Torihashi et al. (1999) noted that blockade of c-Kit signalling induced transdifferentiation of ICC into a smooth muscle phenotype, indicating that this signalling was important not only for differentiation but also for maintenance of the ICC phenotype. Considering this information, it is possible that ongoing differentiation through c-Kit development and signalling is taking place in this last part of the large intestine and is not complete until some time after birth. In the three neonatal animals examined, there appeared to be a uniform distribution of ICC throughout the circular muscle layer in the most distal part of the large intestine, indicating that this differentiation may be complete at this stage. The apparent immaturity at full term of the ICC in this region coupled with the proposed location of the main pacemaker-generating ICC at the submucosal border of the circular muscle layer in the large colon (Durdle et al. 1983; Ward & Sanders, 1990) offers possible insight into the causes of equine neonatal intestinal obstructive disorders such as meconium impaction. Assuming this area is also the primary pacemaker-generating region in the horse, this immaturity of ICC at birth may contribute to retention of meconium in the distal colon and rectum by some neonatal foals. However, it is possible that even immature cells may have functional properties as demonstrated by Torihashi et al. (1997) and hence functional electrophysiological studies are needed in order to determine the onset of normal motility patterns, as well to confirm the location of primary pacemaker sites in the distal large intestine of the foal.
In the colon of some species, including the dog (Berezin et al. 1988) and human (Rumessen, 1996), ICC have been described in association with the nerve fibres of the submuscular plexus on the luminal side of the circular muscle layer. Although no accumulation of ICC in this region has been seen in the current study and a submuscular plexus in the equine colon has yet to be identified, this is an area that warrants further investigation.
In the large intestine, there appeared to be an increased density of ICC in the circular muscle layer as well as in the myenteric plexus region in the areas near the taenial bands irrespective of gestational age or anatomical region. Increased ICC density in these areas has been described previously in adult horses (Hudson et al. 1999). The taenial bands are important as they are likely to be actively involved in peristalsis (Burns, 1992). A second pacemaker-generating area in the myenteric plexus region of the colon has been demonstrated in the dog, initiating myenteric potential oscillations that spread into both the circular and the longitudinal muscle layers (Smith et al. 1987). It is possible that ICC surrounding the myenteric plexus, especially in the region of the taenial bands, represent additional pacemaker-generating areas in the equine colon in addition to those proposed to be located in the circular muscle layer. This may account for the increased ICC density in these areas. A more likely explanation is that the ICC here are involved in mediating neurotransmission (Thuneberg, 1982; Daniel & Posey-Daniel, 1984). The high ICC density in the region of the taenial bands may therefore be related to the dense innervation that has been noted in this area (Burns, 1992).
In summary, this study has demonstrated the presence and distribution of ICC in the equine fetus during the latter half of gestation and in the neonate. It has shown that ICC distribution and density in the small intestine in the full-term fetus appear similar to that of the neonatal animal. However, it appears that ICC development in the distal large intestine continues after birth with further colonization of the inner aspect of the circular muscle region. This study has provided information on ICC development and distribution in the equine fetus and neonate that will provide a foundation for future studies in diseased animals.
Acknowledgments
C.F. is a Horserace Betting Levy Board Research Training Scholar. N.H. is supported by The Dowager Countess Eleanor Peel Trust. We would like to thank Colin Warwick for the illustrations, Gordon Goodall and Neil MacIntyre for technical advice on sample processing and Drs Susan Rhind and Elspeth Milne for advice on tissue sections. We would also like to thank Dr Jenny Ousey and Lorraine Palmer at Rossdale and Partners for help with sample collection and data retrieval.
References
- Berezin I, Huizinga JD, Daniel EE. Interstitial cells of Cajal in the canine colon: a special communication network at the inner border of the circular muscle. J. Comp. Neurol. 1988;273:42–51. doi: 10.1002/cne.902730105. [DOI] [PubMed] [Google Scholar]
- Burns GA. The teniae of the equine intestine. Cornell Vet. 1992;82:187–212. [PubMed] [Google Scholar]
- Daniel EE, Posey-Daniel V. Neuromuscular structures in opossum esophagus: role of interstitial cells of Cajal. Am. J. Phys. 1984;246:G305–G315. doi: 10.1152/ajpgi.1984.246.3.G305. [DOI] [PubMed] [Google Scholar]
- Daniel EE, Wang Y-F, Cayabyab FS. Role of gap junctions in structural arrangements of interstitial cells of Cajal and canine ileal smooth muscle. Am. J. Phys. 1998;274:G1125–G1141. doi: 10.1152/ajpgi.1998.274.6.G1125. [DOI] [PubMed] [Google Scholar]
- Durdle NG, Kingma YJ, Bowes KL, Chambers MM. Origin of slow waves in the canine colon. Gastroenterology. 1983;84:375–382. [PubMed] [Google Scholar]
- Faussone-Pellegrini MS. Morphogenesis of the special circular muscle layer and of the interstitial cells of Cajal related to the plexus muscularis profondus of mouse intestinal muscle coat: an EM study. Anat. Embryol. 1984;169:151–158. doi: 10.1007/BF00303144. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Matini P, Stach W. Differentiation of enteric plexuses and interstitial cells of Cajal in the rat gut during pre- and postnatal life. Acta Anat. 1985;155:113–125. doi: 10.1159/000147796. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS. Cytodifferentiation of the interstitial cells of Cajal of mouse colonic circular muscle layer. Acta Anat. 1987;128:98–109. doi: 10.1159/000146325. [DOI] [PubMed] [Google Scholar]
- Faussone-Pellegrini MS, Matini P, Stach W. Differentiation of enteric plexuses and interstitial cells of Cajal in the rat gut during pre- and postnatal life. Acta Anat. 1996;155:113–125. doi: 10.1159/000147796. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Wershil BK. The c-kit receptor, stem cell factor, and mast cells. Am. J. Pathol. 1993;142:965–974. [PMC free article] [PubMed] [Google Scholar]
- Greet TR. Ileal intussusception in 16 young thoroughbreds. Equine Vet. J. 1992;24:81–83. doi: 10.1111/j.2042-3306.1992.tb02787.x. [DOI] [PubMed] [Google Scholar]
- Hara Y, Kubota M, Szurszewski JH. Electrophysiology of smooth muscle of the small intestine of some mammals. J. Physiol. 1986;372:501–520. doi: 10.1113/jphysiol.1986.sp016022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson NPH, Pearson GT, Kitamura N, Mayhew IG. An immunohistochemical study of interstitial cells of Cajal in the equine gastrointestinal tract. Res. Vet. Sci. 1999;66:265–271. doi: 10.1053/rvsc.1998.0297. [DOI] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malusz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Kenny SE, Connell MG, Rintala RJ, Vaillant C, Edgar DH, Lloyd DA. Abnormal colonic interstitial cells of Cajal in children with anorectal malformations. J. Ped. Surg. 1998a;33:130–132. doi: 10.1016/s0022-3468(98)90379-7. [DOI] [PubMed] [Google Scholar]
- Kenny SE, Vanderwinden JM, Rintala RJ, Connell MG, Lloyd DA, Vanderhaegen JJ, De Laet MH. Delayed maturation of the interstitial cells of Cajal: a new diagnosis for transient neonatal pseudoobstruction. Report of two cases. J. Ped. Surg. 1998b;33:94–98. doi: 10.1016/s0022-3468(98)90370-0. [DOI] [PubMed] [Google Scholar]
- Kenny SE, Connell G, Woodward MN, Lloyd DA, Gosden CM, Edgar DH, Vaillant C. Ontogeny of the interstitial cells of Cajal in the human intestine. J. Ped. Surg. 1999;34:1241–1247. doi: 10.1016/s0022-3468(99)90160-4. [DOI] [PubMed] [Google Scholar]
- Kluppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev. Dyn. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Langer JC, Berezin I, Daniel EE. Hypertrophic pyloric stenosis: ultrastructural abnormalities of enteric nerves and the interstitial cells of Cajal. J. Ped. Surg. 1995;30:1535–1543. doi: 10.1016/0022-3468(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Lecoin L, Gabella G, LeDouarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122:725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- Liu LWC, Thuneberg L, Huizinga JD. Development of pacemaker activity and interstitial cells of Cajal in the neonatal mouse small intestine. Dev. Dyn. 1998;213:271–282. doi: 10.1002/(SICI)1097-0177(199811)213:3<271::AID-AJA4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Palmer JE. Gastrointestinal diseases of foals. Vet. Clin. North Am. 1985;5:151–168. doi: 10.1016/S0749-0739(17)30774-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossdale PD, Ousey JC, Cottrill CM, Chavatte P, Allen WR, McGladdery AJ. Effects of placental pathology on maternal plasma progestagen and mammary secretion calcium concentrations and on neonatal adrenocortical function in the horse. J. Reprod. Fertil. Suppl. 1991;44:579–590. [PubMed] [Google Scholar]
- Rossdale PD. Clinical view of disturbances in equine foetal maturation. Equine Vet. J. Suppl. 1993;14:3–7. doi: 10.1111/j.2042-3306.1993.tb04800.x. [DOI] [PubMed] [Google Scholar]
- Rumessen JJ, Peters S, Thuneberg L. Light- and electron microscopical studies of interstitial cells of Cajal and muscle cells at the submucosal border of human colon. Lab. Invest. 1993;68:481–494. [PubMed] [Google Scholar]
- Rumessen JJ. Ultrastructure of interstitial cells of Cajal at the colonic submuscular border in patients with ulcerative colitis. Gastroenterology. 1996;111:1147–1155. doi: 10.1016/s0016-5085(96)70005-7. [DOI] [PubMed] [Google Scholar]
- Sanders KM, Stevens R, Burke EP, Ward SM. Slow waves actively propagate at submucosal surface of circular layer in canine colon. Am. J. Physiol. 1990;259:G258–G263. doi: 10.1152/ajpgi.1990.259.2.G258. [DOI] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Smith TK, Reed JB, Sanders KM. Origin and propagation of electrical slow waves in circular muscle of canine proximal colon. Am. J. Physiol. 1987;252:C215–C224. doi: 10.1152/ajpcell.1987.252.2.C215. [DOI] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells. Adv. Anat. Embryol. Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Nishikava S-I, Nishi K, Kobayashi S, Sanders KM. C-kit-dependent development of interstitial cells and electrical activity in the murine gastrointestinal tract. Cell Tissue Res. 1995;280:97–111. doi: 10.1007/BF00304515. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Sanders KM. Development of c-kit-positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Nishi K, Tokutomi Y, Nishi T, Ward SM, Sanders KM. Blockade of kit signaling induces transdifferentiation of interstitial cells of Cajal to a smooth muscle phenotype. Gastroenterology. 1999;117:140–148. doi: 10.1016/s0016-5085(99)70560-3. [DOI] [PubMed] [Google Scholar]
- Vaala WE. Peripartum asphyxia. Vet. Clin. North Am. 1994;10:187–218. doi: 10.1016/s0749-0739(17)30374-7. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM. Intimate relationship between interstitial cells of Cajal and enteric nerves in the guinea-pig small intestine. Cell Tissue Res. 1999;295:247–256. doi: 10.1007/s004410051231. [DOI] [PubMed] [Google Scholar]
- Wang XY, Sanders KM, Ward SM. Relationship between interstitial cells of Cajal and enteric motor neurons in the murine proximal colon. Cell Tissue Res. 2000;302:331–342. doi: 10.1007/s004410000272. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Pacemaker activity in septal structures of canine colonic circular muscle. Am. J. Physiol. 1990;258:G264–G273. doi: 10.1152/ajpgi.1990.259.2.G264. [DOI] [PubMed] [Google Scholar]
- Ward SM, Keller R, Sanders KM. Structure and organization of the electrical activity of the canine distal colon. Am. J. Physiol. 1991;260:G724–G735. doi: 10.1152/ajpgi.1991.260.5.G724. [DOI] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical activity in the murine gastrointestinal tract. J. Physiol. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Harney SC, Bayguyinov JR, McLaren GJ, Sanders KM. Development of electrical rhythmicity in the murine gastrointestinal tract is specifically encoded in the tunica muscularis. J. Physiol. 1997;505:241–258. doi: 10.1111/j.1469-7793.1997.241bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester T, Eriksson L, Olsson Y, Olsen L. Interstitial cells of Cajal in the human fetal small bowel as shown by c-kit immunohistochemistry. Gut. 1999;44:65–71. doi: 10.1136/gut.44.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JH, Cudd TA. Section three: common gastrointestinal diseases. In: Koterba AM, Drummond WH, Kosch PC, editors. Equine Clinical Neonatology. Malvern, PA: Lea & Febiger; 1990. pp. 412–430. [Google Scholar]
- Yamataka A, Ohshiro K, Kobayashi H, Lane GL, Yamataka T, Fujiwara T, Sunagawa M, Miyano T. Abnormal distribution of intestinal pacemaker (c-kit)-positive cells in an infant with chronic idiopathic intestinal pseudoobstruction. J. Ped. Surg. 1998;33:859–862. doi: 10.1016/s0022-3468(98)90660-1. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Southwell BR, Newgreen DF. Origin of the interstitial cell of Cajal in the mouse intestine. Dev. Biol. 1996;180:97–107. doi: 10.1006/dbio.1996.0287. [DOI] [PubMed] [Google Scholar]