Abstract
The vestibular, cochlear and facial nerves have a common course in the internal auditory canal (IAC). In this study we investigated the average number of nerve fibres, the average cross-sectional areas of the nerves and nerve fibres, and the apparent connections between the facial, cochlear and vestibular nerve bundles within the IAC, using light and scanning electron microscopy. The anatomical localization of the nerves within the IAC was not straightforward. The general course showed that the nerves rotated anticlockwise in the right ear from the inner ear end towards the brainstem end and vice versa for the left ear. The average number of fibres forming vestibular, cochlear, and facial nerves was not constant during their courses within the IAC. The superior and the inferior vestibular nerves showed an increase in the number of nerve fibres from the inner ear end towards the brainstem end of the IAC, whereas the facial and the cochlear nerves showed a reduction in the number of fibres. This suggests that some of the superior and inferior vestibular nerve bundles may receive fibres from the facial and/or cochlear nerves. Scanning electron microscopic evaluations showed superior vestibular–facial and inferior vestibular–cochlear connections within the IAC, but no facial–cochlear connections were observed. Connections between the nerves of the IAC can explain the unexpected vestibular disturbances in facial paralysis or persistence of tinnitus after cochlear neurectomy in intractable tinnitus cases. The present study offers morphometric and scanning electron microscopic data on the fibre connections of the nerves of the IAC.
Keywords: cochlear nerve, connections, facial nerve, fibre counts, internal auditory canal, vestibular nerve
Introduction
The internal auditory canal (IAC) is 10–17 mm in length and contains the facial, vestibular and cochlear nerves, surrounding blood vessels and a common dural sheath (Bergström, 1973a). Anatomical relations between the nerves in the IAC have revealed variations from the inner ear end to the brainstem end of the IAC (Silverstein, 1984; Rubinstein et al. 1996).
The vestibular, cochlear and facial nerves do not have common functions but have a common anatomical course within the IAC. In early studies, a number of connections or anastomoses between these nerve bundles within the IAC were described. Vestibulo-cochlear connections were first described by Oort (1918) in a series of temporal bone dissections. Paturet (1951) described consistent and inconsistent patterns of nerve connections in the IAC. The consistent pattern was found between the nerve of Wrisberg (a branch of the facial nerve) and the vestibular nerve. Paturet also reported a constant pattern connecting two or three fibres from the genu of the facial nerve to the Scarpa's ganglion. Moreover, he described more inconsistent connections between the facial and vestibular nerve bundles. Fibre connections between the facial and vestibular nerve bundles were observed during surgical procedures (House, 1961; Fisch, 1973).
Vestibulo-facial connections may be important clinically because some vertiginous patients develop spontaneous nystagmus as a side-effect of facial nerve block for the treatment of hemifacial spasm (Wakasugi, 1972). The presence of anastomotic connections between the facial and vestibular nerves in the internal auditory canal is reported to contribute to the vestibular disturbance in facial paralysis (Fisch & Esslen, 1972). The presence of these connections causes a higher incidence of vestibular disturbance in patients with Bell's palsy. Additionally, the persistence of vertigo after vestibular neurectomy or the persistence of tinnitus after cochlear neurectomy in some patients may be the consequence of the presence of connections between vestibular and the cochlear nerve bundles (Natout et al. 1987; Pulec, 1995). Therefore connecting branches between the nerve bundles of the IAC may be important during both surgery and in the explanation of unexpected disturbances.
The aim of the present study was to show the average number of nerve fibres, and the connections between the facial, cochlear and vestibular nerve bundles within the IAC, using light and scanning electron microscopy.
Materials and methods
Nine formalin-fixed adult cadaveric heads (one female and eight male) were used in this study (light microscopy n = 4, and scanning electron microscopy n = 5). The age range of the cadavers was between 39 and 62. Cadavers with intracranial pathologies were excluded from this study.
Surgical procedure
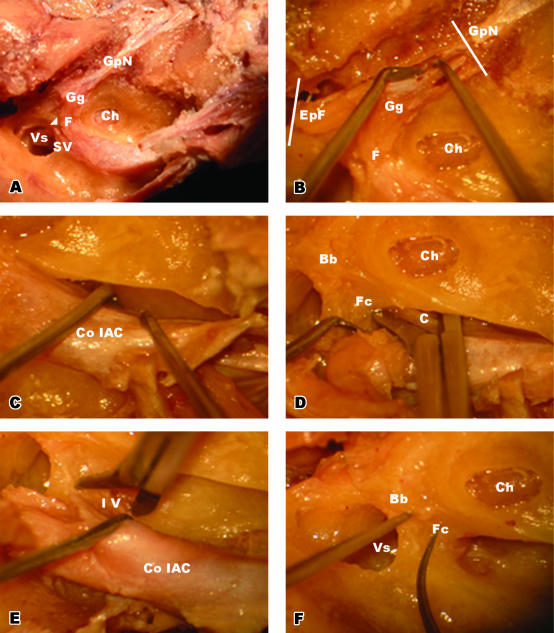
Calvaria were removed using a routine autopsy dissection technique, and after removing the cerebrum and cerebellum the entrance sites of vestibulo-cochlear and facial nerve bundles into the brainstem were identified. The nerve bundles were resected from the brainstem at both sides, by leaving a part of the brainstem at the nerve ends to prevent changing of the anatomical positions. The middle meningeal artery and arcuate eminence were identified as major landmarks at the superior aspect of the petrous bone. The greater petrosal nerve was found anterior to the arcuate eminence and approximately 1 cm medial to the middle meningeal artery. It was followed postero-laterally by unroofing the postero-superior aspect of the petrous part of the temporal bone to localize the geniculate ganglion. The facial nerve was followed postero-laterally from the geniculate ganglion towards epitympanum and postero-medially towards the IAC by drilling out the overlying bone over the facial nerve with an Aesculap Microtron. After a sufficient amount of bone was elevated, the bony structure separating the superior vestibular nerve from the facial nerve, namely Bill's Bar (vertical crest), was identified. The roof of the internal auditory canal was drilled out medially to expose the IAC content wrapped in dura (Fig. 1A).
Fig. 1.
(A)Postero-superior view of the IAC. Bill's bar (arrowhead) is marked between the facial and superior vestibular nerves. (B) Dissection of ganglionic end of the facial nerve. The greater petrosal nerve and epitympanic end of the facial nerve were cut at the planes indicated with the white bars and released towards the IAC. (C) Removal of the IAC content within the dura from the bony structures. (D) Releasing process of the cochlear nerve at the inner end of the IAC. At the level of the cochlea, the cochlear nerve was cut after retracting the facial nerve posteriorly. (E) Releasing process of the inferior vestibular nerve at the inner end of the IAC. At the level of the vestibulum, the inferior vestibular nerve was cut after further retraction of the facial nerve. (F) Bony landmarks (Bill's bar and falciform crest) were identified after removing the content of the IAC. Abbreviations: Bb, Bill's bar; C, cochlear nerve; Ch, cochlea; Co IAC, content of internal auditory canal; EpF, epitympanic end of the facial nerve; F, facial nerve; Fc, falciform crest; Gg, geniculate ganglion; GpN, greater petrosal nerve; IV, inferior vestibular nerve; SV, superior vestibular nerve; Vs, vestibulum.
The epitympanic end of the facial nerve and greater petrosal nerve were cut and the geniculate ganglion was released from bony structures towards the IAC (Fig. 1B). The ampullary end of the superior vestibular nerve was cut by sharp dissection at its ampullary origin, and the falciform crest (transverse crest) was identified by retracting the superior vestibular and facial nerves. The falciform crest separates the facial nerve from the cochlear nerve and the superior vestibular nerve from the inferior vestibular nerve.
During the separation process of the dura from the IAC, the cochlear end of the cochlear nerve and the ampullary end of the inferior vestibular nerve were cut with surgical scissors, and the IAC content wrapped with dura was removed from the bony structures. All these surgical procedures were made using a Carl-Zeiss Opmi 99 surgical microscope (Fig. 1C–F).
Histological procedure
Eight (four left and four right) nerve specimens obtained from cadavers were post-fixed in 10% formalin for 48 h, dehydrated in an ascending ethanol series and embedded in paraffin wax. Five sections from both ends, by taking every 50th section, were used for evaluation of each nerve of the IAC. Selected specimens were stained with haematoxylin and eosin.
Each slide was photographed under 1200 magnification (×40 lens, ×10 eyepiece, ×3 optic zoom) for calculating the average nerve fibre counts and the average cross-sectional areas of nerve fibres. In addition, under 120 magnification (×4 lens, ×10 eyepiece, ×3 optic zoom) the average cross-sectional areas of the nerves were calculated. Images were loaded on a computer.
The nerve fibre counts and nerve fibre densities were calculated from three randomly selecting areas, on each nerve, with a sampling size of 7920 µm2. All nerve fibres within this area were marked with a black dot using the computer program Adobe Photoshop 6.0. The marking process was performed according to a two-dimensional quantifying method (Gundersen et al. 1988). Nerve fibre counts within these areas were made with the aid of the Clemex Vision PE Demo Version computer program. The data from the three sampling sides of each nerve were statistically examined using the Friedman test (non-parametric anova for related samples).
The average cross-sectional areas of the nerves and nerve fibres were calculated by drawing a fine outline along the boundaries of the nerves and nerve fibres in Adobe Photoshop 6.0. Nerve fibres and cross-sectional areas of the nerves were calculated with the aid of the Clemex Vision PE Demo Version computer program. All procedures were performed by the same researcher. Calculations are given in Table 1.
Table 1.
The average nerve fibre counts, nerve fibre densities, cross-sectional areas of nerves and nerve fibres for both sides of four cases at the inner ear and brain stem ends of the IAC of cochlear, facial, inferior vestibular and superior vestibular nerves
| Average no. of nerve fibres | Average cross-sectional areas of nerves (mm2) | Average cross-sectional areas of nerve fibres (µm2) | Average nerve fibre density (mm−2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | Location | Nerve | R | L | R | L | R | L | R | L |
| 1 | Brainstem | C | 29453 ± 992 | 25154 ± 1036 | 2.09 ± 0.07 | 2.12 ± 0.06 | 62.3 ± 9.8 | 63.9 ± 13.3 | 14064 ± 22 | 11887 ± 800 |
| end | F | 11358 ± 1777 | 11306 ± 37 | 1.30 ± 0.18 | 1.30 ± 0.02 | 84.2 ± 5.7 | 83.8 ± 9.0 | 8893 ± 2587 | 8806 ± 1295 | |
| IV | 9440 ± 619 | 9268 ± 230 | 0.89 ± 0.14 | 0.88 ± 0.15 | 68.4 ± 3.2 | 69.4 ± 0.9 | 10651 ± 931 | 10740 ± 2041 | ||
| SV | 12481 ± 873 | 11614 ± 579 | 1.15 ± 0.07 | 1.16 ± 0.07 | 96.2 ± 4.1 | 94.1 ± 5.0 | 10865 ± 114 | 9995 ± 133 | ||
| Inner ear | C | 30640 ± 545 | 27700 ± 1773 | 1.78 ± 0.01 | 1.77 ± 0.00 | 31.9 ± 1.5 | 32.2 ± 0.4 | 17258 ± 438 | 15628 ± 967 | |
| end | F | 11818 ± 769 | 11748 ± 1207 | 1.32 ± 0.13 | 1.33 ± 0.14 | 56.7 ± 2.2 | 55.1 ± 0.6 | 8936 ± 263 | 8821 ± 12 | |
| IV | 8952 ± 139 | 8765 ± 904 | 0.71 ± 0.01 | 0.71 ± 0.00 | 39.3 ± 2.7 | 38.3 ± 8.7 | 12632 ± 388 | 12369 ± 1340 | ||
| SV | 12372 ± 449 | 11514 ± 2253 | 1.06 ± 0.07 | 1.05 ± 0.07 | 50.3 ± 2.8 | 50.6 ± 5.6 | 11706 ± 340 | 11060 ± 2930 | ||
| 2 | Brainstem | C | 23256 ± 580 | 28768 ± 825 | 2.19 ± 0.04 | 2.19 ± 0.03 | 71.0 ± 7.8 | 70.4 ± 11.1 | 10605 ± 72 | 13115 ± 551 |
| end | F | 10311 ± 612 | 12670 ± 194 | 1.35 ± 0.07 | 1.34 ± 0.02 | 87.6 ± 6.7 | 88.3 ± 4.2 | 7634 ± 37 | 9431 ± 317 | |
| IV | 9956 ± 579 | 10096 ± 480 | 0.95 ± 0.05 | 0.93 ± 0.03 | 74.5 ± 3.4 | 72.5 ± 1.2 | 10551 ± 1167 | 10926 ± 923 | ||
| SV | 11805 ± 344 | 12235 ± 473 | 1.22 ± 0.03 | 1.21 ± 0.06 | 98.0 ± 4.5 | 97.5 ± 2.8 | 9664 ± 532 | 10108 ± 109 | ||
| Inner ear | C | 25305 ± 433 | 29711 ± 814 | 1.80 ± 0.01 | 1.81 ± 0.03 | 34.7 ± 1.2 | 34.6 ± 2.8 | 14056 ± 170 | 16463 ± 724 | |
| end | F | 11047 ± 266 | 13247 ± 418 | 1.40 ± 0.03 | 1.37 ± 0.05 | 57.3 ± 3.0 | 57.4 ± 1.9 | 7869 ± 41 | 9670 ± 36 | |
| IV | 8958 ± 81 | 9315 ± 272 | 0.73 ± 0.03 | 0.73 ± 0.01 | 41.3 ± 1.6 | 44.2 ± 3.4 | 12217 ± 340 | 12687 ± 570 | ||
| SV | 11327 ± 512 | 11367 ± 1571 | 1.12 ± 0.03 | 1.12 ± 0.05 | 52.5 ± 2.4 | 51.9 ± 2.1 | 10112 ± 169 | 10199 ± 1856 | ||
| 3 | Brainstem | C | 23028 ± 1673 | 22859 ± 1582 | 2.16 ± 0.01 | 2.16 ± 0.07 | 62.8 ± 3.7 | 64.0 ± 5.9 | 10673 ± 735 | 10615 ± 1056 |
| end | F | 11769 ± 298 | 12065 ± 532 | 1.31 ± 0.05 | 1.33 ± 0.08 | 85.4 ± 4.6 | 85.6 ± 2.4 | 8997 ± 6585 | 9097 ± 163 | |
| IV | 8938 ± 263 | 8530 ± 452 | 0.86 ± 0.02 | 0.87 ± 0.08 | 68.2 ± 2.2 | 68.7 ± 1.1 | 10421 ± 585 | 9866 ± 357 | ||
| SV | 12255 ± 850 | 11122 ± 1253 | 1.20 ± 0.01 | 1.17 ± 0.03 | 92.8 ± 2.2 | 93.4 ± 2.6 | 10219 ± 6627 | 9508 ± 1282 | ||
| Inner ear | C | 27862 ± 1042 | 23657 ± 991 | 1.77 ± 0.02 | 1.77 ± 0.00 | 32.3 ± 1.4 | 31.7 ± 0.8 | 15731 ± 759 | 13380 ± 588 | |
| end | F | 12133 ± 327 | 12865 ± 424 | 1.37 ± 0.03 | 1.36 ± 0.06 | 54.1 ± 1.6 | 56.8 ± 0.5 | 8848 ± 16 | 9480 ± 127 | |
| IV | 8164 ± 276 | 8080 ± 177 | 0.71 ± 0.01 | 0.72 ± 0.01 | 36.3 ± 4.8 | 36.2 ± 4.4 | 11560 ± 520 | 11275 ± 26 | ||
| SV | 10376 ± 235 | 10705 ± 779 | 1.05 ± 0.04 | 1.06 ± 0.01 | 50.8 ± 0.4 | 50.5 ± 2.3 | 9845 ± 148 | 10074 ± 631 | ||
| 4 | Brainstem | C | 25292 ± 1826 | 23936 ± 958 | 2.01 ± 0.01 | 2.00 ± 0.02 | 59.4 ± 5.7 | 57.2 ± 7.1 | 12618 ± 1003 | 11978 ± 348 |
| end | F | 11840 ± 303 | 10122 ± 360 | 1.23 ± 0.04 | 1.22 ± 0.04 | 76.9 ± 1.9 | 76.8 ± 3.4 | 9655 ± 541 | 8333 ± 18 | |
| IV | 8401 ± 361 | 9041 ± 714 | 0.81 ± 0.02 | 0.83 ± 0.02 | 65.6 ± 1.8 | 66.9 ± 0.9 | 10408 ± 162 | 10883 ± 1133 | ||
| SV | 10390 ± 379 | 10566 ± 593 | 1.08 ± 0.03 | 1.11 ± 0.04 | 90.9 ± 2.6 | 91.2 ± 3.1 | 9614 ± 122 | 9488 ± 197 | ||
| Inner ear | C | 26558 ± 834 | 25751 ± 707 | 1.74 ± 0.08 | 1.75 ± 0.01 | 29.3 ± 1.2 | 30.3 ± 1.3 | 15260 ± 410 | 14742 ± 323 | |
| end | F | 11997 ± 558 | 10784 ± 356 | 1.23 ± 0.01 | 1.27 ± 0.04 | 51.4 ± 0.7 | 51.2 ± 1.5 | 9775 ± 572 | 8517 ± 18 | |
| IV | 8779 ± 519 | 8933 ± 250 | 0.69 ± 0.02 | 0.69 ± 0.02 | 35.6 ± 3.2 | 34.6 ± 3.8 | 12678 ± 324 | 12972 ± 65 | ||
| SV | 11481 ± 669 | 10720 ± 1197 | 1.00 ± 0.29 | 1.00 ± 0.03 | 49.6 ± 1.7 | 49.1 ± 0.8 | 11552 ± 1005 | 10678 ± 891 | ||
| Average | Brainstem | C | 25257 ± 2942 | 25179 ± 2530 | 2.11 ± 0.08 | 2.12 ± 0.09 | 63.9 ± 7.1 | 63.9 ± 8.9 | 11990 ± 1614 | 11899 ± 1098 |
| end | F | 11319 ± 978 | 11541 ± 1048 | 1.30 ± 0.09 | 1.30 ± 0.10 | 83.5 ± 5.8 | 83.6 ± 6.1 | 8795 ± 1288 | 8917 ± 666 | |
| IV | 9183 ± 716 | 9233 ± 712 | 0.88 ± 0.08 | 0.88 ± 0.07 | 69.2 ± 4.1 | 69.4 ± 2.3 | 10508 ± 618 | 10604 ± 1064 | ||
| SV | 11733 ± 1002 | 11384 ± 887 | 1.16 ± 0.07 | 1.16 ± 0.05 | 94.5 ± 4.0 | 94.0 ± 3.6 | 10090 ± 627 | 9775 ± 578 | ||
| Inner ear | C | 27591 ± 2190 | 26704 ± 2556 | 1.77 ± 0.02 | 1.77 ± 0.03 | 32.0 ± 2.3 | 32.2 ± 2.0 | 15576 ± 1282 | 15053 ± 1328 | |
| end | F | 11749 ± 597 | 12161 ± 1160 | 1.33 ± 0.09 | 1.33 ± 0.08 | 54.9 ± 3.0 | 55.1 ± 2.7 | 8857 ± 760 | 9122 ± 506 | |
| IV | 8713 ± 417 | 8773 ± 608 | 0.71 ± 0.02 | 0.71 ± 0.02 | 38.1 ± 3.5 | 38.3 ± 5.7 | 12272 ± 567 | 12326 ± 881 | ||
| SV | 11389 ± 843 | 11077 ± 1234 | 1.06 ± 0.06 | 1.06 ± 0.06 | 50.8 ± 1.9 | 50.5 ± 2.7 | 10804 ± 980 | 10503 ± 1437 | ||
Scanning electron microscopic investigations were performed on ten (five right and five left) nerve specimens obtained from cadavers. Before the procedures for SEM investigation the superior vestibular nerve was marked with a silk ligature, the inferior vestibular nerve by making an oblique incision, the facial nerve by keeping the geniculate ganglion intact and the remainder was determined as the cochlear nerve. These structures were fixed and dehydrated in an ascending ethanol series, dried with liquid CO2 under pressure with a critical point dryer (Bio-Rad E 3000, Hertfordshire, UK) and sputter-coated with gold (Bio-Rad SC 502). The samples were observed under a JEOL JSM scanning electron microscope (Tokyo, Japan).
Results
Light microscopic evaluations
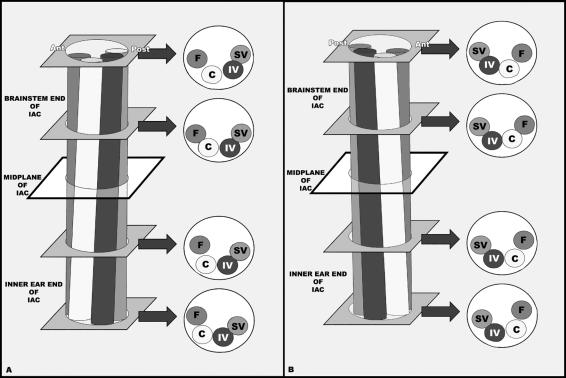
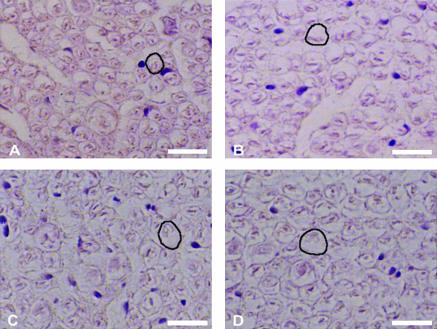
Anatomical relations between the nerves within the IAC were not straightforward. The general course showed that the nerves rotated anticlockwise in the right ear from the inner ear towards the brainstem end and vice versa for the left ear (Fig. 2A,B). The superior vestibular nerve was consistently positioned above the inferior vestibular nerve and the cochlear nerve was situated below the facial nerve throughout its course in the IAC. The fibres of the cochlear nerve were smaller in cross-sectional area and densely packed, whereas the fibres of the superior vestibular, inferior vestibular and facial nerves were larger and relatively loose (Fig. 3A–D).
Fig. 2.
Schematic illustration of the variable anatomical relations of the nerves within the IAC, (A) showing an anticlockwise rotation from the inner ear end towards the brainstem end of the IAC at the right side and (B) showing a clockwise rotation from the inner ear end towards the brainstem end of the IAC at the left side. Abbreviations: Ant, anterior; C, cochlear nerve; F, facial nerve; IAC, internal auditory canal; IV, inferior vestibular nerve; Post, posterior; SV, superior vestibular nerve.
Fig. 3.
Correlation of the average cross-sectional areas of nerve fibres forming the superior vestibular, inferior vestibular, cochlear and facial nerves within the IAC. All sections were obtained from the inner ear end of the IAC and the sections were stained with haematoxylin and eosin and photographed at ×1200 magnification (×40 lens, ×10 eyepiece, ×3 optic zoom). (A) Cochlear nerve, (B) inferior vestibular nerve, (C) superior vestibular nerve and (D) facial nerve. From each section a nerve fibre was marked with a black outline. Scale bar, 12.5 µm.
At the inner ear end of the IAC
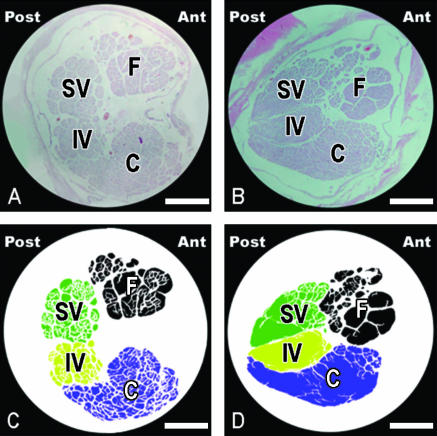
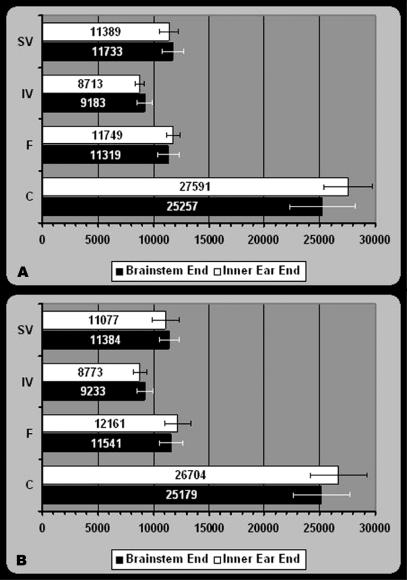
At the inner ear end of the IAC the facial nerve was located anterior and slightly superior to the vestibulo-cochlear nerve (Fig. 4A,C). The average number of nerve fibres (Fig. 5A,B), nerve fibre densities, and cross-sectional areas of nerves and nerve fibres from the inner ear end of the IAC were determined for each case (Table 1).
Fig. 4.
Photographs and schematic illustrations of the vestibulo-cochlear and facial nerves of the left side showing the clockwise rotation from the inner ear end towards the brainstem end of the IAC. Facial nerve is located antero-superior to the vestibulo-cochlear nerve at the inner ear end and anterior to the vestibulo-cochlear nerve at the brainstem end of the IAC. Histological sections were stained with haematoxylin and eosin and photographed at ×120 magnification (×4 lens, ×10 eyepiece, ×3 optic zoom). (A) Histological section and (C) schematic illustration from the inner ear end; (B) histological section and (D) schematic illustration from the brainstem end of the IAC. Scale bars, 50 µm. Abbreviations: Ant, anterior; C, cochlear nerve; F, facial nerve; IV, inferior vestibular nerve; Post, posterior; SV, superior vestibular nerve.
Fig. 5.
Histogram showing the average number of nerve fibres forming the superior vestibular, inferior vestibular, cochlear and facial nerves at the brainstem and inner ear ends of the IAC on (A) the right and (B) left sides. Error bars represent standard deviations. Abbreviations: C, cochlear nerve; F, facial nerve; IV, inferior vestibular nerve; SV, superior vestibular nerve.
At the brainstem end of the IAC
At the brainstem end of the IAC the superior and inferior vestibular nerves ascended and the facial nerve slightly descended, positioned anterior to the vestibulo-cochlear nerve (Fig. 4B,D). The cochlear nerve had a crescentic shape at the brainstem end of the IAC (Fig. 4B,D). The average number of nerve fibres (Fig. 5A,B), nerve fibre densities, and the cross-sectional areas of nerves and nerve fibres from the brainstem end of the IAC were determined for each case (Table 1).
The nerve fibres of the superior and the inferior vestibular nerves increased in number, whereas the nerve fibres of the facial and the cochlear nerves decreased in number from the inner ear end towards the brainstem end of the IAC (Table 1, Fig. 5A,B).
The average cross-sectional areas of the nerve fibres increased towards the brainstem end of the IAC, whereas the average cross-sectional areas of nerves increased towards the brainstem end of the IAC, except for the facial nerve (Table 1). The average cross-sectional area for the right and the left of each nerve were calculated as C > F > SV > IV for both brainstem and inner ear ends.
Scanning electron microscope evaluation
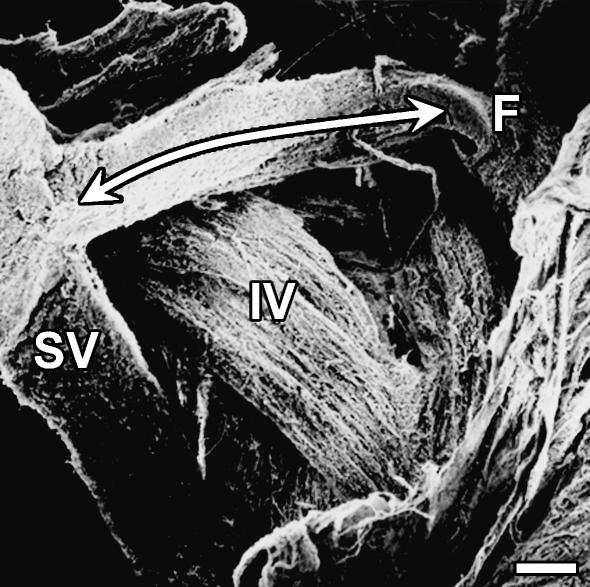
Scanning electron microscopy revealed connections between the superior vestibular and the facial nerve bundles, and between the inferior vestibular and the cochlear nerve bundles within the IAC (Figs 6 and 7). Connections between the facial and the cochlear nerve bundles were not observed.
Fig. 6.
Scanning electron microscopic appearance of the fibre connections between the superior vestibular and the facial nerve bundles within the IAC. Scale bar, 300 µm. Abbreviations: F, facial nerve; IV, inferior vestibular nerve; SV, superior vestibular nerve.
Fig. 7.
Scanning electron microscopic appearance of the fibre connections between the inferior vestibular and the cochlear nerve bundles within the IAC. Scale bar, 300 µm. Abbreviations: C, cochlear nerve; IV, inferior vestibular nerve.
Discussion
The anatomy of the neural structures in the IAC has been the subject of numerous histological, surgical and radiological investigations owing to its clinical and functional importance (Silverstein, 1984; Schefter & Harner, 1986; Natout et al. 1987; Rubinstein et al. 1996; Kim et al. 1998; Mitsuoka et al. 1999; Ryu et al. 1999; Terasaka et al. 2000).
The present study showed that the vestibular, cochlear and facial nerve bundles did not have straightforward courses within the IAC. An anticlockwise rotation of the nerves was observed on the right ear from the inner ear end towards the brainstem end and vice versa was observed on the left ear. Silverstein (1984) described a similar rotation of the nerves in a surgical study. Furthermore, the results of magnetic resonance imaging (MRI) studies showed that the facial nerve was located anterior to the vestibulo-cochlear nerve and as the nerve travels laterally in the IAC, it changes its position from the anterosuperior to the vestibulo-cochlear nerve, which was in accordance with the present study (Mitsuoka et al. 1999). The MRI study by Ryu et al. (1999) also showed spatial changes of the nerves of the IAC, in which the inferior vestibular and the cochlear nerves passed below the superior vestibular nerve rather than rotating towards the brainstem. Kim et al. (1998) observed via MRI posteroinferior displacement of the facial and vestibulo-cochlear nerves in the IAC. There were some differences between the results of the present study and the MRI studies. Although particular care was taken during harvesting of the specimens from the IAC, some displacement may have occurred and may have disturbed the normal anatomical positions of the nerves. Furthermore, discrepancies between MRI and cadaver studies can result from the absence of cerebrospinal fluid and blood pressure in cadaver studies.
Early studies, using various techniques, counted the number of fibres forming the vestibulo-cochlear nerves. Rasmussen (1940) calculated the number of vestibular nerve fibres as being 18 900; Naufal & Schuknecht (1972) found 18 439 in Scarpa's ganglion, and Fujii et al. (1990) found a total of 17 727. Bergström (1973b) counted the number of fibres forming the vestibular nerves in three different age groups in autopsy cases. A reduction of approximately 37% of the number of vestibular nerve fibres was found in the older age group. In contrast to Bergstöm's study, Fujii et al. (1990) found no difference in the number of nerve fibres with age. In the present study we did not evaluate the number of fibres in relation to age due to the limited sample size (n = 4). We obtained slightly higher vestibular fibre counts, which may be due to the limited sample size (n = 4) and the computer programs used for fibre counts. The results obtained via a computer should be more reliable then those obtained using an eyepiece graticule with a microscope. The above studies counted the vestibular fibres at various regions, as the number of fibres in the vestibular nerve bundle was not constant within the IAC; it was difficult to correlate our data with the former studies.
Additionally, Rasmussen (1940) counted the number of cochlear fibres in human adult cadavers and showed that the number varied between 24 000 and 40 000. Fujii et al. (1990) calculated the average number of cochlear fibres as 25 098. Our results were in accordance with the above results. There have been no previous studies of the number of fibres forming the facial nerve within the IAC; therefore we were unable to correlate our data.
The present study showed that the number of fibres forming the vestibular, cochlear and facial nerve bundles varied between the inner ear and the brainstem ends of the IAC, which might suggest connections between nerve bundles within the IAC.
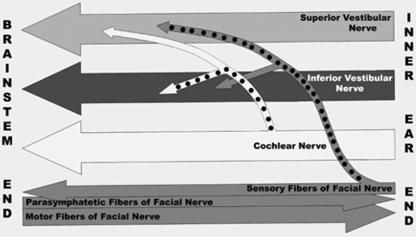
The superior vestibular and the inferior vestibular nerve bundles showed an increase in the number of nerve fibres from the inner ear end towards the brainstem end of the IAC, whereas the facial and cochlear nerve bundles showed a reduction in the number of nerve fibres on both sides. Some of the superior vestibular and inferior vestibular nerve bundles may receive fibres from the facial and/or cochlear nerve bundles (Fig. 8).
Fig. 8.
Schematic illustration of the results of the present study showing the fibre connections between the nerve bundles of the IAC (the connections are marked with black dots).
The existence of vestibulo-cochlear and vestibulo-facial connections within the IAC have been described in early studies using electrophysiology, histological examinations, radiological techniques, autopsy cases and cadavers (Oort, 1918; Rasmussen, 1940; House, 1961; Fisch & Esslen, 1972; Wakasugi, 1972; Bergström, 1973a, b; Fisch, 1973; Kim et al. 1998; Nageris et al. 2000). The vestibulo-cochlear connections were demonstrated in electrophysiological studies, in which the vestibular end-organ responded to acoustic stimulation (Burian et al. 1989). Rasmussen's (1940) histological studies showed that a variable number of vestibular fibres were localized within the cochlear nerve. Arnesen (1984) calculated the connecting branches between the vestibular and cochlear nerves, and found 1360 fibres. Hardy (1934) demonstrated the presence of some vestibular fibres, including the saccular fibres, within the cochlear part of the nerve in humans. Additionally, connecting fibres between the inferior vestibular and cochlear nerves were demonstrated via MRI (Kim et al. 1998). Bergström (1973a) showed that the fibres of the vestibulo-facial anastomoses were generally much thinner than those of the vestibular nerve, and that these anastomotic fibres contained approximately 700 fibres. Silverstein (1984) and Nageris et al. (2000) described connecting fibres between the superior vestibular and facial nerves in their temporal bone dissections.
Our SEM observations clearly showed superior vestibular–facial and inferior vestibular–cochlear connections. We have not defined any facial–cochlear connection. Our morphometric data confirm the SEM observations and explain the unexpected vestibular disturbance in facial paralysis and the persistence of tinnitus after cochlear neurectomy in intractable tinnitus cases. However, our results did not explain the persistence of vertigo after vestibular neurectomy. We suggest that some anastomoses between the vestibular and cochlear nerves may occur before entering or after exiting the IAC. In addition, Bukowska (2002) showed connections between the two nerves at nuclear levels, which may also be the cause of persistent vertigo.
In conclusion, the present study has presented SEM and morphometric data on the connections of the nerves of the IAC that may help to increase the success of diagnostic evaluation and surgical approaches to the region.
References
- Arnesen AR. Fiber population of the vestibulocochlear anastomosis in humans. Acta Otolaryngol. 1984;98:501–518. doi: 10.3109/00016488409107591. [DOI] [PubMed] [Google Scholar]
- Bergström B. Morphology of the vestibular nerve. I. Anatomical studies of the vestibular nerve in man. Acta Otolaryngol. 1973a;76:162–172. doi: 10.3109/00016487309121495. [DOI] [PubMed] [Google Scholar]
- Bergström B. Morphology of the vestibular nerve. II. The number of myelinated vestibular nerve fibers in man at various ages. Acta Otolaryngol. 1973b;76:173–179. doi: 10.3109/00016487309121496. [DOI] [PubMed] [Google Scholar]
- Bukowska D. Morphological evidence for secondary vestibular afferent connections to the dorsal cochlear nucleus in the rabbit. Cells Tissues Organs. 2002;170:61–68. doi: 10.1159/000047921. [DOI] [PubMed] [Google Scholar]
- Burian M, Gstoettner W, Zundritsch R. Saccular afferent fibers to the cochlear nucleus in the guinea pig. Arch. Otorhinolaryngol. 1989;246:238–241. doi: 10.1007/BF00463563. [DOI] [PubMed] [Google Scholar]
- Fisch U, Esslen E. Total intratemporal exposure of the facial nerve. Pathologic findings in Bell's palsy. Arch. Otolaryngol. 1972;95:335–341. doi: 10.1001/archotol.1972.00770080529008. [DOI] [PubMed] [Google Scholar]
- Fisch UP. Excision of the Scarpa's ganglion. Arch. Otolaryngol. 1973;97:147–149. doi: 10.1001/archotol.1973.00780010153011. [DOI] [PubMed] [Google Scholar]
- Fujii M, Goto N, Kikuchi K. Nerve fiber analysis and the aging process of the vestibulocochlear nerve. Ann. Otol. Rhinol. Laryngol. 1990;99:863–870. doi: 10.1177/000348949009901103. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bendtsen TF, Korbo L, Marcussen N, Møller A, Nielsen K, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Hardy M. Observations on the innervation of the macula sacculi in man. Anat. Rec. 1934;59:403–418. [Google Scholar]
- House WF. Surgical exposure of the internal auditory canal and its contents through the middle cranial fossa. Laryngoscope. 1961;71:1363–1385. doi: 10.1288/00005537-196111000-00004. [DOI] [PubMed] [Google Scholar]
- Kim HS, Kim DI, Chung IH, Lee WS, Kim KY. Topographical relationship of the facial and vestibulocochlear nerves in the subarachnoid space and internal auditory canal. AJNR Am. J. Neuroradiol. 1998;19:1155–1161. [PMC free article] [PubMed] [Google Scholar]
- Mitsuoka H, Arai H, Tsunoda A, Okuda O, Sato K, Makita J. Microanatomy of the cerebellopontine angle and internal auditory canal: study with new magnetic resonance imaging technique using three-dimensional fast spin echo. Neurosurgery. 1999;44:561–567. doi: 10.1097/00006123-199903000-00069. [DOI] [PubMed] [Google Scholar]
- Nageris B, Braverman I, Kalmanowitz M, Segal K, Frenkiel S. Connections of the facial and vestibular nerves: an anatomic study. J. Otolaryngol. 2000;29:159–161. [PubMed] [Google Scholar]
- Natout MAY, Terr LI, Linthicum FH, House WF. Topography of the vestibulocochlear nerve fibers in the posterior cranial fossa. Laryngoscope. 1987;97:954–958. [PubMed] [Google Scholar]
- Naufal PM, Schuknecht HF. Vestibular, facial and oculomotor neuropathy in diabetes mellitus. Arch. Otolaryngol. 1972;96:468–474. doi: 10.1001/archotol.1972.00770090700016. [DOI] [PubMed] [Google Scholar]
- Oort H. Über die verästelung des nervus octavus bei Säugetieren. (Modell des utriculus und sacculus des kaninchens) Anat. Anz. 1918;51:272–280. [Google Scholar]
- Paturet G. Traite d’Anatomie Humaine. Vol. 1. Paris: Masson et Cie; 1951. [Google Scholar]
- Pulec JL. Cochlear nerve section for intractable tinnitus. Ear Nose Throat J. 1995;74:468–476. [PubMed] [Google Scholar]
- Rasmussen AT. Studies of the VIII'th cranial nerve of man. Laryngoscope. 1940;50:67–83. [Google Scholar]
- Rubinstein D, Sandberg EJ, Cajade-Law AG. Anatomy of the facial and vestibulocochlear nerves in the internal auditory canal. AJNR Am. J. Neuroradiol. 1996;17:1099–1105. [PMC free article] [PubMed] [Google Scholar]
- Ryu H, Tanaka T, Yamamoto S, Uemura K, Takehara Y, Isoda H. Magnetic resonance cisternography used to determine precise topography of the facial nerve and three components of the eighth cranial nerve in the internal auditory canal and cerebellopontine cistern. J. Neurosurg. 1999;90:624–634. doi: 10.3171/jns.1999.90.4.0624. [DOI] [PubMed] [Google Scholar]
- Schefter RP, Harner SG. Histologic study of the vestibulocochlear nerve. Ann. Otol. Rhinol. Laryngol. 1986;95:146–150. doi: 10.1177/000348948609500207. [DOI] [PubMed] [Google Scholar]
- Silverstein H. Cochlear and vestibular gross and histologic anatomy (as seen from postauricular approach) Otolaryngol. Head Neck Surg. 1984;92:207–211. doi: 10.1177/019459988409200213. [DOI] [PubMed] [Google Scholar]
- Terasaka S, Sawamura Y, Fukushima T. Topography of the vestibulocochlear nerve. Neurosurgery. 2000;47:162–168. doi: 10.1097/00006123-200007000-00034. [DOI] [PubMed] [Google Scholar]
- Wakasugi B. Facial nerve block in the treatment of the facial spasm. Arch. Otolaryngol. 1972;95:356–359. doi: 10.1001/archotol.1972.00770080550013. [DOI] [PubMed] [Google Scholar]