Abstract
Using stereological methods, especially the optical disector for unbiased estimation of nuclear number, our recent study demonstrated that long-term (6 or 12 months) vasectomy in the rhesus monkey had no significant effects on spermatogenesis (Peng et al. Reproduction 2002, 124, 847–856). This study aimed to determine the scenario in the rabbit using the same morphometric methodology. Three groups of normal male Japanese white rabbits (aged 4–5 months) were subjected to unilateral vasectomy; 10 days, 6 months and 12 months later both testes and epididymides were removed. Testicular and epididymal methacrylate-embedded sections were obtained for stereology. Vasectomy-induced damage to spermatogenesis was observed, primarily sloughing of spermatogenic cells with a greater reduction in the number of advanced (adluminal) cells. The damage was most severe at 10 days, occurring in all the testes on the vasectomized side and involving sloughing of even type A spermatogonia, the number of which returned to normal at 6 and 12 months. Damage was less severe at 6 and 12 months, being found in half of the testes of the vasectomy side, in which the total numbers of later germ cell types were 24.0–59.1% (spermatocytes) and 0.3–11.6% (spermatids) of control at 6 months, and 20.1–22.1% (spermatocytes) and 0.4–12.0% (spermatids) of control at 12 months. By contrast, Sertoli cell number per testis was unchanged following vasectomy in any group. Epididymis on the vasectomy side, especially at 10 days and 6 months, appeared larger than on the contralateral side, but this difference was not statistically significant, and no sperm granuloma was seen in the epididymis.
Keywords: epididymis, histology, morphometry, rabbit, spermatogenesis, stereology, testis, vasectomy
Introduction
Many studies have reported spermatogenic damage following vasectomy whereas numerous others have not (Peng et al. 2002). Sloughing or smaller numbers of spermatids and spermatocytes has often been reported associated with this vasectomy-induced damage (Gupta et al. 1975; Croft & Bartke, 1976; Urry et al. 1976; Neaves, 1978; Perera, 1978; Barratt & Cohen, 1988; Singh & Chakravarty, 2000; Whyte et al. 2000). However, it was unclear whether type A spermatogonia and Sertoli cells were involved in the damage and what was the pattern of damage in terms of severity, extent and time course, which was partly attributable to the lack of detailed quantitative data on changes of different stages of germ cells in most previous studies. Using sophisticated stereological methods, we recently demonstrated that vasectomy in the monkey for 6 or 12 months induced formation of sperm granuloma in the epididymis but had no considerable impact on the testicular structures (Peng et al. 2002). In the rabbit, Liu et al. (1990) and Wang et al. (1992) reported extensive degeneration of seminiferous tubules 10 months after vasectomy, and apparent damage to spermatogenesis, despite considerable individual differences, was also observed in our pilot study (Wen et al. 2001). Using a rabbit model and contemporary stereological methodology this study was therefore undertaken to determine the morphometric changes, if any, of all germ cell types and Sertoli cells at 10 days, 6 months and 12 months following vasectomy, thus further clarifying the spermatogenic effects of vasectomy in animals.
Materials and methods
Animals and design
Nineteen normal male Japanese white rabbits, aged 4–5 months and obtained in October from the Animal Center sponsored by the Sichuan Administrative Committee of Experimental Animals, underwent unilateral (left or right, alternatively chosen) vasectomy. All operations were performed by an experienced surgeon (X.-Z.D.) and anaesthesia was induced by intra-abdominal injection of ketamine hydrochloride. A scrotal incision (1–2 cm in length) was made to expose the vas deferens and a segment of it (2–3 mm in length) was excised with the two ends being ligated. A scrotal hypodermic cut was made on the contralateral side to serve as a sham operation. Ten days (10d group), 6 months (6m group) and 12 months (12m group) following the operation, both the vasectomized (organs on the vasectomized side) and the contralateral control (organs on the contralateral nonvasectomized side) testes and epididymides were removed from six, six and seven randomly chosen animals, respectively. [Our pilot study showed that apparent damage to spermatogenesis was not observed in three out of eight rabbits 3 months after bilateral vasectomy (Wen et al. 2001). Therefore, a pair-design, i.e. unilateral vasectomy with the contralateral non-vasectomized side serving as a control, was chosen to give a better indication of vasectomy-induced changes, if any, of spermatogenesis.]
Tissue processing
Organ removal was performed after perfusion with first 300 mL normal saline and then 500 mL 4% paraformaldehyde in PBS via the heart under anaesthesia. The organs were further then immersion fixed for 24–48 h and immersed in 70% ethanol for a few days before the intact organs were weighed to calculate their volume by dividing the weight by the density, which was consistently found to be 0.93 g mL−1 (Zhang et al. 2002).
Testes and epididymides were cut into parallel slices of 1–2 mm thickness perpendicular to the long axis of the organs. Three testicular slices were sampled in a systematic random manner (evenly spaced). Three epididymal slices from the head, middle and tail parts of the epididymis were sampled; in some cases two slices from the head and tail parts of the epididymis were sampled. Slices were cut into smaller blocks and one block was randomly sampled from each slice for embedding in hydroxyethyl-methacrylate (Historesin, Leica Microsystems Nussloch GmbH, Nussloch, Germany) after dehydration in ethanol and butanol. One section was cut from each block at 25 µm thickness (average section area: ∼20 mm2 for testicular sections and ∼29 mm2 for epididymal sections) and stained with periodic acid–Schiff's reagent and haematoxylin (testis) or haematoxylin alone (epididymis).
Stereology
Stereological methods used in the present study were largely as previously described (Wen & Yang, 2000; Peng et al. 2002; Zhang et al. 2002, 2003 ).
Cell number
Spermatogenic cells were grouped as type A spermatogonia, type B spermatogonia, spermatocytes (including primary and secondary), early (round and elongating) spermatids and late (elongated) spermatids; these cells and Sertoli cells (nuclei) were identified as previously described (Zhang et al. 2002). Cell number was assumed to equate to the nuclear number, which was estimated using the stereological optical disector tool. Briefly, sections were observed using an oil lens on a computer screen at final magnification of ×2677, fields were sampled at a fixed space (approximately 375 µm between fields) along the x- or y-axis with the help of a motorized stage, and six rectangular counting frames (each with size 17 µm × 20 µm) were generated on the screen image, with all frames being used to count spermatogonia, secondary spermatocytes and Sertoli cells, and only one of the six frames used to count other spermatogenic cells because of their much greater number. The upper surface of the section was brought into focus and the upper 3 µm was traversed to avoid possible surface imperfections. The next 10 µm of the section was examined by focusing down step by step (distance between adjacent optical sections was adjusted to be 1 µm) with a computer-assisted motorized stage, and nuclei were counted as they came into focus in the counting frame according to the disector principle. The numerical density of nuclei was calculated by dividing the total number of nuclei counted per testis by the total volume of the disectors (each with volume 17 × 20 × 10 = 3400 µm3) used for the counting. The absolute number of nuclei per testis was further calculated by multiplying the numerical density by the testicular volume. It took about 1 h to count one section and around 590 nuclei were counted per testis on average.
The numbers of spermatozoa and other non- spermatozoal cells (i.e. those cells without the typical shape of the spermatozoa, mostly immature round spermatids) in the epididymal duct were also estimated with the optical disector as described above. To facilitate counting of the elongated and more densely packed spermatozoa in the epididymis, the distance between adjacent optical sections was adjusted to be 0.5 µm in focusing and observing optical sections step by step, and smaller counting frames (each 5 µm × 6 µm) were used. An average of 91 nuclei were counted per epididymis.
Volume of seminiferous tubules and epididymal duct
The upper left corner of each counting frame was regarded as a test point, and test points hitting different structures (the parenchyma, seminiferous tubules or epididymal duct, and the interstitium) were counted at one focal plane of the section just prior to nuclear counting as described above. The percentage of points hitting the parenchyma is the estimate of its volume fraction (percentage volume) in the organ, and its total volume per organ was then calculated by multiplying the fraction by the volume of the organ.
Diameter and length of seminiferous tubules
All the testicular sections were re-observed at a lower magnification (×268). Round or elliptical tubule profiles with a clear lumen were sampled using a test frame according to the unbiased forbidden-line rule, and their diameter (diameter of a circular profile or length of the shortest axis of an elliptical profile) was measured. The mean area of the tubule cross-section was calculated by multiplying the mean of squared diameters by a constant (π/4). The tubule length per testis was calculated by dividing the total volume of the tubules per testis by the cross-sectional area of the tubules. A total of 60 tubules were sampled and measured per testis. (Owing to the irregularity in shape and large intra-organ variation in the size of the epididymal duct, its diameter and length were not measured in the present study.)
Thickness of the basement membrane of the seminiferous tubules
All the testicular sections were re-observed using an oil lens on a computer screen at a final magnification of ×2677. A straight test line was superimposed on the image. When the test line intersected the basement membrane and the nucleus of the peritubular (myoid) cells lying closely on the basement membrane, the length of the part of the test line inside the membrane (i.e. the distance between the outer edge of the seminiferous epithelial cells and the inner side of the peritubular nucleus) was measured (cf. Huang et al. 2001). (The reason why the length was measured in the membrane only around the peritubular nucleus was that it was usually at this location where the outer edge of the membrane could be unambiguously identified.) Thirty such lengths were measured per testis. The arithmetic mean thickness of the membrane was estimated by dividing the mean of the lengths (i.e. line-sampled intercepts in stereological terminology) by a constant 2 (Yang & Wei, 2003).
Statistics
Data in the text and tables are shown as mean ± SEM. Vasectomized organs were compared with the contralateral non-vasectomized organs using a paired t-test to detect the significance of the effects of vasectomy.
Results
General effects of vasectomy
The body weights (kg) of the animals at death were 2.4 ± 0.1 (10d group), 3.5 ± 0.1 (6m group) and 3.6 ± 0.1 (12m group), and the values at 6 or 12 months were significantly greater than that at 10 days (as shown by one-way analysis of variance in conjunction with the Student–Newman–Keuls method for multiple comparisons).
Unexpectedly, in three unilaterally vasectomized animals at 12 months, the testis and ipsilateral epididymis on the vasectomy side appeared missing in the scrotal sac, i.e. the organs were not readily palpable or separated with confidence, and their weights or volumes were therefore unavailable. Tissue samples were obtained from inside the scrotal sac and processed for sectioning, from which small numbers of seminiferous tubule and epididymal duct profiles were indeed observed: the epididymal duct was filled with fluid but without spermatozoa; all seminiferous tubules (diameters of ∼65–81 µm) were highly atrophied, with only Sertoli cells and type A spermatogonia being seen. (By contrast, the contralateral organs on the non-vasectomy side of these three animals were of normal size and spermatogenesis.) Owing to the extremity of the damage, a lack of data on the volumes of the organs and uncertainty regarding whether it might be the result of factors other than vasectomy, all data obtained from these three animals in the 12m group were discarded for further analysis.
Marked damage to spermatogenesis occurred in all the vasectomized testes at 10 days, but at 6 or 12 months the damage was less severe and only found in half of the vasectomized testes (Fig. 1). Apoptotic (with condensed nucleus) and multinucleated giant cells (germ cells) were common in five of the six vasectomized testes in the 10d group, their nuclear numbers accounting for 3.81% and 5.65% of the total germ cell number, respectively. However, these cells were only occasionally counted or observed in the 6m and 12m groups, accounting for less than 0.15% of the total germ cells. Sertoli cell number was unchanged following vasectomy in any group, but the basement membrane of the seminiferous tubules was markedly thickened in all the six vasectomized testes (compared with the contralateral ones) in the 10d group, five of the six vasectomized testes in the 6m group and all the four vasectomized testes in the 12m group (Tables 1–3).
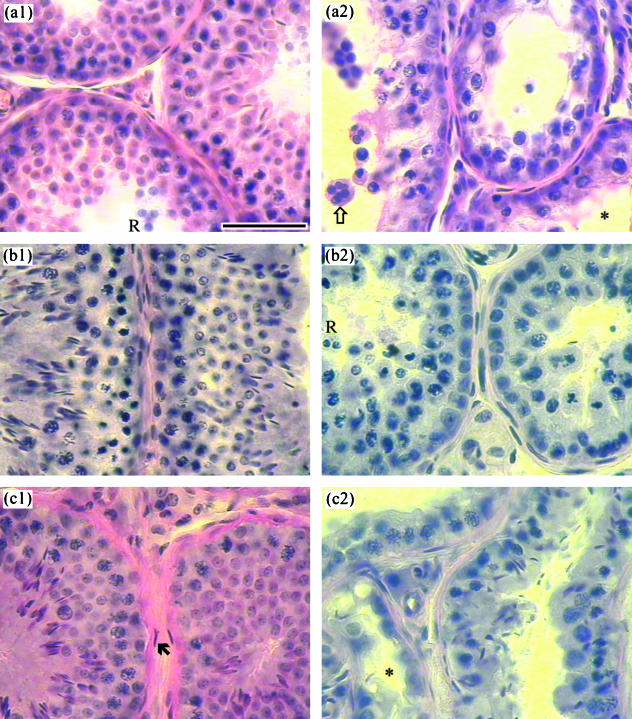
Fig. 1.
Typical testicular micrographs of 25-µm-thick methacrylate-embedded sections of rabbit testis. Sections were from the non-vasectomized testis (a1) and the contralateral vasectomized testis (a2) of an animal at 10 days after unilateral vasectomy; and from vasectomized testes at 6 (b) and 12 (c) months after unilateral vasectomy, where (b1) and (c1) are from vasectomized testes with essentially normal spermatogenesis, whereas (b2) and (c2) are from apparently atrophied testes. *Lumen of the seminiferous tubule; Ò: multinucleated giant cell; „: nucleus of peritubular cell (note the thick basement membrane between peritubular cells and spermatogenic cells); R: sloughed round spermatids. Scale bar represents 50 µm.
Table 1.
Testis and epididymis morphometry in the 10-day group (mean ± SEM, n = 6, range in square brackets)
| Parameters | Control | Vasectomy |
|---|---|---|
| Testis | ||
| Volume of testis (cm3) | 2.09 ± 0.17a[1.50–2.74] | 1.03 ± 0.09 [0.76–1.37] |
| Total volume of seminiferous tubules (cm3) | 1.40 ± 0.12a[1.04–1.78] | 0.44 ± 0.05 [0.30–0.61] |
| Mean diameter of seminiferous tubules (µm) | 169 ± 7a[147–189] | 105 ± 5 [88–117] |
| Total length of seminiferous tubules (m) | 62.3 ± 1.7a[57.7–67.9] | 49.9 ± 3.5 [40.2–58.6] |
| Mean thickness of basement membrane (µm) | 0.99 ± 0.09a[0.66–1.24] | 1.85 ± 0.20 [1.26–2.71] |
| Total no. of type A spermatogonia (106) | 46.8 ± 3.6a[34.8–56.3] | 28.4 ± 3.2 [16.3–37.5] |
| Total no. of type B spermatogonia (106) | 27.5 ± 5.0a[13.5–45.4] | 10.4 ± 3.1 [1.0–23.5] |
| Total no. of spermatocytes (106) | 349 ± 41a[290–525] | 25 ± 7 [11–54] |
| Total no. of early spermatids (106) | 758 ± 104a[350–1063] | 1 ± 1 [0–4] |
| Total no. of late (elongated) spermatids (106) | 228 ± 64a[6–449] | 2 ± 1 [0–6] |
| Total no. of Sertoli cells (106) | 66.8 ± 7.2 [49.1–99.4] | 73.3 ± 4.6 [52.5–84.6] |
| Epididymis | ||
| Volume of epididymis (cm3) | 0.428 ± 0.050b[0.311–0.625] | 0.558 ± 0.050 [0.371–0.692] |
| Total volume of epididymal duct (cm3) | 0.169 ± 0.028 [0.115–0.272] | 0.235 ± 0.039 [0.112–0.365] |
| Total no. of spermatozoa (106) | 116 ± 64 [1–406] | 83 ± 34 [0–209] |
Significantly different from vasectomy (paired t-test, P < 0.05);
P = 0.057.
Table 3.
Testis and epididymis morphometry in the 12-month group (mean ± SEM, n = 4, range in square brackets)
| Parameters | Control | Vasectomy |
|---|---|---|
| Testis | ||
| Volume of testis (cm3) | 2.10 ± 0.46 [1.34–3.39] | 2.06 ± 0.95 [0.53–1.57, 1.30–4.85] |
| Total volume of seminiferous tubules (cm3) | 1.31 ± 0.31a[0.68–2.08] | 0.82 ± 0.34 [0.17–0.66, 0.68–1.77] |
| Mean diameter of seminiferous tubules (µm) | 157 ± 6 [142–168] | 139 ± 15 [94–153, 144–164] |
| Total length of seminiferous tubules (m) | 66.5 ± 14.1 [42.5–97.5] | 46.4 ± 13.0 [24.2–36.0, 41.8–83.7] |
| Mean thickness of basement membrane (µm) | 0.94 ± 0.07a[0.82–1.07] | 2.30 ± 0.13 [2.20–2.70, 2.13–2.19] |
| Total no. of type A spermatogonia (106) | 47.8 ± 11.6 [31.8–82.3] | 60.9 ± 23.3 [34.4–41.5, 37.0–130.8] |
| Total no. of type B spermatogonia (106) | 33.3 ± 12.2 [13.6–68.9] | 30.0 ± 25.8 [0.0–0.1, 13.2–106.8] |
| Total no. of spermatocytes (106) | 455 ± 154 [175–840] | 232 ± 127 [35–126, 166–603] |
| Total no. of early spermatids (106) | 845 ± 208a[381–1309] | 365 ± 227 [3–133, 304–1022] |
| Total no. of late (elongated) spermatids (106) | 471 ± 140b[224–804] | 120 ± 55 [0–67, 161–253] |
| Total no. of Sertoli cells (106) | 46.4 ± 2.2 [44.1–52.9] | 44.7 ± 6.1 [34.5–62.3, 40.8–41.2] |
| Epididymis | ||
| Volume of epididymis (cm3) | 0.80 ± 0.13 [0.57–1.16] | 1.72 ± 0.55 [0.45–2.56, 1.15–2.72] |
| Total volume of epididymal duct (cm3) | 0.28 ± 0.06 [0.20–0.47] | 0.62 ± 0.21 [0.15–0.48, 0.68–1.17] |
| Total No. of spermatozoa (106) | 608 ± 145 [273–947] | 433 ± 363 [0–221, 0–1509] |
Data in square brackets in the vasectomy column are two subranges obtained from vasectomized testes with (n = 2) and without (n = 2) apparent spermatogenic damage, respectively.
Significantly different from vasectomy (paired t-test, P < 0.05);
P = 0.055.
Epididymis on the vasectomy side, especially at 10 days and 6 months, appeared larger than the contralateral side, but no statistically significant difference was detected in epididymal volumes (Tables 1 and 2), and no sperm granuloma was seen on the epididymal sections.
Table 2.
Testis and epididymis morphometry in the 6-month group (mean ± SEM, n = 6, range in square brackets)
| Parameters | Control | Vasectomy |
|---|---|---|
| Testis | ||
| Volume of testis (cm3) | 2.85 ± 0.13a[2.29–3.25] | 2.17 ± 0.31 [1.15–1.69, 2.75–2.98] |
| Total volume of seminiferous tubules (cm3) | 2.04 ± 0.12 [1.71–2.44] | 1.46 ± 0.29 [0.54–1.03, 2.05–2.15] |
| Mean diameter of seminiferous tubules (µm) | 206 ± 3 [198–217] | 170 ± 15 [125–149, 196–214] |
| Total length of seminiferous tubules (m) | 60.9 ± 2.1 [53.3–66.1] | 59.7 ± 4.4 [43.8–68.8, 57.1–71.5] |
| Mean thickness of basement membrane (µm) | 1.04 ± 0.08a[0.79–1.37] | 1.46 ± 0.14 [1.32–1.87, 0.98–1.65] |
| Total no. of type A spermatogonia (106) | 56.7 ± 6.0 [41.4–82.1] | 55.7 ± 8.2 [32.6–72.1, 38.7–79.7] |
| Total no. of type B spermatogonia (106) | 28.2 ± 7.8 [8.9–65.2] | 16.7 ± 4.5 [5.4–23.7, 9.4–34.7] |
| Total no. of spermatocytes (106) | 460 ± 25 [376–542] | 332 ± 69 [90–284, 417–553] |
| Total no. of early spermatids (106) | 988 ± 129 [688–1493] | 566 ± 227 [2–170, 869–1200] |
| Total no. of late (elongated) spermatids (106) | 382 ± 47 [223–501] | 212 ± 97 [0–8, 359–532] |
| Total no. of Sertoli cells (106) | 54.8 ± 3.8 [44.6–64.7] | 56.4 ± 3.4 [41.1–60.8, 56.5–66.5] |
| Epididymis | ||
| Volume of epididymis (cm3) | 1.06 ± 0.06 [0.85–1.25] | 1.91 ± 0.49 [[0.96–1.62, 0.87–3.84] |
| Total volume of epididymal duct (cm3) | 0.57 ± 0.10 [0.23–0.82] | 1.15 ± 0.37 [0.35–1.20, 0.33–2.61] |
| Total no. of spermatozoa (106) | 982 ± 432 [18–2488] | 1541 ± 923 [0–18, 325–4634] |
Data in square brackets in the vasectomy column are two subranges obtained from vasectomized testes with (n = 3) and without (n = 3) apparent spermatogenic damage, respectively.
Significantly different from vasectomy (paired t-test, P < 0.05).
Spermatogenic effects of vasectomy in the 10d group
In the contralateral control organs in the 10d group, late elongated spermatid number in the testis and spermatozoal number in the epididymis were, at 10 days after vasectomy on the other side, 6 and 4 × 106 per organ, respectively, in one animal; in another animal, they were 100 and 1 × 106, respectively; and in the other four animals, they were 248–449 and 53–406 × 106, respectively. These numbers indicated that all kinds of spermatogenic cells might well have been developed in the testes of these animals prior to vasectomy. In response to the surgical occlusion of the vas deferens, dramatic and consistent changes occurred: germ cells, especially spermatids and spermatocytes, sloughed off the seminiferous epithelium, many spermatids and even some spermatocytes being in groups and forming multinucleated giant cells (Fig. 1a2). All seminiferous tubules became much smaller in diameter, with many tubules having only Sertoli cells and spermatogonia in the seminiferous epithelium. The volume fraction (percentage volume) of the tubules in the testis was significantly reduced to 64.3 ± 6.2% of control.
Compared with the contralateral control, the testicular volume decreased by 50.4 ± 1.1%, tubule diameter by 37.1 ± 3.2% and tubule length per testis by 20.2 ± 3.8% (Table 1). In parallel, germ cell numbers per testis decreased markedly: type A spermatogonia decreased to 62.4 ± 7.2% (28.9–77.6%) of control, type B spermatogonia to 37.2 ± 10.6% (7.7–83.7%), spermatocytes to 8.1 ± 3.0% (3.6–22.6%), early spermatids to 0.2 ± 0.1% (0–0.7%) and late spermatids to 0.5 ± 0.3% (0–1.4%). The reduction in the late spermatid, early spermatid or spermatocyte number was more severe than that in the type B spermatogonial number, and the reduction in type B spermatogonial number was more than that in type A spermatogonial number (one-way repeated measures analysis of variance in conjunction with the Student–Newman–Keuls method for multiple comparison).
The volume of the epididymis on the vasectomy side was consistently higher than that on the contralateral side, the ratio between the volumes being 1.36 ± 0.18, but this difference was not statistically significant (Table 1). In one case, on two epididymal sections sampled from one epididymis on the vasectomy side, spermatozoal leakage was seen in three places, where part of the epididymal wall (epithelium) was absent and a patch of spermatozoa (without wall) was seen running out of the tubule into the interstitial tissue.
Few spermatocytes were observed in the epididymal duct. Although 30.8 ± 11.4 (11.0–84.7) × 106 round spermatids and 2.5 ± 1.4 (0–8.1) × 106 other non-spermatozoal nuclei (per epididymis) were counted on the vasectomy side, these were not significantly different from those [17.7 ± 5.5 (3.4–35.4), and 2.4 ± 1.2 (0–8.4), respectively] on the contralateral side.
Spermatogenic effects of vasectomy in the 6m group
Essentially normal spermatogenesis was seen in three vasectomized testes (Fig. 1b1). In the other three vasectomized testes, the major changes were thinner seminiferous tubules, smaller volume fraction of tubules (47.3–62.6%), a looser arrangement of spermatogenic cells and smaller number or absence of spermatids. Sloughing of spermatids and spermatocytes and presence of multinucleated giant cells were also observed but not as common as in the 10d group (Fig. 1b2). In the testes with apparent spermatogenic damage, the numbers of spermatocytes, early spermatids and late spermatids fell to 41.4 ± 10.1% (24.0–59.1%), 6.3 ± 3.9% (0.3–13.6%) and 1.1 ± 0.9% (0–2.8%) of control, respectively (Table 2). However, the numbers of both type A and B spermatogonia were not significantly different from controls.
Round spermatids were counted in one of the epididymides on the vasectomy side; the number per epididymis was 141 × 106, and the number of spermatozoa in the organ was 18 × 106 (Table 3). Round spermatids were occasionally seen in the epididymis on the non-vasectomy side, but were not counted during the counting procedure owing to their very low number.
Spermatogenic effects of vasectomy in the 12m group
The volume fraction of seminiferous tubules was significantly reduced to 66.8 ± 7.1% of control. Two vasectomized testes had a largely normal spermatogenesis in terms of germ cell numbers (Fig. 1c1 and Table 3), with only a few atrophied tubules being seen on occasion. Damage to spermatogenesis (Fig. 1c2) in the other two vasectomized testes was similar to that described in the 6m group: the numbers of spermatocytes, early spermatids and late spermatids were 21.2%, 6.6% and 5.5% of control, respectively (Table 3).
Round spermatids were counted in one epididymis on the vasectomy side and one epididymis (with largely normal testicular spermatogenesis) on the contralateral non-vasectomy side, the numbers per organ being 80.6 and 15.9 × 106, respectively.
Discussion
The present study demonstrated that vasectomy in the rabbit led to early severe damage to spermatogenesis; this damage gradually recovered almost completely in some animals, but only partially in other animals in which spermatogenesis was subsequently maintained at somewhat lower than normal levels. The spermatogenic damage was primarily germ cell sloughing off the seminiferous epithelium, with a greater reduction in the number of advanced (adluminal) cells. The damage was most severe at 10 days, occurring in all the vasectomized testes and involving sloughing of even type A spermatogonia, and became less severe at 6 and 12 months, when damage was found in only half of the vasectomized testes. In these testes with apparent spermatogenic damage the total numbers of later germ cell types were 24.0–59.1% (spermatocytes) and 0.3–11.6% (spermatids) of control at 6 months, and 20.1–22.1% (spermatocytes) and 0.4–12.0% (spermatids) of control at 12 months. By contrast, type A spermatogonia returned to normal ranges in all animals at 6 and 12 months, and Sertoli cell number per testis was unchanged following vasectomy in any group. This is the first study to report the finding that (i) vasectomy-induced early germ cell sloughing went deep into the level of type A spermatogonia in the seminiferous epithelium, but its number was restored at later stages; and (ii) vasectomy-induced damage to spermatogenesis was not complicated by a reduction in the number of Sertoli cells. (Note that if the extreme atrophy of testis on the vasectomized side, occurring at 12 months in the three animals excluded in the data analysis, were the result of vasectomy per se, the conclusion would be that spermatogenic damage would be observed in most vasectomized animals in the longer term, and the damage could be either moderate, as described above, or progressively severe.)
Multinucleated giant cells and tubular atrophy were common in vasectomy-induced spermatogenic damage, probably as a result of germ cell sloughing (Hutson et al. 1976; Urry et al. 1976; Neaves, 1978; Perera, 1978; Tung & Alexander, 1980; Singh & Chakravarty, 2000). While germ cell sloughing would apparently lead to tubular atrophy and spermatogenic cell degeneration, it might also induce formation of multinucleated giant cells, which were formed mostly by spermatids as a result of fusion of spermatids due to alterations in the intercellular bridges (Singh & Chakravarty, 2000), or due to poor formation of the intercellular bridges or junctions before or after the cells sloughed off the seminiferous epithelium. The mechanism of germ cell sloughing requires investigation. The damage is probably pressure-mediated rather than immunological (Bedford, 1976; McDonald, 2000), because dramatic damage occurred by 10 days after vasectomy. Although the intraseminiferous tubule pressure might not be elevated at some stages after vasectomy (Johnson & Howards, 1975; Flickinger et al. 1995), such measurements were not commonly used. It may also be argued that the pressure might have been increased prior to measurement but decreased later with the presence of spermatogenic damage (McDonald, 2000).
Sloughing of round spermatids was apparent in the testis after vasectomy, but an increased number of round spermatids in the ipsilateral epididymis was not found. This might be ascribed to the continual development and/or clearance of round spermatids. Macrophages were found in the degenerated seminiferous tubules after vasectomy, which might contribute to clearance of sloughed germ cells to some degree (McDonald, 2000; Singh & Chakravarty, 2000).
The present study emphasized that a major histological change in response to vasectomy was germ cell sloughing. Such change was observed in previous studies but was not stressed, probably due to the lack of reliable quantitative data and the use of paraffin-embedded sections. In staining paraffin sections, paraffin will be dissolved, which may result in artefacts such as empty spaces between single or groups of cells. Methacrylate-embedded sections give better microscopy and the resin is not dissolved in staining.
Post-vasectomy, widespread spermatogenic degeneration was observed by 3 weeks after vasectomy in dogs (Urry et al. 1976), 1 month in men (Gupta et al. 1975), 5 weeks to 3 months in rats (Whyte et al. 2000; Kubota et al. 2001; Shiraishi et al. 2001), 3 months in rabbits (Liu et al. 1990; Wang et al. 1992) or 6 months in mice (Barratt & Cohen, 1988; Singh & Chakravarty, 2000). The more acute (by 10 days after vasectomy) and severe damage observed in the present study may be partly explained by, among other factors such as species difference, the fact that the seminiferous epithelium of the normal rabbit testis was prone to sloughing at the age of 4–5 months (Fig. 1a1). We previously speculated that spermatid sloughing might occur more frequently after puberty or when spermatogenesis starts to occur actively in the normal New Zealand white rabbit (Zhang et al. 2002). Morton et al. (1986) also noted a frequent finding of spermatid giant cells in normal New Zealand white rabbits 15 weeks of age and older. As shown in the present study, a considerable number of round spermatids was observed in the epididymis on the non-vasectomy side at 10 days after the experiment, and the cells appeared sparser at 6 or 12 months.
Species difference was evident in terms of spermatogenic damage after vasectomy. It appeared that humans (Gupta et al. 1975) and non-human primates (Hadley & Dym, 1983; Peng et al. 2002) were more resistant than other mammals in this respect. The volume of testis in men, as measured in vivo, was unchanged 5 days, 1–6 months or 1–13 years after vasectomy (Dias, 1983; Guo et al. 1987). This provided additional evidence for the resistance in men, because spermatogenic damage was usually associated with an atrophied testis. In other mammals, however, there seemed always to be some degree of spermatogenic damage after vasectomy, although individual variation was considerable. Hutson et al. (1976) reported that 13 of 30 vasectomized (for 1–6 months) guinea-pigs had spermatogenic damage. McDonald & Scothorne (1988) reported five (out of eight) normal vasectomized testes (weights 1.2–1.6 g) after vasectomy (for 6 months) in rats, but the other three vasectomized testes, atrophied with weights of 0.7–1.0 g, were not studied. These atrophied testes might well have severe spermatogenic changes.
It was observed that vasectomy resulted in thickening of the basement membrane of the seminiferous tubules in the guinea-pig (Aitken et al. 2000), human (Gupta et al. 1975; Jarow et al. 1985) and rat (Whyte et al. 2000). Such thickening was also confirmed in the current study. To our knowledge this is the first study to have used a stereological intercept method to obtain quantitative data on the thickness of the basement membrane. As demonstrated, the thickness increased soon after vasectomy and remained so at later stages in almost all animals, although spermatogenesis may recover with time in some animals.
By means of special staining and image analysis, Shiraishi et al. (2002) proposed that interstitial fibrosis was a key lesion in the vasectomized human testis. On the rabbit sections used in the present study, however, interstitial fibrosis could hardly be observed as a prominent feature, although the volume fraction of the interstitial tissue did tend to increase after vasectomy.
With respect to the correlation between sperm granuloma formation and spermatogenic damage, granuloma was not observed in the rabbit epididymis after vasectomy, as shown in the present and previous studies (Liu et al. 1990; Wang et al. 1992), and damage to spermatogenesis was severe. However, different scenarios have also been reported: (i) absence of sperm granuloma coupled with mild spermatogenic damage in mice (Croft & Bartke, 1976) or guinea-pigs (Aitken et al. 1999); (ii) presence of sperm granuloma coupled with mild spermatogenic damage in mice (Barratt & Cohen, 1988), rats (McDonald & Scothorne, 1988) or monkeys (Peng et al. 2002); and (iii) presence of sperm granuloma coupled with severe spermatogenic damage in rams (Perera, 1978), hamsters (Sun et al. 1992) or mice (Singh & Chakravarty, 2000). Thus, whether sperm granuloma is formed or not is an unreliable indicator of spermatogenic changes. Granuloma formation probably depends largely on the distensiblity of the epididymal duct and is not invariably related to a higher or lower intra-epididymal or even seminiferous tubule pressure (McDonald, 2000).
This is the first study to report the number of spermatozoa in the rabbit epididymis as estimated by unbiased stereological methods, and a large individual variation was shown in the parameter (Tables 1–3), suggesting that it would be inefficient to use data obtained from the epididymis alone as a key indicator of spermatogenic status in the rabbit testis.
Without separate control (sham) groups of animals, we will not be able to demonstrate the effects of the unilateral operation (vasectomy) itself on the contralateral scrotal testis. To study the effects of vasectomy, however, it will suffice to compare the unilaterally vasectomized testis with the contralateral scrotal testis only. Such pair-design will be important (efficient) because a considerable individual difference was found in bilaterally vasectomized rabbits in our pilot study (Wen et al. 2001). Our previous study showed that the testis made unilaterally cryptorchid for 13 weeks generally had no effect on the spermatogenesis in the contralateral scrotal testis (Zhang et al. 2002). So we did not include a separate sham group at 10 days. However, we did include separate sham groups at 6 and 12 months (three animals in each group); all the data listed in Tables 2 and 3 except the basement membrane thickness were also obtained, and no significant difference was found in these data between the sham group (non-treated animals) and the contralateral control (treated animals) at either 6 or 12 months (data not shown).
Acknowledgments
This study was supported by grants from the Sichuan Committee of Family Planning (#99-4-2), the Sichuan Department of Education (Chuan Jiao Ji [2000] 25), and the Sichuan Youth Foundation of Science and Technology (Chuan Ke Ji [2001] 2).
References
- Aitken H, Kumarakuru S, Orr R, Reid O, Bennett NK, McDonald SW. Effect of long-term vasectomy on seminiferous tubules in the guinea pig. Clin. Anat. 1999;12:250–263. doi: 10.1002/(SICI)1098-2353(1999)12:4<250::AID-CA3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Aitken H, Kumarakuru S, Reid O, Milne EW, Bennett NK, McDonald SW. Degenerated tubules in the guinea pig testis after long-term vasectomy or sham operation. Clin. Anat. 2000;13:6–10. doi: 10.1002/(SICI)1098-2353(2000)13:1<6::AID-CA2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Barratt CL, Cohen J. Quantitative effects of short- and long-term vasectomy on mouse spermatogenesis and sperm transport. Contraception. 1988;37:415–424. doi: 10.1016/0010-7824(88)90118-7. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Adaptations of the male reproductive tract and the fate of spermatozoa following vasectomy in the rabbit, rhesus monkey, hamster and rat. Biol. Reprod. 1976;14:118–142. doi: 10.1095/biolreprod14.2.118. [DOI] [PubMed] [Google Scholar]
- Croft BT, Bartke A. Quantitative study of spermatogenesis in vasectomized mice. Int. J. Fertil. 1976;21:61–64. [PubMed] [Google Scholar]
- Dias PL. The effects of vasectomy on testicular volume. Br. J. Urol. 1983;55:83–84. doi: 10.1111/j.1464-410x.1983.tb07086.x. [DOI] [PubMed] [Google Scholar]
- Flickinger CJ, Howards SS, Herr JC. Effects of vasectomy on the epididymis. Microsc. Res. Tech. 1995;30:82–100. doi: 10.1002/jemt.1070300107. [DOI] [PubMed] [Google Scholar]
- Guo XK, Chen BS, Lu XP. Changes of testicular volume after vasectomy. Clin. J. Urol. Surg. 1987;2:54–55. (in Chinese) [Google Scholar]
- Gupta AS, Kothari LK, Bapna RB. Surgical sterilization by vasectomy and its effects on the structure and function of the testis in man. Br. J. Surg. 1975;62:59–63. doi: 10.1002/bjs.1800620114. [DOI] [PubMed] [Google Scholar]
- Hadley MA, Dym M. Spermatogenesis in the vasectomized monkey: quantitative analysis. Anat. Rec. 1983;205:381–386. doi: 10.1002/ar.1092050403. [DOI] [PubMed] [Google Scholar]
- Huang AP, Zhang RD, Yang ZW. Quantitative (stereological) study of placental structures in women with pregnancy iron-deficiency anemia. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001;97:59–64. doi: 10.1016/s0301-2115(00)00480-2. [DOI] [PubMed] [Google Scholar]
- Hutson JC, Gardner PJ, Lacy SS. Changes in testis of guinea pig after vasectomy. Urology. 1976;7:287–291. doi: 10.1016/0090-4295(76)90460-x. [DOI] [PubMed] [Google Scholar]
- Jarow JP, Budin RE, Dym M, Zirkin BR, Noren S, Marshall FF. Quantitative pathologic changes in the human testis after vasectomy. A controlled study. N. Engl. J. Med. 1985;313:1252–1256. doi: 10.1056/NEJM198511143132003. [DOI] [PubMed] [Google Scholar]
- Johnson AL, Howards SS. Intratubular hydrostatic pressure in testis and epididymis before and after vasectomy. Am. J. Physiol. 1975;228:556–564. doi: 10.1152/ajplegacy.1975.228.2.556. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Sasaki S, Kubota H, Tatsura H, Kohri K. A study on the mechanism of the spermatogenic damage after vasectomy in rats. Nippon Hinyokika Gakkai Zasshi (Japan) 2001;92:13–22. doi: 10.5980/jpnjurol1989.92.13. [DOI] [PubMed] [Google Scholar]
- Liu FX, Zhao XJ, Wang ZS, Lin H, Zhao D, Zhao YL, Hu JD, Sun YQ. Observation of histological changes of testes in vasectomized rabbits with PAP staining. J. Norman Bethune University Med. Sci. 1990;16:37–39. (in Chinese) [Google Scholar]
- McDonald SW, Scothorne RJ. A quantitative study of the effects of vasectomy on spermatogenesis in rats. J. Anat. 1988;159:219–225. [PMC free article] [PubMed] [Google Scholar]
- McDonald SW. Cellular responses to vasectomy. Int. Rev. Cytol. 2000;199:295–339. doi: 10.1016/s0074-7696(00)99006-5. [DOI] [PubMed] [Google Scholar]
- Morton D, Weisbrode SE, Wyder WE, Maurer JK, Capen CC. Spermatid giant cells, tubular hypospermatogenesis, spermatogonial swelling, cytoplasmic vacuoles, and tubular dilatation in the testes of normal rabbits. Vet. Pathol. 1986;23:176–183. doi: 10.1177/030098588602300211. [DOI] [PubMed] [Google Scholar]
- Neaves WB. The effect of vasectomy on the testes of inbred Lewis rats. J. Reprod. Fertil. 1978;54:405–411. doi: 10.1530/jrf.0.0540405. [DOI] [PubMed] [Google Scholar]
- Peng B, Zhang RD, Dai XS, Deng XZ, Wan Y, Yang ZW. Quantitative (stereological) study of the effects of vasectomy on spermatogenesis in rhesus monkeys (Macaca mulatta) Reproduction. 2002;124:847–856. doi: 10.1530/rep.0.1240847. [DOI] [PubMed] [Google Scholar]
- Perera BM. Changes in the structure and function of the testes and epididymides in vasectomized rams. Fertil. Steril. 1978;29:354–359. doi: 10.1016/s0015-0282(16)43166-3. [DOI] [PubMed] [Google Scholar]
- Shiraishi K, Naito K, Yoshida K. Vasectomy impairs spermatogenesis through germ cell apoptosis mediated by the p53-Bax pathway in rats. J. Urol. 2001;166:1565–1571. [PubMed] [Google Scholar]
- Shiraishi K, Takihara H, Naito K. Influence of interstitial fibrosis on spermatogenesis after vasectomy and vasovasostomy. Contraception. 2002;65:245–249. doi: 10.1016/s0010-7824(01)00311-0. [DOI] [PubMed] [Google Scholar]
- Singh SK, Chakravarty S. Histologic changes in the mouse testis after bilateral vasectomy. Asian J. Androl. 2000;2:115–120. [PubMed] [Google Scholar]
- Sun YB, Qiu Y, Wang ZX. Vasectomy and spermatic granuloma in hamsters. Contraception. 1992;45:177–185. doi: 10.1016/0010-7824(92)90051-t. [DOI] [PubMed] [Google Scholar]
- Tung KS, Alexander NJ. Monocytic orchitis and aspermatogenesis in normal and vasectomized rhesus macaques (Macaca mulatta) Am. J. Pathol. 1980;101:17–30. [PMC free article] [PubMed] [Google Scholar]
- Urry RL, Dougherty KA, Cockett AT. Vasectomy and vasovasostomy. I. Timing of histologic changes in immature and mature dog testis after vasectomy. Fertil. Steril. 1976;27:937–944. doi: 10.1016/s0015-0282(16)42022-4. [DOI] [PubMed] [Google Scholar]
- Wang ZS, Zhao XJ, Lin H, Wang TL, Zhao JB. Probing the pathogenesis of epididymal stasis and spermatic granuloma in epididymides after vasectomy. J. Norman Bethune University Med. Sci. 1992;18:30–33. (in Chinese) [Google Scholar]
- Wen XH, Yang ZW. Quantitative (stereological) study on the spermatozoal storage capacity of epididymis in rats and monkeys. Asian J. Androl. 2000;2:73–77. [PubMed] [Google Scholar]
- Wen XH, Deng XZ, Wan Y, Huang AP, Yang ZW. Effects of spermatogenesis three months after vasectomy in rabbits. Sichuan J. Anat. 2001;9:44. in Chinese. [Google Scholar]
- Whyte J, Sarrat R, Cisneros AI, Whyte A, Mazo R, Torres A, Lazaro J. The vasectomized testis. Int. Surg. 2000;85:167–174. [PubMed] [Google Scholar]
- Yang ZW, Wei GW. Comparison of point-, line- and boundary-sampled intercepts inside a circle or sphere. Int. J. Pattern Recognition Artificial Intelligence. 2003;17:319–330. [Google Scholar]
- Zhang RD, Wen XH, Kong LS, Deng XZ, Peng B, Huang AP, Wan Y, Yang ZW. A quantitative (stereological) study of the effects of experimental unilateral cryptorchidism and subsequent orchiopexy on spermatogenesis in adult rabbit testis. Reproduction. 2002;124:95–105. doi: 10.1530/rep.0.1240095. [DOI] [PubMed] [Google Scholar]
- Zhang RD, Peng B, Deng XZ, Wan Y, Yang ZW. A stereological study of the effects of experimental inguinal cryptorchidism and subsequent orchiopexy on spermatogenesis in adult monkeys. Int. J. Androl. 2003;26:180–186. doi: 10.1046/j.1365-2605.2003.00416.x. [DOI] [PubMed] [Google Scholar]