Abstract
Two embryological fates for cells of the neural tube are well established. Cells from the dorsal part of the developing neural tube emigrate and become neural crest cells, which in turn contribute to the development of the peripheral nervous system and a variety of non-neural structures. Other neural tube cells form the neurons and glial cells of the central nervous system (CNS). This has led to the neural crest being treated as the sole neural tube-derived emigrating cell population, with the remaining neural tube cells assumed to be restricted to forming the CNS. However, this restriction has not been tested fully. Our investigations of chick, quail and duck embryos utilizing a variety of different labelling techniques (DiI, LacZ, GFP and quail chimera) demonstrate the existence of a second neural tube-derived emigrating cell population. These cells originate from the ventral part of the cranial neural tube, emigrate at the exit/entry site of the cranial nerves, migrate in association with the nerves and populate their target tissues. On the basis of its site of origin and route of migration we have named this cell population the ventrally emigrating neural tube (VENT) cells. VENT cells also differ from neural crest cells in that they emigrate considerably after the emigration of neural crest cells, and lack expression of the neural crest cell antigen HNK-1. VENT cells are multipotent, differentiating into cell types belonging to all four basic tissues in the body: the nerve, muscle, connective and epithelium. Thus, the neural tube provides at least two cell populations – neural crest and VENT cells – that contribute to the development of the peripheral nervous system and various non-neural structures. This review describes the origin of the idea of VENT cells, and discusses evidence for their existence and subsequent fates.
Keywords: cranial nerves, cranial neural tube, development, neural crest, ventral neural tube cell emigration
Introduction
The nervous system is derived from the ectoderm, one of the three germ layers in the embryo. Not all ectodermal cells, however, give rise to the nervous system. As a result of neurulation (the process by which a flat neural plate is gradually converted into a tubular structure called the neural tube), ectodermal cells segregate into the surface ectoderm, neural crest and neural tube (Fig. 1A; Schoenwolf & Smith, 1990; Moore & Persaud, 1998; Casanova et al. 2000). The neural crest cells arise from the dorsal part of the developing neural tube, and contribute to the development of the peripheral nervous system (PNS) and to a wide variety of non-neural structures throughout the body (Fig. 1B; Moore & Persaud, 1998; Le Douarin & Kalcheim, 1999; Gammill & Bronner-Fraser, 2003). The remaining cells of the neural tube form the neurons and glial cells of the central nervous system (CNS) (Moore & Persaud, 1998; Le Douarin & Kalcheim, 1999). The possibility that some of these remaining cells could also emigrate and assume different fates has not been fully explored, and the neural crest cells are commonly treated as the sole emigrating neural tube-derived cells that participate in the development of non-CNS tissues. Accordingly, the results of studies aimed at understanding the basic mechanisms of normal development, and the underlying causes of congenital malformations, are interpreted within this conceptual framework. In contrast to this view, our investigations of avian embryos (chick, quail and duck) have demonstrated the existence of a second neural tube-derived cell population that participates in the development of the PNS and a variety of non-neural structures. On the basis of its site of origin and route of migration, we have named this cell population the ventrally emigrating neural tube (VENT) cells (Ali et al. 1999; Sohal et al. 1999b,c). The contribution of VENT cells will need to be integrated into the existing conceptual framework.
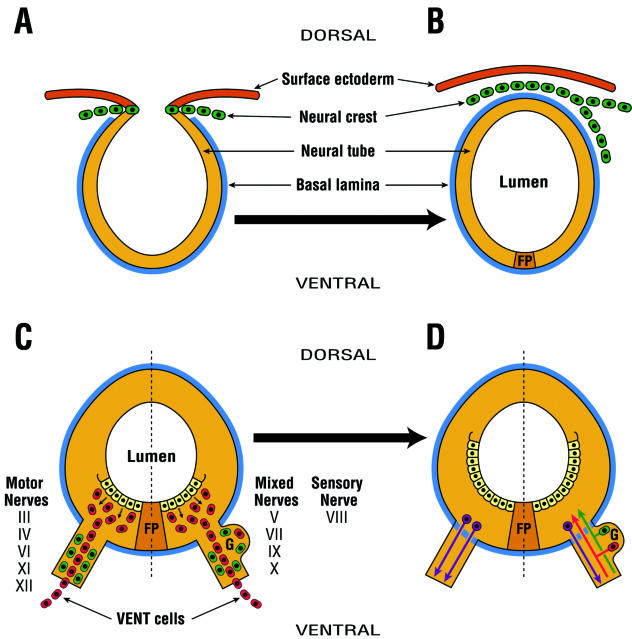
Fig. 1.
The concept of emigration of two separate and distinct neural tube-derived cell populations: neural crest and VENT cells. (A) Neural crest cells (green) arise from the dorsal region of the developing neural tube. They are HNK-1+. (B) Neural crest cells emigrate from the dorsal midline, and contribute to the development of the PNS and a variety of neural and non-neural tissues. This occurs in the cranial neural tube of the chick embryo between HH stages 9 and 11 (about E2). (C) VENT cells (red) arise from the ventral region of the neural tube. They are HNK-1−. VENT cells emigrate at the exit/entry site of all cranial nerves of the midbrain and hindbrain through a transient breach in the basal lamina (blue) surrounding the neural tube. In the chick, this begins to occur at about HH stage 16 (E3), i.e. considerably after the emigration of neural crest, but before the outgrowth/ingrowth of axons from the neural tube to the nerves. Prior to the emigration of VENT cells, the cranial nerves contain neural crest and/or placodally derived HNK-1+ cells. After this emigration, the nerves also contain HNK-1− VENT cells. Most VENT cells continue their migration to target tissues of these cranial nerves. (D) About 1 day later (i.e. E4), the transient breach is sealed. By E5, some of the VENT cells (red) that remain in the ganglia, and neural crest and/or placodally derived cells (green), differentiate into sensory neurons, with axons entering the neural tube through a fenestrated basal lamina. Motor neuron (purple) axons similarly exit the neural tube. FP, floor plate.
The concept of VENT cells
Our concept is that after the emigration of the neural crest, a second cell population emigrates from the neural tube. These cells originate from the ventral part of the cranial neural tube and emigrate at the exit/entry site of all cranial nerves of the midbrain (cranial nerves III and IV) and hindbrain (cranial nerves V–XII) (Fig. 1C). This emigration takes place through a transient breach in the basal lamina surrounding the neural tube. This breach occurs prior to the ingrowth/outgrowth of axons through the entry/exit sites on the neural tube in motor, sensory and mixed cranial nerves (Fig. 1C). Most VENT cells migrate in association with the nerves and contribute to the development of their target tissues (Fig. 1C). Some VENT cells remain in the ganglia of the sensory and mixed cranial nerves, and differentiate into neurons (Fig. 1D). VENT cells differ from neural crest cells in at least four ways: they originate in the ventral (not the dorsal) neural tube; they emigrate at the sites of nerve exit/entry (not the dorsal midline); in the chick they emigrate at Hamburger & Hamilton (1951) stage 16 onwards (not stages 9–11) (Johnston, 1966; Noden, 1975; Tosney, 1982); and they do not express the neural crest (and placodal cell) antigen HNK-1 (Vincent & Thiery, 1984; see below). Thus, VENT cells represent a second neural tube-derived emigrating cell population, whose origin and migration are topographically and temporally distinct from the neural crest. Therefore, the fate of all neural tube cells is not restricted to forming the CNS.
The origin of the idea of VENT cells
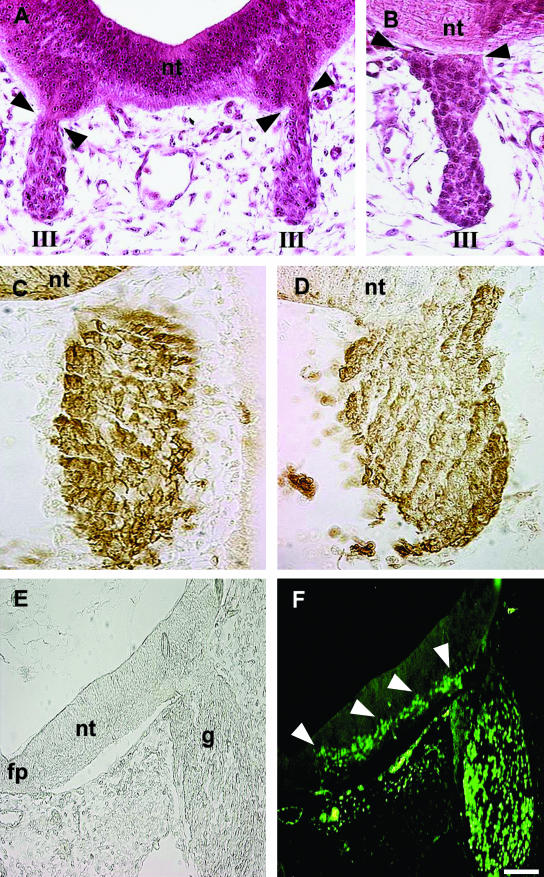
The idea of VENT cells originated from three different types of observations from histological sections of normal (i.e. unmanipulated) embryos. The first line of circumstantial evidence was that, shortly after the cranial nerves become morphologically identifiable, the ventral part of the neural tube is directly connected with the nerve, as if there was no physical barrier between their cell populations (Sohal et al. 1996; Fig. 2A, cranial nerve III, motor). The ventral neural tube cells are continuous with the cells in the nerve and they appear to be morphologically identical (Fig. 2A). This continuity is only apparent in 3–4 consecutive sections, and is easily missed. Such physical and cellular continuity is transient, lasting about a day (Fig. 2B, cranial nerve III, motor). Basal lamina is thought to separate nerves from the neural tube, and continuity most likely arises from a transient breach (Erickson, 1987; Niederlander & Lumsden, 1996). This observation raised the possibility that some ventral neural tube cells could emigrate through the exit/entry site of the nerves during the transient period of physical continuity.
Fig. 2.
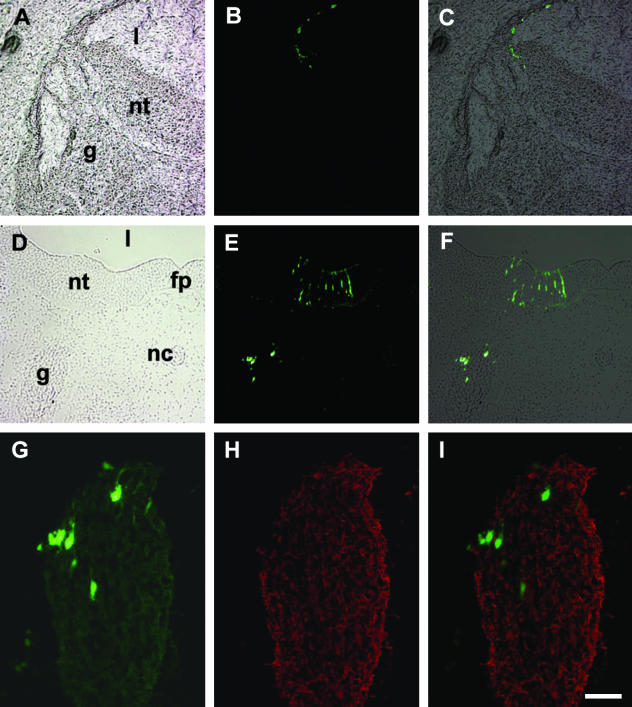
Circumstantial evidence for the VENT cells from normal (i.e. experimentally unmanipulated) embryos. The ventral neural tube is directly connected transiently with the cranial nerves. (A,B) H&E-stained cross-sections of quail embryos. nt, midbrain neural tube; III, the third cranial nerve. Continuity between the ventral neural tube and the nerve is seen at E3 (A, arrowheads) but not at E4 (B, arrowheads). Differential distribution of the HNK-1 antigen. (C,D) Sections through the hindbrain neural tube and fifth cranial nerve of quail embryos at E2.5 (C) and E3 (D), immunostained with anti-HNK-1 antibody. HNK-1+ cells are dark brown, whereas HNK-1− cells are unstained. nt, neural tube. Prior to continuity with the neural tube, the ganglion comprises HNK-1+ cells (C). After continuity is established at E3, the ganglion also contains regions of HNK-1− cells, continuous with the neural tube (D). The HNK-1− cells are mainly in the central regions, whereas the HNK-1+ cells are mainly in the periphery of the ganglion. (E,F) Expression pattern of Islet-1. A section through the hindbrain neural tube and fifth cranial nerve of an E3.5 chick embryo is shown in bright-field (E) and fluorescence (F) after immunostaining with anti-Islet-1 antibody. fp, floor plate; g, ganglion; nt, neural tube. Arrowheads point to a trail of Islet-1+ cells in the ventral neural tube, extending into the ganglion. Scale bar, (A) 32 µm; (B) 28 µm; (C) 32 µm; (D) 29 µm; (E, F) 75 µm.
The second line of circumstantial evidence came from the pattern of expression of the HNK-1 antigen. HNK-1 is generally considered to be a good marker for neural crest and placodally derived cells (Vincent & Thiery, 1984). Prior to the physical continuity between the ventral neural tube and the cranial nerves of the midbrain and hindbrain described above (and before axon ingrowth/outgrowth), the cranial nerves contain predominantly or exclusively HNK-1+ cells (Fig. 2C, cranial nerve V, mixed). For motor nerves, these HNK-1+ cells are neural crest-derived precursors of Schwann and supporting cells. For sensory and mixed nerves, the HNK-1+ cells are both neural crest and placodally derived. The neural crest cells give rise to neurons, Schwann and supporting cells, the placodally derived cells to neurons only (Le Douarin & Kalcheim, 1999). After this continuity, the nerves clearly contain a heterogeneous mixture of cells, some HNK-1+ and others HNK-1− (Fig. 2D, cranial nerve V, mixed). Further, the HNK-1+ cells were primarily located in the periphery of the nerves, whereas the HNK-1− cells were primarily located in the central portion (Fig. 2D). It was reasoned that whereas the HNK-1+ cells represent neural crest and/or placodally derived cells (because both cell populations are HNK-1+Vincent & Thiery, 1984), the HNK-1− cells may have emigrated from the ventral part of the neural tube into the nerve during the transient period of physical continuity between them.
The third line of circumstantial evidence came from the expression pattern of the homeobox gene Islet-1. During the period of direct continuity between the ventral neural tube and the trigeminal ganglion, a trail of Islet-1+ cells extends ventrolaterally from the region adjacent to the floor plate up to the attachment point of the ganglion, and into the ganglion (Sohal et al. 1996; Fig. 2E,F; cranial nerve V, mixed), as if some Islet-1+ cells are destined to emigrate into the ganglion.
In summary, the transient lack of a barrier could allow ventral neural tube cells to emigrate into the nerve, and at least some of the HNK-1−, Islet-1+ cells could be the emigrated cells. Such circumstantial evidence and reasoning formed the basis of the idea of VENT cells, which was subsequently investigated experimentally.
Operational definition of VENT cells
Based on the circumstantial evidence and reasoning outlined above, VENT cells were defined operationally as a population of ventral neural tube cells which emigrates considerably after neural crest, emerges from the exit/entry site into the cranial nerves and is HNK-1−. These VENT cells disperse into the target tissues of the nerves. Presently, no specific markers are available for the identification of VENT cells. However, they can be identified based on the operational definition by labelling cells in the ventral part of the neural tube at stage 13 or 14, i.e. considerably after the emigration of neural crest (Johnston, 1966; Noden, 1975; Tosney, 1982), and subsequently monitoring the presence of the labelled cells outside the neural tube. Labelled cells initially appear in the adjacent nerve and later migrate in association with the nerve to progressively further locations. Because the labelling is performed at a stage past neural crest emigration, labelled migrating cells should not be neural crest-derived. This can be confirmed immunohistochemically, as the labelled VENT cells do not express the neural crest cell antigen HNK-1 (Vincent & Thiery, 1984).
Evidence consistent with the idea of VENT cells
Four different types of experimental approaches have been used to test the validity of the idea of VENT cells using the operational definition described above. Cranial neural tube cells were labelled with the vital dye DiI, lacZ-expressing retroviral infection, green fluorescent protein (GFP) gene electroporation and quail chimeras. In the first three approaches, label or marker is introduced into the lumen of the neural tube, and, after an interval, tagged cells are identified in adjacent tissues.
Evidence for VENT cells from DiI labelling
The vital dye DiI is irreversibly incorporated into the plasma membrane of cells that contact the dye (Sims et al. 1974; Honig & Hume, 1986; Collazo et al. 1993). It is not spread from labelled to unlabelled cells (but is diluted by cell division). The position of the dye can be monitored by fluorescence microscopy. A concern of dye labelling techniques is leakage. An advantage to DiI labelling is that leakage can be observed directly and immediately following injection, and such embryos rejected (Ali et al. 2003b).
In initial studies, the ventral neural tube cells in the rostral hindbrain of duck embryos were focally labelled, by using a small piece of DiI-infiltrated paper, after the emigration of neural crest from this region (Sohal et al. 1996). About 12 h later, labelled cells were detected only in the ventral part of the neural tube, in the region of the DiI filter paper. One day after labelling, a trail of labelled cells could be easily followed from the ventral part of the neural tube into the adjacent trigeminal ganglion. The emigration of cells was restricted to the site of attachment of the ganglion. Thus, labelling the ventral part of the neural tube resulted in the subsequent presence of labelled cells in the ganglion, which suggests the emigration of VENT cells. When ventral neural tube cells were labelled in the caudal hindbrain or in the rostral or caudal midbrain, no labelled cells were seen in the trigeminal ganglion. This indicated that ventral emigration of cells into the trigeminal ganglion was limited to the adjacent region associated with the ganglion. When cells of the dorsal part of the neural tube were labelled at the same stage, labelled cells were not seen in the ganglion or outside the neural tube. This indicated that after the emigration of neural crest, cells from the dorsal part of the neural tube do not emigrate (Sohal et al. 1996).
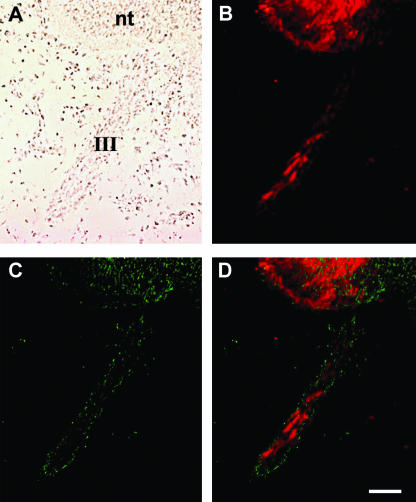
These experiments suggested that after the emigration of neural crest, an injection of the DiI solution into the lumen of the neural tube could be used to label the prospective VENT cells (Ali et al. 2003b). A typical result of DiI labelling is shown in Fig. 3 (cranial nerve III, motor). When DiI was injected into the lumen of the chick midbrain neural tube at stage 14 (after the emigration of the neural crest), numerous DiI-positive cells were subsequently observed in the third cranial nerve, consistent with emigration of the labelled VENT cells from the midbrain neural tube (Fig. 3A,B). The DiI-labelled VENT cells were HNK-1−, indicating that they are not derived from the neural crest (Fig. 3C,D). A neural crest origin of the DiI-labelled cells would not be expected because the neural tube cells were labelled after the emigration of neural crest. As observed in quail embryos during normal development (Fig. 2), the DiI+, HNK-1− VENT cells were localized in the centre of the nerve, whereas the HNK-1+ cells were primarily in the periphery. Similar results were seen for all other cranial nerves of the midbrain and hindbrain (i.e. cranial nerves III–XII).
Fig. 3.
Emigration of DiI-labelled VENT cells from the chick midbrain neural tube into cranial nerve III. DiI was injected into the lumen of the midbrain after the emigration of neural crest cells (E2, stage 14), and fixed at E3.5, as described (Ali et al. 2003b). A–D show images of the same section of the embryo through the neural tube and the third cranial nerve: (A)bright-field image; (B)DiI fluorescence; (C)HNK-1 immunostaining; and (D)merged DiI and HNK-1 images. nt, neural tube; III, third cranial nerve. A trail of red fluorescent emigrating cells is seen in the nerve (B). The pattern of HNK-1 staining (green fluorescence) is not consistent with the DiI-labelled emigrating cells (C and D). Scale bar, 67 µm.
Evidence for VENT cells from recombinant retroviral LacZ labelling
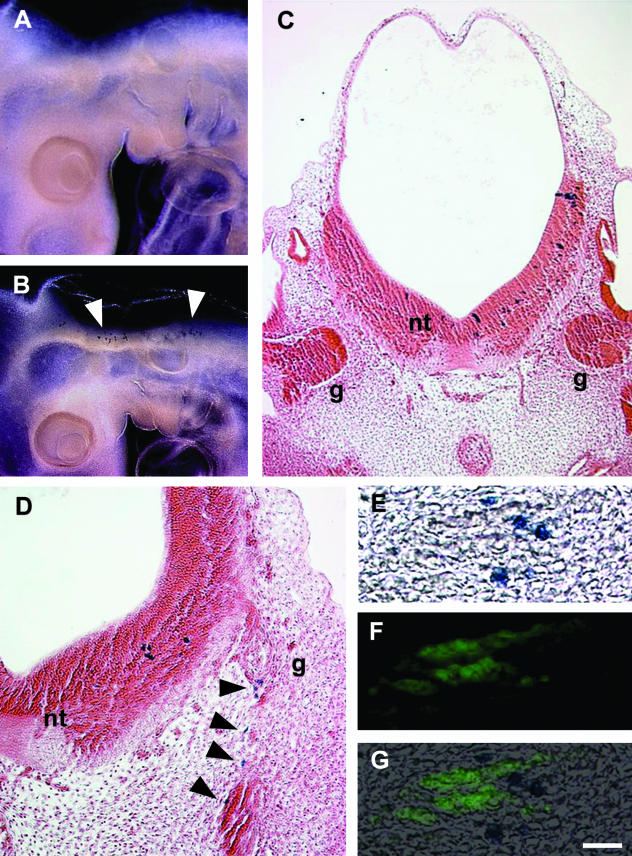
Tagging of cells by LacZ expression has also been employed (Bockman & Sohal, 1998; Sohal et al. 1998b; Ali et al. 2003b). Viral particles infect single cells, and the infected cells are detected histochemically. An advantage of this method over DiI labelling is that the label is not diluted following cell division, so tagged cells can be followed for longer. Two replication-deficient retroviral vectors containing the LacZ gene were utilized (Galileo et al. 1990, 1992). One vector (LZ12) expresses LacZ in the nucleus whereas in the other (LZ14) it is localized in the cytoplasm. LZ12 is derived from Molony murine sarcoma and leukaemia viruses, whereas LZ14 is derived from Rous sarcoma virus. The viral concentrate (0.2 µL) was injected into the lumen of the hindbrain neural tube of chick embryos after the emigration of neural crest (Sohal et al. 1998b). In control embryos, the vector was placed on the dorsal part of the neural tube: this did not label cells anywhere in the embryo (e.g. Fig. 4A). One day after the introduction of the vector into the lumen of the caudal hindbrain, labelled cells were restricted to the ventral neural tube (Fig. 4B),C. Shortly thereafter, labelled cells were seen in the adjacent ganglion of the vagus nerve (Fig. 4D; cranial nerve X, mixed). Thus, labelling the hindbrain neural tube later resulted in the presence of the labelled cells in the adjacent ganglion, indicating emigration of VENT cells. The emigrated cells were HNK-1−, consistent with their non-neural crest origin (Fig. 4E–G). Similar results were seen in all other cranial nerves of the midbrain and hindbrain.
Fig. 4.
Emigration of lacZ-retrovirus-labelled VENT cells from the chick hindbrain neural tube into cranial nerves. (A,B) Whole mounts of embryos histochemically stained for lacZ detection (blue cells). (A) Virus was placed on the dorsal part of the neural tube on E2. No labelled cells were seen on E3. (B) Virus was injected into the lumen of the hindbrain neural tube after the emigration of neural crest cells (E2, stage 14), as described (Sohal et al. 1998b). Labelled cells were restricted to the ventral part of the neural tube 1 day after injection (E3) (arrowheads). (C) No emigrated VENT cells are visible in the adjacent seventh and eighth cranial nerve ganglia 1 day after labelling (E3). g, ganglion; nt, neural tube. (D) Emigrated cells are visible in the adjacent tenth cranial nerve ganglion on E4 (arrowheads). A section adjacent to that shown in D was histochemically stained for lacZ (E), immunostained for HNK-1 (F) and the images merged (G). The pattern of HNK-1 staining (green fluorescence) is not coincident with the lacZ+ emigrated cells. Scale bar, (A and B) 555 µm; (C) 108 µm; (D) 24 µm; (E–G) 33 µm.
Evidence for VENT cells from GFP electroporation
Cells were labelled at different positions along the neural tube by injection of an enhanced GFP expression vector into the lumen followed by electroporation (Nakamura & Funahashi, 2001), after the emigration of neural crest. This technique has two advantages. First, by manipulating the placement of the electrodes, unilateral labelling can be achieved, providing a negative control within the same embryo. Second, by reversing the electrode polarity, the dorsal neural tube can be labelled, providing a control for migration via this route. Dorsal labelling resulted in the subsequent absence of any GFP-positive cells outside the neural tube (Fig. 5A–C). When labelled ventrally, GFP+ cells were subsequently found in the ventral part of the neural tube and the adjacent ganglia (Fig. 5D–F, cranial nerve V, mixed). Thus, labelling the ventral part of the neural tube resulted in the subsequent presence of labelled cells in the corresponding nerve, demonstrating emigration of VENT cells. Immunostaining with the neural crest marker HNK-1 revealed that the emigrated GFP+ cells were HNK-1−, indicating that they were not derived from the neural crest (Fig. 5G–I). Using this method, emigration of VENT cells was confirmed in all cranial nerves of the midbrain and hindbrain except the fourth, which develops later than the period examined (Sohal et al. 1985).
Fig. 5.
Emigration of GFP-labelled VENT cells from ventrally but not dorsally labelled chick neural tube. Neural tube cells were labelled on E2 (stage 14) by electroporation of an enhanced GFP expression vector (pEGFP-N1, Clontech). Vector solution (1.2 µg µL−1 of plasmid DNA in PBS with 0.125% fast green) was injected into the hindbrain neural tube after the emigration of neural crest. Electrodes were placed on either side of the neural tube and six pulses (25 V, 50 ms duration) were applied. Dorsal labelling was obtained by reversing the electrode polarity. Embryos were fixed from E3.5 to E4 in 4% paraformaldehyde and sections were evaluated for GFP-positive cells. (A–C) A dorsally labelled neural tube section with the adjacent cranial nerve ganglion is shown.(A) Bright-field view; nt, neural tube; l, lumen; g, ganglion. (B) Fluorescence view; (C) merged image. No GFP-labelled cells are seen outside the neural tube. (D–F) A ventrally and unilaterally labelled neural tube sectioned through the fifth cranial nerve is shown. (D) Bright-field view; nt, neural tube; l, lumen; g, ganglion; nc, notochord; fp, floor plate. (E)Fluorescence view; (F)merged image. Labelled emigrated cells are seen in the ganglion. (G) Higher magnification of the labelled ganglion shown in E. (H) The same section immunofluorescently stained with an anti-HNK-1 antibody. (I) Merged image of G and H. The labelled, emigrated cells are HNK-1−. Scale bar, (A–C) 73 µm; (D–F) 57 µm; (G–I) 40 µm.
Evidence for VENT cells from quail chimeras
In the fourth approach, quail chimeras were utilized. Portions of the Japanese quail neural tube were transplanted into Bob White quail after the emigration of neural crest, but prior to the emigration of VENT cells. The grafts were enzymatically treated with dispase I to remove any adhering cells, as described (Kontges & Lumsden, 1996). The grafted Japanese quail cells were identified using the species-specific antibody QCPN. Three major advantages of this technique are that it does not involve injection (and therefore issues related to leakage are obviated), all emigrating cells can be detected (not just a tagged subset) and emigration of cells can be examined from either dorsal or ventral halves of the neural tube.
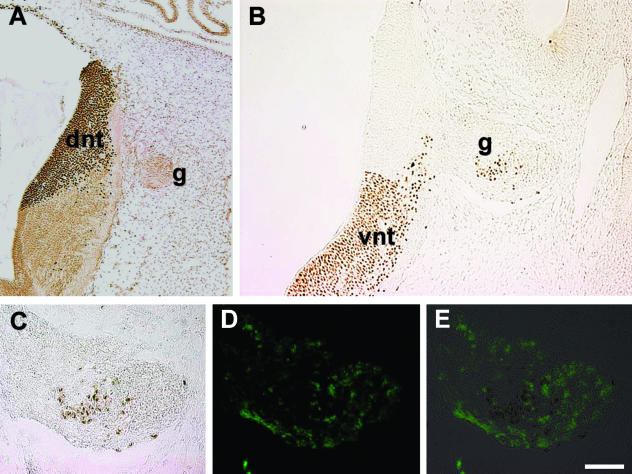
When the dorsal half of the neural tube was grafted, the QCPN-positive cells were not seen in the adjacent ganglion or outside the neural tube (Fig. 6A, cranial nerve V, mixed), indicating that cells from the dorsal part of the neural tube do not emigrate after the emigration of neural crest, as also observed by other labelling techniques. When ventral segments of the Bob White quail neural tube were replaced with the corresponding segments from the Japanese quail, many QCPN-positive Japanese quail cells were consistently detected in the adjacent ganglia (Fig. 6B, cranial nerveVII/VIII mixed/sensory). They were generally located in the centre of the nerve, as observed for other labelling techniques (see above). The emigrated cells were HNK-1− (Fig. 6C–E). This demonstrated emigration of VENT cells. Because dorsal transplants do not produce emigrating cells, but ventral transplants do, complete neural tubes were transplanted after the emigration of neural crest, which is technically easier. This approach showed that VENT cells emigrate at all cranial nerves of the midbrain and hindbrain. Using quail-chick chimeras, Boot et al. (2003) also reported emigrated QCPN+ quail cells in the cranial nerve ganglia of the hindbrain following transplantation of the neural tube after neural crest emigration.
Fig. 6.
Emigration of VENT cells from ventral but not dorsal neural tube transplants in quail chimeras. Dorsal or ventral segments of the Bob White quail neural tube were replaced with the equivalent region of the Japanese quail neural tube after the emigration of neural crest (E2, stage 13), as described (Ali et al. 2003b). Embryos were fixed in Bouin's at E3.5–E4, sectioned and immunostained with the species-specific monoclonal antibody QCPN. This antibody stains cells of the Japanese quail but not of the Bob White quail (Ali et al. 2003b). (A)Dorsal transplant; (B)ventral transplant. dnt, dorsal neural tube; vnt, ventral neural tube; g, ganglion. Emigrating cells are only seen with the ventral transplant. (C)Enlarged view of the ventrally labelled ganglion seen in B. (D)The same section immunofluorescently stained with anti-HNK-1 antibody. (E) Merged image of C and D. The QCPN+ emigrating cells are HNK-1−. Scale bar, (A) 110 µm; (B) 64 µm; (C–E) 40 µm.
Technical issues in the detection of VENT cells
At least two issues relate to any labelling method: (1) does the label reach the target cell population, and how efficiently does it label them; and (2) is the labelling restricted to the target cell population? Following injection into the lumen of the neural tube, label must diffuse through the cell layers to reach the target cells. It might be anticipated that viral particles would diffuse more slowly than dye, infecting cells along their path, and therefore have a higher probability of infecting cells adjacent to the lumen than more peripheral cells within the neural tube. At present, we do not know the exact location of the VENT cell progenitor population within the ventral neural tube at the time of labelling. If the VENT cell progenitor location is in the periphery of the ventral neural tube at the time of labelling, then viral and DiI labelling (and probably electroporation) are likely to be relatively inefficient. Over 3500 embryos have now been injected with lacZ-expressing retrovirus. As previously reported (Bockman & Sohal, 1998; Sohal et al. 1998b, 1999b, 2002), even at the higher viral titres used (107 infectious particles mL−1; 0.2 µL injected), about 20–58% of the embryos did not show incorporation into neural tube cells. At lower viral titres (105 infectious particles mL−1; 0.2 µL injected), almost 100% of the embryos failed to show incorporation. Of the embryos that did show incorporation, only a proportion (roughly 20%) had significant labelling of the VENT cell precursor population due to the random nature of the infection, site of precursor cells, etc. About 14 embryos showing incorporation would need to be examined for a 95% probability of finding at least one (about three expected) with VENT cell precursor population labelling. Furthermore, the precise timing of VENT cell emigration has not been fully defined. Thus, VENT cell emigration is likely to be missed in a proportion of appropriately labelled embryos. Similar arguments apply to DiI labelling. Therefore, relatively large numbers of embryos (as employed by Bockman & Sohal, 1998; Sohal et al. 1998b, 1999b, 2002) are required to detect or exclude VENT cells with statistical reliability.
Boot et al. (2003), using a replication-incompetent retrovirus containing the bacterial lacZ gene to label chick neural tube cells, failed to find evidence for VENT cells migrating into the heart. They suggested that the labelled cells in the myocardium found by Sohal and co-workers (see below) were the result of virus leakage during injection, leading to labelling of mesenchymal cells. However, labelling is limited to the hindbrain when the vector is injected into the lumen, and there is no feasible route for virus to traffic to the mesenchyme. In addition, viral labelling tagged cells in the myocardium, but not the epicardium or endocardium (Sohal et al. 1999b; see below), which are all classically thought to develop from mesodermal mesenchyme. Moreover, leakage does not explain why deliberate placement of virus on the dorsal part of the neural tube failed to label cells in the embryo in a manner mimicking VENT cell labelling (Sohal et al. 1998b; Ali et al. 2003b). It should be noted that relatively few embryos (up to ten) were examined by Boot et al. (2003). As pointed out earlier, the limitations of the retroviral labelling efficiency require large numbers of embryos to be injected. It is therefore likely that the failure by Boot et al. (2003) to detect VENT cells in the heart was the result of limited sampling.
Based on data compiled from about 1600 electroporated embryos, the survival rate is 27.3% (437/1600). Of the surviving embryos, 60.9% (266/437) showed ventral neural tube labelling, and of these labelled embryos, only 49.3% (131/266) showed one or more labelled nerves. For these efficiencies, at least nine surviving embryos, or five embryos with ventral neural tube labelling, would need to be examined for a 95% probability of observing VENT cells in a cranial nerve in at least one embryo. Put another way, at least seven appropriately labelled embryos would need to be examined to reduce the probability of seeing no VENT cells to 1%.
Yaneza et al. (2002) examined VENT cell emigration using electroporation of a GFP-expressing plasmid injected into the lumen of the chick cranial neural tube at stage 14 (i.e. after neural crest migration has ceased). Fluorescent cells were then followed over a period of 3 days using a fluorescence microscope. GFP-expressing, ventrally emigrating cells were not detected. There are several possible explanations for a failure to detect VENT cells by this approach. First, as mentioned above, a large number of embryos are needed to detect multiple cases of VENT cells by this method. Relatively few embryos (seven or fewer per experiment) were examined by Yaneza et al. (2002). Thus, in some cases the failure to find VENT cells may have been simply due to limited sampling. Second, in some instances the site of labelling in the neural tube may not have labelled effectively the VENT cell progenitor population. At present, the exact location(s) of these populations within the ventral neural tube during the period of labelling are not known. Third, whole live embryos were examined under the fluorescence microscope. Migrating VENT cells may have been unresolved in some cases. Fourth, Yaneza et al. (2002) used a β-actin promoter to drive the expression of GFP. Cell-type-specific expression of β-actin is well known. It is not known if this promoter is active in HNK-1− cells, or in VENT cells. By contrast, we used a promiscuous cytomegalovirus (CMV)-derived promoter to drive GFP expression.
The phenomenon of neural tube cell emigration
Four entirely different experimental strategies have now been used to test the validity of the idea of VENT cells. Bearing in mind the technical issues discussed above, all four gave the same result: VENT cells emigrate into the cranial nerves after the emigration of neural crest. The phenomenon of emigration of VENT cells is unlikely to be related to a specific functional modality subserved by the nerve, because it occurs in all cranial nerves (sensory, motor and mixed) of the midbrain and hindbrain. Furthermore, the presence of a ganglion is not required for the emigration of VENT cells. Therefore, mechanisms governing their emigration are likely to reside within the ventral neural tube itself and/or at the barrier between the neural tube and the exit/entry site of the nerve, which is considered to be composed of basal lamina and the boundary cap cells (Niederlander & Lumsden, 1996; Golding & Cohen, 1997).
As previously noted (Erickson & Weston, 1999), we should perhaps be not too surprised that the neural tube can give rise to emigrating cell populations besides neural crest. The first and second cranial nerves arise from the forebrain neural tube, and some forebrain neural tube cells migrate and contribute to the target tissues of these nerves. The major interneurons in the olfactory bulb form from cells migrating from the forebrain in association with the first (olfactory) cranial nerve (Luskin, 1993; Lois & Alvarez-Buylla, 1994; Pencea & Luskin, 2003). In addition, it has long been known that neural tube cells from the forebrain contribute to the pigmented epithelium and neural retina of the eye via the second (optic) cranial nerve. We have shown emigration of VENT cells from the third to the twelfth cranial nerves, i.e. from the midbrain and hindbrain. There have been several reports indicating the emigration of cells from the developing spinal cord (reviewed by Erickson & Weston, 1999). Cells emigrate at the site of attachment of the dorsal and ventral roots in avian embryos and differentiate into neurons and supporting cells of the PNS, and into melanocytes (Weston, 1963; Lunn et al. 1987; Loring et al. 1988; Sharma et al. 1995). Thus, some non-neural crest cells can leave the neural tube along the entire rostrocaudal length.
Fate of VENT cells
Most VENT cells continue their migration in the nerves and populate their target tissues. We have followed the fate of some of the labelled VENT cells that emigrate into the trigeminal (fifth), vestibulocochlear (eighth) and vagus (tenth) nerves. VENT cell migration in association with the trigeminal and vagus nerves has been followed by tagging with lacZ retroviral vectors (Ali et al. 1999, 2003a; Sohal et al. 1998a,b, 1999a,b,c); migration in association with the vestibulocochlear (eighth cranial) nerve has been studied using DiI and LacZ tagging and quail chimeras (Ali et al. 2003b). It should be noted that the descriptions of target tissues provided below are incomplete because of the limitations of the labelling techniques.
The trigeminal nerve innervates the first arch. In the adult, it carries sensory information from the face and oral cavity, and provides motor innervation to the muscles of mastication. The vestibulocochlear nerve carries sensory information from the hair cells of the vestibular organ and the cochlea. The vagus nerve has multiple targets and functions (sensory and motor), including providing innervation to the heart and to the gastrointestinal tract and associated glands.
The tissues innervated by these cranial nerves are derived collectively from the three germ layers of the embryo (ectoderm, mesoderm and endoderm), and contain cells representing the four basic tissue types of the body (nerve, connective, muscle and epithelial). To date, the first pharyngeal arch mesenchyme has been generally considered to form from mesodermal and neural crest cells only (Le Douarin & Kalcheim, 1999). Craniofacial muscles develop from this arch mesenchyme, which also gives rise to the smooth muscle cells of craniofacial blood vessels (Noden, 1988; Le Douarin & Kalcheim, 1999). Meckel's and quadrate cartilages of the first arch have been believed to develop from neural crest only (Le Douarin & Kalcheim, 1999). The endolymphatic duct, semicircular canals, saccule, utricle and cochlea of the inner ear, and neurons of the vestibulocochlear ganglia develop from the otic vesicle. The otic vesicle has previously been thought to be formed solely from non-neural ectodermal cells (the otic placode), and the inner ear to be mostly derived from these cells with a minor contribution of neural crest cells, which differentiate into melanocytes in the stria vascularis and Schwann cells (Noden & Van De Water, 1986; Fritzsch et al. 1998; Moore & Persaud, 1998; Torres & Giraldez, 1998; Fekete & Wu, 2002). To date, epidermis was believed to develop from surface ectoderm and dermis of the face from neural crest (Le Douarin & Kalcheim, 1999). Traditionally, the heart is considered to be derived from neural crest and mesoderm (Noden, 1991; Fukiishi & Morriss-Kay, 1992; Mikawa et al. 1992; Moore & Persaud, 1998; Kirby, 1999; Le Douarin & Kalcheim, 1999; Yutzey & Kirby, 2002). The liver is considered to develop from endoderm, which gives rise to the hepatocytes, and mesoderm, which provides the precursors of all other cell types found in this organ (Moore & Persaud, 1998). Similarly, the epithelium of the gastrointestinal tract also develops from the endoderm, and the connective tissue and smooth muscle of the walls of the gastrointestinal tract from mesodermal mesenchyme (Moore & Persaud, 1998). The interstitial cells of Cajal (ICC), which act as pacemakers for gastrointestinal tract motility and connect the enteric nervous system (ENS) and the smooth muscle cells, are also thought to develop from this mesenchyme (Lecoin et al. 1996; Kluppel et al. 1998; Young, 1999; Ward & Sanders, 2001a,2b). The neurons and glial cells of the ENS have traditionally been considered to be derived exclusively from neural crest (Yntema & Hammond, 1954; Gershon, 1997; Moore & Persaud, 1998; Le Douarin & Kalcheim, 1999; Burns & Le Douarin, 2001; Young & Newgreen, 2001).
Differentiation of VENT cells into nerve tissue
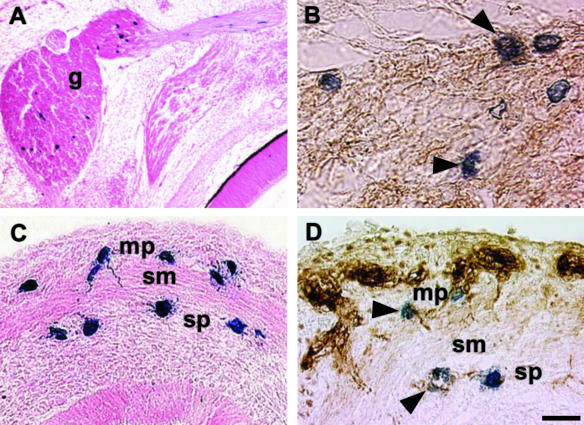
LacZ-tagged VENT cells began to appear in the chick trigeminal ganglion at about embryonic day 3 (E3), with the numbers increasing through E3.5 (Sohal et al. 1998b). Some VENT cells remained in the ganglion, and did not migrate further (Fig. 7A). By E6, some of these cells expressed β-tubulin, a neuronal marker (Fig. 7B). Thus, some VENT cells contribute neurons to the sensory ganglia of the cranial nerves. Therefore, three precursor cell populations provide neurons to the PNS: neural crest, placodes and VENT cells.
Fig. 7.
Differentiation of VENT cells into nerve tissue. Emigration of VENT cells into nerve tissue was monitored by tagging ventral neural tube cells with lacZ retrovirus at stage 14. Labelled cells were subsequently detected histochemically (blue-stained cells, A–D). (A)Some of the VENT cells that had emigrated into the trigeminal ganglion (g) remained in the ganglion by E6. (B)Some of these VENT cells expressed β-tubulin (arrowheads), a marker for neuronal tissue (brown immunostained cells). (C)A section of an E9 duodenum is shown. Some VENT cells migrating in association with the vagus nerve populated the region of the ENS in the gut. mp, myenteric plexus; sp, submucosal plexus; sm, smooth muscle layer. (D)Some of these VENT cells expressed β-tubulin, indicating differentiation into neurons of the ENS (arrowheads). Scale bar, (A) 119 µm; (B) 14 µm; (C) 21 µm; (D) 15 µm.
As for the trigeminal ganglion, by E3.5 a trail of VENT cells was observed extending from the neural tube to the vestibulocochlear nerve, consistent with emigration through the site of attachment of the nerve to the neural tube (Ali et al. 2003b). Immunostaining with an antibody to neurofilaments revealed that some VENT cells in the vestibulocochlear ganglia differentiated into neurons. Thus, neurons of the eighth cranial nerve are derived from two populations, otic placode and VENT cells.
LacZ-tagged VENT cells appeared in the vagus nerve on embryonic day E3, and entered the foregut by E4. By E6, some of these VENT cells were localized to the presumptive myenteric and submucosal plexus regions of the duodenum and stomach (Sohal et al. 2002; Fig. 7C). These cells were HNK-1−, in contrast to the vagal crest-derived cells in the plexuses. By E9, some VENT cells in both plexuses stained well with β-tubulin (a marker for neurons) (Fig. 7D), whereas others stained with GFAP (a marker for glial cells). Thus, some VENT cells had differentiated into neurons and glial cells. Therefore, the ENS develops from two sources of cells, neural crest and VENT cells.
Differentiation of VENT cells into muscle tissue
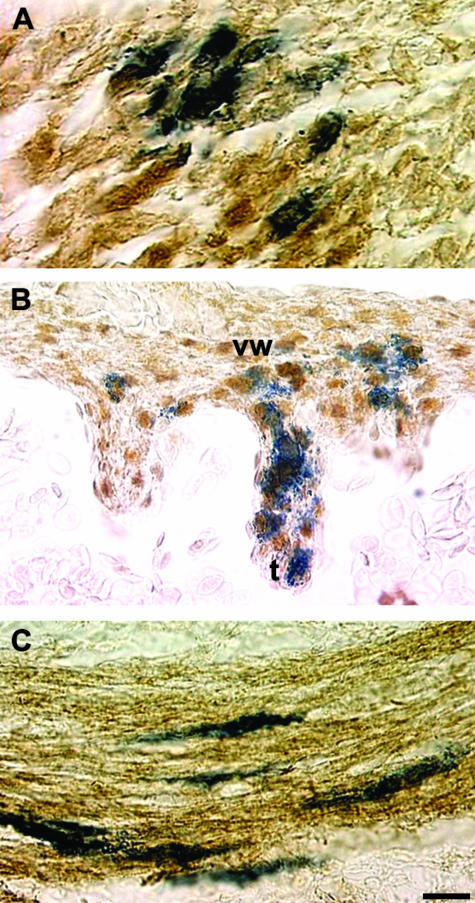
By E4, although some LacZ-tagged VENT cells remained in the trigeminal ganglion, other VENT cells had reached the mesenchyme of the first pharyngeal arch. By E7, VENT cells were seen in the myotubes of the craniofacial muscles (Sohal et al. 1998a). These cells stained with a monoclonal antibody to skeletal muscle fast myosin, indicating their differentiation into muscle (Fig. 8A). α-Actin smooth muscle-positive VENT cells were also detected in larger arteries and veins, indicating differentiation into vascular smooth muscle (Ali et al. 1999). Thus, craniofacial skeletal and smooth muscle cells develop from three sources of cells: mesoderm, neural crest and VENT cells.
Fig. 8.
Differentiation of VENT cells into muscle tissue. Pre-emigratory VENT cells were labelled by injection of LacZ-expressing retrovirus into the lumen of the neural tube of stage 14 chick embryos. Emigrated, labelled cells in tissues were subsequently detected histochemically (blue-stained cells, A–C). (A)By E7, some lacZ-labelled VENT cells have populated craniofacial muscle tissue, and are immunopositive for skeletal muscle fast myosin (brown colour), indicating differentiation into skeletal muscle. (B)By E8, some VENT cells in cardiac muscle are immunopositive for α-actin smooth muscle, which is also expressed in heart tissue, indicating differentiation into cardiac muscle. vw, ventricular wall; t, trabecula. (C)By E11, some VENT cells in the circular smooth muscle layer of the duodenum express α-actin smooth muscle, demonstrating differentiation of VENT cells into smooth muscle cells. Scale bar, (A) 12 µm; (B) 17 µm; (C) 13 µm.
Some of the VENT cells in the vagus nerve subsequently migrated into the heart by E4 (Sohal et al. 1999b; Ali et al. 2003a). VENT cells were detected in a variety of locations in the myocardium and the great vessels of the heart, but not in the epicardium or endocardium. Immunostaining for MF20, which is specific for cardiac myocytes, or for α-actin smooth muscle (which is also expressed in the heart) showed that the VENT cells in the myocardium had differentiated into cardiac myocytes (Sohal et al. 1999b; Fig. 8B). Some of the VENT cells contributed to the smooth muscle cells of the great vessels of the heart. This observation is consistent with a report that ventral neural tube transplants in quail-chick chimeras can give rise to vascular smooth muscle cells (Korn et al. 2002). Some vagal VENT cells were localized to the circular smooth muscle layer of the intestine by E11 (Bockman & Sohal, 1998). Based on α-actin smooth muscle immunostaining, at least some had differentiated into smooth muscle cells (Fig. 8C). Thus, one major fate of VENT cells is to give rise to cells in all three types of muscle in the body: skeletal, smooth and cardiac. Some VENT cells located adjacent to the smooth muscle cells in the gastrointestinal tract differentiated into ICC, as indicated by immunostaining for c-Kit (Sohal et al. 2002), a marker for ICC (Maeda et al. 1992; Huizinga et al. 1995; Torihashi et al. 1997; Epperson et al. 2000).
Differentiation of VENT cells into connective tissue
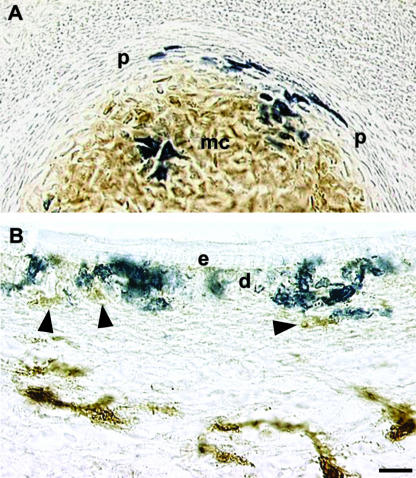
Some VENT cells migrating in association with the trigeminal nerve were subsequently found in the Meckel's cartilage and the surrounding perichondrium (Sohal et al. 1999c). This pattern was distinct from the distribution of neural crest-derived HNK-1+ cells, which were found in the cartilage but not in the perichondrium (Fig. 9A). A similar distribution of VENT cells was observed in the quadrate cartilage (Sohal et al. 1999c). These distributions indicate that VENT cells differentiate into chondrocytes, and play a role during cartilage formation. A mixed neural crest/non-neural crest cell population in Meckel's cartilage was previously observed in a mouse Wnt1-Cre/R26R model for tracking neural crest cell lineages (Chai et al. 2000). They suggested that VENT cells might account for the non-neural crest cells.
Fig. 9.
Differentiation of VENT cells into connective tissue. Pre-emigratory VENT cells were labelled by injection of LacZ-expressing retrovirus into lumen of the midbrain or hindbrain neural tube of stage 14 (E3) chick embryos. Emigrated, labelled cells in tissues were subsequently detected histochemically (blue-stained cells, A and B). (A) By E7, VENT cells were present in Meckel's cartilage (mc) and the surrounding perichondrium (p). HNK-1+ cells (brown immunostain) are absent in the perichondrium but are prominent in the cartilage. The VENT cells are HNK-1−. (B)VENT cells populate the dermal layers (d) of the craniofacial skin by E7. Unlike other cells in the dermis (arrowheads, brown immunostain), they are HNK-1−. e, epidermis. Scale bar, (A) 22 µm; (B) 13 µm.
Some VENT cells migrating in association with the trigeminal ganglia were detected in the craniofacial dermis, indicating a role in skin development (Fig. 9B; Sohal et al. 1999c). These VENT cells were intermingled with HNK-1+ neural crest-derived cells.
Differentiation of VENT cells into epithelium
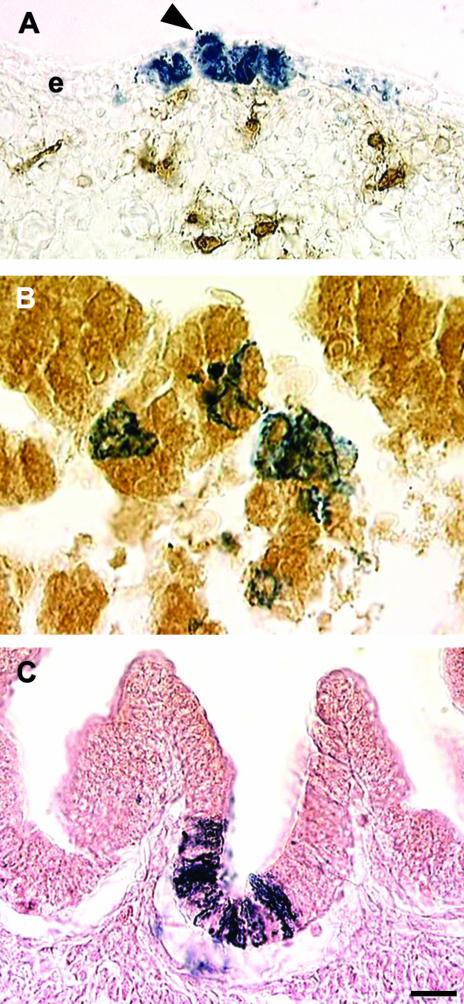
In addition to the dermis, VENT cells migrating in association with the trigeminal nerve were also detected in the craniofacial epidermis (Fig. 10A; Sohal et al. 1999c). In some cases, columns of labelled cells spanning the epidermis were seen. Therefore, VENT cells contribute to all layers of the skin, and the epidermis of the face develops from three sources of cells: surface ectoderm, neural crest and VENT cells.
Fig. 10.
Differentiation of VENT cells into epithelium. Pre-emigratory VENT cells were labelled by injection of LacZ-expressing retrovirus into lumen of the neural tube of stage 14 (E3) chick embryos. (A)VENT cells populate the epidermal layers of the craniofacial skin by E7. Labelled VENT cells (blue) are observed in all layers of the epidermis (e). In some cases columns of labelled cells rise from the basal layers to the superficial layers (arrowhead). Brown immunostained cells in the dermis are HNK-1+, unlike the VENT cells. (B)By E11, many VENT cells in the liver express albumin (brown immunostain), a marker for hepatocytes. (C)Some VENT cells in the duodenum differentiate into epithelial cells. The VENT cells are often located in the deeper layers of the villi, which is where the proliferating stem cells are located. Scale bar, (A) 13 µm; (B) 8 µm; (C) 13 µm.
Some embryos at E3.5 showed VENT cells migrating in association with the vestibulocochlear nerve in the region adjacent to the ventral part of the otic vesicle and in the otic vesicle itself (Ali et al. 2003b). By E4, VENT cells were detected in different parts of the otic vesicle, and by E5, in the endolymphatic duct, semicircular canals, saccule, utricle and cochlea that develop from the otic vesicle. Therefore, the inner ear develops from non-neural surface ectodermal epithelium (otic placode), neural crest and VENT cells.
By E5, some VENT cells migrating in association with the vagus nerve were found in the trabeculae, but not the sinusoids, of the developing liver (Sohal et al. 1999a). These cells had the histological appearance of hepatocytes, and stained with an antibody for albumin, a hepatocyte marker (Fig. 10B). A significant number of cells were found to have migrated into the gastrointestinal tract by E6, where they were restricted to the duodenum and stomach, which develop from the foregut (Bockman & Sohal, 1998; Sohal et al. 2002). As early as E5, some tagged cells had reached the epithelial lining of the lumen of the duodenum and stomach. In the duodenum, the cells were localized to the deepest portions of the villi, where the epithelial stem cells are located in the adult (Fig. 10C). In the proventriculus (the glandular stomach of the chick), some cells were localized in the gastric glands. These locations are consistent with differentiation of the VENT cells into epithelial cells.
Again, it should be emphasized that the list of fates of VENT cells is presently incomplete, owing to the limitations of labelling methods. As noted above, lacZ labelling is relatively inefficient: only 23 out of 120 labelled embryos (19%) showed lacZ-positive cells in the heart (Sohal et al. 1999b), and 27/127 (21%) showed lacZ-positive cells in the gastrointestinal tract. This emphasizes the importance of examining a large number of embryos.
Collectively, the data indicate that the VENT cells constitute a new neural tube-derived cell population that can differentiate into cell types belonging to all four basic tissues in the body: the nerve, muscle, connective and epithelium. Thus, VENT cells are not only multipotent, but also have the ability to contribute to tissues derived from all three germ layers. However, the full range of potential for VENT cells remains to be determined, and final proof of functional differentiation awaits detailed expression analysis. Conversely, we do not know if any VENT cells in tissues retain stem cell-like properties.
Several lines of evidence indicate that the neural tube cells have the capacity to give rise to a variety of cell types. For example, lineage studies have revealed that neural tube cells can give rise to epidermal cells (Selleck & Bronner-Fraser, 1995). They can generate neural crest phenotypes after ablation of the neural crest (Scherson et al. 1993; Sechrist et al. 1995) or after transplantation into the neural crest migratory pathways in the chick (Korade & Frank, 1996). Clonal analysis of rat neural tube cells in culture has shown that both CNS and neural crest phenotypes can arise from a single cell (Kalyani et al. 1997, 1998; Mujtaba et al. 1998; Rao, 1999). Thus, the fate of all neural tube cells does not appear to be as rigid as previously thought. This flexibility in the fate of the neural tube cells is not surprising given the flexibility of adult neural stem cells from the CNS (Bjornson et al. 1999; Clarke et al. 2000). For instance, spinal cord cells have been shown to differentiate into cartilage and muscle-forming cells (mesodermally derived tissues) following tail amputation in the axolotl (Echeverri & Tanaka, 2002). Adult neural stem cells give rise to derivatives of all three germ layers when injected into the chick embryo (Clarke et al. 2000).
Possible roles for VENT cells
At present, the function of VENT cells in development is speculative. However, ablation experiments provide some clues to their importance. Extirpation of the ventral part of the caudal hindbrain neural tube (a source of VENT cells) led to abnormalities of the heart and great vessels, including misshapen and asymmetrical atria, and narrowing of the great vessels (Ali et al. 2003a). The most common defect was persistent truncus arteriosus, in which the truncus arteriosus fails to separate into the aorta and the pulmonary trunk. Extirpation of the ventral part of the rostral hindbrain neural tube (the source of VENT cells for the fifth cranial nerve) led to a variety of craniofacial abnormalities (Sohal et al. 2001).
The craniofacial and heart abnormalities that result from ablation of presumptive VENT cell precursor populations argue for a direct role in development of normal morphologies. This could be by contributing additional cells that differentiate into structural components. However, this leads to the question of why vertebrates would have evolved a mechanism to provide supplemental cells to tissues, apparently duplicating established developmental pathways. Alternatively, VENT cells could be involved in inductive interactions. An interesting possibility that fits with either function is that VENT cells may serve to provide a flexible evolutionary mechanism to modify the morphology of a tissue that does not involve a reprogramming of more universal developmental pathways, such as those involving Hox genes. As a consequence, VENT cells could allow a broader range of phenotypic variation in a trait than might be possible from tolerated variations in other pathway components governing that trait. Conversely, VENT cells could provide a mechanism to ‘balance’ cell populations in developing tissues of variants in other developmental pathways so that an advantageous alteration in one tissue need not be at the expense of another tissue. This is consistent with ablation experiments (e.g. McKee & Ferguson, 1983; Scherson et al. 1993; Hunt et al. 1995; Sechrist et al. 1995; Couly et al. 1996) that show normal (or near normal) development of tissues after removal of neural crest. These roles would have implications for selection of developmental mutations during evolution.
Another possibility is that VENT cells may provide a reservoir of multipotent cells (stem cells) that are involved in tissue repair. A fifth possibility, consistent with the migration pathways followed by VENT cells, is that they may be involved in establishing appropriate connections between the nervous system and target tissues. Which of these possibilities are correct, if any, awaits further discoveries.
Summary and future directions
Neural crest is often considered to be the sole neural tube-derived emigrating cell population; consequently, the fate of the remaining neural tube cells has been assumed to be restricted to forming the CNS. The idea presented here is somewhat different: the fate of all non-neural crest neural tube-derived cells is not restricted to forming the CNS, and the neural tube provides a second emigrating cell population, the VENT cells. The idea of VENT cells originated from three main observations from normal (i.e. experimentally unmanipulated) embryos: (1) the transient continuity between the ventral neural tube and the cranial nerves; (2) after this continuity, the appearance of HNK-1− cells in the nerves; and (3) the trail of Islet-1+ cells extending from the ventral part of the neural tube into the ganglia. These observations were interpreted to mean that some ventral neural tube cells could emigrate into nerves during this period of a transient lack of a barrier. This led to the operational definition of VENT cells: they emigrate considerably later than that of neural crest cell emigration, migrate into cranial nerves and are HNK-1−. Four entirely different labelling methods based on this operational definition confirmed the existence of VENT cells. Therefore, the neural tube provides at least two emigrating cell populations, neural crest and VENT cells, that contribute to the development of the PNS and non-neural structures. These two populations differ in significant ways. Unlike neural crest cells, VENT cells originate in the ventral region of the cranial neural tube, and they emigrate at the exit/entry sites of the cranial nerves. VENT cells emigrate considerably after the emigration of the neural crest, and are HNK-1−.
Many important questions remain. What are the origins of the VENT cell progenitor population? What are the molecular signatures of VENT cells? How does this population differ from other neural tube or neural crest cell populations? What mechanisms guide VENT cell emigration and subsequent migration? How do the two neural tube-derived populations interact? How do VENT cells interact with mesodermally and endodermally derived cells? What is the exact nature of the contribution VENT cells make to the target tissues? There is evidence for differentiation and integration into the normal cell population. How important is this to the development of form? What are the implications for evolution? Do any VENT cells in the adult retain stem cell-like properties? Clearly, specific markers that will allow VENT cells to be tracked more efficiently are needed. Undoubtedly, the existence of VENT cells opens up exciting areas for further investigation. It also points to the need to re-evaluate our current view of the fate of the neural tube cells.
Acknowledgments
We gratefully acknowledge the technical assistance of Gu Zhu, and the artwork of Laura McKie. This work was supported by grants from the National Institutes of Health (DE12471, DC4547 and DK59854).
References
- Ali AA, Ali MM, Dai D, Sohal GS. Ventrally emigrating neural tube cells differentiate into vascular smooth muscle cells. Gen. Pharmacol. 1999;33:401–405. doi: 10.1016/s0306-3623(99)00034-8. [DOI] [PubMed] [Google Scholar]
- Ali MM, Farooqui FA, Sohal GS. Ventrally emigrating neural tube cells contribute to the normal development of heart and great vessels. Vas. Pharmacol. 2003a;40:133–140. doi: 10.1016/s1537-1891(03)00003-x. [DOI] [PubMed] [Google Scholar]
- Ali MM, Jayabalan S, Machnicki M, Sohal GS. Ventrally emigrating neural tube cells migrate into the developing vestibulocochlear nerve and otic vesicle. Int. J. Dev. Neurosci. 2003b;21:199–208. doi: 10.1016/s0736-5748(03)00036-4. [DOI] [PubMed] [Google Scholar]
- Bjornson CRR, Rietze RL, Reynolds BA, Magli MC, Vescovi AL. Turning brain into blood: a hematopoietic fate adopted by adult neural stem cells in vivo. Science. 1999;283:534–537. doi: 10.1126/science.283.5401.534. [DOI] [PubMed] [Google Scholar]
- Bockman DE, Sohal GS. A new source of cells contributing to the developing gastrointestinal tract demonstrated in chick embryos. Gastroenterology. 1998;114:878–882. doi: 10.1016/s0016-5085(98)70306-3. [DOI] [PubMed] [Google Scholar]
- Boot MJ, Gittenberger-de-Groot AC, Iperen L, Poelmann RE. The myth of ventrally emigrating neural tube (VENT) cells and their contribution to the developing cardiovascular system. Anat. Embryol. 2003;206:327–333. doi: 10.1007/s00429-002-0302-5. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Le Douarin NM. development: analysis of the selective developmental potentialities of vagal and sacral neural crest cells using quail-chick chimeras. Anat. Rec. 2001;262:16–28. doi: 10.1002/1097-0185(20010101)262:1<16::AID-AR1007>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Casanova MF, Buxhoeveden D, Sohal GS. Brain development and evolution. In: Ernst M, Rumsey J, editors. Functional Neuroimaging in Child Psychiatry. Cambridge: Cambridge University Press; 2000. pp. 113–136. [Google Scholar]
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Johansson CB, Wilbertz J, et al. Generalized potential of adult neural stem cells. Science. 2000;288:1660–1663. doi: 10.1126/science.288.5471.1660. [DOI] [PubMed] [Google Scholar]
- Collazo A, Bronner-Fraser M, Fraser SE. Vital dye labeling of Xenopus laevis trunk neural crest reveals multipotency and novel pathways of migration. Development. 1993;118:363–376. doi: 10.1242/dev.118.2.363. [DOI] [PubMed] [Google Scholar]
- Couly G, Grapin-Bottom A, Coltey P, Le Dourain NM. The regeneration of the cephalic neural crest, a problem revisited: the regenerating cells originate from the contralateral or from the anterior and posterior neural fold. Development. 1996;122:3393–3407. doi: 10.1242/dev.122.11.3393. [DOI] [PubMed] [Google Scholar]
- Echeverri K, Tanaka EM. Ectoderm to mesoderm lineage switching during axolotl tail regeneration. Science. 2002;298:1993–1996. doi: 10.1126/science.1077804. [DOI] [PubMed] [Google Scholar]
- Epperson A, Hatton WJ, Callaghan B, et al. Molecular components expressed in cultured and freshly isolated interstitial cells of Cajal. Am. J. Physiol. Cell. Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- Erickson CA. Behavior of neural crest cells on embryonic basal laminae. Dev. Biol. 1987;120:38–49. doi: 10.1016/0012-1606(87)90101-1. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Weston JA. VENT cells: a fresh breeze in a stuffy field? Trends Neurosci. 1999;22:486–488. doi: 10.1016/s0166-2236(99)01496-4. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr. Opin. Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. New York: Springer; 1998. pp. 80–145. [Google Scholar]
- Fukiishi Y, Morriss-Kay G. Migration of cranial neural crest cells to the pharyngeal arches and heart in rat embryos. Cell Tissue Res. 1992;268:1–8. doi: 10.1007/BF00338048. [DOI] [PubMed] [Google Scholar]
- Galileo DS, Gray GE, Owens GC, Majors J, Sanes JR. Neurons and glia arise from a common progenitor in chicken optic tectum: demonstration with two retroviruses and cell type specific antibodies. Proc. Natl. Acad. Sci. USA. 1990;87:458–462. doi: 10.1073/pnas.87.1.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galileo DS, Majors J, Horwitz AF, Sanes JR. Retrovirally introduced antisense integrin RNA inhibits neuroblast migration in vivo. Neuron. 1992;9:1117–1131. doi: 10.1016/0896-6273(92)90070-t. [DOI] [PubMed] [Google Scholar]
- Gammill LS, Bronner-Fraser M. Neural crest specification: migrating into genomics. Nature Rev. Neurosci. 2003;4:795–805. doi: 10.1038/nrn1219. [DOI] [PubMed] [Google Scholar]
- Gershon MD. Genes and lineages in the formation of the enteric nervous system. Curr. Opin. Neurobiol. 1997;7:101–109. doi: 10.1016/s0959-4388(97)80127-4. [DOI] [PubMed] [Google Scholar]
- Golding JP, Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Mol. Cell. Neurosci. 1997;9:381–396. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term cultures. J. Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen H, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Hunt P, Ferretti P, Krumlauf R, Thorogood P. Restoration of normal Hox code and branchial arch morphogenesis after extensive deletion of hindbrain neural crest. Dev. Biol. 1995;168:584–597. doi: 10.1006/dbio.1995.1104. [DOI] [PubMed] [Google Scholar]
- Johnston MC. A radioautographic study of the migration and fate of cranial neural crest cells in the chick embryo. Anat. Rec. 1966;156:143–155. doi: 10.1002/ar.1091560204. [DOI] [PubMed] [Google Scholar]
- Kalyani A, Hobson K, Rao MS. Neuroepithelial stem cells from the embryonic spinal cord: isolation, characterization, and clonal analysis. Dev. Biol. 1997;186:202–223. doi: 10.1006/dbio.1997.8592. [DOI] [PubMed] [Google Scholar]
- Kalyani AJ, Piper D, Mujtaba T, Lucero MT, Rao MS. Spinal cord neuronal precursors generate multiple neuronal phenotypes in culture. J. Neurosci. 1998;18:7856–7868. doi: 10.1523/JNEUROSCI.18-19-07856.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby ML. Contribution of neural crest to heart and vessel morphology. In: Harvey RP, Rosenthal N, editors. Heart Development. Boston: Academic Press; 1999. pp. 179–193. [Google Scholar]
- Kluppel M, Huizinga JD, Malysz J, Bernstein A. Developmental origin and Kit-dependent development of the interstitial cells of Cajal in the mammalian small intestine. Dev. Dyn. 1998;211:60–71. doi: 10.1002/(SICI)1097-0177(199801)211:1<60::AID-AJA6>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Kontges G, Lumsden A. Rhombencephalic neural crest segmentation is preserved throughout craniofacial ontogeny. Development. 1996;122:3229–3242. doi: 10.1242/dev.122.10.3229. [DOI] [PubMed] [Google Scholar]
- Korade Z, Frank E. Restriction in cell fates of developing spinal cord cells transplanted to neural crest pathways. J. Neurosci. 1996;16:7638–7648. doi: 10.1523/JNEUROSCI.16-23-07638.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn J, Christ B, Kurz H. Neuroectodermal origin of brain pericytes and vascular smooth muscle cells. J. Comp. Neurol. 2002;442:78–88. doi: 10.1002/cne.1423. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Kalcheim C. The Neural Crest. 2nd edn. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Lecoin L, Gabella G, Le Douarin N. Origin of the c-kit-positive interstitial cells in the avian bowel. Development. 1996;122:725–733. doi: 10.1242/dev.122.3.725. [DOI] [PubMed] [Google Scholar]
- Lois C, Alvarez-Buylla A. Long-distance neuronal migration in the adult mammalian brain. Science. 1994;264:1145–1148. doi: 10.1126/science.8178174. [DOI] [PubMed] [Google Scholar]
- Loring JF, Barker DL, Erickson CA. Migration and differentiation of neural crest and ventral neural tube cells in vitro: implications for in vitro and in vivo studies of the neural crest. J. Neurosci. 1988;8:1001–1015. doi: 10.1523/JNEUROSCI.08-03-01001.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunn ER, Scourfield J, Keynes RJ, Stern CD. The neural tube origin of ventral root sheath cells in the chick embryo. Development. 1987;101:247–254. doi: 10.1242/dev.101.2.247. [DOI] [PubMed] [Google Scholar]
- Luskin MB. Restricted proliferation and migration of postnatally generated neurons derived from the forebrain subventricular zone. Neuron. 1993;11:173–189. doi: 10.1016/0896-6273(93)90281-u. [DOI] [PubMed] [Google Scholar]
- Maeda H, Yamagata A, Nishikawa S, et al. Requirement of c-kit for development of intestinal pacemaker system. Development. 1992;116:369–375. doi: 10.1242/dev.116.2.369. [DOI] [PubMed] [Google Scholar]
- McKee GJ, Ferguson MW. The effects of mesencephalic neural crest cell extirpation on the development of chicken embryos. J. Anat. 1983;139:491–512. [PMC free article] [PubMed] [Google Scholar]
- Mikawa T, Borisov A, Brown AM, Fischman DA. Clonal analysis of cardiac morphogenesis in the chicken embryo using a replication-defective retrovirus. I. Formation of the ventricular myocardium. Dev. Dyn. 1992;193:11–23. doi: 10.1002/aja.1001930104. [DOI] [PubMed] [Google Scholar]
- Moore KL, Persaud TVN. The Developing Human: Clinically Oriented Embryology. 6th edn. Philadelphia: Saunders; 1998. [Google Scholar]
- Mujtaba T, Mayer-Proschel M, Rao MS. A common neural progenitor for the CNS and PNS. Dev. Biol. 1998;200:1–15. doi: 10.1006/dbio.1998.8913. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Funahashi J. Introduction of DNA into chick embryos by in ovo electroporation. Methods. 2001;24:43–48. doi: 10.1006/meth.2001.1155. [DOI] [PubMed] [Google Scholar]
- Niederlander C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–2374. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- Noden DM. An analysis of the migratory behavior of avian neural crest cells. Dev. Biol. 1975;42:106–130. doi: 10.1016/0012-1606(75)90318-8. [DOI] [PubMed] [Google Scholar]
- Noden DM, Van De Water TR. The developing ear: tissue origins and interactions. In: Ruben RJ, Van De Water TR, Rubel EW, editors. The Biology of Change in Otolaryngology. Amsterdam: Elsevier; 1986. pp. 15–46. [Google Scholar]
- Noden DM. Interactions and fates of avian craniofacial mesenchyme. Development. 1988;103(Suppl.):121–140. doi: 10.1242/dev.103.Supplement.121. [DOI] [PubMed] [Google Scholar]
- Noden DM. Origins and patterning of avian outflow tract endocardium. Development. 1991;111:867–876. doi: 10.1242/dev.111.4.867. [DOI] [PubMed] [Google Scholar]
- Pencea V, Luskin MB. Prenatal development of the rodent rostral migratory stream. J. Comp. Neurol. 2003;463:402–418. doi: 10.1002/cne.10746. [DOI] [PubMed] [Google Scholar]
- Rao MS. Multipotent and restricted precursors in the central nervous system. Anat. Rec. 1999;257:137–148. doi: 10.1002/(SICI)1097-0185(19990815)257:4<137::AID-AR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Scherson T, Serbedzijz G, Fraser S, Bronner-Fraser M. Regulative capacity of the cranial neural tube to form neural crest. Development. 1993;118:1049–1062. doi: 10.1242/dev.118.4.1049. [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: traditional viewpoint and recent advances. Development. 1990;109:243–270. doi: 10.1242/dev.109.2.243. [DOI] [PubMed] [Google Scholar]
- Sechrist J, Nieto MA, Zamanian RT, Bronner-Fraser M. Regulative response of the cranial neural tube after neural fold ablation: spatiotemporal nature of neural crest regeneration and upregulation of Slug. Development. 1995;121:4103–4115. doi: 10.1242/dev.121.12.4103. [DOI] [PubMed] [Google Scholar]
- Selleck MA, Bronner-Fraser M. Origins of the avian neural crest: the role of neural plate–epidermal interactions. Development. 1995;121:525–538. doi: 10.1242/dev.121.2.525. [DOI] [PubMed] [Google Scholar]
- Sharma K, Korade Z, Frank E. Late-migrating neuroepithelial cells from the spinal cord differentiate into sensory ganglion cells and melanocytes. Neuron. 1995;14:143–152. doi: 10.1016/0896-6273(95)90248-1. [DOI] [PubMed] [Google Scholar]
- Sims PJ, Waggoner AS, Wang CH, Hoffman JF. Studies on the mechanism by which cyanine dyes measure membrane potential in red blood cells in phosphatidylcholine vesicles. Biochemistry. 1974;13:3315–3330. doi: 10.1021/bi00713a022. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Knox TS, Allen JC, Jr, Arumugam T, Campbell LR, Yamashita T. Development of the trochlear nucleus in quail and comparative study of the trochlear nucleus, nerve, and innervation of the superior oblique muscle in quail, chick and duck. J. Comp. Neurol. 1985;239:227–236. doi: 10.1002/cne.902390209. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Bockman DE, Ali MM, Tsai NT. Dil labeling and homeobox gene Islet-1 expression reveal contributions of ventral neural tube cells to the formation of the avian trigeminal ganglion. Int. J. Dev. Neurosci. 1996;14:419–427. [PubMed] [Google Scholar]
- Sohal GS, Ali AA, Ali MM. Ventral neural tube cells differentiate into craniofacial skeletal muscles. Biochem. Biophys. Res. Commun. 1998a;252:675–678. doi: 10.1006/bbrc.1998.9715. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Ali MM, Galileo DS, Ali AA. Emigration of neuroepithelial cells from the hindbrain neural tube in the chick embryo. Int. J. Dev. Neurosci. 1998b;16:477–481. doi: 10.1016/s0736-5748(98)00049-5. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Ali MM, Ali AA, Bockman DE. Ventral neural tube cells differentiate into hepatocytes in the chick embryo. Cell. Mol. Life. Sci. 1999a;55:128–130. doi: 10.1007/s000180050276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal GS, Ali MM, Ali AA, Dai D. Ventrally emigrating neural tube cells differentiate into heart muscle. Biochem. Biophys. Res. Commun. 1999b;254:601–604. doi: 10.1006/bbrc.1998.0109. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Ali MM, Ali AA, Dai D. Ventrally emigrating neural tube cells contribute to the formation of Meckel's and quadrate cartilage. Dev. Dyn. 1999c;216:37–44. doi: 10.1002/(SICI)1097-0177(199909)216:1<37::AID-DVDY6>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Sohal GS, Ali MM, Farooqui FA. Neural tube stem cells: VENT cells. Int. J. Dev. Neurosci. 2001;19:729. [Google Scholar]
- Sohal GS, Ali MM, Farooqui FA. A second source of precursor cells for the developing enteric nervous system and interstitial cells of Cajal. Int. J. Dev. Neurosci. 2002;20:619–626. doi: 10.1016/s0736-5748(02)00103-x. [DOI] [PubMed] [Google Scholar]
- Torihashi S, Ward SM, Sanders KM. Development of c-Kit positive cells and the onset of electrical rhythmicity in murine small intestine. Gastroenterology. 1997;112:144–155. doi: 10.1016/s0016-5085(97)70229-4. [DOI] [PubMed] [Google Scholar]
- Torres M, Giraldez F. The development of the vertebrate inner ear. Mech. Dev. 1998;71:5–21. doi: 10.1016/s0925-4773(97)00155-x. [DOI] [PubMed] [Google Scholar]
- Tosney KW. The segregation and early migration of cranial neural crest cells in the avian embryo. Dev. Biol. 1982;89:13–24. doi: 10.1016/0012-1606(82)90289-5. [DOI] [PubMed] [Google Scholar]
- Vincent M, Thiery JP. A cell surface marker for neural crest and placodal cells: further evolution in peripheral and central nervous system. Dev. Biol. 1984;103:468–481. doi: 10.1016/0012-1606(84)90334-8. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Interstitial cells of Cajal: primary targets of enteric motor innervation. Anat. Rec. 2001a;262:125–135. doi: 10.1002/1097-0185(20010101)262:1<125::AID-AR1017>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ward SM, Sanders KM. Physiology and pathophysiology of the Interstitial cells of Cajal: from bench to bedside. I. Functional development and plasticity of interstitial cells of Cajal networks. Am. J. Physiol. Gastrointest. Liver Physiol. 2001b;281:G602–G611. doi: 10.1152/ajpgi.2001.281.3.G602. [DOI] [PubMed] [Google Scholar]
- Weston JA. A radioautographic analysis of the migration and localization of trunk neural crest cells in the chick. Dev. Biol. 1963;6:279–310. doi: 10.1016/0012-1606(63)90016-2. [DOI] [PubMed] [Google Scholar]
- Yaneza M, Gilthorpe JD, Lumsden A, Tucker AS. No evidence for ventrally migrating neural tube cells from the mid- and hindbrain. Dev. Dyn. 2002;223:163–167. doi: 10.1002/dvdy.1241. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS. The origin of intrinsic ganglia of trunk viscera from vagal neural crest in the chick embryo. J. Comp. Neurol. 1954;101:515–541. doi: 10.1002/cne.901010212. [DOI] [PubMed] [Google Scholar]
- Young HM. Embryological origin of interstitial cells of Cajal. Microsc. Res. Techn. 1999;47:303–308. doi: 10.1002/(SICI)1097-0029(19991201)47:5<303::AID-JEMT1>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Young HM, Newgreen D. Enteric neural crest-derived cells: origin, identification, migration, and differentiation. Anat. Rec. 2001;262:1–15. doi: 10.1002/1097-0185(20010101)262:1<1::AID-AR1006>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Yutzey KE, Kirby ML. Wherefore heart thou? Embryonic origins of cardiogenic mesoderm. Dev. Dyn. 2002;223:307–320. doi: 10.1002/dvdy.10068. [DOI] [PubMed] [Google Scholar]