Abstract
The present investigation was carried out to analyse, immunohistochemically, in vivo leptin expression in cartilage and bone cells, the latter restricted to the elements of the osteogenic system (stromal cells, osteoblasts, osteocytes, bone lining cells). Observations were performed on the first lumbar vertebra, tibia and femur of four rats and on the humerus, femur and acromion of four patients. Histological sections of paraffin-embedded bone samples were immunostained using antibody to leptin. The results showed that, in growing rat bone, leptin is expressed in chondrocytes and stromal cells, but not in osteoblasts; bone lining cells were not found in the microscopic fields examined. In adult human bone, leptin is expressed in chondrocytes, stromal cells and bone lining cells; osteoblasts were not found in the microscopic fields examined. Osteocytes were found to be leptin positive only occasionally and focally in both rat and human bone. The in vivo findings reported show, for the first time, that leptin appears to be expressed only in the cells of the osteogenic lineage (stromal cells, bone lining cells, osteocytes) that, with respect to osteoblasts, are permanent and inactive, i.e. in those cells that according to our terminology constitute the bone basic cellular system (BBCS). Because the BBCS seems to be primarily involved in sensing and integrating mechanical strains and biochemical factors and then in triggering and driving bone formation and/or bone resorption, it appears that leptin seems to be mainly involved in modulating the initial phases of bone modelling and remodelling processes.
Keywords: bone, chondrocytes, immunohistochemistry, leptin, osteogenic cells
Introduction
Leptin is a 16-kDa hormone, primarily secreted by adipose tissue, which controls body weight through its effects on food intake and energy expenditure by negative feedback at the hypothalamic nuclei (Zhang et al. 1994; Cinti et al. 1997; Ahima & Flier, 2000). Leptin is now known to have actions in the immune system (Lord et al. 1998), reproduction (Considine & Caro, 1999), development (Hoggard et al. 1997), haemopoiesis (Gainsford et al. 1996), angiogenesis (Sierra-Honigmann et al. 1998) and, most recently, in bone metabolism. In fact two well-known clinical observations suggest that there is a link between bone mass, body weight and reproduction: obesity protects against bone loss, while menopause favours it (Riggs & Melton, 1986). However, there is considerable controversy surrounding the putative activity of leptin on bone. In ob/ob- and db/db-mice deficient, respectively, in leptin or its receptor, obesity and hypogonadism result in an increased bone mass (Ducy et al. 2000). With regard to the control of body weight, Ducy et al. (2000) hypothesized a central action for leptin and observed that leptin and its receptor did not appear to be expressed in osteoblasts. Takeda et al. (2002) have demonstrated that leptin's anorexigenic and anti-osteogenic effects are performed by two distinct neuronal pathways and that the sympathetic nervous system relays leptin's downstream signals via norepinephrine, which stimulates peripheral β2 adrenergic receptors on osteoblasts, thus reducing bone formation. By contrast, Steppan et al. (2000) and more recently Yagasaki et al. (2003) have demonstrated that peripheral leptin administration has anabolic effects on bone metabolism in ob/ob mice: the demonstration that human osteoblasts in vitro express leptin receptor suggests that the bone growth-promoting effects of leptin could be direct. A leptin-positive effect on bone has also been suggested by Tamasi et al. (2003) in obese leptin receptor-deficient Zucker rats (fa/fa). In addition, Cornish et al. (2002) have demonstrated, in mice, that leptin tends to reduce bone fragility and that it also could contribute to the high bone mass and low fracture rates in obesity. In line with Steppan et al. (2000), other authors have demonstrated in vivo (Hoggard et al. 1997; Kume et al. 2002) and in vitro (Reseland et al. 2001; Cornish et al. 2002; Kume et al. 2002; Lee et al. 2002; Yagasaki et al. 2003) that leptin and its receptor are expressed on osteoblasts. More recently, Hamrick et al. (2004) have noted that in ob/ob mice the effects of altered leptin signalling on bone differs significantly in axial and appendicular regions of the skeleton.
Reports on the expression of leptin system in bone have mainly been provided by in vitro investigations, whose findings are often contradictory, as they did not allow permit conclusions to be made regarding whether leptin has a direct or indirect effect on osteoblasts. By contrast, very few in vivo studies have been conducted on the leptin system and they only concern murine fetal and neonatal bone (Hoggard et al. 1997; Kume et al. 2002). For this reason, the aim of the present immunohistochemical study under light microscopy (LM) was to analyse in vivo leptin expression in cartilage and in bone cells pertaining to the osteogenic lineage of growing rat and adult human bones.
Materials and methods
Animals
Observations were performed on the first lumbar vertebra, tibia and femur of four male Sprague–Dawley rats (from Charles River, Milan, Italy) aged 19 days.
Patients
Specimens of humerus, femur and acromion were obtained from four patients (two women, two men) aged between 32 and 59 years. All patients underwent surgery for traumatic accident and authorized the biopsies for histological studies.
Antibodies used for immunohistochemistry
Rabbit polyclonal antibody raised against the peptide corresponding to amino acids 137–156 (COOH terminus) of the ob gene product (leptin) of human origin (A-20 lot. I 276; Santa Cruz Biotech, CA, USA) was used as primary antibody.
Immunohistochemistry
Tissue specimens from rat and human biopsies were fixed by immersion in 4% paraformaldehyde in 0.1 m sodium phosphate buffer (PBS), pH 7.4. After washing in PBS, the samples were decalcified with EDTA 10%, dehydrated in a graded series of ethanol and embedded in paraffin (Paraplast). Sections (5 µm thick) were immunostained by means of the avidin–biotin technique (Hsu et al. 1981). They were incubated first with primary rabbit polyclonal anti-leptin antibody (Santa Cruz), diluted 1 : 40 in PBS, then with the corresponding biotinylated anti-rabbit IgG secondary antibody, made in goat, diluted 1 : 200 (Vector) and finally with ABC complex (Vectastain ABC kit, Vector). Peroxidase activity was revealed by diaminobenzidine hydrochloride as chromogen (Sigma, St Louis, MO, USA). Sections were then counterstained with haematoxylin, mounted in Eukitt (Kindler, Germany) and observed under LM (Axiophot, Zeiss).
Method specificity was tested (1) by omitting the primary antibody in the immunostaining procedures, and (2) by incubating the sections with the antiserum saturated with the homologous antigen. For the latter procedure, the antibody had been incubated with a 10-fold excess concentration of the homologous peptide (1 µg mL−1) for 48 h (adsorption test).
Antibody specificity was tested by demonstrating leptin antigen in murine and human tissues: stomach was used as positive control and small intestine as negative control (Bado et al. 1998; Cinti et al. 2000). A further control experiment uncoupling protein-1 (UCP1), a specific marker for brown adipose tissue (Klaus et al. 1991), was performed both in bone samples (negative control) and in rat interscapular brown adipose tissue (positive control). A sheep anti-rat UCP serum (generously provided by Dr D. Ricquier, Meudon, France) was used at a final dilution of 1 : 4000.
Results
Rat bone
In 19-day-old rats, tibiae and femurs display both the diaphyseal primary ossification centre and the epiphyseal secondary centre. The body of the first lumbar vertebra is ossified in the central part, whereas its upper and lower surfaces are covered by growing cartilaginous plates.
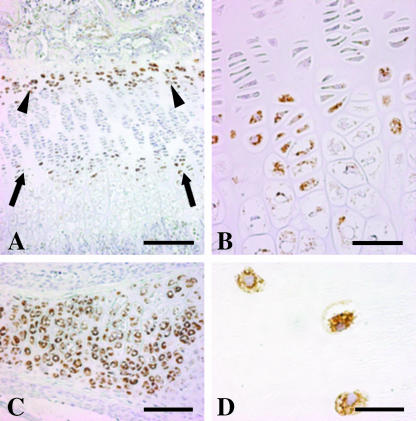
Immunohistochemical analyses showed that leptin is expressed in both cartilage and bone cells. In metaphyseal growth plates, leptin is expressed in hypertrophic chondrocytes and in chondrocytes facing the secondary ossification centre (Fig. 1A,B). In addition, chondrocytes (Fig. 1C) in cartilaginous plates of the vertebral soma were found to be positive for leptin.
Fig. 1.
Light micrographs showing immunoreactivity for leptin expression in chondrocytes of growing cartilaginous plates in proximal metaphysis of tibia (A,B) and in first lumbar vertebra (C) of a 19-day-old rat, and in cartilage of adult human acromion (D). Note in A that chondrocytes are leptin positive in the hypertrophic zone (arrows), enlarged in B, and in the layer facing the secondary ossification centre (arrowheads). Scale bars: A, 200 µm; B, 50 µm; C, 100 µm; D, 20 µm.
For bone, we only took into account the bone cells pertaining to the osteogenic lineage (stromal cells, osteoblasts, osteocytes, bone lining cells) in trabecular bone of both vertebral body and epiphyses as well as in cortical bone of the diaphysis of long bones. All bone surfaces observed appeared completely covered by osteoblast laminae in intense osteoformative activity; bone lining cells were never observed. Osteoblasts were found to be leptin negative, whereas bone marrow stromal or haematopoietic cells as well as white adipocytes (positive internal control) were leptin positive (Figs 2A and 3A). Occasionally, osteocytes were found to be leptin positive, and in a focal manner (Fig. 4A). The negativity of vessels and blood cells constitutes a negative internal control.
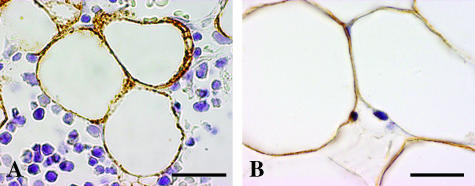
Fig. 2.
Light micrographs showing stromal or haematopoietic cells positive for leptin expression in the marrow space of rat first lumbar vertebra (A) and of human femural neck (B). Note in A the negative osteoblastic lamina (between arrows). Scale bars = 20 µm.
Fig. 3.
Light micrographs showing adipocytes positive for leptin expression in the medullary space of rat tibia (A) and human humerus (B). Scale bars = 20 µm.
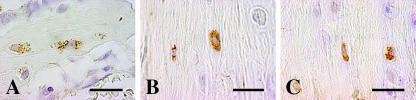
Fig. 4.
Light micrographs showing some osteocytes positive for leptin expression in cortical bone of rat tibia (A) and in human acromion (B,C). Scale bars = 20 µm.
Human bone
Immunohistochemical analyses performed in adult human bones showed that leptin is expressed in both cartilage and bone cells, as in growing rat bone. Chondrocytes in the articular cartilage of the humeral head and acromion were positive for leptin (Fig. 1D).
For bone, no osteogenic surfaces were found, so we had no opportunity to analyse osteoblast immunoreactivity for leptin. The bone surfaces appeared to be in a resting phase and were covered by bone lining cells. Leptin expression was observed in bone lining cells (Fig. 5), bone marrow stromal or haematopoietic cells (Fig. 2B) and white adipocytes (positive internal control) (Fig. 3B). Osteocytes were occasionally leptin positive and in a focal manner (Fig. 4B,C) as in rat bone. The negativity of vessels and blood cells constitutes a negative internal control.
Fig. 5.
Light micrograph (A) showing bone lining cells positive for leptin expression, covering the bone surface in a resting phase, in the proximal end of adult human humerus. A transmission electron micrograph (B) demonstrates that the brownish strip bordering the bone surface in A really corresponds to bone lining cells. Scale bars: A, 20 µm; B, 0.5 µm.
Controls
Leptin antibody stained gastric glandular epithelium (positive control), while all the cellular elements of small intestine were negative (negative control) in murine organs (data not shown). The addition of 1 µg mL−1 leptin in all the positive tests (adsorption test) gave negative results. Using UCP1 antibody, brown adipocytes of rat interscapular brown adipose tissue were positive, while bone cells and bone marrow cells (particularly white adipocytes and stromal or haematopoietic cells) were negative (data not shown).
Discussion
The present immunohistochemical study was performed to analyse in vivo leptin expression in chondrocytes and in bone cells pertaining to the osteogenic lineage.
The positivity for leptin expression we found in chondrocytes appears to be in line with in vivo and in vitro findings recorded by other authors (Kume et al. 2002). Note that chondrocytes have also been found to express leptin receptor (Steppan et al. 2000; Figenschau et al. 2001; Cornish et al. 2002; Kume et al. 2002; Gat-Yablonski et al. 2004). Because leptin has been observed to be present in synovial fluid and in chondrocytes of articular cartilage of patients affected by osteoarthritis as well as in osteophytes, it has been postulated that leptin might exert a modulatory effect on chondrocyte metabolism and consequently on the pathogenesis of osteoarthritis (Dumond et al. 2003).
Our results on leptin expression in bone marrow adipocytes are also in agreement with those obtained from primary culture of human bone marrow adipocytes (Laharrague et al. 1998). Because such adipocytes are close to marrow stromal cells and to the other cells of the osteogenic lineage, we agree with the hypothesis suggested by other authors that adipocyte-derived leptin, in addition to its well-established endocrine role, could serve as a paracrine factor in modulating the activity of the osteogenic cells and the differentiation of haematopoietic precursor cells (Bennet et al. 1996; Gainsford et al. 1996).
In vitro findings on leptin expression appear to be contradictory: according to Ducy et al. (2000) rat osteoblasts are leptin negative, whereas according to Reseland et al. (2001) they are leptin positive. Beacuse the so-called osteoblasts used for in vitro studies are in fact osteoblast-like cells, which are identifiable only on the basis of some specific markers, we do not think that our in vivo results can be compared with those from in vitro investigations. In fact osteoblast-like cells include both osteogenic-active elements (osteoblasts) and osteogenic-inactive elements (osteocytes, bone lining cells, stromal cells), which we found in our in vivo study to be negative and positive, respectively, for leptin expression. Indeed, Hoggard et al. (1997) found in vivo positivity for leptin in fetal cartilage/bone, but failed to attribute leptin expression to a particular differentiated cell type.
The fact that we found all cells of the osteogenic lineage, excluding osteoblasts, to be positive for leptin expression leads us to hypothesize that the expression of this protein might occur in inverse fashion to osteogenic activity. It should be noted in this connection that a similar pattern has been recently demonstrated in adipocytes: leptin was found to be expressed in unilocular brown adipocytes (cells in inactive thermogenic phase) and unexpressed in multilocular brown adipocytes (cells in active thermogenic phase) (Cancello et al. 1998).
The above inverse leptin expression behaviour, as regards bone formation activity, mirrors our recent morphofunctional studies on the cells of the osteogenic lineage. We found that such cells constitute a continuous cytoplasmic network extending from the vascular endothelium to the osteocytes, passing through stromal cells, bone lining cells or osteoblasts (according to whether the surface is resting or growing, respectively). As gap junctions (electrical synapses) connect all these cells, they have been considered a functional syncytium, along which mechanical and metabolic signals can be issued by wiring and volume transmission (Marotti, 1996, 2000; Palazzini et al. 1998; Palumbo et al. 2001; Rubinacci et al. 2002). Note that, of the cells of the osteogenic lineage, osteoblasts are transient elements which appear during bone formation only, whereas the other cells of the osteogenic lineage (stromal cells, bone lining cells, osteocytes), unlike osteoblasts, are permanent elements and in an inactive phase. For this reason we suggested that the cells of the osteogenic lineage, excluding osteoblasts, form a sort of bone basic cellular system (BBCS) capable of sensing and integrating mechanical strains and biochemical factors and then triggering bone formation or bone resorption. The fact that leptin expression is positive on the cells of the BBCS (though occasionally and focally on osteocytes) suggests the hypothesis that such protein could mainly play a role in modulating BBCS activity.
We are aware that the leptin effect, not only on bone metabolism but also over a larger extent, remains controversial, notwithstanding the huge amount of data recorded. In particular, the effect of leptin on bone metabolism might be dependent on concentration and mode of exposure, determined by the source, from which leptin reaches bone cells. A central negative effect (Ducy et al. 2000) could counterbalance that peripheral positive effect (Steppan et al. 2000), the latter being predominant in central leptin resistance occurring during obesity, or when there are high serum leptin levels, a signal of elevated energetic storage. This is supported by a recent study demonstrating a correlation between serum leptin levels and bone mineral density only when leptin values were higher than the threshold of leptin resistance occurrence (Ghazali et al. 2003). Finally, we suggest a paracrine way of leptin action on bone derived from the bone itself and/or bone marrow adipocytes that undoubtedly requires further morphofunctional investigations.
Acknowledgments
This study was supported by funds from the Fondazione Cassa di Risparmio di Modena (CARIMO); by 2002 FAR (ex 60%) for the University of Modena and Reggio Emilia; by 2001 MURST Co-financing and by funds (ex 60%) for the Università Politecnica delle Marche-Ancona
References
- Ahima RS, Flier JS. Leptin. Annu. Rev. Physiol. 2000;62:413–437. doi: 10.1146/annurev.physiol.62.1.413. [DOI] [PubMed] [Google Scholar]
- Bado A, Levasseur S, Attoub S, et al. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- Bennet BD, Solar GP, Yuan JQ, Mathias J, Thomas GR, Matthews W. A role for leptin and its cognate receptor in hematopoiesis. Curr. Biol. 1996;6:1170–1180. doi: 10.1016/s0960-9822(02)70684-2. [DOI] [PubMed] [Google Scholar]
- Cancello R, Zingaretti MC, Sarzani R, Ricquier D, Cinti S. Leptin and UCP1 genes are reciprocally regulated in brown adipose tissue. Endocrinology. 1998;139:4747–4750. doi: 10.1210/endo.139.11.6434. [DOI] [PubMed] [Google Scholar]
- Cinti S, Frederich RC, Zingaretti MC, De Matteis R, Flier JS, Lowell BB. Immunohistochemical localization of leptin and uncoupling protein in white and brown adipose tissue. Endocrinology. 1997;138:797–804. doi: 10.1210/endo.138.2.4908. [DOI] [PubMed] [Google Scholar]
- Cinti S, De Matteis R, Picó C, et al. Secretory granules of endocrine and chief cells of human stomach mucosa contain leptin. Int. J. Obes. Relat. Metab. Disord. 2000;24:789–793. doi: 10.1038/sj.ijo.0801228. [DOI] [PubMed] [Google Scholar]
- Considine RV, Caro JF. Pleiotropic cellular effects of leptin. Curr. Opin. Endocrinol. Diabetes. 1999;6:163–169. [Google Scholar]
- Cornish J, Callon KE, Bava U, et al. Leptin directly regulates bone cell function in vitro and reduces bone fragility in vivo. J. Endocrinol. 2002;175:405–415. doi: 10.1677/joe.0.1750405. [DOI] [PubMed] [Google Scholar]
- Ducy P, Amling M, Takeda S, et al. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell. 2000;100:197–207. doi: 10.1016/s0092-8674(00)81558-5. [DOI] [PubMed] [Google Scholar]
- Dumond H, Presle N, Terlain B, et al. Evidence for a key role of leptin in osteoarthritis. Arthritis Rheum. 2003;48:3118–3129. doi: 10.1002/art.11303. [DOI] [PubMed] [Google Scholar]
- Figenschau Y, Knutsen G, Shahazeydi S, Johansen O, Sveinbjörnsson B. Human articular chondrocytes express functional leptin receptors. Biochem. Biophys. Res. Commun. 2001;287:190–197. doi: 10.1006/bbrc.2001.5543. [DOI] [PubMed] [Google Scholar]
- Gainsford T, Willson TA, Metcalf D, et al. Leptin can induce proliferation, differentiation, and functional activation of hemopoietic cells. Proc. Natl Acad. Sci. USA. 1996;93:14564–14568. doi: 10.1073/pnas.93.25.14564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat-Yablonski G, Ben-Ari T, Shtaif B, et al. Leptin reverses the inhibitory effect of caloric restriction on longitudinal growth. Endocrinology. 2004;145:343–350. doi: 10.1210/en.2003-0910. [DOI] [PubMed] [Google Scholar]
- Ghazali A, Grados F, Oprisiu R, et al. Bone mineral density directly correlates with elevated serum leptin in haemodialysis patients. Nephrol. Dial. Transplant. 2003;18:1882–1890. doi: 10.1093/ndt/gfg268. [DOI] [PubMed] [Google Scholar]
- Hamrick MW, Pennington C, Newton D, Xie D, Isales C. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone. 2004;34:376–383. doi: 10.1016/j.bone.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Hoggard N, Hunter L, Duncan JS, Williams LM, Trayhurn P, Mercer JG. Leptin and leptin receptor mRNA and protein expression in the murine fetus and placenta. Proc. Natl Acad. Sci. USA. 1997;94:11073–11078. doi: 10.1073/pnas.94.20.11073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidine–biotin–peroxidase (ABC) in immunoperoxidase technique: a comparison between ABC and unlabelled antibody (PAP procedure) J. Histochem. Cytochem. 1981;2:577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Klaus S, Casteilla L, Bouillaud F, Ricquier D. The uncoupling protein UCP: a membranous mitochondrial ion carrier exclusively in brown adipose tissue. Int. J. Biochem. 1991;23:791–801. doi: 10.1016/0020-711x(91)90062-r. [DOI] [PubMed] [Google Scholar]
- Kume K, Satomura K, Nishisho S, et al. Potential role of leptin in endochondral ossification. J. Histochem. Cytochem. 2002;50:159–169. doi: 10.1177/002215540205000204. [DOI] [PubMed] [Google Scholar]
- Laharrague P, Larrouy D, Fontanilles AM, et al. High expression of leptin in human bone marrow adipocytes in primary culture. FASEB J. 1998;12:747–752. doi: 10.1096/fasebj.12.9.747. [DOI] [PubMed] [Google Scholar]
- Lee Y-J, Park J-H, Ju S-K, You K-H, Ko JS, Kim H-M. Leptin receptor isoform expression in rat osteoblasts and their functional analysis. FEBS Lett. 2002;528:43–47. doi: 10.1016/s0014-5793(02)02889-2. [DOI] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Marotti G. The structure of bone tissues and the cellular control of their deposition. It. J. Anat. Embryol. 1996;101:25–79. [PubMed] [Google Scholar]
- Marotti G. The osteocyte as a wiring transmission system. J. Musculoskel. Neuron. Interact. 2000;1:133–136. [PubMed] [Google Scholar]
- Palazzini S, Palumbo C, Ferretti M, Marotti G. Stromal cell structure and relationships in perimedullary spaces of chick embryo shaft bones. Anat. Embryol. 1998;197:349–357. doi: 10.1007/s004290050145. [DOI] [PubMed] [Google Scholar]
- Palumbo C, Ferretti M, Ardizzoni A, Zaffe D, Marotti G. Osteocyte–osteoclast morphological relationships and the putative role of osteocytes in bone remodeling. J. Musculoskel. Neuron. Interact. 2001;1:327–332. [PubMed] [Google Scholar]
- Reseland JE, Syversen U, Bakke I, et al. Leptin is expressed in and secreted from primary cultures of human osteoblasts and promotes bone mineralization. J. Bone Miner. Res. 2001;16:1426–1433. doi: 10.1359/jbmr.2001.16.8.1426. [DOI] [PubMed] [Google Scholar]
- Riggs BL, Melton LJ. Involutional osteoporosis. N. Engl. J. Med. 1986;314:1676–1686. doi: 10.1056/NEJM198606263142605. [DOI] [PubMed] [Google Scholar]
- Rubinacci A, Covini M, Bisogni C, et al. Bone as an ion exchange system: evidence for a link between mechanotransduction and metabolic needs. Am. J. Physiol. Endocrinol. Metab. 2002;282:E851–E864. doi: 10.1152/ajpendo.00367.2001. [DOI] [PubMed] [Google Scholar]
- Sierra-Honigmann MR, Nath AK, Murakami C, et al. Biological action of leptin as an angiogenic factor. Science. 1998;281:1683–1686. doi: 10.1126/science.281.5383.1683. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Crawford DT, Chidsey-Frink KL, Ke H, Swick AG. Leptin is a potent stimulator of bone growth in ob/ob mice. Regul. Peptides. 2000;92:73–78. doi: 10.1016/s0167-0115(00)00152-x. [DOI] [PubMed] [Google Scholar]
- Takeda S, Elefteriou F, Levasseur R, et al. Leptin regulates bone formation via the sympathetic nervous system. Cell. 2002;111:305–317. doi: 10.1016/s0092-8674(02)01049-8. [DOI] [PubMed] [Google Scholar]
- Tamasi JA, Arey BJ, Bertolini DR, Feyen JH. Characterization of bone structure in leptin receptor-deficient Zucker (fa/fa) rats. J. Bone Miner. Res. 2003;18:1605–1611. doi: 10.1359/jbmr.2003.18.9.1605. [DOI] [PubMed] [Google Scholar]
- Yagasaki Y, Yamaguchi T, Watahiki J, Konishi M, Katoh H, Maki K. The role of craniofacial growth in leptin deficient (ob/ob) mice. Orthod. Craniofacial Res. 2003;6:233–241. doi: 10.1034/j.1600-0544.2003.00260.x. [DOI] [PubMed] [Google Scholar]
- Zhang YR, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]