Abstract
Testicular development in the harbour porpoise Phocoena phocoena was examined using animals (n = 192) stranded or by-caught off the coast of England, Wales and Scotland. Classification of animals according to their stage of sexual development was undertaken using gonadal morphology and the distribution of cytoskeletal proteins. Smooth muscle actin (SMA) and vimentin proved particularly useful in this respect; SMA was prominent in the myoid peritubular cells of the adult testis, and two stages of peritubular cell SMA expression could be recognized (‘absent’ or ‘incomplete’). The initial appearance of SMA in peritubular cells was associated with significant increases in body length and body weight (P < 0.001), and occurred during the second year of life. Vimentin, which was prominent in prespermatogonia and spermatogonia, sometimes showed a polarized cytoplasmic distribution. This correlated with a developmental stage at which the seminiferous tubule epithelium becomes populated by germ cells (mean age 1.8 years). Several antibodies were tested for their utility as Sertoli cell markers, but none was found to be specific or useful. Nevertheless, immunohistochemical localization of desmin, GATA-4, Ki67 and androgen receptor was possible despite the poor quality of tissue preservation. This study showed that immunohistochemical classification of these individuals provides a robust basis for the recognition of key physiological stages of sexual development in the male harbour porpoise. This may provide an alternative to the estimation of age, body weight and body length in future analyses aimed at detecting possible adverse effects of environmental pollutants on the reproductive potential of wild marine mammals.
Keywords: actin, GATA-4, SMA, spermatogenesis, vimentin
Introduction
The harbour porpoise (Phocoena phocoena) is a cetacean species that lives in shallow coastal and shelf waters, where it feeds at high trophic levels that are often heavily polluted. In the light of current concerns about the adverse effects of endocrine-disrupting chemicals (EDCs) on reproductive development and function (Guillette & Gunderson, 2001; Jobling et al. 2002; Sharpe & Skakkebaek, 2003), some harmful effects on porpoise reproduction might be expected. However, there are a number of factors that make the detection of such effects rather difficult. One major problem in attempting to judge whether any adverse effects have taken place is that it is not possible to classify animals as having been exposed to ‘experimental’ or ‘control’ conditions; being wild animals, they exist in similar and uncontrolled environments. There is also a lack of detailed reference data on key events in reproductive development, such as the age at which the blood testis barrier is formed or that Sertoli cells undergo proliferation. Technical reasons also render such studies unusually difficult. Tissues are usually only available for examination after the animals have been dead for at least 24–48 h. Autolytic tissue degeneration is therefore commonplace, but unavoidable, and high-quality morphological examination is usually impossible.
An initial morphometric study, carried out with less than half of the data currently available (Karakosta et al. 1999), provided initial indications that key stages in the onset of spermatogenesis could be recognized histologically. The analysis showed that males classified as mature on the basis of testicular histology always exceeded 140 cm body length and 40 kg body weight. Animals classified as immature were always less than 135 cm in length and less than 30 kg in weight. These results confirmed similar observations in other populations of harbour porpoise (Gaskin et al. 1984; Sorensen & Kinze, 1994; Lockyer et al. 2001). A number of anomalous cases were identified within our initial dataset, in which testicular histology and somatic parameters appeared to be inconsistent. Such inconsistency was, and remains, difficult to explain. Although adverse effects of EDCs on reproductive development might be an obvious explanation, the effects might also be attributable to differences in somatic growth characteristics between animals from subpopulations that reside in different coastal regions. One objective of the current study was therefore to find alternative and independent methods of assessing reproductive development that might help explain these anomalies. For this purpose we explored the feasibility of using immunocytochemical markers of testicular development, whose value had already been demonstrated in other species. Despite the disadvantages outlined above, developments in immunocytochemical technology enabled us to localize a number of testicular proteins in the samples available.
An influential hypothesis about impaired reproductive development in humans and laboratory animals, the so-called ‘oestrogen hypothesis’ (Sharpe & Skakkebaek, 1993), implicated reduced Sertoli cell proliferation as a possible cause of reduced sperm production in later life. For this reason we tested the use of a variety of antibodies as specific Sertoli cell markers in the harbour porpoise. These included two antibodies against proteins involved in the cell cycle and differentiation; Ki67 a cell proliferation marker commonly used for the identification of tumours (Scholzen & Gerdes, 2000), and the transcriptional regulator GATA-4, which has previously been used to identify immature Sertoli cells in the developing porcine testis (McCoard et al. 2001a,b). Several antibodies against growth factors and hormone receptors were also tested, including transforming growth factor (TGFα), inhibin, follicle-stimulating hormone (FSH) and androgen and oestrogen receptors; unfortunately none of these was satisfactory for this purpose although some have been used to localize Sertoli cells in other species.
Antibodies against a variety of cytoskeletal proteins were also tested in this study as general markers of testis development and growth rather than as means of localizing Sertoli cells, on the basis that these proteins may be more robust in the face of post mortem degeneration. These antibodies included anti-smooth muscle actin, which has previously been used as a marker of the blood–testis barrier, as well as antibodies against vimentin, desmin and different forms of cytokeratin. Desmin and vimentin are components of intermediate filaments, although they are not always found together, and cytokeratin is a component of the cellular keratin network found in cytoplasm.
Materials and methods
Source of materials
Tissues were obtained from harbour porpoises which had become stranded on the coasts of England, Wales and Scotland or by-caught in commercial fishing nets between 1989 and 2000, using a standard post-mortem examination protocol (Law, 1994). These formed part of the collection of post-mortem materials of sea mammals amassed since 1989 at the Institute of Zoology, London, and Scottish Agricultural College (Inverness) as part of a major ongoing survey of UK-stranded marine mammal mortality.
Testis samples were obtained from 192 harbour porpoises (England, n = 100, Wales, n = 50 and Scotland, n = 42) collected between June 1989 and January 2000 and reported ‘freshly dead’ or ‘slightly decomposed’ before fixation. The following information was recorded at the time of post-mortem examination; location found, date of death (or collection), date of post-mortem, body weight, body length (measured as straight line distance from the tip of the upper jaw to the bottom of the notch of the tail flukes), and left and right testis weight (to the nearest gram, and without the epididymides). The testes were fixed in 10% neutral buffered formalin, embedded in paraffin wax and sectioned. A standardized testis sampling protocol was used as far as possible. If the individual testes weighed more than 50 g, a cross-sectional tissue slice about 1 cm thick was taken midway along the length and placed in fixative; smaller testes were fixed in their entirety, but tissues for histological examination were sampled from the mid-length position. Sections (3–6 µm) were mounted and stained with a variety of antibodies after suitable antigen retrieval treatment. One section from each testis was also stained with haematoxylin and eosin for stereological estimation of Sertoli cell numbers.
Classification of testis development
To provide an index of testicular development that was independent of the immunocytochemistry, morphological criteria developed previously by Karakosta et al. (1999) for classification of immature porpoise testes were extended to include two mature categories (Mature A and B, without and with spermatozoa, respectively). All individuals in this study were classified using this system; the classifications were then compared against the other parameters of growth and development.
The classifications described by Karakosta et al. (1999, figure 4 in their paper) were based largely on the relative abundance of prespermatogonia and spermatogonia. They were defined as shown below:
Fig. 4.
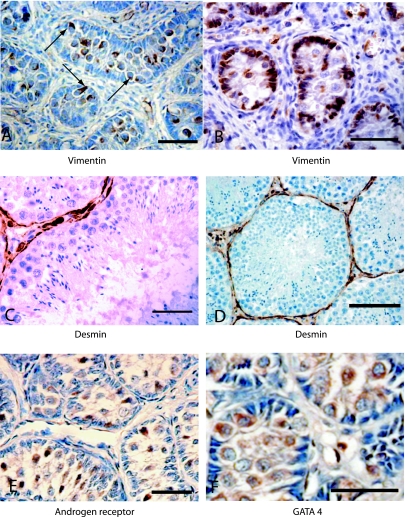
Sections of immature (A, B, E and F; scale bar = 100 µm) and mature testes (C and D) showing localization of: (A)vimentin in prespermatogonia showing polarized cytoplasmic distribution (arrows), (B)uniform vimentin distribution in Sertoli cells and prespermatogonia, (C)desmin in peritubular cells (scale bar = 100 µm), (D)status class Mature B showing spermatozoa and desmin-stained peritubular cells (scale bar = 50 µm), (E)androgen receptor in prespermatogonia and (F)GATA-4 in spermatogonia and Sertoli cells.
Class 1: abundant interstitial tissue and the presence of a few or numerous (Class 1a) prespermatogonia;
Class 2: little interstitial tissue between tubules and few or numerous (class 2a) prespermatogonia;
Class 3: little interstitial tissue but less space between tubules than in class 2, and the presence of a few to numerous (class 3a) prespermatogonia or spermatogonia.
Immunocytochemistry
Paraffin wax was removed from sections by treatment with Histoclear (R. A. Lamb, UK) for 20 min, and the sections were rehydrated through a graded alcohol series and finally washed in water. The sections were then placed in 10% (v/v) hydrogen peroxide for 30 min to block endogenous peroxidase activity, followed by immersion in running water for 10 min. A high-temperature antigen unmasking technique was required for some antibodies. Target retrieval solution (10%, 1.5 mL; Dako, Cambs., UK) was heated in a microwave oven at full power for 5 min; 12 sections at a time were then placed in this solution and heated for 20 min at full power. The sections were left in the solution for a further 10 min and washed through three changes of tris-buffered saline solution (TBS). Non-specific antigens were blocked using ‘protein block’ solution (Dako) for 10 min. Excess blocking solution was poured away and the primary antibodies applied for 1 h at room temperature. Details of the sources of antibodies used and their working dilutions are shown in Table 1. Sections were then washed with three changes of TBS, and primary antibody binding was detected using the Duet A.B.C. kit (Dako) according to the manufacturer's instructions. A stock solution of diaminobenzidine (DAB) (Dako) containing 1 : 1000 (v/v) hydrogen peroxide was applied to the sections for 3 min to visualize the final reaction. Slides were then counterstained with haematoxylin and mounted in Histomount (R. A. Lamb, UK) for examination.
Table 1.
Identity, source and working dilution of antibodies used in this study
| Antibody | Clone | Supplier | Host and type | Antigen retrieval required | Dilution TBS |
|---|---|---|---|---|---|
| Mouse IgG1 (negative control) | IgG1 kappa | Dako Ltd | Mouse monoclonal | Yes | 1 : 50 |
| α smooth muscle (SMA) | 1A4 | Dako Ltd | Mouse monoclonal | No | 1 : 25 |
| Vimentin | V9 | Dako Ltd | Mouse monoclonal | Yes | 1 : 25 |
| Desmin | DE-R-11 | Dako Ltd | Mouse monoclonal | Yes | 1 : 50 |
| Cytokeratin 18 | DC10 | Dako Ltd | Mouse monoclonal | Yes | 1 : 50 |
| Cytokeratin (pan) | AE1 and AE3 | Dako Ltd | Mouse monoclonal | Yes | 1 : 50 |
| Transforming growth factor α (TGF-α) | 213–4.4 | Oncogene Research Products (Cambs., UK) | Mouse monoclonal | Yes | 1 : 100 |
| Follicle stimulating hormone-receptor (FSH) | Biogenesis (Poole, Dorset) | Sheep polyclonal | Yes | 1 : 100 | |
| Oestrogen receptor-β | 14C8-ab288 | Abcam Ltd | Mouse monoclonal | Yes | 1 : 50 |
| Oestrogen receptor-α NCL-ER-LH2 | CC4-5 | Novo Castra (Newcastle, UK) | Mouse monoclonal | Yes | 1 : 50 |
| Inhibin α subunit | R1 | Oxford Bio-Innovation Ltd | Mouse monoclonal | Yes | 1 : 100 |
| Inhibin Beta B subunit | C5 | Oxford Bio-Innovation Ltd | Mouse monoclonal | Yes | 1 : 100 |
| Androgen receptor NCL-Arp (AR) | Novo Castra | Rabbit polyclonal | Yes | 1 : 10 | |
| Ki67 (proliferation marker) | Novo Castra | Rabbit polyclonal | Yes | 1 : 250 | |
| Alpha-Tubulin (sea-urchin) | B-5-1-2 | Sigma | Mouse monoclonal | No | 1 : 100 |
| Tubulin (yeast) | YOL1/34 | Abcam (Cambs., UK) | Rat monoclonal | No | 1 : 400 |
| Transcription factor GATA-4 | C-20 | Santa Cruz Biotechnology | Goat polyclonal | Yes | 1 : 200 |
To confirm that reaction products were not being caused by the non-specific binding of secondary antibodies, all reactions were controlled by the parallel treatment of tissue sections with secondary antibody only.
Developmental studies of the testis
For each porpoise the diameters of 20 seminiferous tubules were measured in transverse section using an ocular micrometer. Seminiferous tubules were randomly selected as far as possible, although those with circular profiles were preferred. The shortest distance between two diametrically opposite points on the peritubular membrane in circular, or nearly circular, profiles was measured (bright field objective, ×40) and the mean (± SEM) for each specimen was calculated. A ‘dot counting’ process was used to sample the relative proportions of seminiferous tubules and interstitium within the testis. Testis sections were observed microscopically using a grid meshwork eyepiece at low magnification (×100). The type of tissue present at 100 intersections of the 10 × 10 grid was identified and recorded. The number of intersections occurring on seminiferous tubules (out of 100 intersections) was described as the proportion of seminiferous tubule tissue in the section and expressed as a percentage of tubules to interstitial tissue (T/I%).
Statistical analyses
Statistical analyses were performed using Statistica 5.5 (Statsoft UK). One-way analyses of variance were used to compare somatic and testicular parameters that were classified by categorical variables mentioned in the text; continuous variables were log transformed for these analyses. Comparisons of frequencies were undertaken using chi-squared tests. Statistical analyses concerning testis weights are presented as ‘right testis weight’ because right, left and total testis weights were strongly correlated.
Results
Smooth muscle actin and vimentin
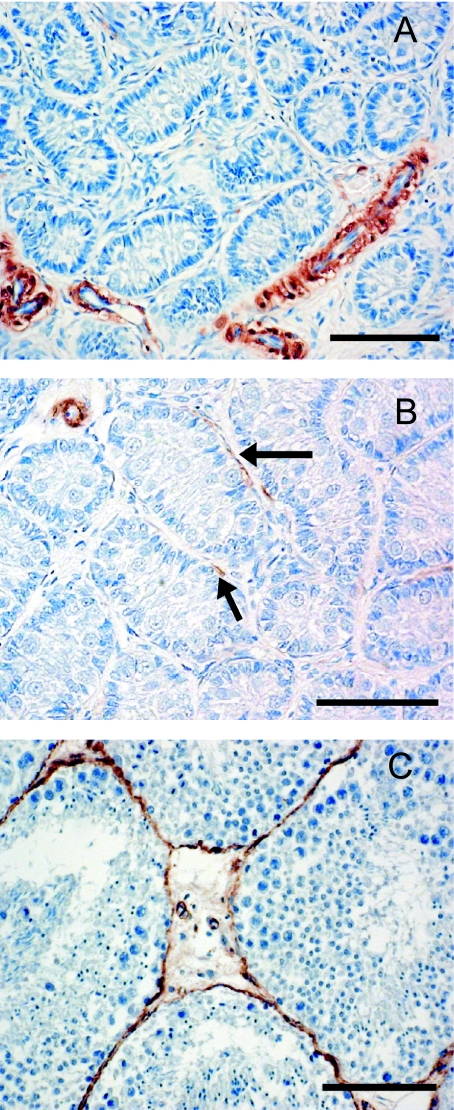
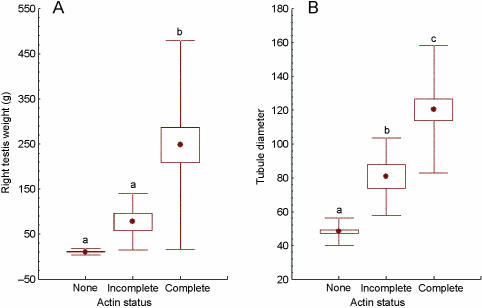
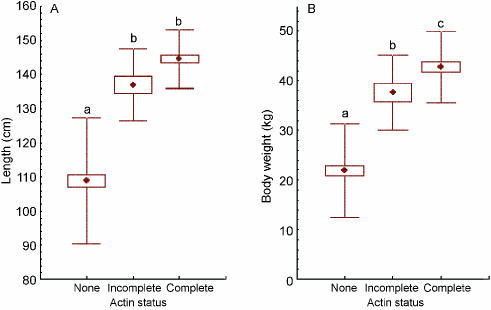
Smooth muscle actin (SMA) was detectable in the peritubular myoid cells that surround the seminiferous tubules and in blood vessels with a muscular coat. SMA was present in the capillary walls at all developmental stages, but was not always present within the myoid cells surrounding the seminiferous tubules. Within the range of samples examined, three clearly distinguishable patterns of distribution within the peritubular myoid cells could be discerned. These were (a) no SMA present (Fig. 1A; least developed testes), (b) present but not completely surrounding the tubules (Fig. 1B) and (c) completely surrounding the tubules (Fig. 1C; fully developed with the spermatocytes present). These categories of localization were correlated with progression through stages of testicular development, particularly the formation of the blood–testis barrier and the appearance of spermatocytes and spermatids in the lumen of seminiferous tubules. We proposed that the SMA localization patterns could be used as independent and categorical markers of testis development. To test this idea, all individual porpoises were categorized on the basis of their SMA score (‘none’, ‘ incomplete’ and ‘complete’), and the scores used as independent grouping variables for the examination of reproductive and somatic parameters by one-way anova. There was no statistically significant difference in weight of the right testis between the ‘none’ and ‘incomplete’ groups, but completion of the peritubular SMA layer (i.e. ‘complete’) was associated with a highly significant increase (P < 0.001) in testis weight (Fig. 2A). Significant incremental differences (P < 0.0001) in seminiferous tubule diameter were apparent between each of these classes (Fig. 2B). A significant increase in body length was associated with the initial appearance of SMA around the seminiferous tubules (P < 0.001), but no further increase in body length was associated with the completion of the peritubular layer of SMA (Fig. 3A). Significantly increased body weight (P < 0.001) was detected at each stage of reproductive development as assessed by SMA production (Fig. 3B).
Fig. 1.
Photomicrographs A, B and C illustrate the three types of testicular SMA distribution used as a basis for classification in this paper; in (A) no peritubular SMA is present, in (B) some sparse peritubular SMA is apparent (arrows) while in (C) SMA-positive peritubular myoid cells surround the seminiferous tubules (scale bar = 100 µm).
Fig. 2.
Box-whisker plots showing differences in right testis weight (A) and seminiferous tubule diameter (B) between groups of porpoises (total n = 95) classified according to the ‘actin status’ (• represents the mean; box represents standard error of the mean and whisker represents standard deviation). Lower-case letters above the whiskers indicate significant differences (P < 0.01) between groups.
Fig. 3.
Box-whisker plots showing differences in body length (A) and body weight (B) between 95 porpoises classified according to the ‘actin status’ (• represents the mean; box represents standard error of the mean and whisker represents standard deviation). Lower-case letters above the whiskers indicate significant differences (P < 0.01) between groups.
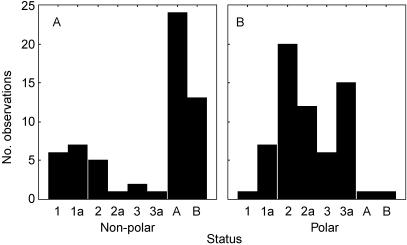
Vimentin was detected in the testis sections and was particularly prominent in the prespermatogonia, spermatogonia and Sertoli cells of immature testes (Fig. 4A,B). Between the individuals, two categories of localization were readily distinguishable, based on polarity of the reaction product within these cells. In some individuals the prespermatogonia contained a highly polarized vimentin distribution, with the vimentin being mainly directed toward the basement membrane (Fig. 4A; arrows); in other cases, where the vimentin was not polarized, the spermatogonia were mostly attached to the basement membrane and some nuclear localization was observed (Fig. 4B). We hypothesized that these represent two stages of early testicular development, when the seminiferous cords become organized by the migration of prespermatogonia from the central region towards the basement membrane. This possibility was supported by a frequency analysis of 122 animals within the dataset that showed that the proportion of individuals showing polar distributions, and also allocated status scores between 2 and 3a, was significantly higher than the non-polar group (see Fig. 5A,B). Thus polarized vimentin was a feature of testes that had advanced beyond status score 1, but had yet to progress beyond 3a.
Fig. 5.
Categorized histogram showing the frequency distribution of 122 individual animals classified by ‘status’ (i.e. histological assessment) and as having (A)non-polarized (n = 59) and (B)polarized (n = 63) vimentin distribution within their spermatogonia and prespermatogonia. Significant differences (χ2 = 67.5; P < 0.0001) between panels A and B are most apparent in status categories 2–3a and (mature) A and B.
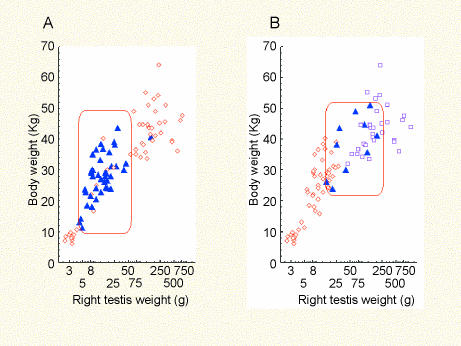
Vimentin and SMA therefore emerged as useful markers of testicular development; SMA was particularly informative about the maturation of the blood–testis barrier while vimentin provided useful information about the initial establishment of seminiferous tubule organization. The polarized distribution of vimentin in prespermatogonia appeared to correspond with a developmental stage in which the basal tubular compartment of the seminiferous tubules was being populated by germ cells and was preferentially associated with status categories 1a−3a. The body weight range (mean ± SEM) of animals undergoing establishment of the blood–testis barrier (i.e. the ‘incomplete’ group) was about 12 kg higher (37.6 ± 1.76 kg) than the group with polar vimentin distribution (25.6 ± 1.17 kg) (Fig. 6A,B); however, the mean estimated age of both groups was identical (1.8 years).
Fig. 6.
Scatterplots showing relationships between right testis weight and body weight. In panel A the points represent 131 individuals showing polarized (▴) and uniform (◊) vimentin in prespermatogonia and spermatogonia. In panel B the points represent 153 individuals distinguished according to the ‘actin status’ (◊ = no actin; ▴ = incomplete actin; □ = complete peritubular actin). Rectangles indicate approximate testis and body weight ranges corresponding to testes in which (A)prespermatogonia are colonizing the basal compartment of the seminiferous tubules and (B)the blood–testis barrier is undergoing development.
Other antibodies
Desmin was localized within the peritubular myoid cells of the seminiferous tubules and, in mature individuals, the localization closely resembled that of SMA (Fig. 4C,D). Androgen receptor and GATA-4 antigen were both detected within the prespermatogonia and spermatogonia of the developing testes (Fig. 4E,F). There was some evidence of androgen receptor and GATA-4 reaction product in the Sertoli cell cytoplasm, but because it was unclear it was not satisfactory as a specific Sertoli cell marker. There was some suggestion that GATA-4 was sometimes localized within the Sertoli cell nucleus and as well as, or instead of, the cytoplasm; although this would be consistent with cyclic Sertoli cell function, the results were not clear enough to merit detailed investigation. Ki67 was detected strongly in prespermatogonia (data not shown), probably as a reflection of their status as proliferating cells. Ki67 was also strongly positive in proliferating spermatocytes of the mature testis; however, spermatogonia within the same tubules, which would be less proliferative, were unstained. No reaction product was detected with antibodies against oestrogen receptor, inhibin, FSH receptor, TGFα receptor, transcription factor c20 and cytokeratin 18; the specific antibodies used are listed in Table 1. The cytokeratin (pan) antibody detected antigen in Sertoli cells (data not shown). The two antitubulin antibodies tested reacted strongly with sperm tails and spermatid cytoplasm in the mature testis (data not shown).
Somatic growth and testicular status
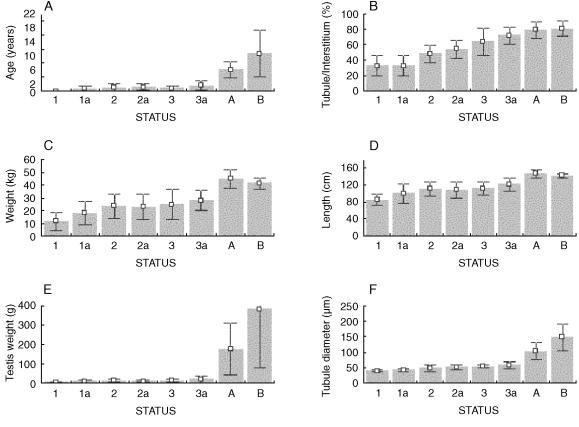
The morphological classification of testes into the status categories defined above proved highly informative in the analysis of reproductive development. All animals classified into status stages 1–3a were less than 4 years of age, and the majority were less than 3 years old (Fig. 7A). The ST/IT ratio was significantly lower in status classes 1 and 1a than any of the other immature status classes (F1,115 = 73.1; P < 0.0001) (Fig. 7B). The mean (± SD) age of mature stage A animals was 6.06 ± 2.2 years, whereas that of mature stage B animals was 10.7 ± 6.73 years. Body length and weight did not show abrupt increases that corresponded to advancement through the various categories (Fig. 7C,D), although both parameters were significantly higher in the mature animals.
Fig. 7.
Panels A–F show a series of bar graphs showing mean (± SD) somatic and reproductive characteristics of porpoises classified according to the histological definition of ‘status’. A, age (n = 80); B, tubule–interstitial tissue ratio (%) (n = 123); C, body weight (n = 128); D, body length (n = 128); E, right testis weight (n = 113); F, tubule diameter (n = 125).
Testis weight was significantly influenced by classification status. No significant difference in testis weight was detected between any of the immature classes (1–3a), but maturation was accompanied by a massive increase in testis weight (Fig. 7E) and a significant increase in seminiferous tubule diameter (Fig. 7F). Testes classified as Mature B (i.e. containing spermatozoa) were significantly heavier than those classified as Mature A (without spermatozoa) (F1,36 = 8.23; P = 0.0068). This difference was almost certainly caused by seasonal effects as well as maturational status; 15/24 individuals classified as Mature A were collected during the winter (i.e. out of the breeding season), and 11/16 individuals classified as Mature B were collected between June and December, a period that includes the breeding season.
Discussion
In order to understand the physiology of environmental disturbance in wild species it is essential to gain some insights into the natural sequence of key reproductive events. In a previous study, Karakosta et al. (1999) used morphometric testicular measurements as markers of reproductive status. Although these proved generally useful in relating reproductive development to somatic growth parameters such as body length and body weight, statistical analyses revealed a number of individual cases in which the reproductive and somatic parameters seemed to be inconsistent. It therefore appeared desirable to develop methods of classification that could be used as independent indicators of testicular development. Contrary to our initial expectations that immunohistochemistry might be out of the question because of tissue degradation, this study demonstrated the feasibility of using immunocytochemistry with antibodies against human proteins for the study of specific antigens in porpoise testes. The cytoskeletal proteins SMA, vimentin and desmin provided particularly robust responses, although other candidates such as GATA-4 and Ki67 were also readily detected.
The presence of F-actin in peritubular myoid cells has previously been reported as a correlate of testicular maturation in mammals, for example in the bovine and ovine testes (Steger & Wrobel, 1994; Wrobel et al. 1995). In these species actin expression represents one aspect of the formation and completion of the blood–testis barrier, an essential feature of seminiferous tubule organization. In contrast, studies of SMA expression in the developing rat testis showed that although SMA is also a correlate of seminiferous tubule development (Palombi et al. 1992), peritubular SMA expression in the rat commences during gestation, while spermatic cords are undergoing organization. Species differences in peritubular actin expression are therefore important and a biological interpretation must be made with caution. However, the data presented here show that onset of SMA expression in peritubular myoid cells, itself a marker of terminal smooth muscle differentiation, is correlated with major increases in seminiferous tubule diameter and testis weight. Myoid peritubular cells engage in a dialogue with Sertoli cells (Hadley et al. 1985), which is crucially important for the structural formation of the blood–testis barrier (Hadley & Dym, 1987). Peritubular myoid cells are known to stimulate total protein production by Sertoli cells and to increase the Sertoli cell production of androgen-binding protein and transferrin. They produce a protein named P-mod-S, that has been shown to be an important regulator of Sertoli cell function in vitro (Anthony et al. 1991) and they are also considered to be highly influential in modulating the effects of androgens on the seminiferous tubule, and therefore upon spermatogenesis itself.
Thus the appearance of SMA seems to be a meaningful and robust marker of sexual development that could be used as a grouping parameter for the recognition of individuals at different stages of testicular development. Formation of the blood–testis barrier is a significant milestone in testicular development, and the estimates of age carried out during this study show that it occurs during the second to third year of life. However, it was apparent from the morphological classification that sexual maturity is not normally reached until animals are in their fourth year. This delay probably implies that the expression of SMA signals the commencement of blood–testis barrier formation rather than its completion.
The presence of a polarized vimentin distribution in prespermatogonia provided similar information, although highlighting a slightly earlier stage of development when body size is still considerably smaller. Vimentin has previously been reported as a marker of prespermatogonia in the ovine testis (Steger & Wrobel, 1994), although the authors noted that it disappeared, rather than became polarized, as these cells matured.
Immunodetection of GATA-4 in the porpoise testis was attempted in an effort to find a specific marker of Sertoli cells. This was based on data from studies in fetal, postnatal and adult domestic boars (McCoard et al. 2001a), where it fulfils this role; however, GATA-4 could not be detected so specifically and abundantly in the porpoise. Although this may have been due to technical problems with tissue preservation, there is a greater likelihood that species differences were responsible. Although McCoard et al. (2001a) showed that GATA-4 was not present in germ cells of the boar testis, we found cytoplasmic staining in prespermatogonia and spermatogonia. GATA-4 is a transcription factor that is intimately involved in the control of reproductive development, both in males and in females and may therefore represent a useful target for future studies. The likelihood that GATA-4 was redistributed from Sertoli cells to spermatogonia as a result of poor tissue preservation is contradicted by the observation that both Ki67, a marker of proliferating spermatogenic cells (Steger et al. 1998), and the androgen receptor were localized as expected.
Around the time this study was originally being designed, in the early 1990s, considerable concern was being expressed over possible deleterious effects of EDCs on human reproductive development. This was catalysed by a review (Carlsen et al. 1992), in which it was suggested that the past 50 years had been associated with an apparent decline in human semen quality. Considerable effort has since been invested in validating or disproving this claim. Although the original data have been reviewed critically in many subsequent publications (Ashby et al. 1997; Toppari & Skakkebaek, 1998; Vos et al. 2000), new and more stringent studies (Swan et al. 2000) continue to confirm the trends that were originally seen. Examination of the available data led Sharpe & Skakkebaek (1993) to suggest that some environmental chemicals are able to bind weakly to oestrogen receptors, thus interfering with key reproductive events including testicular differentiation and spermatogenic output.
Sharpe (2003) has recently updated the ‘oestrogen hypothesis’ in the light of experimental evidence obtained from laboratory animal studies. These studies have led towards the view that a suite of reproductive abnormalities during male development can be described inclusively as ‘testicular dysgenesis syndrome’ (Fisher, 2004). Not only chemicals with oestrogenic activity, but also anti-androgens and androgenic activities are implicated as causative agents in this expanded view, which takes into consideration that developmental disorders may lead to testicular cancer and hypospadias as well as more subtle effects on sperm production.
Within our data set of nearly 200 male porpoises, there was only one case of a testicular tumour, no other anatomical or histological evidence of reproductive abnormalities, and no obvious reason to believe that testicular development is impaired. This seems rather surprising given that porpoises are more likely than humans to experience EDC exposure throughout their life, principally through their diet. Newborn porpoises are exposed to chlorinated hydrocarbons (CHs) such as polychlorinated biphenyls (PCBs), because these lipophillic chemicals, which are stored in their mother's fat reserves, are mobilized and secreted during milk production. Once weaned, porpoises subsequently subsist on a diet that consists mainly of fish, which probably carry their own contaminant load and are thus likely to store and concentrate the chemicals in their own fat reserves. Analyses of the contaminant load in six different types of tissue from harbour porpoises have confirmed that CHs are concentrated within the blubber (Tilbury et al. 1997); however, this study also showed that the testes and brain contained the lowest CH concentrations. The authors of this paper noted that the brain–blood barrier may be important in preventing uptake of contaminants; if true this would suggest that the blood–testis barrier might have the same effect, a prediction supported by experimental studies in rats (Cooke et al. 2001). The results of the present study would suggest that if this is the case in porpoises, prepubertal animals, which do not gain a functional blood–testis barrier until they are 4 or 5 years of age, should lack this protective mechanism during a lengthy period of their development. Although it may be the case that our methods were not sufficiently sensitive to detect any signs of testicular dysgenesis, it may also point towards the porpoise having developed more sophisticated protective mechanisms than humans.
Acknowledgments
We are grateful to WWF-UK who provided the main support for this specific study and to the UK Department for Environment, Food and Rural Affairs (Defra) who have supported the long-term collection and analysis of UK-stranded marine mammals used in this work. Post-mortem examinations were conducted by Dr John Baker, Dr Thijs Kuiken, Tony Patterson, Harry Ross and Victor Simpson and assisted by Bob Reid.
References
- Anthony CT, Rosselli M, Skinner MK. Actions of the testicular paracrine factor (p-mod-s) on Sertoli-cell transferrin secretion throughout pubertal development. Endocrinology. 1991;129:353–360. doi: 10.1210/endo-129-1-353. [DOI] [PubMed] [Google Scholar]
- Ashby J, Houthoff E, Kennedy SJ, et al. The challenge posed by endocrine-disrupting chemicals. Environ. Health Perspect. 1997;105:164–169. doi: 10.1289/ehp.97105164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. Br. Med. J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke GM, Newsome WH, Bondy GS, et al. The mammalian testis accumulates lower levels of organochlorine chemicals compared with other tissues. Reprod. Toxicol. 2001;15:333–338. doi: 10.1016/s0890-6238(01)00126-5. [DOI] [PubMed] [Google Scholar]
- Fisher JS. Environmental ant-androgens and male reproductive health: focus on phthalates and testicular dysgenesis syndrome. Reproduction. 2004;127:305–315. doi: 10.1530/rep.1.00025. [DOI] [PubMed] [Google Scholar]
- Gaskin DE, Smith GJD, Watson AP, Yasui WY, Yurick DB. Reproduction in the Porpoises (Phocoenidae): Implications for Management. 1984. pp. 135–148. Report of the International Whaling Commission.
- Guillette LJ, Jr, Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122:857–864. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- Hadley M, Byers S, Suarez-Quian C, Kleinman H, Dym M. Extracellular matrix regulates Sertoli cell differentiation, testicular cord formation, and germ cell development in vitro. J. Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley MA, Dym M. Immunocytochemistry of extracellular-matrix in the lamina propria of the rat testis – electron-microscopic localization. Biol. Reprod. 1987;37:1283–1289. doi: 10.1095/biolreprod37.5.1283. [DOI] [PubMed] [Google Scholar]
- Jobling S, Coey S, Whitmore JG, et al. Wild intersex roach (Rutilus rutilus) have reduced fertility. Biol. Reprod. 2002;67:515–524. doi: 10.1095/biolreprod67.2.515. [DOI] [PubMed] [Google Scholar]
- Karakosta CV, Jepson PD, Ohira H, Moore A, Bennett PM, Holt WV. Testicular and ovarian development in the harbour porpoise (Phocoena phocoena) J. Zool. (Lond.) 1999;249:111–121. [Google Scholar]
- Law RJ, compiler. In: Collaborative UK Marine Mammal Project: Summary of Data Produced 1988–1992. Lowestoft: MAFF Directorate of Fisheries Research; 1994. Fisheries Research Technical Report 97. [Google Scholar]
- Lockyer C, Heide-Jorgensen MP, Jensen J, Kinze CC, Sorensen TB. Age, length and reproductive parameters of harbour porpoises Phocoena phocoena (L.) from West Greenland. ICES. J. Mar. Sci. 2001;58:154–162. [Google Scholar]
- McCoard SA, Lunstra DD, Wise TH, Ford JJ. Specific staining of Sertoli cell nuclei and evaluation of Sertoli cell number and proliferative activity in Meishan and white composite boars during the neonatal period. Biol. Reprod. 2001a;64:689–695. doi: 10.1095/biolreprod64.2.689. [DOI] [PubMed] [Google Scholar]
- McCoard SA, Wise TH, Fahrenkrug SC, Ford JJ. Temporal and spatial localization patterns of GATA4 during porcine gonadogenesis. Biol. Reprod. 2001b;65:366–374. doi: 10.1095/biolreprod65.2.366. [DOI] [PubMed] [Google Scholar]
- Palombi F, Farini D, Salanova M, Degrossi S, Stefanini M. Development and cytodifferentiation of peritubular myoid cells in the rat testis. Anat. Rec. 1992;233:32–40. doi: 10.1002/ar.1092330106. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J. Cell. Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Sharpe RM. The ‘oestrogen hypothesis’– where do we stand now? Int. J. Androl. 2003;26:2–15. doi: 10.1046/j.1365-2605.2003.00367.x. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Are oestrogens involved in falling sperm counts and disorders of the male reproductive tract? The Lancet. 1993;341:1392–1395. doi: 10.1016/0140-6736(93)90953-e. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, Skakkebaek NE. Male reproductive disorders and the role of endocrine disruption: advances in understanding and identification of areas for future research. Pure Appl. Chem. 2003;75:2023–2038. [Google Scholar]
- Sorensen TB, Kinze CC. Reproduction and reproductive seasonality in Danish harbour porpoises, Phocoena phocoena. Ophelia. 1994;30:159–176. [Google Scholar]
- Steger K, Wrobel KH. Immunohistochemical demonstration of cytoskeletal proteins in the ovine testis during postnatal development. Anat. Embryol. 1994;189:521–530. doi: 10.1007/BF00186825. [DOI] [PubMed] [Google Scholar]
- Steger K, Aleithe I, Behre H, Bergmann M. The proliferation of spermatogonia in normal and pathological human seminiferous epithelium: an immunohistochemical study using monoclonal antibodies against Ki-67 protein and proliferating cell nuclear antigen. Mol. Hum. Reprod. 1998;4:227–233. doi: 10.1093/molehr/4.3.227. [DOI] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934–1996. Environ. Health Perspect. 2000;108:961–966. doi: 10.1289/ehp.00108961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilbury KL, Stein JE, Meador JP, Krone CA, Chan SL. Chemical contaminants in harbor porpoise (Phocoena phocoena) from the north Atlantic coast: tissue concentrations intra- inter-organ distribution. Chemosphere. 1997;34:2159–2181. doi: 10.1016/s0045-6535(97)00076-3. [DOI] [PubMed] [Google Scholar]
- Toppari J, Skakkebaek NE. Sexual differentiation and environmental endocrine disrupters. Baillieres Clin. Endocrinol. 1998;12:143–156. doi: 10.1016/s0950-351x(98)80529-6. [DOI] [PubMed] [Google Scholar]
- Vos JG, Dybing E, Greim HA, et al. Health effects of endocrine-disrupting chemicals on wildlife, with special reference to the European situation. Crit. Rev. Toxicol. 2000;30:71–133. doi: 10.1080/10408440091159176. [DOI] [PubMed] [Google Scholar]
- Wrobel KH, Bickel D, Kujat R. Distribution pattern of F-actin, vimentin and alpha-tubulin in the bovine testis during postnatal development. Acta Anat. 1995;153:263–272. doi: 10.1159/000147727. [DOI] [PubMed] [Google Scholar]