Abstract
Interactions between Wnts, Fgfs and Tbx genes are involved in limb initiation and the same gene families have been implicated in mammary gland development. Here we explore how these genes act together in mammary gland initiation. We compared expression of Tbx3, the gene associated with the human condition ulnar–mammary syndrome, expression of the gene encoding the dual-specificity MAPK phosphatase Pyst1/MKP3, which is an early response to FGFR1 signalling (as judged by sensitivity to the SU5402 inhibitor), and expression of Lef1, encoding a transcription factor mediating Wnt signalling and the earliest gene so far known to be expressed in mammary gland development. We found that Tbx3 is expressed earlier than Lef1 and that Pyst1 is also expressed early but only transiently. Patterns of expression of Tbx3, Pyst1 and Lef1 in different glands suggest that the order of mammary gland initiation is 3, 4, 1, 2 and 5. Consistent with expression of Pyst1 in the mammary gland, we detected expression of Fgfr1b, Fgf8 and Fgf9 in both surface ectoderm and mammary bud epithelium, and Fgf4 and Fgf17 in mammary bud epithelium. Beads soaked in FGF-8 applied to the flank of mouse embryos, at a stage just prior to mammary bud initiation, induce expression of Pyst1 and Lef1 and maintain Tbx3 expression in flank tissue surrounding the bead. Grafting beads soaked in the FGFR1 inhibitor, SU5402, abolishes Tbx3, Pyst1 and Lef1 expression, supporting the idea that FGFR1 signalling is required for early mammary gland initiation. We also showed that blocking Wnt signalling abolishes Tbx3 expression but not Pyst1 expression. These data, taken together with previous findings, suggest a model in which Tbx3 expression is induced and maintained in early gland initiation by both Wnt and Fgf signalling through FGFR1.
Keywords: embryonic mammary gland, Fgfs, T-box, Wnt
Introduction
The development of embryonic mammary glands is not only an important process in its own right but is also a classic example of epithelial–mesenchymal interactions. The later stages are also under hormonal action (Hennighausen & Robinson, 2001; Veltmaat et al. 2003). Relatively little is known about how mammary gland development is initiated in correct locations in different mammals, although there are now several mouse mutants in which absence of mammary glands and/or abnormal development of the glands has been reported (van Genderen et al. 1994; Wysolmerski et al. 1998; Lewis et al. 1999; Bocchinfuso et al. 2000; Lewis et al. 2001; Spencer-Dene et al. 2001; Mailleux et al. 2002; Medina et al. 2002; Satokata et al. 2002; Davenport et al. 2003; Pispa et al. 2003). For other organs, such as limbs, it has been shown that signalling networks involving precise interactions between Wnt, Fgf and Tbx genes are required for initiation (Takeuchi et al. 2003). Members of the same families of genes have also been implicated in mouse mammary gland development (reviewed in Veltmaat et al. 2003). Here, we determine how these genes are integrated in mammary gland initiation.
In mice, the development of mammary glands involves a spacing pattern of repeated structures in which five individual mammary glands (designated Mb1 to Mb5, reading anterior–posterior) form along each of two mammary lines on the ventral side of the body (Turner & Gomez, 1933). The early development of mammary glands begins in the ectoderm of the ventral flanks on both sides of the embryo around E10.5. By E11.5–E12.5, five mammary placodes on each side can be detected. These epithelial thickenings then enlarge into bud-like structures. At E13.5, the mammary buds sink into the underlying mesenchyme (Turner & Gomez, 1933; Veltmaat et al. 2003).
Wnt genes have been suggested to play key roles in glandular development, including the induction of mouse mammary glands (Gavin & McMahon, 1992; Weber-Hall et al. 1994). Evidence in favour of this suggestion comes from the regulated expression of these genes in mammary development as well as their proliferative effects on mammary epithelial cells when overexpressed (Wong et al. 1994; Bradbury et al. 1995). Wnt10b and Wnt12, in particular, have been detected in a presumptive mammary line between the fore- and hind limb of E11.5 mouse embryos (Christiansen et al. 1995) and in the mammary placodes at E12.5 (Veltmaat et al. 2003). Lef1, a gene encoding a transcription factor involved in the canonical Wnt signalling pathway, is expressed in the ectoderm of presumptive placode regions, first as a short line of expression that then resolves to a dot shape via a comet-like intermediate (van Genderen et al. 1994; Mailleux et al. 2002). Later, Lef1 is expressed in mammary mesenchyme (Satokata et al. 2002). In mice carrying the TOPGAL reporter for Wnt signaling, blue cells can be detected in an arc between fore- and hindlimb at E10.5 (DasGupta & Fuchs, 1999; reviewed in Veltmaat et al. 2003). However, Lef1−/– embryos have mammary placodes (van Genderen et al. 1994) but these fail to develop past the bud stage and eventually disappear. Thus, Lef1 does not seem to be essential for placode induction but might participate later in cell fate determination.
Several genes encoding Fgf ligands are known to be expressed during early mammary gland development together with the gene that encodes a receptor, to which they are known to bind. Fgfr2b has been reported to be expressed in mouse mammary ectoderm at E11.5 and E15.5 (Mailleux et al. 2002), Fgf7 in mammary mesenchyme at E12.5 and Fgf10 in mammary placode at E11.5. Mouse embryos lacking Fgf10 gene function fail to develop mammary placodes 1, 2, 3 and 5, whereas placode 4 is unaffected (Mailleux et al. 2002), suggesting that Fgf10 signalling is required to initiate development of mammary glands 1–4.
We recently reported that Pyst1/MKP3, an immediate early gene encoding a negative regulator of FGF signalling (Eblaghie et al. 2003), is expressed in embryonic mouse mammary glands (Dickinson et al. 2002). In the limb, Pyst1/MKP3 is expressed in mesenchyme in response to FGF signalling but is not expressed in ectoderm, suggesting that expression of this gene is mediated via Fgfr1/2c, which is expressed in limb mesenchyme, but not via Fgfr2b, which is expressed in limb ectoderm. The fact that Pyst1 is expressed in mammary glands suggests that FGF signalling via Fgfr1/2c receptors may play a role in mammary gland initiation.
Tbx2 and Tbx3, members of the T-box family of transcription factors, are also expressed in the early mammary rudiment (Chapman et al. 1996). Tbx3 mutations in humans (ulnar–mammary syndrome) lead to severe mammary hypoplasia, or sometimes a complete lack of mammary glands (Bamshad et al. 1997). Moreover, Tbx3 null mice do not form mammary buds and Wnt10b and Lef1 expression is absent (Davenport et al. 2003). This suggests that Tbx3 is upstream of Wnt signalling in mammary gland initiation.
During limb initiation, Tbx genes act in concert with Wnt and Fgf signals in both mesenchyme and ectoderm (Takeuchi et al. 2003). There is evidence that Tbx genes lie upstream of Wnt and Fgf genes and that these signals in turn maintain Tbx gene expression. Consequently, expression of genes encoding Wnt and Fgf signals and Tbx3 is mutually sustained. Indeed, Tbx5 misexpression in the interlimb region in chick embryos induces formation of additional forelimb-like structures with ectopic expression of Fgf10 and Wnt2b in lateral plate mesoderm (Rallis et al. 2003). Not only is there a Wnt–Fgf autoregulatory loop in the presumptive limb mesenchyme, but a similar loop is also involved in formation and maintenance of the apical ectodermal ridge. In chick embryos, Wnt2b can induce Fgf10 in the limb lateral plate mesoderm, and Fgf10 can, in turn, induce and maintain expression of Fgf8 in surface ectoderm (Kengaku et al. 1998) via Wnt3a (Kawakami et al. 2001). Thus, an elegant cascade involving alternating Wnt and FGF signalling is established in early limb bud development.
Here we focus on relationships between Wnt and FGF signalling and Tbx3 in initiation of mammary gland development. We compare early expression patterns of Lef1, Pyst1 and Tbx3. We manipulate FGF signalling by either applying FGFs or blocking FGF signalling with an inhibitor (SU5402) and examine the effects on expression of these genes. We also investigate whether Wnts participate in FGF signalling and maintenance of Tbx3 expression by specifically inhibiting Casein Kinase I, a factor implicated in the canonical Wnt pathway.
Materials and methods
Experimental animals
Adult mice were housed in a temperature-controlled room (22 °C) under artificial illumination (lights on from 05:00 h to 17:00 h) and at 55% relative humidity, with access to food and water ad libitum. B6D2F1 (C57/B6 females × B6 males matings) mouse embryos were obtained from time-mated pregnant mice. The day a vaginal plug was confirmed was designated embryonic day 0.5 (E0.5).
In situ hybridization
In situ hybridization on whole mouse embryos was performed as previously described (Wilkinson & Nieto, 1993; Nieto et al. 1996) and on wax sections using standard protocols. Briefly, embryos were fixed in 4% paraformaldehyde (PFA), embedded in wax and sectioned at 9 µm. Sections were baked at 65 °C, de-waxed in Xylene, rehydrated through a graded series of alcohol washes and post-fixed in 4% PFA. Sections were prehybridized in a humid chamber containing 50% formamide in 2×SSC, at 55 °C, for 30 min. Digoxigenin-labelled RNA probes were prewarmed at 95 °C and hybridized to sections overnight at 75 °C. The following mouse DNA plasmids were used as templates for the synthesis of DIG-labelled RNA probes: a 506-bp Tbx3 probe previously described (Tumpel et al. 2002); a 1.1-kb Pyst1/MKP3 probe provided by Dr Steve Keyse; a 360-bp Lef1 probe (Dr Yi-Ping Chen); Fgf4 (620 bp), Fgf7 (227 bp), Fgf8 (875 bp), Fgf9 (900 bp), Fgf10 (584 bp) and Fgf17 (700 bp) (Dr Gail Martin); Fgfr1 (133 bp) (Dr David Rice). Frozen sections were prepared for photomicrography according to Stern (1993).
In vitro organ culture
B6D2F1 mouse embryos were isolated at stages just prior to mammary bud formation (approximately E10.75). Individual embryos were dissected into left and right halves using a fine tungsten needle to bissect the neural tube. Flank tissues were placed on Whatman Nuclepore Track-etch filter membranes (BDH), laid on top of stainless steel grids (a kind gift of Professor Rolf Zeller) in sterile multiwell culture dishes and cultured at the air–medium interface with the culture medium replaced every 24 h at 37 °C and 7.5% CO2 for 48 h. The culture medium (BGJb, Sigma) was supplemented with 20 µg mL−1 (v/v) ascorbic acid (Sigma) and 1% (v/v) penicillin/streptomycin. Tissues were then fixed and processed for in situ hybridization.
Bead implantation
Heparin beads (Sigma H-5263) were incubated in 1 mg mL−1 FGF8 (R & D Systems) for 1 h at room temperature. PBS-soaked beads were used as controls. AG-1 X2 (Bio-Rad Laboratories) formate-derived beads were incubated in 10 mm SU5402 (a kind gift from Sir Philip Cohen) or 20 mm CKI-7 (Seikagaku, USA) for 1 h at room temperature. DMSO beads were used as controls. Beads were implanted in the flank region along the mammary line of E10.75 mouse embryos.
Histological observation
Cultured tissues were fixed in 4% formamide in PBS (pH 7.4) overnight at 4 °C. After washing in PBS, tissues were treated with 20% sucrose in PBS overnight at 4 °C and subsequently embedded in gelatin. Ten-micrometre-thick frozen sections were cut and de-gelatinized in warm PBS for 30 min. After tap-water, the sections were stained with haematoxylin and eosin. The tissue was mounted using DAKO glycergel mountant (DAKO Corp.).
Results
Tbx3 and Pyst1 expression in early mammary bud development
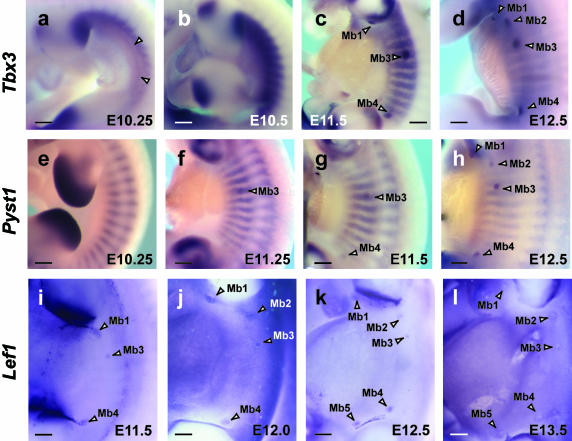
The first signs of mammary gland development can be recognized around E10.5–E11. At E10.25, Tbx3 transcripts are detected in a very thin line, more ventrally, marking what appears to be the mammary line (Fig. 1a). Tbx3 is also expressed more dorsally in a band along the flank between the limb buds. This expression is graded from anterior to posterior, with highest levels of transcripts adjacent to the posterior margin of the forelimb bud (at the level of somite 12), gradually diminishing down the flank to the anterior limit of the hindlimb bud (somite 23). No expression is observed in the flank anterior to the level of the forelimb (somite level 8), although Tbx3 is expressed caudal to the hindlimb. At a similar stage in development, Pyst1 appears as stripes of somitic expression marking epaxial and hypaxial myotome (Dickinson et al. 2002; Fig. 1e).
Fig. 1.
Expression of Tbx3, Pyst1 and Lef1 from E10.25 to E13.5. (a) Tbx3 expressed in a thin line marking what appears to be the mammary line (between two arrowheads). Tbx3 is also expressed in a graded fashion in a thicker band more dorsally with transcripts being more abundant anteriorly. (b) Tbx3 expression at E10.5 is stronger than E10.25 but the line seen at E10.25 cannot be distinguished. (c) E11.5; three pairs of mammary buds expressing Tbx3 become apparent in a dot-like pattern (Mb1, Mb3 and Mb4). (d) E12.5; five pairs of mammary buds expressing Tbx3. (e) E10.25; Pyst1 expressed as stripes marking the somites, and not yet in mammary rudiments. (f) Pyst1 expression first detected in a mammary rudiment (Mb3); at E11.25 it is also in stripes of somitic expression. (g) Pyst1 expression in Mb3 and Mb4 at E11.5. (h) Pyst1 expressed in full complement of mammary buds at E12.5 (Mb1–Mb5). (i) E11.5; Lef1, known as early marker of mammary placode formation, expressed in Mb3 in a dot-like pattern; in Mb1 and Mb4 in a comet-like pattern with a tail. (j) Lef1 expressed separately in Mb2 and Mb1 at E12. (k–l) E12.5 and E13.5; Lef1 expression detected in five pairs of mammary buds. Note that Mb5 is last to form. Mb1–5, mammary buds. Scale bars, 200 µm.
By E10.5, no localized Tbx3 expression is seen in mammary buds but the flank expression of Tbx3 intensifies into a thick, more ventral band (Fig. 1b) and is uniform along the anterior–posterior length of the flank. At this stage, Pyst1 expression remains as stripes marking the somites (data not shown). A few hours of development later (approximately E11), the first mammary bud expressing Tbx3 can be seen in flank ectoderm at the level of somite 16/17, corresponding to the third mammary bud (Mb3) (data not shown). Pyst1 transcripts are also first detected in Mb3 ground (E11.25) (Fig. 1f); at this stage, the stripes of myotomal expression of Pyst1 extend more ventrally along the flank of the embryo marking intercostal muscles.
By E11.5, three mammary buds expressing Tbx3 become apparent – Mb1, Mb3 and Mb4 (Fig. 1c). At this stage, the robust band of Tbx3 expression is reduced to stripes marking what appears to be emerging ribs. Pyst1 expression is first detected in Mb3 at E11.25 (Fig. 1f) then in Mb4 at E11.5 (Fig. 1g). Lef1, reported as one of the earliest genes expressed in early mammary bud rudiments (van Genderen et al. 1994), is also first expressed in Mb3 at E11/11.5, then Mb4 and Mb1 at E11.5/12.
By E12.5, the last mammary buds (Mb2 and Mb5) form. Mb2 emerges in the axillary region at the level of somite 13/14, whereas Mb5 develops as an inguinal rudiment inside the anterior margin of the hindlimb (at the level of somite 23). Both Tbx3 and Pyst1 expression becomes more robust in the full complement of mammary buds (Mb1–Mb5), whereas the stripy expression in the trunk is reduced (Fig. 1d,h). At this stage, mammary bud expression of Tbx3 is more intense. Tbx3 expression persists longer than Pyst1 expression, which is eventually lost shortly after E13. Tbx3 transcripts are still present by E14 (data not shown). Lef1 is still expressed in all five mammary buds at E13.5 (Fig. 1l). Based on these expression patterns, it appears that the mammary buds are initiated along the main body axis in the sequence Mb3, Mb4, Mb1, Mb2 and Mb5.
Expression of Fgf4, Fgf8, Fgf9, Fgf17 and Fgfr1 in embryonic mammary gland
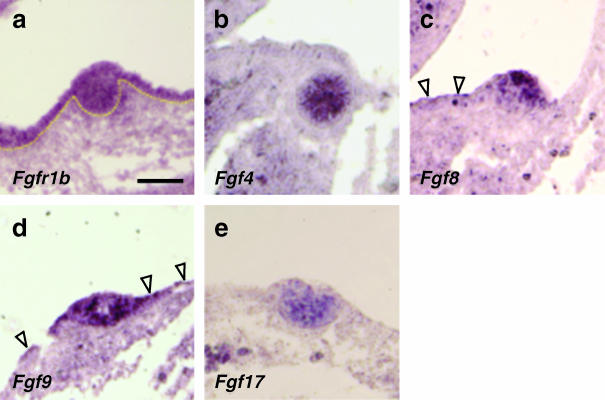
Previous studies on FGF signalling in embryonic mammary gland development focused on the FGF10/FGFR2b signalling pathway (Spencer-Dene et al. 2001; Mailleux et al. 2002). However, the transient expression of Pyst1 in E11.5–E13 mouse mammary buds suggests that a non-FGFR2b signalling pathway may also be involved in early stages of embryonic mammary gland development. We therefore examined expression of Fgfr1 and FGF ligands specific for FGFR1, including Fgf4, Fgf8, Fgf9 and Fgf17 (Ornitz et al. 1996) in sections of mammary glands at early stages of development.
At E12, Fgfr1 is expressed in ectoderm and mammary epithelium (Fig. 2a). Expression of Fgf8 and Fgf9 could also be detected in mammary epithelium and lower-level transcripts in adjacent ectoderm (Fig. 2c,d). Expression of Fgf4 and Fgf17 was detected in mammary epithelium, but not in ectoderm (Fig. 2b,e). We also observed that Fgf7 and Fgf10 are both expressed in mammary epithelium at E12, as previously reported (reviewed in Veltmaat et al. 2003). Fgf7, but not Fgf10, has also been reported to be expressed in surface ectoderm (Mailleux et al. 2002). Fgf7 is also expressed in mesenchyme before it condenses into mammary mesenchyme, whereas mesenchymal Fgf10 expression only appears much later in the fat pad layer at E15.5.
Fig. 2.
Expression of Fgfr1 and FGF ligands specific for FGFR1 in mammary glands at E12. (a) Fgfr1 expression at high levels in ectoderm and mammary epithelium. (b) Fgf4 expression in centre of mammary bud, but not in epidermis. (c) Fgf8 more highly expressed in mammary epithelium than ectoderm (arrowheads). (d) Fgf9 strongly expressed in ectoderm (arrowheads) and in mammary epithelium. (e) Fgf17 expression only detected in mammary epithelium. Scale bars, 100 µm.
FGF8 maintains and/or induces ectopic expression of Tbx3, Pyst1 and Lef1
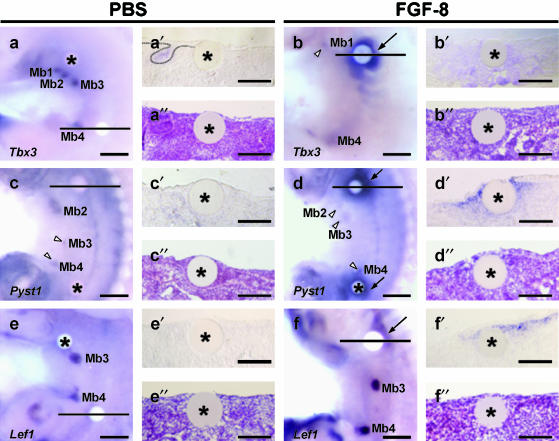
Beads soaked in various FGF proteins, including FGF-1, FGF-2, FGF-4, FGF-8 and FGF-10, can induce ectopic limb buds when grafted into lateral plate mesoderm of early (prelimb bud) chick embryos (Cohn et al. 1995; Ohuchi et al. 1995; Vogel et al. 1996; Crossley et al. 2001) or into mouse flank (Tanaka et al. 2000). In mice, five pairs of mammary epithelial buds emerge at E11.5/12 after limb buds have formed at E9.5 (forelimbs) and E10 (hindlimbs). To test whether exogenous application of FGF on beads could induce ectopic mammary glands, FGF-8 (1 mg mL−1)-soaked beads were grafted onto E10.75 mouse flank just prior to the first signs of mammary gland development. After 48 h of in vitro culture, Tbx3 expression is maintained in flank tissue around FGF-8 bead (7/7 cases; Fig. 3b). A section through the bead reveals Tbx3 in mesenchyme (n = 3; compare Fig. 3b′ with Fig. 3b″). In controls, Tbx3 expression is not maintained around PBS beads (3/3 cases; Fig. 3a) but is confined to mammary buds. Ectopic expression of Pyst1 is also induced in mesenchyme around FGF-8 bead (6/6; Fig. 3d,d′,d″) as is mesenchymal expression of Lef1 (4/6; Fig. 3f,f′,f″). FGF8 induction of Tbx3, Pyst1 and Lef1 occurs predominantly on one side of the FGF bead. However, we could not observe any ectopic mammary buds 48 h after FGF8 bead implantation nor any changes in epithelial morphology.
Fig. 3.
Effects of FGF8 on gene expression in devloping mammary glands. In vitro organotypic cultures 48 h after implanting PBS (a,c,e) or FGF-8 (b,d,f) soaked beads. After whole-mount in situ hybridization for Lef1, Pyst1 and Tbx3 (a,b,c,d,e,f), cultured tissue was sectioned through the bead (a′,b′,c′,d′,e′,f′). Haematoxylin and eosin-stained sections of organ cultures (a″,b″,c″,d″,e″,f″). (a) PBS bead; Tbx3 expression confined to mammary bud. No Tbx3 expression around the bead see also (a′). Ectoderm morphology unaffected by bead (a″). (b) FGF8 bead; Tbx3 expression maintained around FGF-8 bead predominantly dorsally. (b′,b″) Section through the FGF-8 bead reveals Tbx3 in mesenchyme but no change in ectodermal morphology. (c) PBS bead; Pyst1 expression detected in mammary buds but not induced near PBS bead. (c′,c″) No Pyst1 transcripts in either epithelium and mesenchyme near bead and no change in ectoderm. (d) FGF-8 beads; strong Pyst1 expression around the beads (arrows). (d′,d″) Pyst1 expression in mesenchyme and no change in ectodermal morphology. (e) PBS bead; Lef1 expression not induced by PBS bead, but confined to mammary buds. (e′,e″) Section showing no expression of Lef1 in either epithelium and mesenchyme; ectoderm morphology normal. (f) FGF8; Lef1 induced in mesenchyme predominantly dorsal to bead (arrow). (f′,f″) Section through FGF-8 bead; Lef1 expressed in mesenchyme; no change in ectoderm morphology. Mb1–5, mammary buds; arrow, ectopic expression around FGF-8 beads; asterisk, PBS or FGF-8 soaked beads; arrowheads, mammary buds. Scale bars (a–f), 300 µm; (a′–f′, a″–f″), 150 µm.
SU5402 inhibits the effects of adding FGF-8 and affects line-to-bud transition of mammary buds
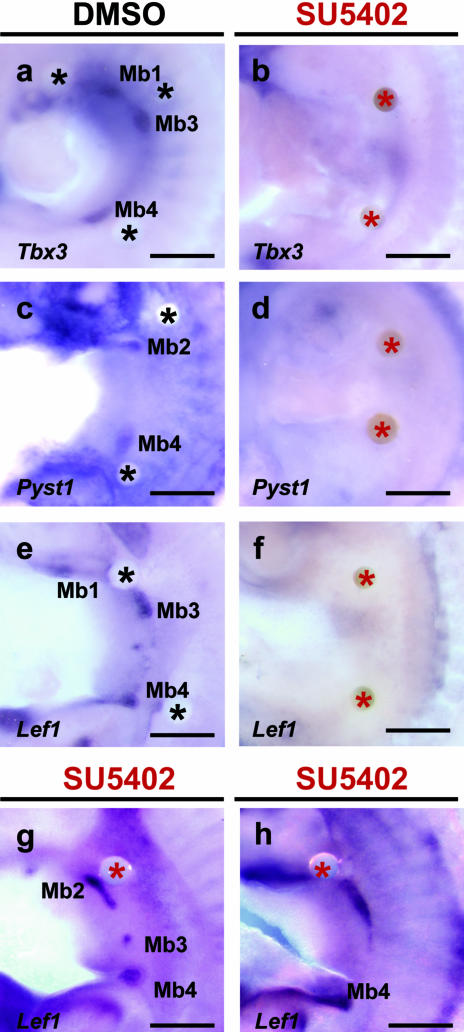
To test the requirement of FGF for maintenance and induction of Tbx3, Pyst1 and Lef1 in mammary gland initiation, we grafted beads soaked in FGFR1 inhibitor, SU5402 (10 mm; Mohammadi et al. 1997) onto the presumptive mammary-forming region of cultured flanks from E10.75 mouse embryos. When two beads were applied for 48 h, Tbx3 expression is completely abolished in flank and mammary buds (3/4 cases; Fig. 4b), compared with DMSO control beads, which have no effect (n = 4; Fig. 4a). Figure 4(a) shows a culture in which three DMSO beads had been grafted and mammary buds 1, 3 and 4 still developed normally. Expression of Pyst1 (n = 5; Fig. 4d) and Lef1 (n = 5; Fig. 4f) also disappears 48 h after SU5402 treatment, whereas Pyst1 (n = 4; Fig. 4c) and Lef1 (n = 4; Fig. 4e) expression is detected in DMSO-treated tissue and corresponds to normal mammary buds. Interestingly, when one SU5402 bead was grafted into flank, Lef1 was expressed in a comet shape (48–72 h, n = 2, Fig. 4g,h). These results indicate that Fgf signalling is required for maintenance of Tbx3, Pyst1 and Lef1 expression in developing mammary buds and may also be involved in the line to bud transition.
Fig. 4.
Effects of FGFR1 inhibitor (SU4502) on gene expression in mammary buds. Flank cultures 48 h after implanting either DMSO beads (a,c,e) or two SU5402 soaked beads (b,d,f). One SU4502 soaked bead applied (g,h). (a) Tbx3 expression; DMSO beads had no effect and Tbx3 expressed normally in mammary buds. (b) Tbx3 expression completely abolished in flank and mammary buds by SU5402 treatment. (c) Pyst1 expressed in mammary buds, but not around DMSO bead. (d) Expression of Pyst1 lost after SU5402 treatment. (e) Lef1 not expressed around DMSO bead. (f) Complete absence of Lef1 expression after SU5402 treatment. (g) One SU5402 bead applied for 48 h to flank; Lef1 in Mb2 remains as comet shape. (h) 72 h culture treated with one SU5402 bead. Lef1 expression in the Mb2 still remains as comet shape. Mb1–5, mammary buds; black asterisk, DMSO soaked beads; red asterisk, SU5402 soaked beads. Scale bars, 300 µm.
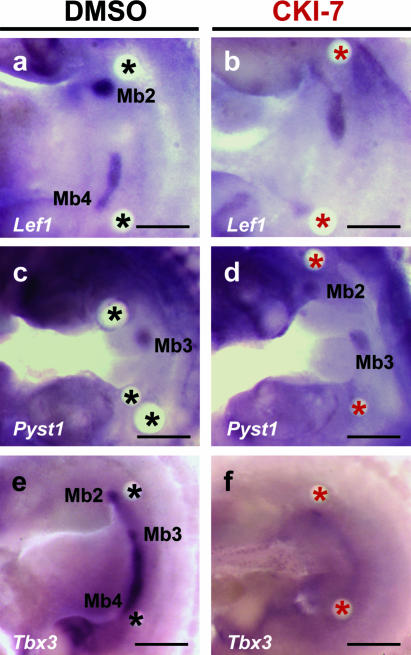
Inhibiting Wnt signalling abolishes Tbx3 expression and mammary bud formation
To determine whether Wnt signalling is involved in maintenance and/or induction of Tbx3 and Pyst1 expression in early mammary bud formation, we treated flanks with beads soaked in Wnt inhibitor, CK1-7 (20 mm), for 48 h. Expression of Lef1, a gene coincident with many known sites of endogenous Wnt signalling and a component of the canonical Wnt pathway, is reduced (compare DMSO control, n = 6, Fig. 5a with CK1-7 bead, 4/6 cases, Fig. 5b). When CK1-7 beads were implanted, Tbx3 expression is completely lost in flank and mammary bud (4/6 cases; Fig. 5f); with DMSO control beads, Tbx3 is expressed in mammary buds as normal (n = 6; Fig. 5e). However, in contrast to the inhibition of Tbx3 expression, Pyst1 expression, an immediate early response to FGFR1 signalling (Eblaghie et al. 2003), is unaffected by implantation of CK1-7 beads (n = 4, Fig. 5d; DMSO control, n = 4, Fig. 5c).
Fig. 5.
Effects of Wnt inhibitor (CK1-7) on gene expression in mammary glands. Flank cultures 48 h after implanting either two DMSO beads (a,c,e) or two CK1-7 beads (b,d,f). (a) Lef1 expressed normally in DMSO treated tissue. (b) Lef1 expression reduced by CK1-7. Note for example weaker expression in Mb4 region. (c) Pyst1 expression; no change with PBS beads. (d) Pyst1 is also not affected by CK1-7 beads. (e) Tbx3 expression; DMSO had no effect. (f) Tbx3 expression completely lost in flank and mammary buds. Mb1–5, mammary buds; black asterisk, DMSO soaked beads; red asterisk, CK1-7 soaked beads. Scale bars, 300 µm.
Discussion
Timing of gene expression in mammary gland initiation
We detected Tbx3 expression in developing mammary glands earlier than expression of Lef1 and Pyst1. Lef1 has previously been reported as the earliest gene to be expressed in mammary gland development (van Genderen et al. 1994). In our study, a line of Tbx3 expression running down the flank and concentrated expression of Tbx3 in the third mammary gland (Mb3) seems to be found at stages prior to expression of Lef1 in Mb1, Mb3 and Mb4 (and Pyst1 in Mb3). The temporal pattern of expression of Tbx3, Lef1 and Pyst1 in the different mammary glands suggests that the sequence of mammary gland initiation is 3, 4, 1, 2, 5. Using Bmp4, Msx1 and Msx2 to visualize mammary buds, we found that the sequence of bud emergence appears to be 3, 1, 2, 4, 5 (data not shown). Based on their observations using Lef1 to visualize mammary glands, Mailleux et al. (2002) reported that mouse mammary buds appear in the order of 3, 4, 5, 1, 2. Of all the genes mentioned, Tbx3 seems to be expressed earliest in developing mammary buds, and the differences in the sequence of mammary bud appearance are probably due to the expression markers used to monitor the development of the glands. Nonetheless, Mb3 is found consistently to be the first to form.
Our analysis of the timing of expression of Tbx3, Lef1 and Pyst1 is consistent with a scheme in which Tbx3 lies upstream of both Wnt and Fgf signalling in mammary gland initiation (see later and Fig. 6). Indeed, in Tbx3 knockout mice, which lack mammary glands, Lef1 and Wnt10b are not expressed (Davenport et al. 2003; see later). Furthermore, in other organs in which Tbx genes are involved in initiation, such as development of limbs (Takeuchi et al. 2003) and lung (Kawashima et al. 1988; Cebra-Thomas et al. 2003; Sakiyama et al. 2003), Tbx genes again seem to lie upstream of Wnt and Fgf signalling. Thus, for example, when dominant negative forms of Tbx5 and Tbx4 are misexpressed in the prospective limb regions of chick embryos, a limbless phenotype results and both Wnt and Fgf expression is repressed (Takeuchi et al. 2003).
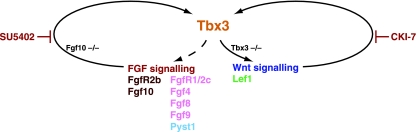
Fig. 6.
Genetic pathway integrating FGF, Wnt and Tbx3 in epithelial–mesenchymal interactions regulating mammary gland initiation. Fgf signalling through FgfR1, blocked by SU5402, maintains Tbx3 expression and leads to expression of Pyst1 and Lef1. Fgf10 signalling is also required for mammary gland development as shown in knock-out mice. Wnt signalling, blocked by CK1-7, also maintains Tbx3 expression. Tbx3 null mutants do not form mammary buds and expression of Wnt10b and Lef1 is absent (Davenport et al. 2003), suggesting that Tbx3 controls their expression. Fgf8, Fgf9, Fgf4, Fgf17 are expressed in ectoderm or mammary knob epithelium. Pyst1 expression depends on signalling through FgfR1.
Tbx3 is initially expressed broadly in ventral mouse flank and then is expressed in a thin line, as has also been reported for Lef1. In contrast, Pyst1 expression is fairly transient and is associated with discrete mammary glands rather than being in a line. The thin line of Tbx3 and Lef1 expression seems likely to represent the so-called mammary line along which discrete mammary glands subsequently develop (reviewed in Veltmaat et al. 2003). The milk line was first seen using scanning electron microscopy in rabbits (Propper, 1978), although this was rather controversial. It is interesting that these patterns of gene expression, together with the line of blue cells seen in TOPGAL mice, provide a visual marker for this structure.
An initial line of gene expression has also been detected in the development of the dorsal feather tract in chick embryos (Jung et al. 1998). A continuous stripe of Shh expression is observed in the dorsal midline of the embryo, where the primary row of feather buds will form. This linear pattern subsequently breaks up into a series of spots of expression. It is not clear whether this change in pattern from a line to a series of spots involves switching off gene expression in intervening cells. Another possibility is that cells expressing the gene aggregate to form spots. In other systems, FGF signalling has been shown to direct cell movement. Interestingly, in some of our experiments in which we inhibited Fgf signalling, the mammary line to spot transition did not occur.
FGFR1 signalling pathway is involved in early mammary gland development
Previous studies have implicated FGF10/FGFR2b signalling in mammary gland development, and expression of genes encoding both ligand and receptor has been reported in mammary epithelium at E11.5 (Mailleux et al. 2002). Our data suggest that FGFR1 signalling is also involved. This possibility was first suggested to us because of the mammary gland expression of Pyst1, which is associated with signalling via FGFR1/FGFR2c in other regions of mouse embryos (Dickinson et al. 2002). Using section in situ hybridization, we were able to detect expression of genes encoding FGF ligands Fgf4, Fgf8, Fgf9 and Fgf17 in the tight mammary knob and/or overlying ectoderm and also expression of the gene encoding the Fgfr1 receptor. Furthermore, the involvement of this Fgf signalling system in mammary gland initiation is supported by the effects of the SU5402 inhibitor on mammary gland development in cultured flanks. This inhibitor, which specifically interferes with FGFR1 signalling, abolished expression of Tbx3, Lef1 and Pyst1 in developing mammary glands. These results suggest that in the mammary gland, as in the developing limb, both FGFR2b and FGFR1/2c signalling are involved in initiation of organ development.
Consistent with a role for FGFR1/2c signalling in mammary gland initiation, we observed ectopic expression of Tbx3, Lef1 and Pyst1 in cultured flanks of mouse embryos in tissue adjacent to beads soaked in FGF8. In the case of Tbx3, this may represent local maintenance of the widespread expression seen prior to mammary gland development. Interestingly, sections showed that the ectopic expression of Tbx3 is in the mesenchyme. Ectopic expression of Lef1 and Pyst1 is also mesenchymal and no ectopic epithelial thickenings were observed. Thus, the issue of whether FGF8 (or other FGFs) can induce ectopic mammary gland formation in the same way as FGF8 (and other FGFs) can induce ectopic limb buds still remains to be resolved (see also Spencer-Dene et al. 2001). The idea that both organs are initiated in the same way is an attractive possibility, but using the same signal to initiate two different structures in a similar region of the embryo during development could present problems. However, work on chick embryos shows that limb induction in response to FGF application can only occur at stages prior to limb development long before mammary glands form.
Mammary gland initiation involves Wnt-dependent regulation of Tbx3
Our results show that inhibition of Wnt signalling abolishes Tbx3 expression in mouse flank region cultured just prior to mammary gland formation. This suggests that Wnt signalling in addition to Fgf signalling is required to induce and/or maintain Tbx3 expression in early mammary glands. Moreover, we found that Pyst1 expression is not affected by the Wnt inhibitor, suggesting that an Fgfr1/2c pathway may be acting independently of a Wnt signal during these early stages of mammary gland development. Although Lef1 expression is reduced when CK1-7 beads are implanted, it does not seem as sensitive to Wnt inhibition as Tbx3 expression. During early tooth development, a canonical Wnt/Lef1 pathway was reported (Kratochwil et al. 2002), with the suggestion that the role of Lef1 is to activate Fgf4 in the dental epithelium, thus connecting the WNT and FGF signalling pathways and allowing sequential and reciprocal communication between epithelium and mesenchyme. It should also be pointed out, however, that the FGF and WNT signalling pathways we are discussing here are found in the epithelial component of the early mammary gland and not in the mesenchyme.
Figure 6 presents a synthesis of our results in the context of previous work. Although there are still many uncertainties, the data point to interactions between Tbx3, Wnt and Fgf signalling being involved in early mammary gland initiation. These interactions are strikingly similar to those that have been dissected out in early limb initiation but that involve Tbx5 and Tbx4 rather than Tbx3. In our scheme, we suggest that Fgf signalling lies both upstream and downstream of Tbx3 expression. It is difficult to distinguish between initiation of Tbx3 expression and maintenance of expression first in the mammary line and then in the bud, but there is good evidence for autoregulation of expression of the Tbx family of genes by Fgf signalling (Isaacs et al. 1994; Schulte-Merker & Smith, 1995; Casey et al. 1998). It is also possible that Wnt signalling also lies both upstream and downstream of Tbx3 expression because in the Tbx3−/– mouse embryos, Wnt10b is not expressed (Davenport et al. 2003). Another common feature between the signalling interactions suggested by results on mammary gland initiation and those in the limb is that FGF signalling through at least two different classes of receptors seems to be involved. FGF signalling not only via FGFR1/2c as we have shown here, but also via FGFR2b (Spencer-Dene et al. 2001; Mailleux et al. 2002), seems to play a role. It is striking that even the same sets of FGF ligands are expressed in both mammary gland and limb, including Fgf4, Fgf8, Fgf9, Fgf10 and Fgf17. In the limb, there is a signalling loop that maintains, reciprocally, Fgf8 and Fgf10 expression. Finally, it is worth bearing in mind that other signalling systems have also been implicated in early mammary gland development, for example Bmp signalling (Phippard et al. 1996; reviewed in Veltmaat et al. 2003). The challenge will be to work out how all these signalling systems act together to initiate mammary gland development.
Acknowledgments
This research was supported by a Royal Society Korea–UK joint project grant to C.T. and H.-S.J. C.T. is also supported by The Royal Society. M.C.E. was supported by a PhD studentship from the Anatomical Society of Great Britain and Ireland, and the British Council. H.-S.J. and S.-J.S. were supported by grant number R13-2003-13 from the Basic Research Programme of the Korea Science & Engineering Foundation.
References
- Bamshad M, Lin RC, Law DJ, Watkins WC, Krakowiak PA, Moore ME, et al. Mutations in human TBX3 alter limb, apocrine and genital development in ulnar–mammary syndrome. Nat. Genet. 1997;16:311–315. doi: 10.1038/ng0797-311. [DOI] [PubMed] [Google Scholar]
- Bocchinfuso WP, Lindzey JK, Hewitt SC, Clark JA, Myers PH, Cooper R, et al. Induction of mammary gland development in estrogen receptor-alpha knockout mice. Endocrinology. 2000;141:2982–2994. doi: 10.1210/endo.141.8.7609. [DOI] [PubMed] [Google Scholar]
- Bradbury JM, Edwards PA, Niemeyer CC, Dale TC. Wnt-4 expression induces a pregnancy-like growth pattern in reconstituted mammary glands in virgin mice. Dev. Biol. 1995;170:553–563. doi: 10.1006/dbio.1995.1236. [DOI] [PubMed] [Google Scholar]
- Casey ES, O'Reilly MA, Conlon FL, Smith JC. The T-box transcription factor Brachyury regulates expression of eFGF through binding to a non-palindromic response element. Development. 1998;125:3887–3894. doi: 10.1242/dev.125.19.3887. [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Bromer J, Gardner R, Lam GK, Sheipe H, Gilbert SF. T-box gene products are required for mesenchymal induction of epithelial branching in the embryonic mouse lung. Dev. Dyn. 2003;226:82–90. doi: 10.1002/dvdy.10208. [DOI] [PubMed] [Google Scholar]
- Chapman DL, Garvey N, Hancock S, Alexiou M, Agulnik SI, Gibson-Brown JJ, et al. Expression of the T-box family genes, Tbx1–Tbx5, during early mouse development. Dev. Dyn. 1996;206:379–390. doi: 10.1002/(SICI)1097-0177(199608)206:4<379::AID-AJA4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Christiansen JH, Dennis CL, Wicking CA, Monkley SJ, Wilkinson DG, Wainwright BJ. Murine Wnt-11 and Wnt-12 have temporally and spatially restricted expression patterns during embryonic development. Mech. Dev. 1995;51:342–350. doi: 10.1016/0925-4773(95)00383-5. [DOI] [PubMed] [Google Scholar]
- Cohn MJ, Izpisua-Belmonte JC, Abud H, Heath JK, Tickle C. Fibroblast growth factors induce additional limb development from the flank of chick embryos. Cell. 1995;80:739–746. doi: 10.1016/0092-8674(95)90352-6. [DOI] [PubMed] [Google Scholar]
- Crossley PH, Martinez S, Ohkubo Y, Rubenstein JL. Coordinate expression of Fgf8, Otx2, Bmp4, and Shh in the rostral prosencephalon during development of the telencephalic and optic vesicles. Neuroscience. 2001;108:183–206. doi: 10.1016/s0306-4522(01)00411-0. [DOI] [PubMed] [Google Scholar]
- DasGupta R, Fuchs E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development. 1999;126:4557–4568. doi: 10.1242/dev.126.20.4557. [DOI] [PubMed] [Google Scholar]
- Davenport TG, Jerome-Majewska LA, Papaioannou VE. Mammary gland, limb and yolk sac defects in mice lacking Tbx3, the gene mutated in human ulnar mammary syndrome. Development. 2003;130:2263–2273. doi: 10.1242/dev.00431. [DOI] [PubMed] [Google Scholar]
- Dickinson RJ, Eblaghie MC, Keyse SM, Morriss-Kay GM. Expression of the ERK-specific MAP kinase phosphatase PYST1/MKP3 in mouse embryos during morphogenesis and early organogenesis. Mech. Dev. 2002;113:193–196. doi: 10.1016/s0925-4773(02)00024-2. [DOI] [PubMed] [Google Scholar]
- Eblaghie MC, Lunn JS, Dickinson RJ, Munsterberg AE, Sanz-Ezquerro JJ, Farrell ER, et al. Negative feedback regulation of FGF signaling levels by Pyst1/MKP3 in chick embryos. Current Biol. 2003;17:1009–1018. doi: 10.1016/s0960-9822(03)00381-6. [DOI] [PubMed] [Google Scholar]
- Gavin BJ, McMahon AP. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell Biol. 1992;12:2418–2423. doi: 10.1128/mcb.12.5.2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Genderen C, Okamura RM, Farinas I, Quo RGP, Arslow TG, Bruhn L, et al. Development of several organs that require inductive epithelial–mesenchymal interations is impaired in LEF-1-deficient mice. Genes Dev. 1994;15:2691–2703. doi: 10.1101/gad.8.22.2691. [DOI] [PubMed] [Google Scholar]
- Hennighausen L, Robinson GW. Signaling pathways in mammary gland development. Dev. Cell. 2001;1:467–475. doi: 10.1016/s1534-5807(01)00064-8. [DOI] [PubMed] [Google Scholar]
- Isaacs HV, Pownall ME, Slack JM. eFGF regulates Xbra expression during Xenopus gastrulation. EMBO J. 1994;3:4469–4481. doi: 10.1002/j.1460-2075.1994.tb06769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HS, Francis-West PH, Widelitz RB, Jiang TX, Ting-Berreth S, Tickle C, et al. Local inhibitory action of BMPs and their relationships with activators in feather formation: implications for periodic patterning. Dev. Biol. 1998;196:11–23. doi: 10.1006/dbio.1998.8850. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Capdevila J, Buscher D, Itoh T, Rodriguez Esteban C, Izpisua Belmonte JC. WNT signals control FGF-dependent limb initiation and AER induction in the chick embryo. Cell. 2001;23:891–900. doi: 10.1016/s0092-8674(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Kawashima T, Tomioka H, Yoshida S. Inhibition of chemical mediator release from human leukocytes and lung fragments by TBX. Arerugi. 1988;37:438–447. [PubMed] [Google Scholar]
- Kengaku M, Capdevila J, Rodriguez-Esteban C, De La Pena J, Johnson RL, Belmonte JC, et al. Distinct WNT pathways regulating AER formation and dorsoventral polarity in the chick limb bud. Science. 1998;280:1274–1277. doi: 10.1126/science.280.5367.1274. [DOI] [PubMed] [Google Scholar]
- Kratochwil K, Galceran J, Tontsch S, Roth W, Grosschedl R. FGF4, a direct target of LEF1 and Wnt signaling, can rescue the arrest of tooth organogenesis in Lef1 (-/-) mice. Genes Dev. 2002;15:3173–3185. doi: 10.1101/gad.1035602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Hui C, et al. The Gli2 transcription factor is required for normal mouse mammary gland development. Dev. Biol. 2001;238:133–144. doi: 10.1006/dbio.2001.0410. [DOI] [PubMed] [Google Scholar]
- Lewis MT, Ross S, Strickland PA, Sugnet CW, Jimenez E, Scott MP, et al. Defects in mouse mammary gland development caused by conditional haploinsufficiency of Patched-1. Development. 1999;126:5181–5193. doi: 10.1242/dev.126.22.5181. [DOI] [PubMed] [Google Scholar]
- Mailleux AA, Spencer-Dene B, Dillon C, Ndiaye D, Savona-Baron C, Itoh N, et al. Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development. 2002;129:53–60. doi: 10.1242/dev.129.1.53. [DOI] [PubMed] [Google Scholar]
- Medina D, Ullrich R, Meyn R, Wiseman R, Donehower L. Environmental carcinogens and p53 tumor-suppressor gene interactions in a transgenic mouse model for mammary carcinogenesis. Environ. Mol. Mutagen. 2002;39:178–183. doi: 10.1002/em.10064. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, McMahon G, Sun L, Tang C, Hirth P, Yeh BK, et al. Structures of the tyrosine kinase domain of fibroblast growth factor receptor in complex with inhibitors. Science. 1997;276:955–960. doi: 10.1126/science.276.5314.955. [DOI] [PubMed] [Google Scholar]
- Nieto MA, Patel K, Wilkinson DG. In situ hybridization analysis of chick embryos in whole mount and tissue sections. Methods Cell Biol. 1996;51:219–235. doi: 10.1016/s0091-679x(08)60630-5. [DOI] [PubMed] [Google Scholar]
- Ohuchi H, Nakagawa T, Yamauchi M, Ohata T, Yoshioka H, Kuwana T, et al. An additional limb can be induced from the flank of the chick embryo by FGF4. Biochem. Biophys. Res. Commun. 1995;209:809–816. doi: 10.1006/bbrc.1995.1572. [DOI] [PubMed] [Google Scholar]
- Ornitz DM, Xu J, Colvin JS, McEwen DG, MacArthur CA, Coulier F, et al. Receptor specificity of the fibroblast growth factor family. J. Biol. Chem. 1996;21:15292–15297. doi: 10.1074/jbc.271.25.15292. [DOI] [PubMed] [Google Scholar]
- Phippard DJ, Weber-Hall SJ, Sharpe PT, Naylor MS, Jayatalake H, Maas R, et al. Regulation of Msx-1, Msx-2, Bmp-2 and Bmp-4 during foetal and postnatal mammary gland development. Development. 1996;122:2729–2737. doi: 10.1242/dev.122.9.2729. [DOI] [PubMed] [Google Scholar]
- Pispa J, Mikkola ML, Mustonen T, Thesleff I. Ectodysplasin, Edar and TNFRSF19 are expressed in complementary and overlapping patterns during mouse embryogenesis. Gene Expr. Patterns. 2003;3:675–679. doi: 10.1016/s1567-133x(03)00092-9. [DOI] [PubMed] [Google Scholar]
- Propper AY. Wandering epithelial cells in the rabbit embryos milk line. Dev. Biol. 1978;67:225–231. doi: 10.1016/0012-1606(78)90311-1. [DOI] [PubMed] [Google Scholar]
- Rallis C, Bruneau BG, Del Buono J, Seidman CE, Seidman JG, Nissim S, et al. Tbx5 is required for forelimb bud formation and continued outgrowth. Development. 2003;130:2741–2751. doi: 10.1242/dev.00473. [DOI] [PubMed] [Google Scholar]
- Sakiyama JI, Yamagishi A, Kuroiwa A. Tbx4–Fgf10 system controls lung bud formation during chicken embryonic development. Development. 2003;130:1225–1234. doi: 10.1242/dev.00345. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 2002;24:391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Smith JC. Mesoderm formation in response to Brachyury requires FGF signalling. Curr. Biol. 1995;5:62–67. doi: 10.1016/s0960-9822(95)00017-0. [DOI] [PubMed] [Google Scholar]
- Spencer-Dene B, Dillon C, Fantl V, Kerr K, Petiot A, Dickson C. Fibroblast growth factor signalling in mouse mammary gland development. Endocr. Relat. Cancer. 2001;8:211–217. doi: 10.1677/erc.0.0080211. [DOI] [PubMed] [Google Scholar]
- Stern K. Court-ordered caesarian sections: in whose interests? Mod. Law. Rev. 1993;56:238–243. doi: 10.1111/j.1468-2230.1993.tb00961.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Suzuki T, Kamimura M, Ogura K, Ogura T. Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade. Development. 2003;130:2729–2739. doi: 10.1242/dev.00474. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Cohn MJ, Ashby P, Davey M, Martin P, Tickle C. Distribution of polarizing activity and potential for limb formation in mouse and chick embryos and possible relationships to polydactyly. Development. 2000;127:4011–4021. doi: 10.1242/dev.127.18.4011. [DOI] [PubMed] [Google Scholar]
- Tumpel S, Sanz-Ezquerro JJ, Isaac A, Eblaghie MC, Dobson J, Tickle C. Regulation of Tbx3 expression by anteroposterior signalling in vertebrate limb development. Dev. Biol. 2002;15:251–262. [PubMed] [Google Scholar]
- Turner CW, Gomez ET. The normal development of the mammary gland of the male and female albino mouse. I. Intrauterine. Mo. Agric. Exp. Stn. Res. Bull. 1933;182:3–20. [Google Scholar]
- Veltmaat JM, Mailleux AA, Thiery JP, Bellusci S. Mouse embryonic mammogenesis as a model for the molecular regulation of pattern formation. Differentiation. 2003;71:117. doi: 10.1046/j.1432-0436.2003.700601.x. [DOI] [PubMed] [Google Scholar]
- Vogel A, Rodriguez C, Izpisua-Belmonte JC. Involvement of FGF-8 in initiation, outgrowth and patterning of the vertebrate limb. Develoment. 1996;122:1737–1750. doi: 10.1242/dev.122.6.1737. [DOI] [PubMed] [Google Scholar]
- Weber-Hall SJ, Phippard DJ, Niemeyer CC, Dale TC. Developmental and hormonal regulation of Wnt gene expression in the mouse mammary gland. Differentiation. 1994;57:205–214. doi: 10.1046/j.1432-0436.1994.5730205.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Nieto MA. Detection of messenger RNA by in situ hybridization to tissue sections and whole mounts. Methods Enzymol. 1993;225:361–373. doi: 10.1016/0076-6879(93)25025-w. [DOI] [PubMed] [Google Scholar]
- Wong GT, Gavin BJ, McMahon AP. Differential transformation of mammary epithelial cells by Wnt genes. Mol. Cell. Biol. 1994;14:6278–6286. doi: 10.1128/mcb.14.9.6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysolmerski JJ, Philbrick WM, Dunbar ME, Lanske B, Kronenberg H, Broadus AE. Rescue of the parathyroid hormone-related protein knockout mouse demonstrates that parathyroid hormone-related protein is essential for mammary gland development. Development. 1998;125:1285–1294. doi: 10.1242/dev.125.7.1285. [DOI] [PubMed] [Google Scholar]