Abstract
This paper describes the initial appearance and distribution of mature T and B cells in the developing immune tissues of the stripe-faced dunnart (Sminthopsis macroura) based on the use of species cross-reactive antibodies to the lymphocyte cell surface markers CD3, CD5 and CD79b. At birth no mature T or B cells were detected in the liver or bone marrow using anti-CD3, anti-CD5 or anti-CD79b antibodies. T cells were detected in the thymus with anti-CD3 by day 12 and anti-CD5 by day 50 postpartum, and T cells in the spleen were detected by day 43 and day 80 postpartum using anti-CD3 and anti-CD5, respectively. B cells were observed in the dunnart spleen by 43 days after birth. CD3- and CD79b-positive cells were detected in the lymph nodes by 50 days and CD5 by day 15 after birth, and in the gut-associated lymphoid tissues by day 50 and anti-CD5 by day 57 postpartum. The development and distribution of T and B cells in the immune tissues of dunnart pouch young is similar to that described in other marsupial species. Low numbers or absence of mature lymphocytes in immune tissues of early pouch young dunnarts further support the proposition that young marsupials are reliant on non-specific defence strategies and/or maternal strategies for a significant period of their time of development in the pouch.
Keywords: development, dunnart, immune tissue, immunohaematopoietic, lymphoid, marsupial, Sminthopsis
Introduction
The stripe-faced dunnart (Sminthopsis macroura) is a Dasyurid. It is a widespread inhabitant of inland central and northern Australia (Dickman & Read, 1992). Although little is known about this marsupial in the wild (Cronin, 1996), observations of captive populations have confirmed that it is nocturnal and feeds on invertebrate prey (Godfrey, 1969). Captive populations of this animal have been used in studies of developmental biology (Selwood & Woolley, 1991; Selwood & Hickford, 1999) and, more recently, immunology (Old et al. 2003a, b, 2004).
In contrast to Eutherians, Metatherians (marsupials) develop for the last two-thirds of organogenesis in a non-sterile external pouch environment (Old & Deane, 2000) and this characteristic makes them unique and readily accessible for developmental studies, particularly of the immune tissues. In addition, the high fecundity of the dunnart and their availability in captive populations makes this Australian native mammal an ideal animal for studying the ontogeny of immune tissues.
Using histological techniques, we previously documented the development of the immune tissues of the stripe-faced dunnart (Old et al. 2003a, 2004). These studies indicate that the pattern of development is similar to that observed in other marsupials (Old & Deane, 2000). This study describes the use of these antibodies to determine the initial time of appearance of mature T and B cells and, in addition, the distribution of these cells in the developing immune tissues of the stripe-faced dunnart. To date, a limited number of antibodies are available that cross-react with marsupial tissues. Anti-CD3 and anti-CD5 recognize the surface markers of T cells and anti-CD79b recognizes B cells (Hemsley & Canfield, 2000). This is the first study to describe the capacity of these antibodies to recognize cell-surface markers in a Dasyurid, and a second Australian Polyprotodont. This study adds to the growing body of information on the maturation of immune tissues in this unique group of mammals.
Materials and methods
Samples of liver, bone marrow, thymus, spleen, lymph nodes, intestine and lung were collected opportunistically from a laboratory colony of stripe-faced dunnarts. The colony was established and maintained by L.S. under permits from the Department of Sustainability and Environment, Victoria. The Australian National Health and Medical Research Council Guidelines for the care and use of animals in research were followed in this study. Samples were obtained from animals of known ages, from 2 days to adulthood. In total, six liver [4, 12 (×3), 43 and 50 days postpartum (dpp)], four bone marrow (12, 15, 40 and 50 dpp), seven thymus [12 (×2), 15, 43, 50 dpp, a juvenile (80 dpp) and an adult], six spleen (15, 43, 50 and 60 dpp, 2.5 months and an adult), four lymph node (15 and 50 dpp, 2.5 months postpartum and an adult), nine intestinal (2, 4, 12, 15, 40, 43, 50, 57 dpp and 2.5 months postpartum) and nine lung samples [12 (×2), 15, 40, 43, 50 and 57 dpp, 2.5 months and an adult] were assessed.
Whole bodies were freshly preserved in 10% neutral buffered formalin. Some whole bodies were stored for a few days, and others were preserved for several years. Tissues were dissected from whole preserved bodies and processed in graded alcohols and xylene, and then embedded in paraffin wax. All wax sections were cut at 4 µm. Immunohistochemistry was conducted using a Streptavidin-biotin kit as described previously (Old & Deane, 2002a). In addition, an avidin/biotin blocking step was also used to decrease background staining due to endogenous biotin in liver and intestinal samples as described previously (Old & Deane, 2003). All slides were viewed using a Leica DMR DAS light microscope and Zeiss Axiovision software.
The antibodies used in this study have been shown to work consistently on a range of other metatherian immune tissues (Hemsley & Canfield, 2000) and included polyclonal anti-CD3 (DAKO Corp., Carpinteria, California, USA) and monoclonal anti-CD3 (Jones et al. 1993), anti-CD5 (Jones et al. 1993) and anti-CD79b (Jones et al. 1993). Anti-CD5 and anti-CD79b were kindly donated by Dr Margaret Jones of the Immunodiagnostic Unit, Radcliffe Hospital, Oxford. The dilutions used for monoclonal anti-CD3 were 1:10 and 1:50, polyclonal anti-CD3 1:500 and 1:1000, anti-CD5 1:50 and 1:100, and anti-CD79b 1:50 and 1:100 as these were shown to work consistently in this and other metatherian species (Old & Deane, 2001, 2002a, b).
A minimum of four sections for each tissue were tested with each antibody with the exception of some lymph node and spleen samples. Primary and secondary negative antibody controls were included with all tests for all sections at the same time and was similar to that described previously (Old & Deane, 2003). In some cases, positive control juvenile tammar wallaby lymph node samples were run in parallel to confirm negative findings.
Results
Liver
Liver samples from six stripe-faced dunnarts [4, 12 (×3), 43 and 50 dpp] were tested using specific antibodies to determine if any lymphocytes expressing surface markers of mature T and B cells were present. Samples from animals older than 50 days were not examined as these had achieved an adult structure (Old et al. 2004) and were therefore not haematopoietic. No staining was observed in any of the liver samples tested (data not shown).
Bone marrow
Immunohistochemistry was conducted on four bone marrow samples from animals aged 12, 15, 40 and 50 dpp. Histological staining of these samples had revealed large numbers of haematopoietic cells; however, anti-CD3, anti-CD5 and anti-CD79b failed to recognize any cells in these samples (data not shown).
Samples from animals older than 50 days were not examined as these had achieved an adult structure with only small numbers of lymphocyte-like cells observed (Old et al. 2003a, 2004).
Thymus
Seven stripe-faced dunnart thoracic thymus samples were collected from animals aged 12 (×2), 15, 43 and 50 dpp as well as a juvenile (aged 80 days) and an adult.
The majority of cells detected in all the thymus samples investigated were defined as T cells with CD3+ cells first observed in 12-day-old individuals (data not shown). No CD5 or CD79b positively stained cells were observed at this age (data not shown). CD3+ cells were detected in all older samples. CD5+ cells were first observed on day 50 but were not present in 43-day-old individuals (data not shown).
CD3+ T cells were present initially only in the medulla but as the animals matured, stained cells appeared in both the cortex and the medulla. Older samples had more T cells stained in the inner cortical areas rather than the medulla.
In juveniles and adults, where defined cortical and medullary regions were still observed, anti-CD3 and anti-CD5 (Fig. 1) stained most cells within the medullary regions with only a few scattered cells stained in the cortical regions. In adult thymus samples, where involution had started to occur, scattered CD3+ and CD5+ cells remained. By contrast, extremely rare CD79b+ cells were detected in some thymus samples (data not shown).
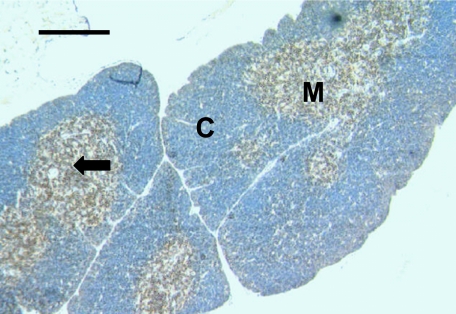
Fig. 1.
Thymus sample from a 6.5- to 7-month-old dunnart showing lymphocytes stained with anti-CD3. Lymphocytes (arrow) are mainly stained throughout the medullary (M) areas, but a few are stained in the cortical (C) regions. Scale bar, 200 µm.
All negative controls were unstained (data not shown).
Spleen
Samples were obtained from animals aged 15, 43, 50 and 60 dpp, a juvenile (2.5 months) and an adult sample. The extremely small size of some of the pouch young samples and the essential inclusion of negative control slides reduced the number of samples available for testing. Some spleen samples were also extremely friable.
CD3+ lymphocytes were not detected in samples from animals aged 15 days, but were present in a sample from a 43-day-old animal (data not shown). CD3+ lymphocytes were observed in all older samples tested. In animals aged from 43 days with defined red and white pulp areas, CD3+ cells were detected in the white pulp (Fig. 2). A similar distribution of cells was observed, using anti-CD5 in the spleen samples. However, in contrast to anti-CD3 staining, the youngest sample tested that stained positive for CD5 was aged 2.5 months (data not shown). No CD5+ cells were detected in a 60-day-old dunnart spleen however; this may have been due to the extreme friability of the tissue (data not shown).
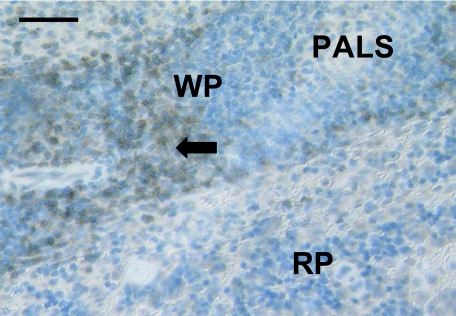
Fig. 2.
Spleen sample from an adult dunnart stained with anti-CD3 showing positively stained cells (arrow) scattered through the white pulp (WP). The periarterial lymphatic sheath (PALS) in the white pulp area and the red pulp (RP) are also apparent. Scale bar, 100 µm.
CD79b+ cells were detected in samples from dunnarts aged 43 and 50 days, but not in dunnarts aged 60 days and 2.5 months, and were again present in all samples from older animals (data not shown). Anti-CD79b stained a few cells in the red pulp areas, the mantles of secondary follicles and some in the marginal zone separating the red and white pulp area in adult samples (data not shown).
Lymph nodes
Owing to the small size of the lymph nodes in the stripe-faced dunnarts it was extremely difficult to locate them macroscopically. Often they were located only after staining and sectioning in association with other organs being investigated, such as the gut and liver. They were usually found surrounded by large amounts of adipose tissue. Only four lymph node samples were tested for the presence of CD3, CD5 and CD79b. One 15-day-old lymph node sample had CD5+ cells (data not shown); however, due to the small amount of available tissue it could not be tested for the presence of CD3 and CD79b.
CD3+, CD5+ and CD79b+ cells were detected in a lymph node from a 50-day-old dunnart (data not shown) as well as juvenile and adult lymph nodes. A 2.5-month-old lymph node stained with anti-CD3 and anti-CD5 (Fig. 3a) stained lymphocytes mainly in the cortex as well as rare lymphocytes at the periphery of germinal centres and in the medullary cords and sinuses. No stained lymphocytes were observed in the mantle of the secondary follicle with anti-CD3 or anti-CD5, whereas with anti-CD79b scattered lymphocytes were stained in the mantle of secondary follicles (Fig. 3b) as well as in the medulla. The adult lymph node was stained similarly to the juvenile lymph node (data not shown).
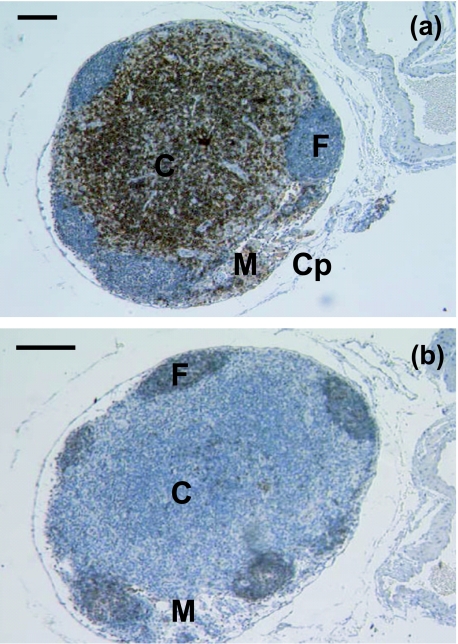
Fig. 3.
(a) A 2.5-month-old lymph node is essentially mature in histological appearance, having a well-defined medulla (M) and cortex (C) within the capsule (Cp). Follicles (F) are prominent. Anti-CD5 heavily stains the cortical areas, with a few scattered lymphocytes stained in the medullary cords and sinuses. Scale bar, 100 µm. (b) Anti-CD79b stained the follicles (F) in lymph node sample from a 2.5-month-old dunnart. Note the well-defined medulla (M) and cortex (C). Scale bar, 100 µm.
All negative controls were unstained (data not shown).
Gut-associated lymphoid tissues
Adequate tissue was available to examine the gut for the presence of CD3, CD5 and CD79b positively stained lymphocytes. Nine whole gut samples from animals of different ages were tested (2, 4, 12, 15, 40, 43, 50, 57 days and 2.5 months postpartum). Samples from animals aged 2 and 4 days showed background staining (data not shown) despite the use of the avidin/biotin blocking step. No samples from animals aged 12–43 days after birth stained positive using anti-CD3, anti-CD5 or anti-CD79b (data not shown). A gut sample from an animal aged 50 days was the youngest to have CD3+ and CD79b+ cells (data not shown). CD5+ cells were not observed in the gut until day 57 (data not shown). CD79b+ cells were heavily stained in primary follicles (Fig. 4) observed in a gut sample from an animal aged 57 dpp.
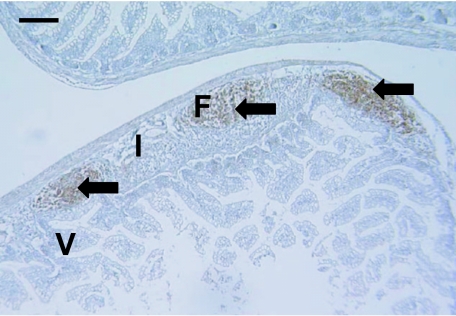
Fig. 4.
Anti-CD79b clearly stained lymphocytes (arrows) in a 57-day-old gut sample. These areas in the submucosa resembled follicles (F). Interfollicular zones (I) and villi (V) were not stained. Scale bar, 100 µm.
In a juvenile stripe-faced dunnart sample, anti-CD3 stained lymphocytes throughout the villi and in the submucosa heavily stained the interfollicular areas (data not shown). Anti-CD5 stained lymphocytes throughout the villi but these were much reduced in numbers compared with the numbers stained with anti-CD3 (data not shown). Anti-CD5 also heavily stained the interfollicular areas (data not shown). Anti-CD79b stained lymphocytes throughout the primary follicles and in the mantles of secondary follicles. Extremely rare CD79b+ lymphocytes were observed in the villi (data not shown).
No stained cells were observed in any of the negative control samples (data not shown).
Bronchus-associated lymphoid tissue (BALT)
Nine lung samples from pouch young (12, 12, 15, 40, 43, 50 and 57 dpp), a juvenile and an adult were investigated using immunohistochemistry. Some positively stained cells were observed specifically in the blood vessels of lungs in some of the sections (data not shown). One sample from an animal aged 57 days had a small amount of BALT and CD3+ cells were present (data not shown).
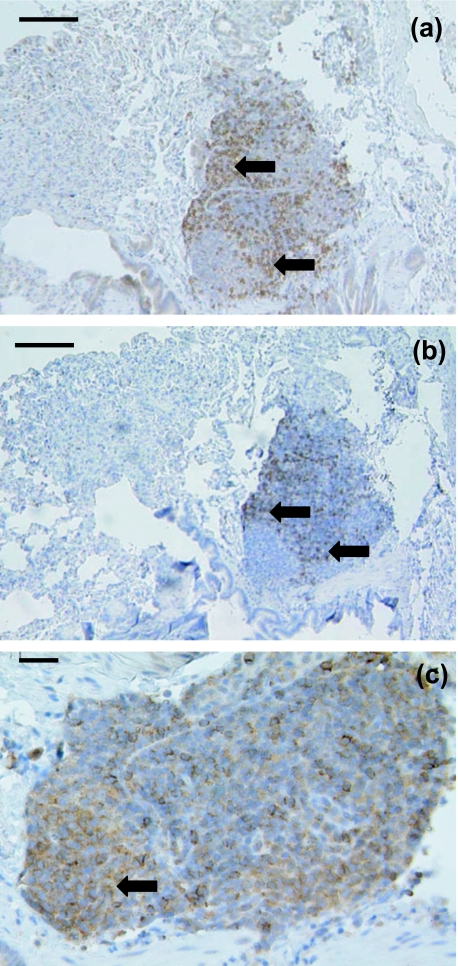
Only a random scattering of cells stained in the lungs of the juvenile and adult stripe-faced dunnart using anti-CD3 (Fig. 5a) and anti-CD5 (Fig. 5b). Both stained a similar number of cells in similar locations. However, anti-CD79b stained cells in different areas, despite there being no defined regions visible using histological techniques. One area in an adult dunnart BALT sample stained a small accumulation of lymphocytes (Fig. 5c). This may have been an early primary follicle.
Fig. 5.
(a)This adult dunnart BALT sample has lymphocytes stained (arrows) with anti-CD3 throughout. Note the lack of secondary follicles. Scale bar, 100 µm. (b)Anti-CD5 stained lymphocytes (arrows) throughout this adult dunnart BALT sample. Scale bar, 100 µm. (c)A high-magnification view of an adult dunnart BALT sample stained showing CD79b+ stained cells (arrow). Scale bar, 20 µm.
Discussion
This study has documented the appearance of mature lymphocyte populations in the immune tissues of the stripe-faced dunnart. The key milestones in this development are summarized in Table 1. The timing and pattern of development are consistent with what has been reported for other Metatherians (Table 2).
Table 1.
Age at which specific lymphocyte populations were initially detected in the immune tissues of the stripe-faced dunnart
| Lymphocytes initially stained in stripe-faced dunnart | |||
|---|---|---|---|
| Tissue | CD3+ | CD5+ | CD79b+ |
| Liver | NS (4 days) | NS (4 days) | NS (4 days) |
| Bone marrow | NS (12 days) | NS (12 days) | NS (12 days) |
| Thymus | 12 days (12 days) | 50 days (12 days) | Adult (12 days) |
| Spleen | 43 days (15 days) | 2.5 months (60 days) | 43 days (43 days) |
| Lymph nodes | 50 days (50 days) | 15 days (15 days) | 50 days (50 days) |
| GALT | 50 days (12 days) | 57 days (12 days) | 50 days (12 days) |
| BALT | 57 days (57 days) | Juvenile (57 days) | Juvenile (57 days) |
NS, no staining at any age.
Age given in parentheses indicates youngest sample tested, with the exception of the age given for BALT that does not include the younger lung only samples.
Table 2.
Summary of the age at which specific lymphocyte populations were initially detected in the immune tissues of marsupials
| Lymphocytes initially stained in stripe-faced dunnart | |||||
|---|---|---|---|---|---|
| Tissue | Species | CD3+ | CD5+ | CD79b+ | Reference |
| Liver | Stripe-faced dunnart | NS | NS | NS | this study |
| Tammar wallaby | NS | NS | NS | Old & Deane (2003) | |
| Bone marrow | Stripe-faced dunnart | NS | NS | NS | this study |
| Tammar wallaby | NS | NS | NS | Old & Deane (2003) | |
| Thymus | White bellied opossum* | 14 mm CRL | NA | NA | Coutinho et al. (1995) |
| Stripe-faced dunnart | by 12 days | by 50 days | rare | this study | |
| Brushtail possum | day 2 | NA | NA | Baker et al. (1999) | |
| Tammar wallaby | by day 12 | by day 12 | by day 23 (rare) | Old & Deane (2003) | |
| Spleen | White bellied opossum* | 80 mm CRL | NA | weanling | Coutinho et al. (1995) |
| Stripe-faced dunnart | by 43 days | by 21/2 months | by 43 days | this study | |
| Brushtail possum | by day 25 | NA | NA | Baker et al. (1999) | |
| Tammar wallaby | by day 21 | by day 30 | by day 21 | Old & Deane (2003) | |
| Lymph nodes | White bellied opossum* | 75 mm CRL | NA | weanling | Coutinho et al. (1995) |
| Stripe-faced dunnart | by 50 days | by 15 days | by 50 days | this study | |
| Tammar wallaby | by day 24 | by day 24 | by day 35 | Old & Deane (2003) | |
| GALT | Stripe-faced dunnart | by 50 days | by 57 days | by 50 days | this study |
| White bellied opossum* | 24 mm CRL | NA | > 60 mm CRL | Coutinho et al. (1994) | |
| Brushtail possum | from day 2 | NA | NA | Baker et al. (1999) | |
| Tammar wallaby | day 12 (rare) | day 74 (rare) | day 7 (rare) | Old & Deane (2003) | |
| BALT | Stripe-faced dunnart | juvenile | juvenile | juvenile | this study |
| Tammar wallaby | no BALT | no BALT | no BALT | Old & Deane (2003) | |
NS, no staining at any age; NA, not attempted; CRL, crown–rump length.
Growth charts have not been constructed for this species (Didelphis albiventris).
In all metatherians studied, including the dunnart, the liver is initially an important site for haematopoiesis (Block, 1964; Basden et al. 1996; Cisternas & Armati, 1999; Old et al. 2004). No mature T or B cells were detected in any of the dunnart liver or bone marrow samples and is similar to findings for the tammar wallaby (Macropus eugenii), a macropod (Old & Deane, 2003).
The marsupial thymus has been presumed to function in a similar way to the eutherian thymus and probably acts as a T cell development area; this is based on the large number of T cells stained and the distribution of unstained immature lymphocytes (thymocytes) maturing (expressing CD3 and CD5) in the cortex and moving to the medulla as observed in eutherians including humans (von Gaudecker, 1991). In the study of the stripe-faced dunnart, mature T cells were initially detected in the medulla of the thymus, but as the thymus matured T cell areas were identified in the medulla and cortico-medullary junction. The distribution of mature T cells in the thymus was similar, and follows a similar developmental pattern, to that observed in other metatherian species (Canfield et al. 1996; Old & Deane, 2002a) (Table 2).
Mature B cells were extremely rare or absent in thymus samples from animals of all ages. Large numbers of B cells were not expected and the distribution and abundance of B cells in the thymus of the dunnart was similar to that for the tammar wallaby (Old & Deane, 2003) as well as other marsupials (Canfield et al. 1996; Baker et al. 1999; Cisternas & Armati, 2000).
A failure to stain immature lymphocytes in the thymus may also indicate that the lymphocytes observed in the bone marrow are also not yet mature and therefore not expressing the CD79b marker. At present, however, there are no antibodies available that can recognize the early lymphocyte markers of marsupials.
Because of the fragility and size of the spleen in younger animals, the numbers of sections available for study was extremely low and resulted in only a few immunohistochemical tests being performed on this tissue. A lack of staining in the spleen sections at some ages is presumably due to poor tissue quality rather than an absence of expression, although further samples are required to confirm this. Further studies are therefore required to provide a more accurate picture of when mature lymphocytes first appear in the spleen of the stripe-faced dunnart.
The appearance of the mature spleen resembled that reported for other adult marsupials (Hemsley et al. 1995; Baker et al. 1999; Cisternas & Armati, 2000; Old & Deane, 2001) with T cells mostly stained in the white pulp areas and some in the outer germinal centres, whereas B cells were observed mainly in the mantle of follicles and some in the red pulp areas. T cells present in the outer germinal centre may be T helper cells (Poppema et al. 1981) as is the case in eutherians, although further studies are required to confirm the existence of T helper cells in marsupials.
Lymph nodes have been hard to locate in several metatherian species (Poskitt et al. 1984; Basden et al. 1996). Despite this, the histological and immunohistological appearance of the dunnart lymph nodes investigated in this study were similar to other reports on marsupial lymph nodes (Hanger & Heath, 1994; Hemsley et al. 1995, 1996; Baker et al. 1999; Cisternas & Armati, 1999). Further samples are required to provide more information regarding the initial appearance of lymphocytes in the lymph nodes of the stripe-faced dunnart but these results suggest that the lymph nodes may mature earlier than the spleen and GALT.
Histologically the GALT were the last lymphoid tissues to mature fully in the stripe-faced dunnart (Old et al. 2003a) as in other metatherian species (Coutinho et al. 1994; Basden et al. 1997; Baker et al. 1999). One similarity observed in metatherian and eutherian GALT was the presence of a large number of mature T cells among the villi (Old & Deane, 2001, 2002b). The stripe-faced dunnart had more CD3- than CD5-expressing T cells among the villi. The large number of mature T cells observed among the villi suggests that the T cells present may be T helper cells because large numbers of T helper cells (recognized by specific eutherian T helper cell antibodies) are observed among the villi in the eutherian gut (Poppema et al. 1981); more specific markers are required to confirm this in metatherians as to date no T or B cell subsets have been identified, only implied.
Anti-CD79b stained extremely rare cells throughout the villi at all ages and may suggest mature B cells are not present in the gut (other than in follicles) or unable to be recognized by the antibodies used in this study, presumably due to a lack of expression of the CD79b antigen.
Only older stripe-faced dunnart gut samples had Peyer's patches with germinal centres and follicles apparent. Immunohistochemistry revealed that the follicular mantles of the secondary follicles were rich in CD79b-expressing B cells. This is in contrast to the findings for the tammar wallaby, which lacked follicles (Basden et al. 1997), but was similar to the observations in other metatherians studied to date (Hemsley et al. 1996). In the dunnart gut, interfollicular areas were heavily stained using anti-CD3 and anti-CD5.
When lymphocytes were first stained in the GALT, it was between the time of first teat release (40 days) and the time of weaning (70 days). This may suggest that a response to an increase in antigen load, via the gastrointestinal tract, stimulates GALT development. Changes in the milk composition observed in the brushtail possum (Adamski & Demmer, 1999) presumably also occur in the dunnart, and may also be a contributing factor to the change in gut tissues, or perhaps the development of the gut is reliant on the development or partial maturation of some of the other lymphoid tissues.
A few studies have described the histological appearance of metatherian lungs (Gemmell, 1986; Gemmell & Little, 1982; Gemmell & Selwood, 1994) but no studies have mentioned BALT until recently (Old et al. 2003a; Young et al. 2003). BALT in the stripe-faced dunnart was only found in juvenile and adult animals with the exception of one 57-day-old animal (Old et al. 2003a). Immunohistochemistry provided further information about the compartmentalization of BALT in the dunnart. There were no secondary follicles identified in any of the BALT samples despite clearly identified secondary follicles (mantles and germinal centres) in the spleen, lymph node and GALT samples. Some aggregations in the BALT consisted largely of T cells, whereas others were largely B cells. A larger number of BALT samples is required to confirm the compartmentalization that appears in these samples.
There is considerable variability in the reported presence of BALT in eutherian species; in some this appears to be dependent on antigenic challenge (Pabst & Gehrke, 1990). In healthy tammar wallabies of all ages no BALT has been located (Old & Deane, 2003) but its presence cannot be ruled out because tissues from unhealthy tammar wallabies were not investigated. BALT has been located in adult rufous hare-wallaby (Lagorchestes hirsutus); however, it is not known whether the appearance of BALT occurs in all rufous hare-wallabies or if it was due to antigenic challenge (Young et al. 2003). In the dunnarts, it is not known if BALT in adult animals was observed due to antigenic challenge or if it only occurs in some animals at adulthood.
This is the first study to describe the development of the immune tissues in a Dasyurid using antibodies to CD3, CD5 and CD79b. This study was, however, limited by the amount of suitable tissue and has therefore limited the conclusions that can be made. Despite this, the lymphoid and immunohaematopoietic tissues of the stripe-faced dunnart develop in a similar pattern to that observed histologically (Old et al. 2003a, b, 2004) and was comparable with other metatherian species (Coutinho et al. 1995; Baker et al. 1999; Cisternas & Armati, 2000), although this study provides some more specific details on the timing of maturation. The young stripe-faced dunnart has no mature T and B cells at the time of birth (Table 1), although T cells were present in the thymus and lymph nodes in the earliest samples tested (day 12 and day 15, respectively), and no mature T and B cells were present in the youngest spleen or GALT samples tested. This suggests that dunnarts do not have mature lymphocytes in these tissues until the end of the second week after birth; in the case of the thymus and lymph nodes, however, T cells may be present before the end of the second week. Regardless of this, B cells were lacking in several of the tissues for longer periods of time in the dunnart compared with other marsupials (Table 2).
A lack of mature T and B cells in the newborn dunnart suggests that the neonate is unable to mount immune defences shortly after birth. This is consistent with reports on immune function in a small number of other marsupials. Grey short-tailed opossums (Monodelphis domestica) and quokkas (Setonix brachyurus) are unable to mount inflammatory immune responses to skin wounds (Rowlands, 1970) and quokkas are unable to reject skin allografts (Waring et al. 1978) shortly after birth. In the neonatal dunnart protection via immunoglobulins and immunological cells in the milk may occur as observed in quokkas, tammar wallabies, koalas and the Virginian opossum (Didelphis virginiana) (Yadav & Eadie, 1973; Deane & Cooper, 1984; Jansen et al. 1994; Young et al. 1997; Young & Deane, 2001). Clearly, more studies are required to investigate the survival strategy of the dunnart from the time the dunnart is born until the immune tissues develop and it is able to mount its own immune responses. In addition, further studies on a greater number of ideally preserved samples will presumably provide a more precise time at which mature lymphocytes appear in the immune tissues of the stripe-faced dunnart.
Acknowledgments
We would like to thank Dr Paula Cisternas (Sydney University, Sydney, Australia) for her kind donation of dunnart specimens and Ms Lisa Masini, Ms Caroline Wilson and Ms Anne Peck for their technical assistance. The support of the ARC to L.S. is acknowledged as well as the helpful comments provided by the reviewers.
References
- Adamski FM, Demmer J. Two stages of increased IgA transfer during lactation in the marsupial, Trichosurus vulpecula (Brushtail possum) J. Immunol. 1999;162:6009–6015. [PubMed] [Google Scholar]
- Baker ML, Gemmell E, Gemmell RT. Ontogeny of the brushtail possum, Trichosurus vulpecula. Anat. Rec. 1999;256:354–365. doi: 10.1002/(SICI)1097-0185(19991201)256:4<354::AID-AR3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Basden K, Cooper DW, Deane EM. Development of the blood-forming tissues of the tammar wallaby Macropus eugenii. Report Fert. Dev. 1996;8:989–994. doi: 10.1071/rd9960989. [DOI] [PubMed] [Google Scholar]
- Basden K, Cooper DW, Deane EM. Development of the lymphoid tissues of the tammar wallaby Macropus eugenii. Report Fert. Dev. 1997;9:243–254. doi: 10.1071/r96032. [DOI] [PubMed] [Google Scholar]
- Block M. The blood forming tissues of the newborn opossum (Didelphys virginiana). I. Normal development through about the one hundredth day of life. Ergeb. Anat. Entwick. 1964;37:1–237. [PubMed] [Google Scholar]
- Canfield P, Hemsley S, Connolly J. Histological and immunological study of the developing and involuting superficial cervical thymus in the koala (Phascolarctos cinereus) J. Anat. 1996;189:159–169. [PMC free article] [PubMed] [Google Scholar]
- Cisternas PA, Armati PJ. Development of the thymus, spleen, lymph nodes and liver in the marsupial, Isoodon macrourus (Northern brown bandicoot, Peramelidae) Anat. Embryol. 1999;200:433–443. doi: 10.1007/s004290050293. [DOI] [PubMed] [Google Scholar]
- Cisternas PA, Armati PJ. Immune system cell markers in the northern brown bandicoot, Isoodon macrourus. Dev. Comp. Immunol. 2000;24:771–782. doi: 10.1016/s0145-305x(00)00030-6. [DOI] [PubMed] [Google Scholar]
- Coutinho H, Nogueira J, King G, Coutinho V, Robalinho T, Amorim A, et al. Immunocytochemical study of the ontogeny of Peyer's patches in the Brazilian marsupial Didelphis albiventris. J. Anat. 1994;185:347–354. [PMC free article] [PubMed] [Google Scholar]
- Coutinho HB, Sewell HF, Tighe P, King G, Nogueira JC, Robalinho TI, et al. Immunocytochemical study of the ontogeny of the marsupial Didelphis albiventris immune system. J. Anat. 1995;187:37–46. [PMC free article] [PubMed] [Google Scholar]
- Cronin L. Key Guide to Australian Mammals. Port Melbourne: Reed books; 1996. [Google Scholar]
- Deane EM, Cooper DW. Immunology of pouch young marsupials. I. Levels of immunoglobulin transferrin and albumin in the blood and milk of euros and wallaroos (Hill kangaroos: Macropus robustus, Marsupialia) Dev. Comp. Immunol. 1984;8:863–876. doi: 10.1016/0145-305x(84)90069-7. [DOI] [PubMed] [Google Scholar]
- Dickman CR, Read DG. Species Management Report No11: the Biology and Management of Dasyurids of the Arid Zone in N.S.W. Sydney: New South Wales National Parks and Wildlife Service; 1992. [Google Scholar]
- von Gaudecker B. Functional histology of the human thymus. Anat. Embryol. 1991;183:1–15. doi: 10.1007/BF00185830. [DOI] [PubMed] [Google Scholar]
- Gemmell RT, Little GJ. The structure of the lung of the newborn marsupial bandicoot, Isoodon macrourus. Cell Tissue Res. 1982;223:445–453. doi: 10.1007/BF01258501. [DOI] [PubMed] [Google Scholar]
- Gemmell RT. Lung development in the marsupial bandicoot, Isoodon macrourus. J. Anat. 1986;148:193–204. [PMC free article] [PubMed] [Google Scholar]
- Gemmell RT, Selwood L. Structural development in the newborn marsupial, the striped-faced dunnart, Sminthopsis macroura. Acta Anat. 1994;149:1–12. doi: 10.1159/000147549. [DOI] [PubMed] [Google Scholar]
- Godfrey GK. Reproduction in a laboratory colony of the marsupial mouse Sminthopsis larapinta (Marsupialia: Dasyuridae) Aust. J. Zool. 1969;17:637–654. [Google Scholar]
- Hanger JJ, Heath TJ. The arrangements of gut-associated lymphoid tissue and lymph pathways in the koala, Phascolarctos cinereus. J. Anat. 1994;185:129–134. [PMC free article] [PubMed] [Google Scholar]
- Hemsley SW, Canfield PJ, Husband AJ. Immunohistological staining of lymphoid tissue in four Australian marsupial species using species cross-reactive antibodies. Immunol. Cell Biol. 1995;73:321–325. doi: 10.1038/icb.1995.49. [DOI] [PubMed] [Google Scholar]
- Hemsley SW, Canfield PJ, Husband AJ. Histological and immunohistological investigation of alimentary tract lymphoid tissue in the koala (Phascolarctos cinereus), brushtail possum (Trichosurus vulpecula) and ringtail possum (Pseudocheirus peregrinus) J. Anat. 1996;188:279–288. [PMC free article] [PubMed] [Google Scholar]
- Hemsley S, Canfield PJ. The roles of histology and immunohistology in the investigation of marsupial disease and normal lymphoid tissue. Dev. Comp. Immunol. 2000;24:455–471. doi: 10.1016/s0145-305x(00)00009-4. [DOI] [PubMed] [Google Scholar]
- Jansen AM, Madeirea FB, Deane MP. Trypanosoma cruzi infection in the opossum Didelphis marsupialis: absence of neonatal transmission and protection by maternal antibodies in experimental infections. Mem. Inst. Os. Cruz. 1994;89:41–45. doi: 10.1590/s0074-02761994000100008. [DOI] [PubMed] [Google Scholar]
- Jones M, Cordell J, Beyers A, Tse A, Mason D. Detection of T and B cells in many animal species using cross-reactive anti-peptide antibodies. J. Immunol. 1993;150:5429–5435. [PubMed] [Google Scholar]
- Old JM, Deane EM. Development of the immune system and immunological protection in marsupial pouch young. Dev. Comp. Immunol. 2000;24:445–454. doi: 10.1016/s0145-305x(00)00008-2. [DOI] [PubMed] [Google Scholar]
- Old JM, Deane EM. Histology and immunohistochemistry of the gut-associated lymphoid tissue of the Eastern Grey Kangaroo, Macropus giganteus. J. Anat. 2001;199:657–662. doi: 10.1046/j.1469-7580.2001.19960657.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old JM, Deane EM. Immunohistochemistry of the lymphoid tissues of the tammar wallaby, Macropus eugenii. J. Anat. 2002a;201:257–266. doi: 10.1046/j.1469-7580.2002.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old JM, Deane EM. The gut-associated lymphoid tissues of a juvenile Northern brown bandicoot (Isoodon macrourus) Dev. Comp. Immunol. 2002b;26:841–848. doi: 10.1016/s0145-305x(02)00031-9. [DOI] [PubMed] [Google Scholar]
- Old JM, Deane EM. The detection of mature T- and B-cells during development of the lymphoid tissues of the tammar wallaby (Macropus eugenii) J. Anat. 2003;203:123–131. doi: 10.1046/j.1469-7580.2003.00207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Old JM, Selwood L, Deane EM. A histological investigation of the adult immune tissues of the stripe-faced dunnart (Sminthopsis macroura) Cell Tissues Org. 2003a;173:115–121. doi: 10.1159/000068946. [DOI] [PubMed] [Google Scholar]
- Old JM, Selwood L, Deane EM. A histological investigation of the development of the lymphoid tissues of the adult stripe-faced dunnart (Sminthopsis macroura) Cell Tissues Org. 2003b;175:192–201. doi: 10.1159/000074941. [DOI] [PubMed] [Google Scholar]
- Old JM, Selwood L, Deane EM. A developmental investigation of the liver, bone marrow and spleen of the stripe-faced dunnart (Sminthopsis macroura) Dev. Comp. Immunol. 2004;28:347–355. doi: 10.1016/j.dci.2003.08.004. [DOI] [PubMed] [Google Scholar]
- Pabst R, Gehrke I. Is the bronchus-associated lymphoid tissue (BALT) an integral structure of the lung in normal mammals, including humans? Am. J. Resp. Cell Mol. Biol. 1990;3:131–135. doi: 10.1165/ajrcmb/3.2.131. [DOI] [PubMed] [Google Scholar]
- Poppema S, Bhan AK, Reinherz EL, McCluskey RT, Schlossman SF. Distribution of T cell subsets in human lymph nodes. J. Exp. Med. 1981;153:30–41. doi: 10.1084/jem.153.1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poskitt DC, Duffey K, Barnett J, Kimpton WG, Muller HK. The gut-associated lymphoid system of two species of Australian marsupial mice, Antechinus swainsonii and Antechinus stuartii. Distribution, frequency and structure of Peyer's patches and lymphoid follicles in the small and large intestine. Aust. J. Exp. Biol. Med. Sci. 1984;62:89–99. doi: 10.1038/icb.1984.8. [DOI] [PubMed] [Google Scholar]
- Rowlands RT. The immune response of adult opossums (Didelphis virginiana) to the bacteriophage f2. Immunology. 1970;18:149–155. [PMC free article] [PubMed] [Google Scholar]
- Selwood L, Woolley PA. A timetable of embryonic development and ovarian and uterine changes during pregnancy in the stripe-faced dunnart Sminthopsis macroura (Marsupialia: Dasyuridae) J. Reprod. Fertil. 1991;91:213–227. doi: 10.1530/jrf.0.0910213. [DOI] [PubMed] [Google Scholar]
- Selwood L, Hickford D. Early cell lineages in marsupial embryos. In: Moody SA, editor. Cell Fate and Cell Determination. New York: Academic Press; 1999. pp. 505–519. [Google Scholar]
- Waring H, Holmes R, Cockson A, Ashman RB, Stanley NF. Induction of long-term tolerance in the quokka (Setonix brachyurus) by thymus and skin allografts into early pouch young. Aust. J. Exp. Biol. Med. Sci. 1978;56:597–604. doi: 10.1038/icb.1978.66. [DOI] [PubMed] [Google Scholar]
- Yadav M, Eadie M. Passage of maternal immunoglobulins to the pouch young of a marsupial, Setonix brachyurus. Aust. J. Zool. 1973;21:171–181. [Google Scholar]
- Young L, Basden K, Cooper D, Deane E. Cellular components of the milk of the tammar wallaby (Macropus eugenii) Aust. J. Zool. 1997;45:423–433. [Google Scholar]
- Young LJ, Deane EM. Cellular composition of the late milk of the koala (Phascolarctos cinereus) Aust. J. Zool. 2001;49:195–202. [Google Scholar]
- Young LJ, McFarlane R, Slender AL, Deane EM. Histological and immunohistological investigation of the lymphoid tissue in normal and mycobacteria-affected specimens of the Rufous Hare-wallaby (Lagorchestes hirsutus) J. Anat. 2003;207:315–325. doi: 10.1046/j.1469-7580.2003.00165.x. [DOI] [PMC free article] [PubMed] [Google Scholar]