Abstract
Ghrelin is a newly identified gastric peptide hormone that has various important functions, including growth-hormone release and appetite stimulation. Ghrelin-immunoreactive cells (ghrelin cells) are characterized by X-type endocrine cells in the rat stomach. In the present study, we analysed ghrelin cells in fundi of stomach from ICR mice and Syrian hamsters immunohistochemically, immunoelectron microscopically and morphometrically, and compared the results with those from Wistar rats. Immunohistochemistry revealed that ghrelin cells were sparsely distributed in the proper gastric glands in all species. The number of ghrelin cells per unit area in hamsters was significantly lower than that in rats. Immunoelectron microscopy detected ghrelin immunolabelling in granules in the X-type endocrine cells. However, the diameter of granules in the hamsters was significantly smaller than that in the mice and rats. Gastric ghrelin contents were determined by radioimmunoassay, and levels in the hamsters were significantly lower than those in mice and rats. The results from mice were identical to those from rats. In conclusion, gastric ghrelin cells in mice and hamsters are characterized by X-type endocrine cells, as has been observed in rats. However, the data indicated that gastric ghrelin production was lower in hamster than in mouse or rat.
Keywords: ghrelin, hamster, mouse, rat, stomach
Introduction
Ghrelin is a peptide hormone that has been recently isolated from rat and human stomach (Kojima et al. 1999). This 28-amino acid peptide is recognized as the main endogenous ligand for growth hormone secretagogue receptors, and it plays important roles in growth-hormone release and appetite stimulation (Kojima et al. 2001; Kojima & Kangawa, 2002). In addition to these functions, ghrelin plays various physiological roles, including control of gastric motility and acid secretion, and it exhibits cardiovascular effects and influences pancreatic activity (De Ambrogi et al. 2003; Gualillo et al. 2003).
Several types of endocrine cells have been identified in the glandular mucosa of the stomach based on the ultrastructural characteristics of the secretory granules (Capella et al. 1971; Vassallo et al. 1971). Seven types (enterochromaffin-like (ECL), P, D, enterochromaffin (EC), X (A-like), D1 and non-granulated) and three types (ECL, D and X) of endocrine cells have been identified in human and rat stomach, respectively (Bordi et al. 2000). Although the main secretory products of ECL, D and EC cells have been determined previously to be histamine-ECL, somatostatin-D and serotonin-EC (Bordi et al. 2000), the products of other types of endocrine cells remained unclear. However, recent immunohistochemical, immunoelectron microscopical and in situ hybridization analyses indicated that X cells from rat stomach produce ghrelin (Date et al. 2000; Dornonville de la Cour et al. 2001). In another report, ghrelin-immunoreactive cells (ghrelin cells) from humans were identified as P/D1 cells, although those of rat and dog were identified as X cells (Rindi et al. 2002). Although localization of ghrelin cells in the stomach of pig, horse, cow and sheep has been investigated immunohistochemically (Hayashida et al. 2001), ultrastructural typing of ghrelin cells has not been previously studied. In addition, species differences in ghrelin cells have not been fully evaluated quantitatively.
The aim of the present study was to assess the ultrastructural cell-type and species differences in gastric ghrelin cells in experimental rodents. We analysed the fundi from mouse, rat and hamster stomachs immunohistochemically, immunoelectron microscopically and morphometrically.
Materials and methods
All experimentation in the present study was conducted in accordance with the Guidelines for Animal Experimentation of Kagoshima University, Japan.
Animals
Three-month-old male mature Slc:ICR mice, Slc:Wistar rats and Slc:Syrian hamsters were used in the present study and were housed in an open system room with a one-way airflow system (temperature, 22 ± 1 °C; humidity, 55 ± 10%; light period, 07:00–19:00 h; ventilation, 12 cycles h−1) at the Division of Laboratory Animal Science, Research Center for Life Science Resources, Kagoshima University. All animals were given an autoclaved commercial diet (CE-2; Japan CLEA, Tokyo, Japan) and tap water ad libitum, and samplings were performed between 13:00 h and 15:00 h. Three animals of each species were used for light and electron microscopic studies, and six animals of each species were used for radioimmunoassay. All animals were killed by exsanguination under anaesthesia using a mixture of ketamine and medetomidine, and the stomachs were quickly removed.
Light microscopy
The fundi of the stomach were fixed in Zamboni's solution for 2 days at 4 °C. After thorough washing in 0.1 m phosphate buffer (pH 7.4) at 4 °C, fundi were routinely embedded in paraffin, and 3-µm-thick sections were cut every 30 µm. Immunohistochemical procedures were as follows: (1) deparaffinization and rehydration, (2) treatment with 0.3% H2O2 in methanol for 30 min, (3) washing in 0.01 m phosphate-buffered saline (PBS, pH 7.4), (4) blocking with 1.5% normal goat serum/PBS for 60 min, (5) incubation with rabbit antiserum anti-rat ghrelin [13–28] (Kojima et al. 1999) diluted 1 : 15 000 overnight at 4 °C, (6) washing in PBS, (7) incubation with biotinylated goat anti-rabbit IgG (Vector Laboratories, Burlingame, USA) diluted 1 : 200 for 30 min, (8) washing in PBS, (9) incubation with peroxidase-conjugated avidin–biotin complex (Elite ABC kit; Vector Laboratories) for 30 min, (10) washing in PBS, (11) detection of immunoreactivity with a 0.025% 3,3′-diaminobenzidine−0.003% H2O2 solution, and (12) stopping the reaction with distilled water. The sections were counterstained with Mayer's haematoxylin. Negative control studies were performed with anti-ghrelin antisera that had been absorbed by 10 µg synthesized rat ghrelin (Kojima et al. 1999).
Electron microscopy
The fundi of the stomach were cut into approximately 1-mm3 pieces, then fixed in a mixture of 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 m cacodylate buffer (CB, pH 7.4) with 2% saccharose for 2 h at 4 °C. The specimens were thoroughly washed in CB at 4 °C and then post-fixed in 1% osmium tetroxide in CB for 2 h at 4 °C. After dehydration through a graded ethanol series, the samples were embedded in Epon 812. Ultrathin sections were mounted on nickel grids. Immunoelectron microscopic procedures were as follows: (1) etching with 5% sodium metaperiodate for 30 min, (2) washing with distilled water and then PBS, (3) blocking with 1.5% normal goat serum/PBS containing 1% bovine serum albumin for 60 min, (4) incubation overnight at 4 °C with rabbit antiserum anti-ghrelin [13–28] diluted 1 : 6000, (5) washing with PBS, (6) incubation with biotinylated goat anti-rabbit IgG diluted 1 : 200 for 30 min, (7) washing with PBS, (8) incubation with gold (10 nm)-conjugated streptavidin (British Biocell International, Cardiff, UK) for 60 min, (9) washing with PBS, (10) treatment with 1.25% glutaraldehyde/PBS for 5 min, and (11) washing with distilled water. Both immunostained and non-immunostained sections were double-stained with uranyl acetate and lead citrate. After air drying, grids were coated with carbon and observed under a transmission electron microscope (H-7000KU, Hitachi, Tokyo, Japan). Negative control studies were performed with anti-ghrelin antisera that had been absorbed by 10 µg rat ghrelin.
Morphometry
For light microscopic morphometry, three sections from each animal were observed at random using an ocular micrometer. The number of ghrelin cells per unit area of glandular portions (mm2) was counted. For electron microscopic morphometry, more than ten electron micrographs of ghrelin cells in the gastric mucosa were taken from each animal at a primary magnification of ×20 000 and printed at a final magnification of ×50 000. These photographs were degrouped, and then approximately 100 round immunogold-labelled granules were selected for each animal and their diameters were measured.
Radioimmunoassay (RIA) for ghrelin content
Stomach samples were washed twice in PBS. After measuring the wet weights of each sample, the stomach tissue was diced and boiled for 5 min in a 10-fold volume of water to inactivate intrinsic proteases. After cooling on ice, boiled samples were adjusted to 1 m acetic acid−20 mm HCl. Peptides were extracted following homogenization with a polytron mixer (PT 6100, Kinematica AG, Littan-Luzern, Switzerland). Extract supernatants, isolated following a 15-min centrifugation at 15 000 r.p.m. (12 000 g), were lyophilized and stored at −80 °C. The lyophilized samples were redissolved in assay buffer prior to ghrelin RIA. RIA specific for ghrelin was performed as previously described (Kojima et al. 1999; Hosoda et al. 2000). Polyclonal antibody was raised in rabbits against C-terminal [Gln13–Arg28] fragments of rat ghrelin. RIA incubation mixtures were composed of 100 µL of either standard ghrelin or an unknown sample and 200 µL of antiserum diluted in RIA buffer (50 mm sodium phosphate buffer (pH 7.4), 0.5% BSA, 0.5% Triton-X100, 80 mm NaCl, 25 mm EDTA-2Na, and 0.05% NaN3) containing 0.5% normal rabbit serum. Anti-rat ghrelin [13–28] antiserum was used at final dilutions of 1 : 20 000. After a 12-h incubation at 4 °C, 100 µL of 125I-labelled ligand (30 000 c.p.m.) was added for an additional 36 h pf incubation. Then, 100 µL of anti-rabbit goat antibody was added. After incubation for 24 h at 4 °C, free and bound tracers were separated by centrifugation at 3000 r.p.m. for 30 min. Pellet radioactivity was quantified in a gamma counter (ARC-600, Aloka, Tokyo, Japan). All assays were performed in duplicate at 4 °C. The anti-rat ghrelin [13–28] antiserum equally recognized des-acyl and all acylated forms of ghrelin peptide. In the following sections, the RIA system using the antiserum against the C-terminal fragment [13–28] is termed C-RIA. Ghrelin-like immunoreactivity measured by C-RIA, which reflects the total (des-acyl and acyl-modified) ghrelin immunoreactivity, is termed ghrelin C-LI.
Statistics
Results were regrouped and expressed as the mean ± standard error (SE), and analysed statistically using one-way analysis of variance (Bonferroni/Dounn test). Statistical significance was defined as P < 0.05.
Results
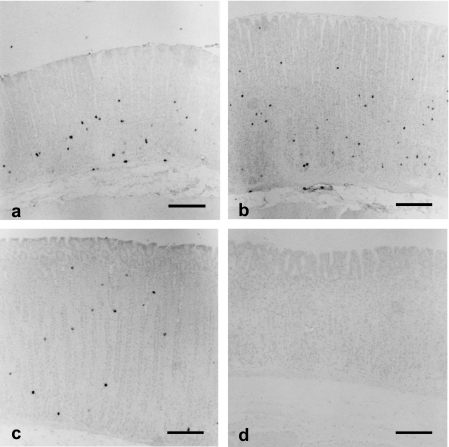
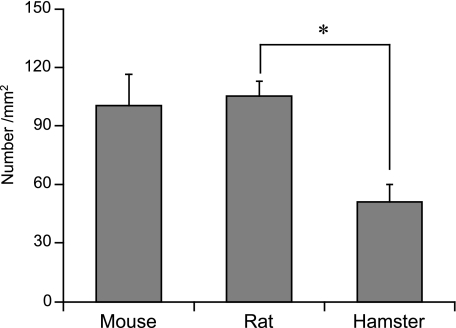
Immunohistochemistry demonstrated the presence of ghrelin cells near the proper gastric glands (Fig. 1). These cells were sparsely distributed from the neck to the fundi, where they were moderately abundant. These cells were also detected in the submucosa. No positive cells were observed in the gastric superficial epithelium. The localization of ghrelin cells in the stomach was similar in mice, rats and hamsters. No immunoreactivities were detected in any animals when antiserum absorbed by excessive ghrelin was used. Figure 2 shows the number of ghrelin-immunoreactive cells per unit area of proper gastric glands in mice, rats and hamsters. Although abundance in mice (100.4 ± 16.2 mm−2) and rats (105.6 ± 7.1 mm−2) was similar, that in hamsters (51.4 ± 9.1 mm−2) was low. The differences in abundance between hamsters and rats were statistically significant.
Fig. 1.
Localization of ghrelin-immunoreactive cells (ghrelin cells) in the fundi of the stomach: (a) mouse, (b) rat, (c) hamster, (d) negative control section from a mouse. Localization of ghrelin cells is similar in mice, rats and hamsters. Scale bars = 200 µm.
Fig. 2.
Number of ghrelin cells per unit area of proper gastric glands. Values are mean ± SE. *Significant difference (P < 0.05).
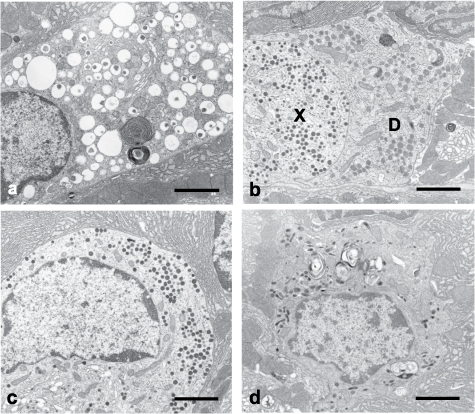
Electron microscopic observation of proper gastric glands identified several types of endocrine cells based on the ultrastructural characteristics of secretory granules. Three types of endocrine cells were observed in rats, as described previously (Bordi et al. 2000): briefly, ECL cells, which were characterized by membrane bounded vacuolar granules containing a small dense eccentric core, D cells, which were characterized by round granules with medium density, and X cells, which were characterized by round and compact dense granules. In mice and hamsters, EC cells, which were characterized by pleomorphic and dense granules, were rarely observed in addition to the three types described above (Fig. 3).
Fig. 3.
Electron micrographs of gastric endocrine cells in hamster: (a) entero-chromaffin (ECL) cell, (b) D and X cells, (c) X cell, (d) entero-chromaffin (EC) cell. Scale bars = 2 µm.
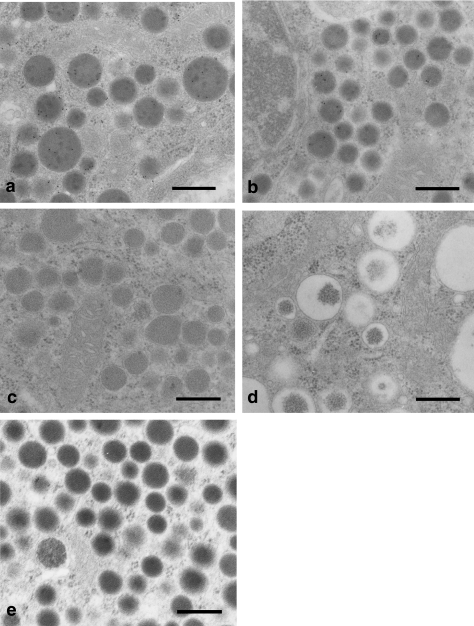
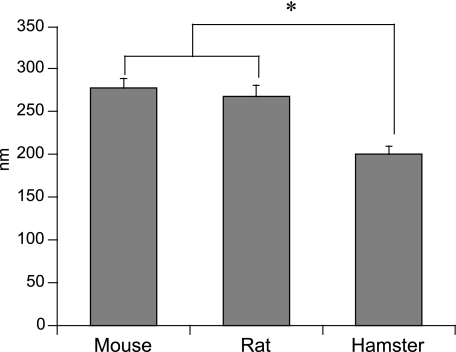
Immunoelectron microscopic observation of proper gastric glands revealed ghrelin-positive gold-immunolabelling in the granules of X cells in all species, whereas granules of other types of endocrine cells were negative (Fig. 4). Gold-immunolabelling was not detected in the granules of any type of endocrine cell when antiserum absorbed by excessive ghrelin was used. Figure 5 shows the diameter of ghrelin-positive granules in mice, rats and hamsters. Although the diameters in mice (277.7 ± 11.1 nm) and rats (268.8 ± 13.0 nm) were similar, those in hamsters (200.8 ± 8.8 nm) were significantly smaller than in mice or rats.
Fig. 4.
Immunolectron micrographs of gastric endocrine cells: (a) X cell in mouse, (b) X cell in hamster, (c) D cell in hamster, (d) ECL cell in hamster, (e) X cell in hamster negative control section. Immunogold (10-nm particles) labelling for ghrelin is observed in the round and compact dense-granules of X cells (panels a and b). Secretory granules in other types of endocrine cells are unreactive for ghrelin antibody (panels c and d). Scale bars = 400 nm.
Fig. 5.
Diameter of ghrelin-immunoreactive cell granules. Values are mean ± SE. *Significant difference (P < 0.05).
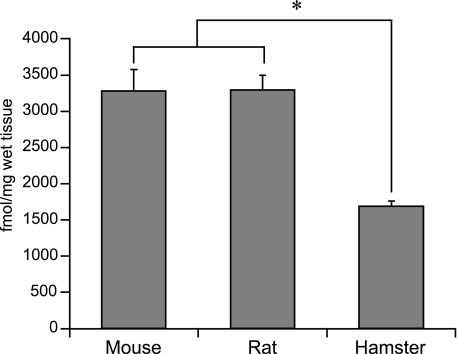
Ghrelin contents of the stomach were similar in mice (3275.2 ± 291.9 fmol g−1) and rats (3296.2 ± 203.6 fmol g−1). However, levels in the hamsters (1673.0 ± 71.8 fmol g−1) were significantly lower than those in the mice and rats (Fig. 6).
Fig. 6.
Ghrelin content in the stomach tissue. Values are mean ± SE. *Significant difference (P < 0.05).
Discussion
Ultrastructural characterization of ghrelin cells in the stomach of experimental rodents has been previously reported for the rat (Date et al. 2000). In the present study, we also analysed the stomach of Wistar rats, and the immunohistochemical and immunoelectron microscopic findings were similar to those of Date et al. (2000). Briefly, ghrelin-immunoreactive cells were localized from the neck to fundi in the proper gastric glands, and ultrastructural immunolabelling was detected in the round and compact dense granules of X-type endocrine cells.
A novel gastric peptide hormone named motilin-related peptide (MTLRP) was recently identified in the mouse stomach and characterized (Tomasetto et al. 2000). The amino acid sequence of MTLRP was subsequently identified as that of ghrelin (Coulie & Miller, 2001; Del Rincon et al. 2001). Although MTLRP/ghrelin cells in the mouse stomach were investigated immunoelectron microscopically by using the Lowicryl-embedding procedure, Tomasetto et al. (2000) suggested that ECL, EC and D cells could synthesize and secrete MTLRP/ghrelin. In the present study, we used the standard Epon-embedding procedure with post-fixation by osmium tetroxide because of improved preservation of ghrelin antigenicity and contrast in secretory granules (Date et al. 2000; Rindi et al. 2002). We found that localization and number of ghrelin cells, ultrastructural cell-type, and the diameter of ghrelin-immunolabelling granules in the ICR mice were identical to those in Wistar rats. These findings suggested that true ghrelin cells in the mouse proper gastric glands are X-type endocrine cells.
Characterization of ghrelin cells has not been previously attempted in the hamster. In the present study, X cells of proper gastric glands in Syrian hamsters were also identified as ghrelin cells; however, the number of ghrelin cells in hamsters was significantly lower than in rats. In addition, the diameter of ghrelin-imunoreactive secretory granules in hamsters was significantly smaller than that in mice and rats. To estimate whether these morphological differences between species correlate with gastric ghrelin production, ghrelin contents in the stomach were determined by radioimmunoassay. As a result, species differences in gastric ghrelin contents were well matched to the morphological findings, and levels in the hamsters were significantly lower than those in the mice or rats. Therefore, low-level gastric ghrelin production was indicated in the hamsters.
Energy homeostasis is accomplished by mechanisms that control food intake, energy expenditure and energy storage, and it is well known that ingestive behaviour of hamsters differs from that of mice and rats (Rowland, 1985; Schneider et al. 2002). A compensatory increase in food intake is commonly observed after a period of food deprivation in many species, including mouse and rat. However, fasted Syrian or Siberian hamsters show an increase in food hoarding rather than food intake (Bartness & Clein, 1994; Schneider et al. 2002). Such behaviour in hamsters is suppressed by leptin and stimulated by neuropeptide Y (NPY) and agouti-related protein (AgRP) (Schneider et al. 2002; Buckley & Schneider, 2003; Day & Bartness, 2004). Ghrelin is strongly involved in the regulation of energy homeostasis as an orexigenic factor, and a recent study indicated that ghrelin increases feeding activity in rat by stimulating NPY- and AgRP-containing neurons in the hypothalamus to promote the production and secretion of NPY and AgRP peptides (Nakazato et al. 2001). It was also demonstrated that ghrelin induces positive energy balance in mouse and rat by decreasing fat utilization without changing energy expenditure or locomotor activity (Tschöp et al. 2000). The stomach is the main source of ghrelin, and our present findings indicated that gastric ghrelin-production was low in hamster compared with that in mouse or rat. Whether this finding correlates with the species differences in energy homeostasis remains unclear. Thus, future investigations to assess the ghrelin functions in hamster are required.
References
- Bartness TJ, Clein MR. Effects of food deprivation and restriction, and metabolic blockers on food hoarding in Siberian hamsters. Am. J. Physiol. 1994;266:R1111–R1117. doi: 10.1152/ajpregu.1994.266.4.R1111. [DOI] [PubMed] [Google Scholar]
- Bordi C, D’Adda T, Azzoni C, Ferraro G. Classification of gastric endocrine cells at the light and electron microscopical levels. Microsc. Res. Techn. 2000;48:258–271. doi: 10.1002/(SICI)1097-0029(20000301)48:5<258::AID-JEMT3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Buckley CA, Schneider JE. Food hoarding is increased by food deprivation and decreased by leptin treatment in Syrian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003;285:R1021–R1029. doi: 10.1152/ajpregu.00488.2002. [DOI] [PubMed] [Google Scholar]
- Capella C, Vassallo G, Solcia E. Light and electron microscopic identification of the histamine-storing argyrophil (ECL) cell in murine stomach and of its equivalent in other mammals. Z. Zellforsc. Mikrosk. Anat. 1971;118:68–84. doi: 10.1007/BF00331767. [DOI] [PubMed] [Google Scholar]
- Coulie BJ, Miller LJ. Identification of motilin-related peptide. Gastroenterology. 2001;120:588–589. doi: 10.1053/gast.2001.22165. [DOI] [PubMed] [Google Scholar]
- Date Y, Kojima M, Hosoda H, et al. Ghrelin, a novel growth hormone-releasing acylated peptide, is synthesized in a distinct endocrine cell type in the gastrointestinal tracts of rats and humans. Endocrinology. 2000;141:4255–4261. doi: 10.1210/endo.141.11.7757. [DOI] [PubMed] [Google Scholar]
- Day DE, Bartness TJ. Agouti-related protein increases food hoarding more than food intake in Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:R38–R45. doi: 10.1152/ajpregu.00284.2003. [DOI] [PubMed] [Google Scholar]
- De Ambrogi M, Volpe S, Tamanini C. Ghrelin: central and peripheral effects of a novel peptydil hormone. Medical Sci. Monit. 2003;9:217–224. RA. [PubMed] [Google Scholar]
- Del Rincon JP, Thorner MO, Gaylinn BG. Motilin-related peptide and ghrelin: lessons from molecular techniques, peptide chemistry, and receptor biology. Gastroenterology. 2001;120:587–588. doi: 10.1053/gast.2001.22164. [DOI] [PubMed] [Google Scholar]
- Dornonville de la Cour C, Bjorkqvist M, Sandvik AK, et al. A-like cells in the rat stomach contain ghrelin and do not operate under gastrin control. Regul. Pept. 2001;99:141–150. doi: 10.1016/s0167-0115(01)00243-9. [DOI] [PubMed] [Google Scholar]
- Gualillo O, Lago F, Gomez-Reino J, Casanueva FF, Dieguez C. Ghrelin, a widespread hormone: insights into molecular and cellular regulation of its expression and mechanism of action. FEBS Lett. 2003;552:105–109. doi: 10.1016/s0014-5793(03)00965-7. [DOI] [PubMed] [Google Scholar]
- Hayashida T, Murakami K, Mogi K, et al. Ghrelin in domestic animals: distribution in stomach and its possible role. Domest. Anim. Endocrinol. 2001;21:17–24. doi: 10.1016/s0739-7240(01)00104-7. [DOI] [PubMed] [Google Scholar]
- Hosoda H, Kojima M, Matsuo H, Kangawa K. Ghrelin and des-acyl ghrelin: two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000;279:909–913. doi: 10.1006/bbrc.2000.4039. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends. Endocrinol. Metab. 2001;12:118–122. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- Kojima M, Kangawa K. Ghrelin, an orexigenic signaling molecule from the gastrointestinal tract. Curr. Opin. Pharmacol. 2002;2:665–668. doi: 10.1016/s1471-4892(02)00220-5. [DOI] [PubMed] [Google Scholar]
- Nakazato M, Murakami N, Date Y, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- Rindi G, Necchi V, Savio A, et al. Characterisation of gastric ghrelin cells in man and other mammals: studies in adult and fetal tissues. Histochem. Cell. Biol. 2002;117:511–519. doi: 10.1007/s00418-002-0415-1. [DOI] [PubMed] [Google Scholar]
- Rowland NE. Ingestive behaviour of Syrian hamsters: advantages of the comparative approach. Brain Res. Bull. 1985;15:417–423. doi: 10.1016/0361-9230(85)90010-3. [DOI] [PubMed] [Google Scholar]
- Schneider JE, Buckley CA, Blum RM, et al. Metabolic signals, hormones and neuropeptides involved in control of energy balance and reproductive success in hamsters. Eur. J. Neurosci. 2002;16:377–379. doi: 10.1046/j.1460-9568.2002.02118.x. [DOI] [PubMed] [Google Scholar]
- Tomasetto C, Karam SM, Ribieras S, et al. Identification and characterization of a novel gastric peptide hormone: the motilin-related peptide. Gastroenterology. 2000;119:395–405. doi: 10.1053/gast.2000.9371. [DOI] [PubMed] [Google Scholar]
- Tschöp M, Smiley DL, Helman ML. Ghrelin induces adiposity in rodents. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]
- Vassallo G, Capella C, Solcia E. Endocrine cells of the human gastric mucosa. Z. Zellforsch. Mikrosk. Anat. 1971;118:49–67. doi: 10.1007/BF00331766. [DOI] [PubMed] [Google Scholar]