Abstract
Iberian red deer need to be conserved for their economic role and for their genetic importance as an important component of the ecosystem. Modifications currently being made to traditional management systems require a better understanding of the structure, function and development of their alimentary system. Here we describe a histomorphometric and immunohistochemical analysis of the stomach of 25 red deer embryos and fetuses from 30 days of gestation until birth (235 days). Differentiation of the reticular compartment from the primitive gastric tube begins at 67 days, forming a three-layered structure: epithelium, pluripotential blastemal tissue and serosa. The primitive reticular cells are initiated as small epithelial evaginations (primary ribs) at 117 days. At 142 days, lateral growths appear from the primary reticular ribs, forming the corneum papillae. The secondary reticular ribs form at 142 days as growths from the primary ribs. The uneven height of primary and secondary reticular ribs leads to the formation of cells of varying size. Growth of the reticular ribs involves the lamina propria but not the submucosa, so clear separation of these layers is maintained during histodifferentiation. Formation of the tunica muscularis from the pluripotential blastemal tissue begins at 67 days of intrauterine life, as two layers of longitudinally and circularly arranged myoblasts. Differentiation of the muscularis from the mucosa occurs at approximately 205 days, as longitudinal projections of the internal bundles of the tunica muscularis form the musculature of the primary ribs. The secretion of neutral and acid mucopolysaccharides by the reticular epithelial layer begins at 67 days, establishing the gradual adaptation of the mucosa to its protective function in postnatal life. Neuroendocrine (non-neuron enolase) and glial cells (glial fibrillary acidic protein and vimentin) were detected by immunohistochemistry, in a similar localization and intensity to that reported in the rumen. The neuropeptides vasoactive intestinal peptide and neuropeptide Y showed a positive immunoreaction in the reticular epithelium from 142 days of prenatal life, again earlier than reported for the rumen. In comparison with domestic ruminants, deer were shown to be less precocious with regard to development of gastric tube, in their capacity to secrete neutral mucopolysaccharides, and in their neuroendocrine nature, as determined by the detection of positive neuroedocrine and/or glial cells.
Keywords: immunohistochemistry, prenatal development, red deer, reticulum
Introduction
Red deer in the Iberian Peninsula represent a hunting species that need to be conserved for two main reasons: on the one hand, for their genetic importance as the bearers of a vital heritage (Carranza, 1999), and on the other, for their economic importance (as a species to be both hunted and eaten) in Mediterranean forests and dehesas (open oak forests) in the south-west of the peninsula. Its economic importance has led to the introduction of modifications to the traditional management and feeding systems of this species (Carranza, 1999).
The current research is set within a wide range of investigation on the development of the stomach of ruminants (Franco et al. 1992, 1993a,b, c; Regodón et al. 1996). There have been no previous studies of the prenatal development of the gastric compartments of the Iberian red deer. As a continuation of the work carried out on the rumen of red deer (Franco et al. 2004) during intrauterine life, we have designed a method of analysis of the reticulum. This study has been carried out in an extensive deer farming system, without food supplements, and the objectives of this study were as follows: (1) to analyse the differentiation of the reticular compartment from the primitive gastric tube, and determine its histological evolution during intrauterine life; and (2) to determine the morphometric, histochemical and immunohistochemical modifications of the reticular mucosa during prenatal development.
Materials and methods
Animals
Deer embryos and fetuses (n = 25) from the initial prenatal stages until birth were studied. The specimens were divided into five groups of five animals each, with reference to the most relevant histomorphogenic characteristics (Table 1). These histomorphogenic characteristics were: group I (30–60 days of gestation), the stomach was still a single cavity; group II (67–90 days of gestation), the reticular compartment initiated its differentiation from the primitive gastric tube; group III (97–135 days of gestation), reticulum showed stratification of epithelial layer and primitive reticular ribs; group IV (142–191 days), the reticular epithelial layer reached its definitive stratification and the corneum papillae and the secondary reticular ribs were visible; group V (205–235 days), fetuses at term, the muscularis mucosae had penetrated the primary reticular ribs. To obtain embryos and fetuses at various stages of development, a total of 125 Caesarian sections on the same number of females were performed. The females were taken from ten hunting grounds from extensive and non-enclosed-type estates from the Sierra of San Pedro (north-east of the Province of Cáceres, Spain).
Table 1.
Neuropeptides present in the reticulum of red deer during prenatal development
| Group I CRL (cm) (1.4–3.6) 30–60 days | Group II CRL (cm) (4.5–7.2) 67–90 days | Group III CRL (cm) (8–19) 97–135 days | Group IV CRL (cm) (21–33) 142–191 days | Group V CRL (cm) (36–40) 205–235 days | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | E | LP-S | TM | S | |
| NNE | – | – | – | – | – | – | – | – | – | + | + | – | – | ++ | ++ | – | – | ++ | ++ | – |
| GFAP | – | – | – | – | – | – | – | – | – | – | – | – | – | ++ | ++ | ++ | – | ++ | ++ | ++ |
| VIM | – | – | – | – | – | + | + | + | ++ | ++ | ++ | ++ | – | ++ | ++ | ++ | – | +++ | +++ | +++ |
| VIP | – | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | ++ | – | – | – |
| NPY | – | – | – | – | – | – | – | – | – | – | – | + | – | – | – | +++ | – | – | – | |
–, No immunoreactivity; +, low immunoreactivity; ++, moderate immunoreactivity; +++, high immunoreactivity.E = epithelium; LP-S = lamina propria-submucosa; T = tunica muscularis; S = serosa.
Sampling and processing
Once the reticulum had been separated, it was analysed by visual and stereomicroscopic inspection. The colour and consistency of the reticular mucosa were determined. Specimens measuring 1.5 × 0.5 cm were taken from the medial region of the reticulum of each red deer. Tissue for histological study was fixed in 4% buffered formaldehyde, processed by usual paraffin-embedding methods, and sections 5 µm thick were cut in transversal direction and treated with haematoxylin and eosin (H-E); Periodic Acid-Schiff (pH 7.2) and PAS-alcian blue (pH 7.2) for specific differentiation of neutral and acid mucopolysaccharides; Von Giesson (VG); and Reticuline of Gomori (RG).
Morphometric analysis
Specimens for morphometric analysis were embedded in paraffin, stained with H-E, and viewed through a microscope (Optiphot, Nikon Inc., Tokyo, Japan) equipped with a video camera. The image was reflected onto the screen of a semi-automatic image analyser (Vid IV, Rego and Cía, Madrid, Spain). Variables studied were height of various tissue strata (epithelium, lamina propria and submucosa, tunica muscularis and serosa) and total wall thickness. Eight specimens (sections) were selected for each group, and 50 measurements were made for each tissue stratum and specimen.
The results are shown as the mean ± SEM. The data were analysed using analysis of the variance. In the cases where the anova was significant, a post hoc (Tukey) analysis was carried out in order to study the significant differences among the distinct groups. A value of P = 0.05 was considered significant.
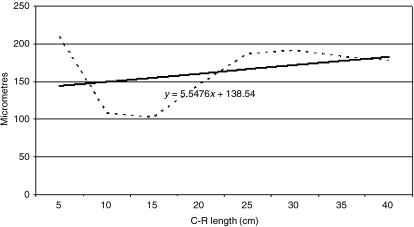
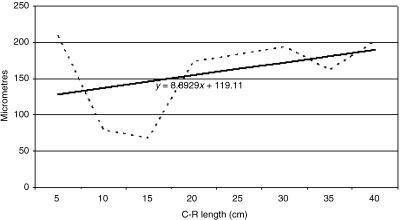
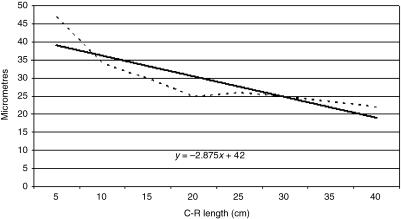
Tissue growth models were created, using a personal computer and statistics program (Statgraphics V 2.1 (1986)). In Figs 21–25 the graphs represent the averages of the real growth values next to the adjusted line of regression. The goodness of fit of this adjustment was measured using the rate of determination r2. In all cases, embryo body length (crown to rump; CRL (cm)) was used as the independent variable; the thickness of each tissue stratum served as the dependent variable.
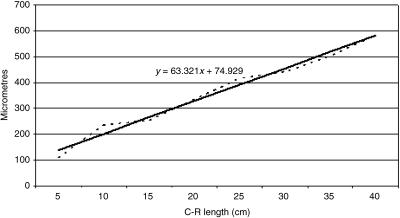
Fig. 21.
Mathematical model of reticulum growth (epithelium).
Fig. 25.
Mathematical model of reticulum wall growth.
Immunocytochemical analysis
ExtrAvidin Peroxidase Staining (EAS) was performed on deparaffinized reticular sections to detect the neuroendocrine cell marker [non-neuron enolase (NNE)]; glial cells markers [glial fibrillary acidic protein (GFAP) and vimentin (VIM),] and markers of peptidergic innervation [neuropeptide Y (NPY) and vasoactive intestinal peptide (VIP)]. Tissue was deparaffinized, hydrated and treated sequentially with 0.5% hydrogen peroxide for 30 min in order to block endogenous peroxidase activity. Non-specific tissue binding sites were blocked by incubation in 1% normal goat serum for 30 min. Samples were incubated with the following dilution in PBS of primary antisera: 1 : 200 monoclonal anti-human NNE (Sigma/Aldrich Química, Madrid, Spain, no. S5768); 1 : 400 monoclonal anti-human GFAP (Sigma/Aldrich Química, no. G-3893); 1 : 20 monoclonal anti-human VIM (Sigma/Aldrich Química, no. V-5255); 1 : 200 monoclonal anti-human NPY (Sigma/Aldrich Química, no. N9528); and 1 : 20 monoclonal anti-human VIP (Sigma/Aldrich Química, no. V3508) for 3 h at 20 °C. Biotinylated goat anti-mouse IgG (1 : 200 dilution) (Sigma/Aldrich Quimica, no. B7151) was then added to the sections for 30 min. Sections were finally incubated with diluted (1 : 50) ExtrAvidin-Horseradish Peroxidase (Sigma/Aldrich Quimica, Madrid, Spain no. E2886) for 1 h. After diaminobenzidine reaction, nuclear counterstaining with Mayer hematoxylin was applied. Finally the sections were mounted with Entellan (Merck 7961).
The specificity of the staining reaction was determined in control experiments. These comprised substitution of the primary antibody by PBS or normal mouse serum 1 : 100, omission of both primary and secondary antibodies, and prior absorption of the primary antibody (overnight pre-incubation of the primary antisera with the respective peptide 50–100 µm). Next, the antibody/peptide mixture was applied to sections in the identical manner and concentration of the primary antibody.
Results
Macroscopic findings
From the differentiation of the reticulum as an individualized compartment, its surface was macroscopically smooth, in particular in Group II; in Group III, small elevations corresponding to the incipient ribs and reticular papillae were detected. In Groups IV and V, an increase in thickness and length was observed in the reticular ribs and in the corneum papillae. The reticular cells, delimited by ribs and the presence of corneum papillae, gave the surface of the reticulum an irregular appearance, and this was clearer in Group V. The colouring of the reticular mucosa was nacreous whitish from differentiation of the primitive stomach until 191 days of fetal life, and pale pink at perinatal stages.
Reticular histomorphogenesis
Group I (1.4–3.6 cm CRL, 30–60 days, 1–25% of gestation)
All conclusions drawn for the rumen (Franco et al. 2004) are valid for the reticulum, given that in this group the compartmentalization of the primitive gastric tube had not yet been produced (Figs 1 and 2).
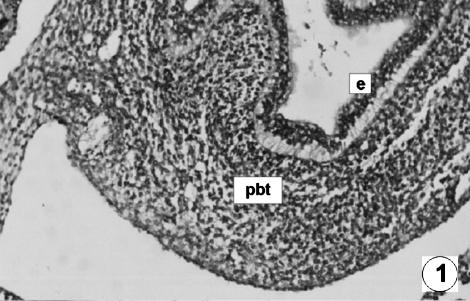
Fig. 1.
Photomicrograph of a transversal direction section of the undifferentiated stomach at 1.4 cm CRL, 30 days. The wall was composed of two layers: epithelium (e) and pluripotential blastemic tissue (pbt). H-E, ×180.
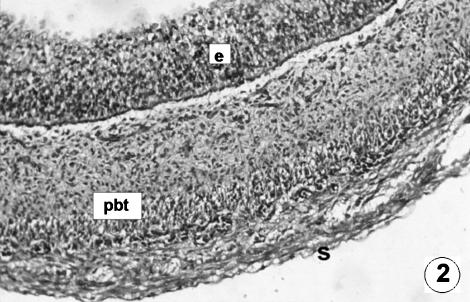
Fig. 2.
Photomicrograph of a transversal direction section of the reticular wall at 3.6 cm CRL, 60 days. Three layers are visible: epithelium (e), pluripotential blastemic tissue (pbt) and serosa (s). H-E, ×250.
Group II (4.5–7.2 cm CRL, 67–90 days, 25–35% of gestation)
At 4.5 cm (Figs 3 and 4) the primitive outline of the reticular compartment appears. The reticular wall (370 ± 16 µm) was made up of three layers.
Fig. 3.
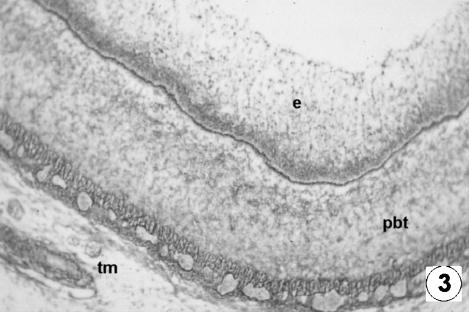
Photomicrograph of a transversal direction section of the reticular wall at 4.5 cm CRL, 67 days. Epithelium (e), pluripotential blastemic tissue (pbt) and tunica muscularis (tm) were observed. RG, ×250.
Fig. 4.
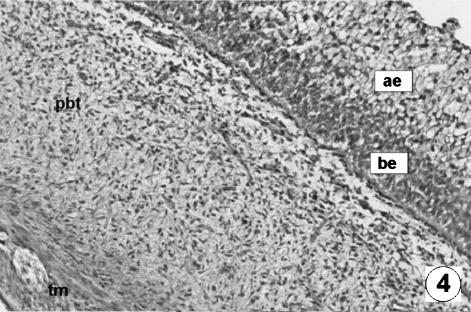
Photomicrograph of a transversal direction section of the reticular wall at 7.2 cm CRL, 97 days. Epithelia layer with a dark basal (be) and lifht apical (ae) zone. Vascularized pluripotential blastemic tissue (pbt). Thin tunica muscularis (tm). VG, ×350.
The epithelial layer of the differentiated reticulum was stratified and bizonal with a dark-staining basal zone rich in germinativum cells and another more external apical zone of globular cells of clear cytoplasm (Figs 3 and 4). It had a thickness of 114 ± 8 µm.
The pluripotential blastemic tissue, separated from the epithelial layer by a clearly defined basal layer, was highly vascularized and in its depth (212 ± 17 µm) a thin tunica muscularis, comprising two layers of supposed myoblasts, circularly and longitudinally arranged, began to appear (Figs 3 and 4).
The serosa (44 ± 5 µm), situated immediately below the previous layer, was composed of a connective-type mesothelium and a subserosa.
Group III (8–19 cm CRL, 97–135 days, 35–50% of gestation)
The wall of the reticulum (454 ± 25 µm) at 8 cm – 97 days of gestation – comprised four well-defined layers: mucosa, submucosa, tunica muscularis and serosa (Figs 5–7). The mucosa was formed by an epithelial layer and a lamina propria. The epithelial layer (248 ± 21 µm) was made up of a basal zone, or stratum germinativum, formed by three or four layers of oval cells and of basophilic cytoplasm, together with an apical layer consisting of polyhedral cells of clear cytoplasm arranged in a mosaic pattern. The lamina propria (104 ± 7 µm), thinner than in its previous stage, was formed by mesenchymatous pluripotential tissue of high vascularization and rich in fibroblasts and collagen fibres.
Fig. 5.
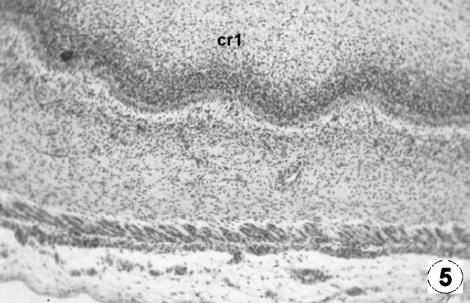
Photomicrograph of a transversal direction section of the reticular wall at 8 cm CRL, 97 days. Primary reticular ribs (cr1) containing evaginations of the epithelial basal stratum. H-E, ×250.
Fig. 7.
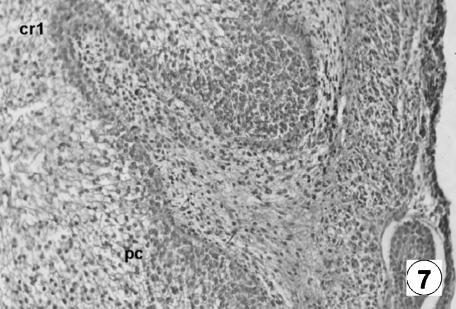
Photomicrograph of a transversal direction section of the reticular wall at 19 cm CRL, 135 days. Presence of ribs (cr1) and corneum papillae (pc). VG, ×350.
The highly cellular submucosa (104 ± 7 µm) appears between ground substance and cells, in contrast to the equilibrium witnessed in the previous stage.
In the tunica muscularis (71 ± 12 µm), the myoblasts were arranged in two bundles, one internal or circular and the other external or longitudinal; between the two was a fine layer of pluripotential mesenchymatous tissue with numerous capillaries and nerve endings.
The serosa (31 ± 5 µm) appeared, made up of a lax subserosa, a continuation of the intervascular pluripotential mesenchymatous tissue. Within this, nerve fibres and vascular structures were observed, covered with a smooth mesothelium.
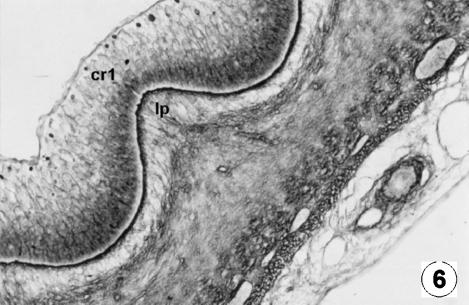
At 97 days of prenatal development (Figs 5 and 6) an undulation of the epithelial germinativum stratum appeared, as the first differentiation of the future cells of the reticulum: the first primitive reticular ribs. As intrauterine development progressed, the reticulum showed the ribs of the cells as evaginations, becoming more and more prominent, from the basal stratum towards the light zone of the epithelium, at more or less regular intervals (Figs 5–7). With growth, these primary ribs pulled with them the lamina propria, without affecting the submucosa.
Fig. 6.
Photomicrograph of a transversal direction section of the reticular wall at 13 cm CRL, 115 days. Growth of the primary rib (cr1) involving the lamina propria (lp).RG, ×250.
Group IV (21–33 cm CRL, 142–191 days, 50–70% of gestation)
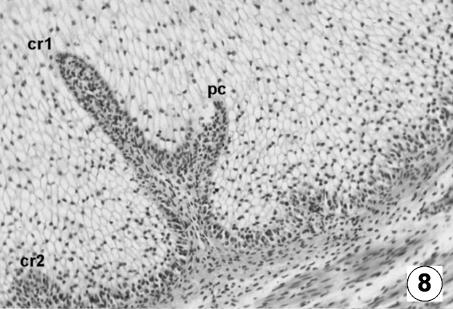
The reticular epithelial layer (427 ± 29 µm) (Figs 8 and 9) was finally formed by the following strata: (1) the basal stratum, or stratum germinativum, formed by a unique layer of dark-staining cells; (2) the granulosum, wider and formed by numerous layers of large polyhedral cells of a clear cytoplasm; and (3) the corneum, the most superficial layer, formed by a unique layer of flat anuclear cells.
Fig. 8.
Photomicrograph of a section of the reticular wall at 21 cm CRL, 142 days. Presence of primary ribs (cr1), secondary ribs (cr2) and corneum papillae (pc). H-E, ×250.
Fig. 9.
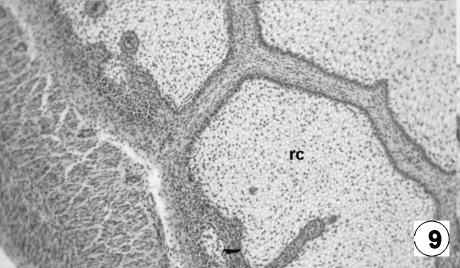
Photomicrograph of a transversal direction section of the reticular wall at 33 cm CRL, 191 days. Fusion of the reticular ribs forming the reticular cells (rc). H-E, ×250.
The lamina propria (189 ± 7 µm) (Figs 8 and 9) was structured with a highly cellular fibrous-type mesenchymatous tissue (mostly fibroblasts), with clear modelling visible in its fibres. It consisted of a highly vascularized tissue, particularly at the level of the ribs.
The submucosa, rich in stellate cellular elements, showed a spatial separation with the lamina propria, resulting from the pull that the latter suffered with growth of the primary ribs from the epithelial stratum germinativum.
The tunica muscularis (188 ± 12 µm) (Fig. 8) was shaped by its two bundles, a circular and oblique, and a longitudinal external bundle.
The external serosa (25 ± 3 µm) was similar to that described in the previous stage.
The primary reticular ribs continued to move deeper towards the epithelial surface from the basal membrane. From the ribs, the corneum papillae emerge as lateral growths of a papillary type, originating from growth and proliferation of the basal cells of the stratum germinativum of the epithelial layer. During this stage, we witnessed the appearance of secondary reticular ribs, from the basal membrane, moving towards the epithelial surface: they were always smaller in size than the primary ribs.
Group V (36–40 cm CRL, 205–235 days, 75–100% of gestation)
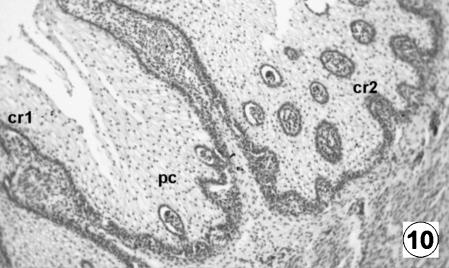
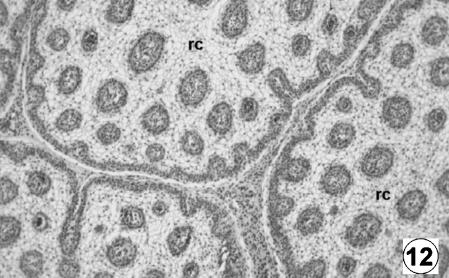
The epithelium was keratinized squamos stratified, corresponding to a tegumentary-type mucosa. It had a thickness of 556 ± 29 µm and, in contrast to the previous stage, showed the differentiation of the lucidum–spinosum stratum, made up of condensed cells of dark cytoplasm (Fig. 10). During this stage, the epithelium was lost in the spaces among the primary ribs, resulting in the appearance of internally delimited reticular cells (Figs 11 and 12).
Fig. 10.
Photomicrograph of a transversal direction section of the reticular wall at 36 cm CRL, 205 days. Presence of primary crests (cr1), secondary (cr2) and corneum papillae (pc). H-E, ×250.
Fig. 11.
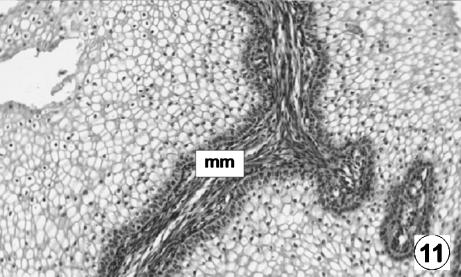
Photomicrograph of a transversal direction section of the reticular wall at 36 cm CRL, 205 days. Presence of the muscularis of the mucosa (mm) in the primary ribs. H-E, ×350.
Fig. 12.
Photomicrograph of a transversal direction section of the reticular wall at 40 cm CRL, 235 days. Fusion of the reticular ribs formed the reticular cells (rc). H-E, ×250.
The lamina propria (185 ± 9 µm) was highly cellular, with an abundant vascularization and a considerable number of elastic and collagen fibres. The submucosa was more lax, with fusiform and stellate elements immersed in an abundant intercellular substance with little fibrillar content.
The muscularis of the mucosa stood out in the primary ribs (Fig. 11). In the joints between the ribs, the muscular bundles pass from one crest to another, thus forming a continual network of muscular tissue in all of the reticular mucosa. The remaining layers that make up the reticular wall (953 ± 47 µm) were similar in terms of their characteristics to those described in the previous stage.
Histochemical behaviour of the epithelium
Histochemical reaction to the neutral and acid mucopolysaccharides mucins and mucoid compounds began at 67 days of intrauterine life, and was similar in localization and intensity to that described for the rumen (Franco et al. 2004).
Immunohistochemical observations
Table 1 shows the neuropeptides present in the reticulum of red deer during prenatal development. Immunohistochemical findings in the reticulum of the five groups of red deer studied are summarized in Figs 13–20.
Fig. 13.
Photomicrograph of a transversal direction section of the reticular wall at 33 cm CRL, 191 days. Presence of neuroendocrine cells (NNE) in interfascicular space of the tunica muscularis (arrow). EAS, ×250.
Fig. 20.
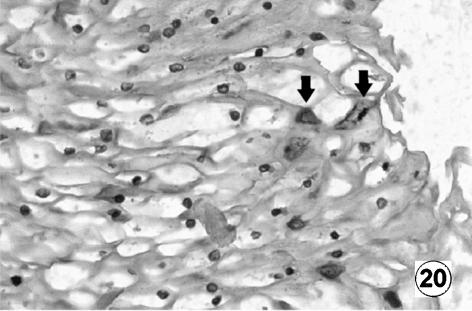
Photomicrograph of a transversal direction section of the reticular wall at 40 cm CRL, 235 days. Positive immunoreaction of VIP (arrow) in the reticular epithelial cells. EAS, ×350.
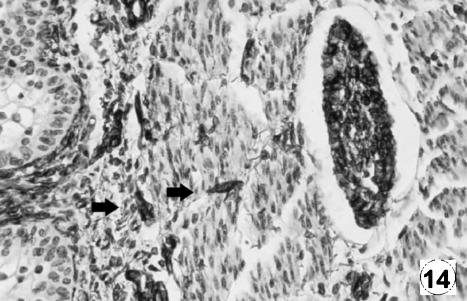
The immunodetection of NNE and GFAP was similar in localization and intensity to that reported in the rumen (Franco et al. 2004). The immunodetection of NNE was positive from 97 days of gestation in the lamina propria and tunica muscularis and was prolonged until birth. A positive immunoreaction for GFAP appeared from 142 days of fetal development in the lamina propria, tunica muscularis and serosa.
With regard to VIM, in comparison with the rumen, there was a more intense immunohistochemical reaction from the moment of its appearance in Group II. VIM was noted over 67 days of gestation in the lamina propria, tunica muscularis and serosa and in an identical location, immunopositivity was increaseling during prenatal development.
With reference to VIP and NPY, positive immunoreaction in the reticular epithelial cells in the animals of group IV was evident. There was also a higher immunopositivity than that detected in rumen for the reticular epithelial cells in the animals of Group V. The positive immunoreaction of the epithelial layer increased by 142 days to reach maximum values at term.
Histomorphometric observations
Table 2 shows the tissue layer thickness in the reticulum of red deer during prenatal development. Each tissue stratum was fitted to mathematical growth models (Figs 21–25), using the corresponding growth equation.
Table 2.
Morphometric and statistical findings of the tissue layer thickness in the reticulum of red deer during prenatal development (µm)
| Group I CRL (cm) (1.4–3.6) 30–60 days | Group II CRL (cm) (4.5–7.2) 67–90 days | Group III CRL (cm) (8–19) 97–135 days | Group IV CRL (cm) (21–33) 142–191 days | Group V CRL (cm) (36–40) 205–235 days | |
|---|---|---|---|---|---|
| Epithelium | 62 ± 5 | 114 ± 8a | 248 ± 21a | 427 ± 29a | 556 ± 29a |
| Lp + Sb | pbt | pbt | 104 ± 7 | 189 ± 7b | 185 ± 9b |
| Tm | 195 ± 10* | 212 ± 17* | 71 ± 12 | 188 ± 12b | 186 ± 16b |
| Serosa | 56 ± 3 | 44 ± 5a | 31 ± 5a | 25 ± 3a | 26 ± 4a |
| Wall | 313 ± 16 | 370 ± 16a | 454 ± 25a | 729 ± 41a | 953 ± 47a |
Lp + Sb = lamina propria and submucosa; Tm = tunica muscularis; pbt = pluripotential blastemic tissue.
The pluripotential blastemic tissue of groups I and II, which will later give rise to the lamina propria and submucosa, were not statistically compared owing to the fact that one structure will give rise to various others.
P < 0.005 vs. Group I;
P < 0.005 vs. Group III.
A factorial anova indicated that the mean value of Group I epithelium was significantly minor than in Groups II to V (F = 16.48; Tukey test P = 0.002). On the other hand, the mean value of serosa of Group I was significantly higher than in Groups II to V (F = 6.29; Tukey test P = 0.001) and the mean value of wall of Group I was also significantly higher than in Groups II to V (F = 8.40; Tukey test P = 0.001). As indicated by main factor analysis in factorial anova, the lamina propria and submucosa of Group III was significantly different than these layers of Groups IV and V (F = 13.70; P = 0.003) and the tunica muscularis of Group III was also significantly different from that of Groups IV and V (F = 17.80; P = 0.0003).
The morphometric evolution of the epithelial layer, the lamina propria and submucosa, the tunica muscular, the serosa and the integral reticular wall displayed identical growth phases, stabilization and regression to those described in rumen (Franco et al. 2004).
Discussion
The reticular compartment initiated its differentiation from the primitive gastric tube at 4.5 cm CRL, 67 days of prenatal development, 25% of gestation, forming a three-layered structure: epithelial layer, pluripotential blastemic tissue and serosa. In comparison with ruminal differentiation in deer (Franco et al. 2004), reticular differentiation occurred later. In relation to the differentiation of the reticulum in other species of ruminants, it was found to be similar to that described in sheep (Del Rio Ortega, 1973; Franco et al. 1993c), where it was placed at around 22% of gestation, and in goat (Molinari & Jorquera, 1988), 24% of gestation. In cow, Vivo et al. (1990), reported it very early at 1.7 cm CRL, equivalent to a 30-day or 11% gestation period.
From 67 days of intrauterine life, 25% gestation, the epithelial layer appeared bistratified. This bizonal stratification was previously reported in prenatal development in the rumen of buffalo (Panchamukhi & Srivastava, 1980), in the rumen and reticulum of sheep (Franco et al. 1992, 1993b) and in the rumen of deer (Franco et al. 2004). The stratification of the epithelial layer of the reticulum of deer during development was complete with the appearance of the corneum stratum at 142 days of gestation (50%), and the lucidum-spinosum stratum at 205 days of gestation (75%). In comparison with other species of ruminants, the corneum stratum was placed at around 83 days of prenatal development (55% of gestation) in the reticulum of sheep (Franco et al. 1993b), and in relation to the lucidum-spinosum stratum, its appearance in sheep was placed during perinatal stages (Groenewald, 1993). Authors such as Tiwari & Jamdar (1970) and Osman & Berg (1981), in buffalo, do not describe it at all.
One area in which there does seem to be unanimity is in the keratinization of the reticular epithelium, in pre- as well as post-natal development in ruminants. Although differences exist in the degree of keratinization, it is dependent on its localization; Yamamoto et al. (1998), in goat, thus demonstrated higher keratinization in the tips of the reticular papillae than in the rest of the epithelium.
The final structure of the epithelial layer resulted in the formation of the primitive reticular cells. We observed them in their first differentiation as small evaginations of the basal zone toward the apical zone of the epithelium (primary ribs) at 117 days of prenatal life (40% gestation). In sheep, Del Rio Ortega (1973), Fath el Bab et al. (1983) and Franco et al. (1993c) estimated their appearance at 72 (48% of gestation), 69 (46%) and 95 days (63%), respectively. In goat, Molinari & Jorquera (1988) established an appearance range between 69 and 102 days (46–68% of gestation). In buffalo Panchamukhi & Sravastava (1980) and Osman & Berg (1981) placed their appearance at 70 and 200 days (22 and 62% of gestation), respectively.
At 142 days (50% of gestation) some lateral growths that formed the corneum papillae appeared from the primary reticular ribs. The differentiation of these structures in sheep was reported at around 55% of gestation (Franco et al. 1993c); at 63% (Fath el Bab et al. 1983) and in perinatal stages (Molinari & Jorquera, 1988).
The secondary reticular ribs appeared at 142 days, at approximately 50% of gestation, as a growth of the primary ribs, although they never reached the same size (Franco et al. 1989). Just as with the primary ribs, there was no unanimity in terms of their histodifferentiation. Thus, they were situated at approximately 40% of gestation in buffalo (Panchamukhi & Siravastava, 1980); in the range 55–70% in sheep (Del Rio Ortega, 1973; Fath el Bab et al. 1983; Franco et al. 1993c); and at 69% in goat (Molinari & Jorquera, 1988).
The characteristic cells of the reticulum require two types of rib growth for their formation: longitudinal, from the basal membrane and toward the epithelial surface (responsible for the growth in length of the ribs), and transversal or an expansion of the ribs toward the adjacent ribs, joining with them to form the cells. The uneven height of both primary and secondary reticular ribs could explain the formation of cells of varying size (Franco et al. 1993c).
The histodifferentiation of the lamina propria and submucosa developed in a similar manner to that described in the rumen of deer (Franco et al. 2004). The clear separation of both structures barely stood out, related to the fact that the growth of the reticular ribs involved the lamina propria without affecting the submucosa. This fact was pointed out by Franco et al. (1993c) in the prenatal development of the ovine reticulum.
The tunica muscularis was formed from the pluripotential blastemic tissue at 67 days of intrauterine life (25% of gestation), comprising two layers of longitudinally and circularly arranged myoblasts. In the prenatal development of the reticulum of sheep, its appearance is described at 33 days (2% of gestation) (Duncan & Philipson, 1955; Franco et al. 1993c), and at 50 days (33% of gestation) (Del Rio Ortega, 1973; Fath el Bab et al. 1983).
The differentiation of the muscularis of the mucosa was estimated at around 205 days (75% of gestation). It was formed by longitudinal projections of the internal bundles of the tunica muscularis towards the primary ribs, becoming condensed within these, and constituting the musculature of the ribs. In ruminants, the appearance was placed in perinatal stages in buffalo (Panchamukhi & Siravastava, 1980); and in sheep, at approximately 75% of gestation (Franco et al. 1993c) or during postnatal development (Wardrop, 1961; Kitamura et al. 2003).
The histodifferentiation of the serosa did not differ from that described for the rumen of deer (Franco et al. 2004).
The secretory characteristics of the reticular epithelial layer were as described for the rumen (Franco et al. 2004). The histochemical reaction was conditioned by the gradual adaptation of the mucosa throughout histomorphogenesis to a protective function in postnatal life (mechanical – from the corneum stratum – and histochemical – due to the presence of neutral and acid mucopolysaccharides – of the deepest layers of the epithelium). Even during prenatal life, the reticular tegumentary mucosa will provide a protective function against the possible ingestion of amniotic fluid (Franco et al. 1993b).
The immunodetection of neuroendocrine cells was similar in localization and intensity to that reported for the reticulum of sheep (Groenewald, 1994) and of bovine reticulum (Kitamura et al. 1993), and for the rumen of deer (Franco et al. 2004). With regard to the presence of glial cells, it is worthwhile pointing out the more intense immunohistochemical reaction for VIM from the moment of its appearance at 67 days of prenatal life with respect to the rumen of deer (Franco et al. 2004). Yamamoto et al. (1995), in perinatal sheep, and Teixeira et al. (1998), in bovine reticulum, described immunoreactivity for glial cells in the reticular papillae, suggesting that some of the functions of the reticular mucosa could be intrinsically regulated by the submucosal plexus.
With regard to VIP and NPY, the positive immunoreaction in the reticular epithelium from 142 days of prenatal is of interest. These observations agree with those described by Franco et al. (2004) in the prenatal development of the rumen of deer, although the immunoreaction from 205 days of gestation was more intense that that detected in the ruminal epithelium. The presence of the neuropeptides VIP and NPY has also been described in the reticulum of sheep at the level of myenteric and submucosal plexus (Vergara-Esteras et al. 1990; Groenewald 1994; Yamamoto et al. 1994; Pfannkuche et al. 2003), and in the stomach of guinea-pig (Mawe & Gershon, 1989), of pig (Timmermans et al. 1990) and of cow (Kitamura et al. 1986).
In comparison with domestic ruminants, deer were shown as being less precocious with regard to development of gastric tube, in their capacity to secrete neutral mucopolysaccharides, and in their neuroendocrine nature, as determined by the detection of positive neuroedocrine and/or glial cells.
Fig. 14.
Photomicrograph of a transversal direction section of the reticular wall at 40 cm CRL, 235 days. Presence of neuroendocrine cells (NNE) in lamina propria and submucosa (arrow). EAS, ×350.
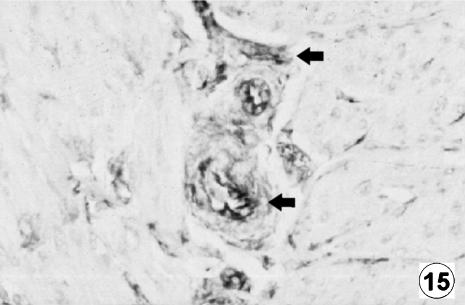
Fig. 15.
Photomicrograph of a transversal direction section of the reticular wall at 33 cm CRL, 191 days. Presence of GFAP-positive cells (arrow) in lamina propria and submucosa. EAS, ×350.
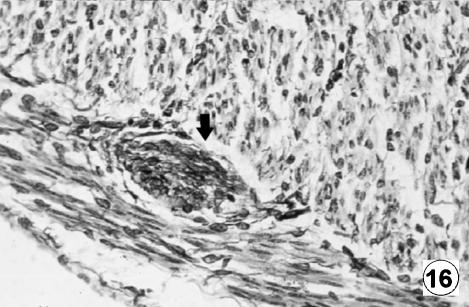
Fig. 16.
Photomicrograph of a transversal direction section of the reticular wall at 40 cm CRL, 235 days. Presence of GFAP-positive cells (arrows) in tunica muscularis. EAS, ×350.
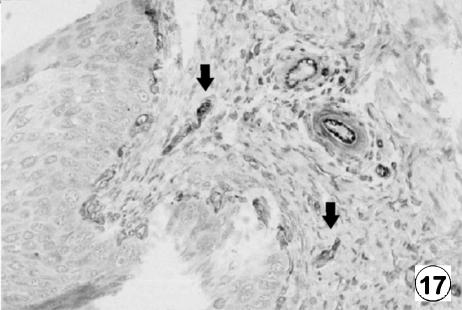
Fig. 17.
Photomicrograph of a transversal direction section of the reticular wall at 19 cm CRL, 135 days. Presence of VIM-positive cells (arrows) in lamina propria and submucosa. EAS, ×350.
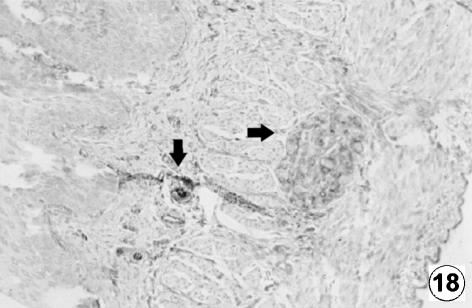
Fig. 18.
Photomicrograph of a transversal direction section of the reticular wall at 40 cm CRL, 235 days. Presence of VIM positive cells (arrows) in the interior of the myenteric plexus. EAS, ×250.
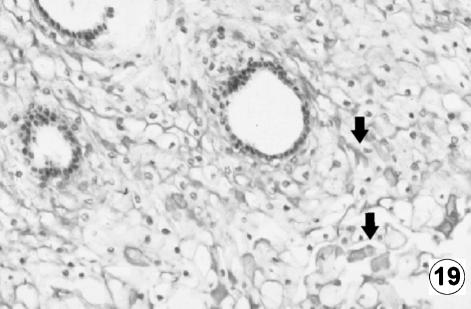
Fig. 19.
Photomicrograph of a transversal direction section of the reticular wall at 40 cm CRL, 235 days. Positive immunodetection of VIP (arrow) in the reticular epithelial cells of the stratum lucidum and spinosum of reticular tegumentary mucosa. EAS, ×250.
Fig. 22.
Mathematical model of reticulum growth (lamina propria and submucosa).
Fig. 23.
Mathematical model of reticulum growth (tunica muscularis).
Fig. 24.
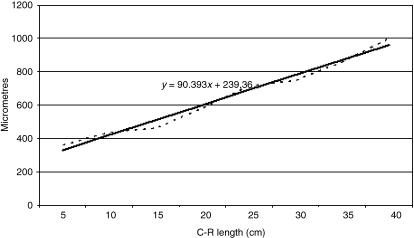
Mathematical model of reticulum growth (serosa).
Acknowledgments
We would like to thank D. Juan Luis Rodríguez Cruz for his invaluable technical assistance and other contributions.
References
- Carranza J. Aplicaciones de la etología al manejo de las poblaciones de ciervo en el suroeste de la Península Ibérica: producción y conservación. Etología. 1999;7:5–18. [Google Scholar]
- Del Rio Ortega S. Doctoral thesis. 1973. Desarrollo prenatal del estómago de la oveja. Facultad de Veterinaria, Zaragoza. [Google Scholar]
- Duncan DL, Phillison AT. The development of motor responses in the stomach of the foetal sheep. J. Exp. Biol. 1955;28:32–40. doi: 10.1242/jeb.28.1.32. [DOI] [PubMed] [Google Scholar]
- Fath el Bab, Schwart R, Ali AMA. Micromorphological studies on the stomach of sheep during prenatal life. Anat. Histol. Embryol. 1983;12:139–153. doi: 10.1111/j.1439-0264.1983.tb01010.x. [DOI] [PubMed] [Google Scholar]
- Franco A, Vivo JM, Guillén MT, Regodón S, Robina A. Evolución parietal del retículo ovino de raza merina desde los 68 días de gestación hasta el nacimiento. Histol. Med. 1989;5:57–58. [Google Scholar]
- Franco A, Regodón S, Robina A, Redondo E. Histomorphometric analysis of the rumen of the sheep during development. Am. J. Vet. Res. 1992;53:1209–1217. [PubMed] [Google Scholar]
- Franco A, Robina A, Regodón S, Vivo JM, Masot AJ, Redondo E. Histomorphometric analysis of the omasum of sheep during development. Am. J. Vet. Res. 1993a;54:1221–1229. [PubMed] [Google Scholar]
- Franco A, Robina A, Guillén MT, Mayoral AI, Redondo E. Histomorphometric analysis of the abomasum of sheep during development. Anat. Anzeiger. 1993b;175:119–125. doi: 10.1016/s0940-9602(11)80164-0. [DOI] [PubMed] [Google Scholar]
- Franco A, Robina A, Regodón S, Vivo JM, Masot AJ, Redondo E. Histomorphometric analysis of the reticulum of the sheep during development. Histol. Histopathol. 1993c;8:547–556. [PubMed] [Google Scholar]
- Franco A, Masot AJ, Gómez L, Redondo E. Immunohistochemical and morphometric study of the rumen of red deer during prenatal development. J. Anat. 2004;204:501–513. doi: 10.1111/j.0021-8782.2004.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groenewald HB. Ultrastructure of the epithelium of the rumen, reticulum and omasum of grey, white and black Karakul lambs. Onderstepoort J. Vet. Res. 1993;60:197–204. [PubMed] [Google Scholar]
- Groenewald HB. Neuropeptides in the myenteric ganglia and nerve fibres of the forestomach and abomasum of grey, white and black Karakul lambs. Onderstepoort J. Vet. Res. 1994;61:207–213. [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamashita T. Immunohistochemical study on the distribution of neuron-specific enolase and peptide-containing nerves in the reticulorumen and the reticular groove of the cattle. J. Compar. Neurol. 1986;248:223–234. doi: 10.1002/cne.902480205. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yamada J, Yamamoto Y, Yamashita T. Substance P-immunoreactive neurons of the bovine forestomach mucosa: their presumptive role in a sensory mechanism. Arch. Histol. Cytol. 1993;56:399–410. doi: 10.1679/aohc.56.399. [DOI] [PubMed] [Google Scholar]
- Kitamura N, Yoshiki A, Sasaki M, et al. Immunohistochemical evaluation of the muscularis mucosae of the ruminant forestomach. Anat. Histol. Embryol. 2003;32:175–178. doi: 10.1046/j.1439-0264.2003.00456.x. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Gershon MD. Structure, afferent innervation, and transmitter content of ganglia of the guinea pig gallbladder: relationship to the enteric nervous system. J. Comp. Neurol. 1989;283:374–390. doi: 10.1002/cne.902830306. [DOI] [PubMed] [Google Scholar]
- Molinari E, Jorquera B. Intrauterine development stages of the gastric compartments of the goat (Capra hircus) Anat. Histol. Embryol. 1988;17:121–137. doi: 10.1111/j.1439-0264.1988.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Osman AHR, Berg R. Studies on the histogenesis of the tunica mucosa of the stomach of the egyptian water buffalo (Bos bubbalis)2. Histogenesis of the reticular mucosa. Anat. Anzeiger. 1981;150:516–520. [PubMed] [Google Scholar]
- Panchamukhi BG, Srivastava HC. Histogenesis of the reticulum of the buffalo (Bubalus bubalis) stomach. Indian J. Anim Sci. 1980;50:1064–1070. [Google Scholar]
- Pfannkuche H, Schellhorn C, Schemann M, Gabel G. Reticular groove and reticulum are innervated by myenteric neurons with different neurochemical codes. Anat. Rec. 2003;274A:917–922. doi: 10.1002/ar.a.10104. [DOI] [PubMed] [Google Scholar]
- Regodón S, Franco A, Masot AJ, Redondo E. Estudio ontogénico comparativo de la lámina epitelial de los compartimentos gástricos no glandulares en ovinos merinos. Anat. Histol. Embriol. 1996;25:233–241. doi: 10.1111/j.1439-0264.1996.tb00086.x. [DOI] [PubMed] [Google Scholar]
- Teixeira AF, Wedel T, Krammer HJ, Kuhnel W. Structural differences of the enteric nervous system in the cattle forestomach revealed by whole mount immunohistochemistry. Anat. Anz. 1998;180:393–400. doi: 10.1016/S0940-9602(98)80099-X. [DOI] [PubMed] [Google Scholar]
- Timmermans JP, Scheuermann DW, Stach W, Adriansen D, De Groot-Lasseel MHA. Distinct distribution of CGRP-, enkephalin-, galanin-, neuromedin U-, neuropeptide Y-, somastotatin-, substance P-, VIP- and serotonin-containing neurons in the two submucosal ganglionic neural netwoks of the porcine small intestine. Cell Tisue Res. 1990;260:367–379. doi: 10.1007/BF00318639. [DOI] [PubMed] [Google Scholar]
- Tiwari GP, Jamdar NM. Studies of the gross and histological structure and development on the forestomach of indian water buffalo calf in early postnatal life with reference to normal feeding. 2. Reticulum. Indian J. Anim. Sci. 1970;57:335–340. [Google Scholar]
- Vergara-Esteras P, Harrison FA, Brown D. The localization of somatostatin-like immunoreactivity in the alimentary tract of the sheep with observations of the effect of an infection with the parasite Haemonchus contortus. Exp. Physiol. 1990;75:779–789. doi: 10.1113/expphysiol.1990.sp003460. [DOI] [PubMed] [Google Scholar]
- Vivo JM, Robina A, Regodón S, Guillén MT, Franco A, Mayoral AI. Histogenetic evolution of bovine gastric compartments during prenatal period. Histol. Histopathol. 1990;5:461–476. [PubMed] [Google Scholar]
- Wardrop JD. Some preliminary observations on the histological development on the forestomach of the lamb. I. Histological changes due to age in the period from 46 days of foetal life to 77 days of postnatal life. J. Agric. Sci. 1961;57:335–341. [Google Scholar]
- Yamamoto Y, Kitamura N, Yamada J, Yamashita T. Immunohistochemical study of the distributions of the peptide and catechol-containing nerves in the omasum of the seep. Acta Anat. 1994;149:104–110. doi: 10.1159/000147564. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Atoji Y, Suzuki Y. Morphological study of the submucosal and mucosal plexuses of the sheep forestomach. Anat. Anz. 1995;177:405–412. doi: 10.1016/S0940-9602(11)80145-7. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Atoji Y, Agungpriyono S, Suzuki Y. Morphological study of the forestomach of the Japanese serow (Capricornis crispus) Anat. Histol. Embryol. 1998;27:73–81. doi: 10.1111/j.1439-0264.1998.tb00160.x. [DOI] [PubMed] [Google Scholar]