Abstract
The bone morphogenetic protein (BMP) family is emerging as playing a crucial role in regulating normal follicle growth and determining ovulation rate. BMPs exert their effects via BMP receptors (BMPR-IA, -IB and -II). However, there is a paucity of information relating to the expression of the BMPRs within the ovary of large polyovular species such as the pig. Furthermore, there is a lack of information on the expression of BMPRs by fetal ovaries of any species. The purpose of this study was to investigate temporal and spatial expression of the BMPRs in the porcine ovary, at different developmental stages. Immunohistochemistry for BMPR-IA, BMPR-IB and BMPR-II was performed using sections from paraffin wax-embedded ovaries, obtained from fetal (n = 15), prepubertal (n = 3) and cycling postpubertal (n = 4) pigs. Results confirmed the presence of all three receptors in the fetal egg nests and in the granulosa cell layer of follicles ranging from primordial to late antral stages. Immunostaining was also observed in oocytes, theca layer, corpus luteum and ovarian surface epithelium. The expression of BMPRs by fetal ovaries may be related to follicle formation, whereas expression in pre- and post-pubertal animals indicates BMPs are involved in regulating porcine ovarian follicle growth.
Keywords: egg nests, granulosa, growth factor, oocyte, pig
Introduction
The bone morphogenetic proteins (BMPs) are members of the TGF-β superfamily. They are prototypically involved in the formation of bone but have also been shown to be multifunctional cytokines capable of regulating cell proliferation, differentiation, morphogenesis and apoptosis (Hogan, 1996; Reddi, 1998). Currently, there are approximately 20 known BMPs, including proteins also identified as members of the growth and differentiation factor (GDF) family and anti-Müllerian hormone (AMH) as a result of their high sequence homology with the BMPs (Yamashita et al. 1996). Of these factors, several are expressed within the mammalian ovary, including BMPs-4, -6, -7, 15 (GDF-9B) and GDF-9 (Shimasaki et al. 1999; Otsuka et al. 2000; Duffy, 2003; Erickson & Shimasaki, 2003; Nilsson & Skinner, 2003).
As with other members of the TGF-β superfamily, the actions of the BMPs are exerted through cell surface receptors – the BMP receptors (BMPRs). Transduction of a signal requires the formation of a hetero-oligomeric complex of BMPR type II (BMPR-II) and BMPR type IA or IB (for a review, see Massague, 1998; Miyazono et al. 2001). BMPR-II expresses constitutive kinase activity and is thought to be responsible for the activation of the type I receptor once the complex has been formed. Once receptor activation has occurred, a signalling cascade is initiated that involves the phosphorylation, heterodimerization and migration of the Smad proteins to the nucleus (for a review, see Miyazono, 2000). Type I receptor activation depends upon ligand–receptor specificity as different BMPs have differing affinities for the three identified BMPRs. Two of the three type I receptors appear to be exclusively BMP receptors (BMPR-IA/ALK3 and BMPR-IB/ALK6) and a third type I receptor is also bound by activin (ActRI/ALK2; Yamashita et al. 1996). Given that ActRI/ALK2 activation may be brought about by ligands other than the BMPs, this paper will focus upon the two prototypical type I, -IA and -IB, in addition to BMPR-II.
The type I receptors are between 50 and 64 kDa (Tendijke et al. 1993) whereas BMPR-II is approximately 80 kDa in size (Liu et al. 1995). The receptors are structurally similar, possessing a relatively short extracellular domain, a single transmembrane domain and an intracellular domain that expresses intrinsic serine/threonine kinase activity (Yamashita et al. 1994, 1996; Kawabata et al. 1998).
The BMP system has been shown to regulate primordial germ cell formation (Lawson et al. 1999; Ying et al. 2000), and is known to be active in both embryonic and adult tissues. However, a lack of information exists regarding the expression of the components of the BMP system in the fetal ovary, where this system could be involved in the initial stages of follicle development. Post-natal BMPR expression has been identified in mammalian ovaries. Receptors have been localized in the mouse ovary (Yi et al. 2001), the rat ovary (Shimasaki et al. 1999; Erickson & Shimasaki, 2003) and the ovine ovary (Wilson et al. 2001; Souza et al. 2002). In these species the functionality of the ovarian BMP system has also been demonstrated in vitro. Furthermore, both naturally occurring mutations and induced alterations in the ovine BMP system have been shown to lead to alterations in ovarian function and ovulation rate (McNatty et al. 2002; Juengel et al. 2004). Therefore, the apparent importance of the BMP family and its receptors in the regulation of ovarian function is clear. However, there is a paucity of information regarding the expression of BMP system components in the porcine ovary. Furthermore, there are species-dependent variations with regard to the temporal and spatial expression of the BMPRs (Shimasaki et al. 1999; Wilson et al. 2001; Yi et al. 2001). Therefore, the aims of this study were two-fold: first, to confirm the presence of BMPR-IA, -IB and -II in the porcine ovary at different developmental stages: fetal, pre- and post-pubertal, and second, to elucidate the location(s) of BMPR expression using immunohistochemistry.
Materials and methods
All chemicals were obtained from Sigma, Poole, UK, unless otherwise stated.
Tissue and section preparation
Both ovaries were removed from fetal pigs (n = 15, Large White hybrid) within 30 min of maternal death. Crown–rump lengths of all female fetuses contained within every litter were measured to ensure that those selected for the study were not visibly intrauterine growth-restricted and also to estimate gestation age (Martinat-Botté et al. 2000). A range of developmental stages was obtained, between approximately days 46 and 114 of gestation. Day 46 was taken as the lower age limit – the final histological differentiation of the fetal porcine ovary is not complete until around day 44 of gestation (Pailhoux et al. 2001). Both ovaries were collected from pre-pubertal (n = 3), post-pubertal (n = 4) pigs (Large White hybrid) and transported to the laboratory on ice within 1 h as described by Shuttleworth et al. (2002). In all cases ovaries were fixed in neutral buffered formol saline (NBFS) for 24 h (fetal) or 72 h (all other developmental stages), before being processed and wax embedded. Sections of 8 µm thickness were cut and mounted on SuperFrost Plus microscope slides (BDH). Each slide carried two tissue sections with the exception of the larger post-pubertal ovaries where one section was used per slide. Mounted sections were left overnight, at room temperature, in a vertical position, in accordance with the slide manufacturer's recommendations. The following day the slides were placed in a dry oven and baked overnight at 37 °C. Sections of porcine kidney were also fixed in NBFS for 72 h and were processed, wax embedded and sectioned as with the ovarian tissue for use as positive controls.
Immunohistochemistry
Immunohistochemistry was performed using the DAKO EnVision+® Peroxidase System (Dako, Cambridge, UK). The protocol used was developed from an existing protocol (Shuttleworth et al. 2002) in accordance with the manufacturer's instructions. Briefly, sections were heated in 0.01 m citrate buffer for 15 min, at medium power in a domestic microwave oven (850 W), before endogenous peroxidase activity was blocked using a 20-min wash in 3% (v/v) hydrogen peroxide in methanol. The rabbit antisera against BMPR-IA, -IB and -II were kind gifts from Professor C. H. Heldin (Hayashi et al. 1997; Onishi et al. 1998) and were diluted to 1 : 1000, 1 : 100 and 1 : 100, respectively, in 0.2% (v/v) normal goat serum (NGS; University Of Nottingham) in 0.05 m Tris-buffered saline (TBS; pH 7.8). The primary antibody incubation was overnight at 4 °C. The goat anti-rabbit IgG secondary antibody polymer was received as part of the EnVision+® system. Sections from these ovaries have been shown previously not to give falsely positive results in the presence of rabbit non-immune serum (Shuttleworth et al. 2002). Therefore, negative controls comprised both ovary and kidney, processed as above and incubated with 0.2% (v/v) NGS in 0.05 m TBS only.
Three staining runs were performed for each primary antibody (BMPR-IA, -IB and -II). Staining runs were designed to ensure that sections from every ovary underwent two separate staining runs to ensure consistency of staining. Every staining run included a negative control and a positive control. Porcine kidney was chosen as the positive control as recent research has shown the receptors of interest are present in the kidney (Bosukonda et al. 2000; Kopp, 2000).
The intensity of immunostaining for ovarian structures was scored from negative (−) to strongly positive (++++) and an average score obtained for each ovarian structure. Comparisons were made only between tissue samples treated with the same BMPR antibody – no comparisons were made between antibody groups. Counts of germ cells were performed on the fetal tissue to allow the proportions of positively stained cells to be calculated. Sections were randomly labelled to avoid counter bias. Two sections were selected per fetal ovary and within these two microscope fields, cells were counted. Images were captured using Image-Pro® Plus version 5.0 for Windows (Media Cybernetics, Inc., MD, USA).
Results
All positive controls stained for either BMPR-IA, -IB and -II (data not shown). Positive staining for all three receptors was observed in the epithelium of the convoluted tubules, as has been observed previously in the rat (Bosukonda et al. 2000). All negative controls failed to show DAB-positive immunostaining and are presented with the appropriate figures. The relative intensity and location of the staining is summarized in Table 1.
Table 1.
Average immunostaining score of structures within fetal (n = 15), pre-pubertal (n = 3) and post-pubertal (n = 4) ovarian tissue sections. Staining is designated by (–) where staining was absent up to (++++) maximal staining (NA, not applicable)
| Ovary | Follicle status | Ovarian structure | BMPR-IA | BMPR-IB | BMPR-II |
|---|---|---|---|---|---|
| Fetal | NA | Egg nests | +++ | +++ | +++ |
| Primordial | Oocyte | +++ | +++ | +++ | |
| Primordial | Pre-granulosa cells | +++ | +++ | +++ | |
| NA | Surface epithelium | ++ | ++ | ++ | |
| NA | Stroma | +/– | +/– | +/– | |
| Prepubertal | All | Oocyte | +++ | +++ | +++ |
| All | Zona pellucida | + | + | + | |
| Preantral | Granulosa cells | ++ | ++ | ++ | |
| Preantral | Theca cells | +/– | +/– | +/– | |
| Antral | Granulosa cells | +++ | +++ | +++ | |
| Antral | Theca cells | +/– | +/– | +/– | |
| NA | Blood vessels | ++ | ++ | ++ | |
| NA | Stroma | +/– | +/– | +/– | |
| Postpubertal | All | Oocyte | ++ | ++ | ++ |
| All | Zona pellucida | +/– | +/– | +/– | |
| Preantral | Granulosa cells | ++ | ++ | ++ | |
| Preantral | Theca cells | +/– | +/– | +/– | |
| Antral | Granulosa cells | +++ | +++ | +++ | |
| Antral | Theca cells | +/– | +/– | +/– | |
| Luteinizing | Granulosa cells | ++++ | ++++ | ++++ | |
| Luteinized | Corpus luteum (large cells) | +++ | +++ | +++ | |
| Luteinized | Corpus luteum (small cells) | +/– | +/– | +/– | |
| NA | Blood vessels | ++ | ++ | ++ | |
| NA | Stroma | +/– | +/– | +/– |
Fetal ovaries
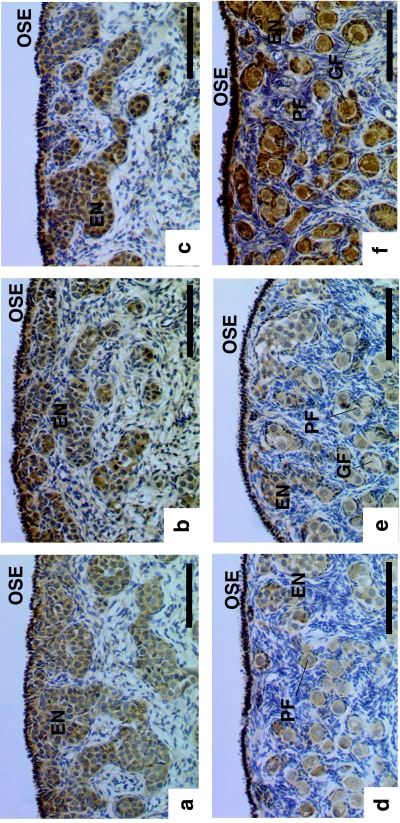
Primordial germ cells were observed to be present within ‘egg nests’, also known as ‘germ cell cords’ (Byskov et al. 1986) and ‘ovigerous cords’ (McNatty et al. 2000; Juengel et al. 2002; Sawyer et al. 2002). The cells contained within these egg nests showed positive immunostaining for all three BMPRs (Fig. 1a–c). Primordial follicles were also present and appeared to be undergoing formation towards the medullary extremes of the egg nests (Fig. 1e,f). Both the oocytes and the granulosa cells of the primordial follicles were observed to stain positively for receptors BMPR-IA, -IB and -II. A high proportion of oocytes were observed to stain positively for all three BMP receptors at all stages of fetal development examined, although there was no correlation between the proportion of oocytes stained and age (P > 0.10 for all). The ovarian surface epithelium was also observed to stain positively for the BMPRs.
Fig. 1.
Immunohistochemical localization of the BMP receptors in the porcine fetal ovary during (a–c) early development (approximately day 46 post-conception (pc)) and (d–f) close to term (approximately day 114 pc). Immunostaining for BMP: (a,d) R-IA; (b,e) R-IB; (c,f) R-II. EN, egg nests; GF, growing follicle; OSE, ovarian surface epithelium; PF, primordial follicle. Scale bars = 100 µm.
Prepubertal ovaries
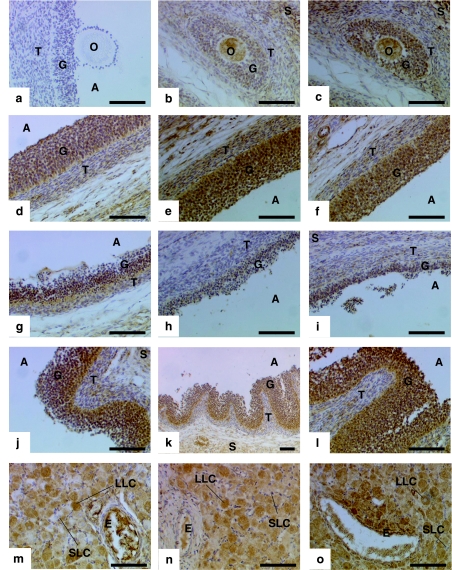
Positive immunostaining for the BMPR-IA, -IB and -II was observed in the oocyte, granulosa cells and theca cells of prepubertal ovaries. Granulosa cells of pre-antral follicles (Fig. 2b,c) were not as intensely stained as those of antral follicles (Fig. 2d–f). This trend was also observed in the thecal layer – cells within the theca interna of both pre-antral (Fig. 2b,c) and antral follicles (Fig. 2d–f) stained positively for the BMPRs. However, the staining was more intense in the theca layer of antral as opposed to pre-antral follicles. Immunolocalization of the BMPRs was also observed in the follicle walls of atretic antral follicles (Fig. 2g,h). Staining was also observed within the oocyte (Fig. 2b,c). Some staining was also observed in regions of the ovarian stroma and blood vessels.
Fig. 2.
Immunohistochemical localization of the BMP receptors in the porcine ovary. Immunostaining for BMP: (d,g,j,m) R-IA; (b,e,h,k,n) R-IB; (c,f,i,l,o) R-II. (a) Negative control. Ovarian structures positively stained included: (b,c) pre-antral follicles; (d–f) healthy antral follicle wall; (g–i) atretic follicle wall; (j–l) follicle wall in early stages of luteinization; (m–o) luteal cells and blood vessels. A, antral space; E, blood vessel endothelial layer; G, granulosa cells; O, oocyte; S, stromal tissue; T, theca cells. Scale bars = 100 µm.
Postpubertal ovaries
Positive staining for all three BMPRs was again observed. Staining was localized within the oocyte, granulosa and theca cells, and was also observed in the stroma and blood vessels therein. The pattern of receptor immunostaining for pre-antral and antral follicles in post-pubertal sections is comparable with that of pre-pubertal sections. In the case of early luteinizing follicles (characterized by the invagination of the follicle wall; Fig. 2j–l) the granulosa cells were more intensely stained than in antral follicles. Theca staining remained relatively pale in both instances. Positive immunostaining for the BMPRs was observed in the large luteal cells, but not all of the small luteal cells of corpora lutea (CLs) and was also observed in the blood vessels (Fig. 2m–o).
Discussion
The results of this study indicate that porcine ovaries at different development stages express receptors for the BMPs. In vitro studies have shown that members of the ovarian BMP family are capable of activating BMPRs (Ebisawa et al. 1999; Vitt et al. 2002). Furthermore, the distribution of these receptors within the ovary implies that the BMPs may be crucially involved in the processes of folliculogenesis and steroidogenesis.
The fetal tissue results provide novel data regarding potential signalling systems present during the initial stages of follicle formation and development. The staining allowed the visualization of distinct populations of germ cells, also termed ‘naked oocytes’, within the fetal ovary organized in ovigerous cords, or fetal egg nests (McNatty et al. 2000; Juengel et al. 2002; Sawyer et al. 2002). A very high proportion of the oocytes and oogonia contained within porcine fetal ovary expressed the BMP receptors from an early stage in development, approximately day 46 of gestation. Currently very little is known regarding the cues that promote egg nest formation and the processes that result in the induction of meiosis and the subsequent formation of primordial follicles. Thus the expression of BMP receptors by oogonia and oocytes within the egg nests from this early stage suggests that BMP signalling may be involved in oogonial proliferation and/or differentiation and, speculatively, the formation of the fetal egg nests.
Primordial follicles were observed to form from approximately day 60 of gestation in the fetal ovaries examined in this study, in accordance with other reports (Mauleon & Mariana, 1977; Christenson et al. 1985). The continued expression of the BMP receptors by both oocytes and pre-granulosa cells during the period of follicle breakout in the pig suggests that the BMP signalling pathway may play a role in this process. Indeed, evidence from a study conducted using neonatal mouse ovaries and an anti-BMP-4 antibody has provided evidence that BMP-4 may be involved in promoting the survival and development of primordial follicles (Nilsson & Skinner, 2003). Positively stained activated primordial follicles were observed – characterized by cuboidal granulosa cells surrounding the oocyte. The presence of positively stained oocytes and (pre)granulosa cells in resting and activated primordial follicles suggests a role for BMPR signalling during follicle activation, as has been suggested in other species (Wilson et al. 2001; Erickson & Shimasaki, 2003). Ovine pre-granulosa cells are thought to be ovarian surface epithelium-derived (Sawyer et al. 2002). Thus, it is of interest that ovarian surface epithelial cells were observed to stain positively for the BMP receptors in this study. It is possible that BMP signalling may be involved in the proliferation of the mesothelial cells and their recruitment as pre-granulosa cells around the oocyte.
Granulosa cells were one of the major sites of BMPR expression at all developmental stages. Localization of BMPRs to granulosa cells suggests a potential role for BMP signalling pathways in the growth and/or steroidogenic function of this cell type. Further evidence suggests that this is true because known and potential ligands for BMPRs, BMPs-2, -6 and -15, have been shown to alter porcine granulosa cell proliferation and steroid production in serum-free culture (Brankin et al. 2003). The exact complement of potential BMPR ligands expressed by porcine follicles throughout the oestrous cycle remains to be determined, although members of the BMP family, such as BMP-15, GDF-9, BMP-6, BMP-4 and BMP-7, have been identified within specific ovarian cell types of a number of other mammalian species (Bodensteiner et al. 1999; Shimasaki et al. 1999; Elvin et al. 2000; Otsuka et al. 2000; Erickson & Shimasaki, 2003). Immunopositive cells were also observed in the theca interna of porcine follicles, although the majority of theca cells did not stain. Similar reports from other species suggest that although BMPR mRNA and protein have been detected in the theca, it is not a site of intense BMPR expression (Shimasaki et al. 1999; Wilson et al. 2001; Yi et al. 2001; Souza et al. 2002; Erickson & Shimasaki, 2003). Localization of BMPRs to theca cells again suggests a role for the BMP system in the control of follicle function. Unlike the theca of other mammals, porcine theca is capable of synthesizing oestradiol (Tonetta et al. 1988; Hunter et al. 1994; Miller et al. 1998; Shores et al. 2000) and evidence gleaned from in vitro cultures has shown that production of this steroid, and also of progesterone and androstenedione, by theca cells was inhibited in response to BMPs-2 or -6, whereas BMP-15 stimulated production of these steroids (Quinn et al. 2004). The difference in BMPR staining intensity between granulosa and theca cells is not yet understood. However, it is clear that both cell types alter steroidogenesis in response to known ligands of the BMPRs. Therefore, signalling via BMPRs in theca may occur in addition to signalling via other TGF-β family receptors, such as the activin receptors (ActR), which have been shown to bind BMPs in osteoprogenitor cells (Aoki et al. 2001). Alternatively, BMP signalling through the less abundant theca cell BMPRs may occur with alternative affinities to that in granulosa cells and in doing so may exert different effects than signalling through the more abundant granulosa cell BMPRs.
Expression of BMPRs by granulosa cells in both healthy and atretic follicles indicates that BMPR expression is constitutive irrespective of cell fate. This trend has also been observed in the atretic follicles of both the rat and the sheep (Shimasaki et al. 1999; Wilson et al. 2001; Erickson & Shimasaki, 2003) and suggests that the BMPs may be involved in the induction/regulation of follicular atresia.
The expression of BMPRs during the early stages of luteinization suggests that BMPs are involved in the regulation of this process. It would also appear that luteal cell expression of BMPRs appears to be related to the cells from which they are derived. In all CLs examined, the large luteal cells stained positively for the BMPRs. However, not all of the small luteal cells showed positive staining. The function of this differential staining is unknown but it is not unexpected as large luteal cells are thought to be derived from granulosa cells (BMPR positive) and small luteal cells derived from theca cells (mostly BMPR negative; O'Shea, 1987; Pitzel et al. 1990; Hansel et al. 1991).
Porcine oocytes were observed to express BMPRs at all developmental stages, indicating a potential role for BMPs in regulating oocyte growth and maturation. In keeping with this, oocyte expression of BMPR mRNAs/proteins have also been observed in other species (Shimasaki et al. 1999; Wilson et al. 2001; Yi et al. 2001; Souza et al. 2002; Erickson & Shimasaki, 2003).
Additionally, BMPR expression was observed in ovarian blood vessels. This is in keeping with reports within the ovary (mRNA; Erickson & Shimasaki, 2003) and in other organs (Atkinson et al. 2002). Investigation into the BMP signalling pathways in endothelial cells has revealed that they are potent stimulators of endothelial cell migration and tube formation in vitro (Valdimarsdottir et al. 2002) and BMPs are known to be capable of stimulating angiogenesis indirectly through production of VEGF-A by osteoblasts (Deckers et al. 2002). Therefore, it is likely that the BMPRs and their ligands contribute to the neovascularization that occurs within the theca during follicle development and during the transformation of follicles into corpora lutea.
In summary, BMPR-IA, -IB and -II have been identified and localized to distinct ovarian structures at different developmental stages, including oocytes, granulosa cells, certain theca cells, luteal cells and blood vessels. Fetal egg nests were localized and shown to express BMPRs in what appears to be a developmental feature. The locations of expression did not differ greatly between receptors. When considered in the wider context of the literature, these results indicate that the BMP receptors, and by default those proteins that activate them, have a number of roles in the mammalian ovary including folliculogenesis, steroidogenesis and oocyte development.
Acknowledgments
We would like to thank J. Corbett for tissue collection, J. Slevin for the provision of NGS and the BBSRC for funding.
References
- Aoki H, Fujii M, Imamura T, Yagi K, Takehara K, Kato M, et al. Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction. J. Cell Sci. 2001;114:1483–1489. doi: 10.1242/jcs.114.8.1483. [DOI] [PubMed] [Google Scholar]
- Atkinson C, Stewart S, Upton PD, Machado R, Thomson JR, Trembath RC, et al. Primary pulmonary hypertension is associated with reduced pulmonary vascular expression of type II bone morphogenetic protein receptor. Circulation. 2002;105:1672–1678. doi: 10.1161/01.cir.0000012754.72951.3d. [DOI] [PubMed] [Google Scholar]
- Bodensteiner KJ, Clay CM, Moeller CL, Sawyer HR. Molecular cloning of the ovine growth/differentiation factor-9 gene and expression of growth/differentiation factor-9 in ovine and bovine ovaries. Biol. Reprod. 1999;60:381–386. doi: 10.1095/biolreprod60.2.381. [DOI] [PubMed] [Google Scholar]
- Bosukonda D, Shih MS, Sampath KT, Vukicevic S. Characterization of receptors for osteogenic protein-1/bone morphogenetic protein-7 (OP-1/BMP-7) in rat kidneys. Kid. Internat. 2000;58:1902–1911. doi: 10.1111/j.1523-1755.2000.00362.x. [DOI] [PubMed] [Google Scholar]
- Brankin V, Quinn RL, McGarr C, Webb R, Hunter MG. BMPs 2, 6 and 15 are regulators of porcine granulosa cell function in vitro. Reprod. Abstract Series. 2003;30:45. [Google Scholar]
- Byskov AG, Hoyer PE, Bjorkman N, Mork AB, Olsen B, Grinsted J. Ultrastructure of germ cells and adjacent somatic cells correlated to initiation of meiosis in the fetal pig. Anat. Embryol. 1986;175:57–67. doi: 10.1007/BF00315456. [DOI] [PubMed] [Google Scholar]
- Christenson RK, Ford JJ, Redmer DA. Maturation of ovarian follicles in the prepubertal gilt. J. Reprod. Fertil. Suppl. 1985;33:21–36. [PubMed] [Google Scholar]
- Deckers MML, van Bezooijen RL, van der Horst G, Hoogendam J, van der Bent C, Papapoulos SE, et al. Bone morphogenetic proteins stimulate angiogenesis through osteoblast-derived vascular endothelial growth factor A. Endocrinology. 2002;143:1545–1553. doi: 10.1210/endo.143.4.8719. [DOI] [PubMed] [Google Scholar]
- Duffy DM. Growth differentiation factor-9 is expressed by the primate follicle throughout the periovulatory interval. Biol. Reprod. 2003;69:725–732. doi: 10.1095/biolreprod.103.015891. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, et al. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. 1999;112:3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Elvin JA, Yan CN, Matzuk MM. Oocyte-expressed TGF-beta superfamily members in female fertility. Mol. Cell. Endocrinol. 2000;159:1–5. doi: 10.1016/s0303-7207(99)00185-9. [DOI] [PubMed] [Google Scholar]
- Erickson GF, Shimasaki S. The spatiotemporal expression pattern of the bone morphogenic protein family in rat ovary cell types during the estrous cycle. Report Biol. Endocrinol. 2003;1:9. doi: 10.1186/1477-7827-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansel W, Alila HW, Dowd JP, Milvae RA. Differential origin and control mechanisms in small and large bovine luteal cells. J. Reprod. Fertil. Suppl. 1991;43:77–89. [PubMed] [Google Scholar]
- Hayashi K, Ishidou Y, Yonemori K, Nagamine T, Origuchi N, Maeda S, et al. Expression and localization of bone morphogenetic proteins (BMPs) and BMP receptors in ossification of the ligamentum flavum. Bone. 1997;21:23–30. doi: 10.1016/s8756-3282(97)00080-x. [DOI] [PubMed] [Google Scholar]
- Hogan BLM. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hunter MG, Biggs C, Pickard AR, Faillace LS. Differences in follicular aromatase-activity between Meishan and Large-White hybrid gilts. J. Reprod. Fertil. 1994;101:139–144. doi: 10.1530/jrf.0.1010139. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Sawyer HR, Smith PR, Quirke LD, Heath DA, Lun S, et al. Origins of follicular cells and ontogeny of steroidogenesis in ovine fetal ovaries. Mol. Cell. Endocrinol. 2002;191:1–10. doi: 10.1016/s0303-7207(02)00045-x. [DOI] [PubMed] [Google Scholar]
- Juengel JL, Hudson NL, Whiting L, McNatty KP. Effects of immunization against bone morphogenetic protein 15 and growth differentiation factor 9 on ovulation rate, fertilization, and pregnancy in ewes. Biol. Reprod. 2004;70 doi: 10.1095/biolreprod.103.023333. in press. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Histokine Growth Factor Rev. 1998;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kopp JB. BMP receptors in kidney. Kid. Internat. 2000;58:2237–2238. doi: 10.1111/j.1523-1755.2000.00402.x. [DOI] [PubMed] [Google Scholar]
- Lawson KA, Dunn NR, Roelen BA, Zeinstra LM, Davis AM, Wright CV, et al. BMP4 is required for the generation of primordial germ cells in the mouse embryo. Genes Dev. 1999;13:424–436. doi: 10.1101/gad.13.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Ventura F, Doody J, Massague J. Human Type-II receptor for bone morphogenic proteins (BMPs) – extension of the 2-kinase receptor model to the BMPs. Mol. Cell. Biol. 1995;15:3479–3486. doi: 10.1128/mcb.15.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinat-Botté F, Renaud G, Madec F, Costiou P, Terqui M. Ultrasonography and Reproduction in Swine. Paris: INRA; 2000. Gestation; pp. 50–69. [Google Scholar]
- Massague J. TGF-beta signal transduction. Ann. Rev. Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Mauleon P, Mariana JC. Oogenesis and folliculogenesis. In: Cole HH, Cupps PT, editors. Reproduction in Domestic Animals. New York: Academic Press; 1977. pp. 175–202. [Google Scholar]
- McNatty KP, Fidler AE, Juengel JL, Quirke LD, Smith PR, Heath DA, et al. Growth and paracrine factors regulating follicular formation and cellular function. Mol. Cell. Endocrinol. 2000;163:11–20. doi: 10.1016/s0303-7207(99)00235-x. [DOI] [PubMed] [Google Scholar]
- McNatty KP, Juengel JL, Wilson T, Galloway SM, Davis GH, Hudson NL, et al. Oocyte-derived growth factors and ovulation rate in sheep. Reprod. Suppl. 2002;61:339–351. [PubMed] [Google Scholar]
- Miller AT, Picton HM, Craigon J, Hunter MG. Follicle dynamics and aromatase activity in high-ovulating Meishan sows and in Large-White hybrid contemporaries. Biol. Reprod. 1998;58:1372–1378. doi: 10.1095/biolreprod58.6.1372. [DOI] [PubMed] [Google Scholar]
- Miyazono K. TGF-beta signaling by Smad proteins. Histokine Growth Factor Rev. 2000;11:15–22. doi: 10.1016/s1359-6101(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Miyazono K, Kusanagi K, Inoue H. Divergence and convergence of TGF-beta/BMP signaling. J. Cell. Phys. 2001;187:265–276. doi: 10.1002/jcp.1080. [DOI] [PubMed] [Google Scholar]
- Nilsson EE, Skinner MK. Bone morphogenetic protein 4 acts as an ovarian follicle survival factor and promotes primordial follicle development. Biol. Reprod. 2003;69:1265–1272. doi: 10.1095/biolreprod.103.018671. [DOI] [PubMed] [Google Scholar]
- O'Shea JD. Heterogeneous cell types in the corpus luteum of sheep, goats and cattle. Reprod. Suppl. 1987;34:71–85. [PubMed] [Google Scholar]
- Onishi T, Ishidou Y, Nagamine T, Yone K, Imamura T, Kato M, et al. Distinct and overlapping patterns of localization of bone morphogenetic protein (BMP) family members and a BMP type II receptor during fracture healing in rats. Bone. 1998;22:605–612. doi: 10.1016/s8756-3282(98)00056-8. [DOI] [PubMed] [Google Scholar]
- Otsuka F, Yao ZX, Lee TH, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 – Identification of target cells and biological functions. J. Biol. Chem. 2000;275:39523–39528. doi: 10.1074/jbc.M007428200. [DOI] [PubMed] [Google Scholar]
- Pailhoux E, Mandon-Pepin B, Cotinot C. Mammalian gonadal differentiation: the pig model. Reprod. Suppl. 2001;58:65–80. [PubMed] [Google Scholar]
- Pitzel L, Jarry H, Wuttke W. Effects of oxytocin on in vitro steroid release of midstage small and large porcine luteal cells. Endocrinology. 1990;126:2343–2349. doi: 10.1210/endo-126-5-2343. [DOI] [PubMed] [Google Scholar]
- Quinn RL, Brankin V, McGarr C, Webb R, Hunter MG. BMPs-2-6 and -15 are regulators of porcine theca cell function in vitro. Reprod. Abstract. Series. 2004;31:17. [Google Scholar]
- Reddi AH. Role of morphogenetic proteins in skeletal tissue engineering and regeneration. Nat. Biotechnol. 1998;16:247–252. doi: 10.1038/nbt0398-247. [DOI] [PubMed] [Google Scholar]
- Sawyer HR, Smith P, Heath DA, Juengel JL, Wakefield SJ, McNatty KP. Formation of ovarian follicles during fetal development in sheep. Biol. Reprod. 2002;66:1134–1150. doi: 10.1095/biolreprod66.4.1134. [DOI] [PubMed] [Google Scholar]
- Shimasaki S, Zachow RJ, Li DM, Kim H, Iemura S, Ueno N, et al. A functional bone morphogenetic protein system in the ovary. Proc. Natl. Acad. Sci. USA. 1999;96:7282–7287. doi: 10.1073/pnas.96.13.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shores EM, Picton HM, Hunter MG. Differential regulation of pig theca cell steroidogenesis by LH, insulin-like growth factor I and granulosa cells in serum-free culture. J. Reprod. Fertil. 2000;118:211–219. [PubMed] [Google Scholar]
- Shuttleworth G, Hunter MG, Robinson G, Broughton F. Immunohistochemical localization of angiotensin II receptor subtypes 1 and 2 in the porcine fetal, prepubertal and postpubertal ovary. J. Anat. 2002;201:267–274. doi: 10.1046/j.1469-7580.2002.00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza CJH, Campbell BK, McNeilly AS, Baird DT. Effect of bone morphogenetic protein 2 (BMP2) on oestradiol and inhibin A production by sheep granulosa cells, and localization of BMP receptors in the ovary by immunohistochemistry. Reproduction. 2002;123:363–369. [PubMed] [Google Scholar]
- Tendijke P, Ichijo H, Franzen P, Schulz P, Saras J, Toyoshima H, et al. Activin receptor-like kinases – a novel subclass of cell- surface receptors with predicted serine threonine kinase- activity. Oncogene. 1993;8:2879–2887. [PubMed] [Google Scholar]
- Tonetta SA, Devinna RS, Dizerega GS. Effects of follicle regulatory protein on thecal aromatase and 3-beta-hydroxysteroid dehydrogenase-activity in medium-sized and large-sized pig follicles. J. Reprod. Fertil. 1988;82:163–171. doi: 10.1530/jrf.0.0820163. [DOI] [PubMed] [Google Scholar]
- Valdimarsdottir G, Goumans MJ, Rosendahl A, Brugman M, Itoh S, Lebrin F, et al. Stimulation of Id1 expression by bone morphogenetic protein is sufficient and necessary for bone morphogenetic protein-induced activation of endothelial cells. Circulation. 2002;106:2263–2270. doi: 10.1161/01.cir.0000033830.36431.46. [DOI] [PubMed] [Google Scholar]
- Vitt UA, Mazerbourg S, Klein C, Hsueh AJW. Bone morphogenetic protein receptor type II is a receptor for growth differentiation factor-9. Biol. Reprod. 2002;67:473–480. doi: 10.1095/biolreprod67.2.473. [DOI] [PubMed] [Google Scholar]
- Wilson T, Wu XY, Juengel JL, Ross IK, Lumsden JM, Lord EA, et al. Highly prolific Booroola sheep have a mutation in the intracellular kinase domain of bone morphogenetic protein IB receptor (ALK-6) that is expressed in both oocytes and granulosa cells. Biol. Reprod. 2001;64:1225–1235. doi: 10.1095/biolreprod64.4.1225. [DOI] [PubMed] [Google Scholar]
- Yamashita H, Tendijke P, Franzen P, Miyazono K, Heldin CH. Formation of hetero-oligomeric complexes of type-I and type-II receptors for transforming growth-factor-beta. J. Biol. Chem. 1994;269:20172–20178. [PubMed] [Google Scholar]
- Yamashita H, TenDijke P, Heldin CH, Miyazono K. Bone morphogenetic protein receptors. Bone. 1996;19:569–574. doi: 10.1016/s8756-3282(96)00259-1. [DOI] [PubMed] [Google Scholar]
- Yi SE, LaPolt PS, Yoon BS, Chen JYC, Lu JKH, Lyons KM. The type I BMP receptor BmprIB is essential for female reproductive function. Proc. Natl. Acad. Sci. USA. 2001;98:7994–7999. doi: 10.1073/pnas.141002798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying Y, Liu XM, Marble A, Lawson KA, Zhao GQ. Requirement of Bmp8b for the generation of primordial germ cells in the mouse. Mol. Endocrinol. 2000;14:1053–1063. doi: 10.1210/mend.14.7.0479. [DOI] [PubMed] [Google Scholar]