Abstract
Retinal hypoxia occurs in many conditions that cause vascular disease in the eye and is an important stimulus to new vessel formation. However, the adult retina can also become hypoxic when there is systemic hypoxaemia such as occurs in chronic lung diseases, congenital cardiac disease and residence at high altitude. Little is known about the adaptive responses of the retinal vasculature in such circumstances. Previous research in the retinopathy of prematurity model may not apply to the adult tissue given the different mechanisms controlling angiogenesis in developing and mature circulations. We tested the hypothesis that chronic systemic hypoxia leads to angiogenesis in the adult retinal circulation, in the absence of pre-existing vascular disease. Adult male Sprague–Dawley rats (n = 9) were exposed to a fraction of inspired oxygen of 0.10 for 2 weeks while control animals (n = 10) were exposed to room air. Stereological techniques were used to quantify the vascular volume, endothelial surface area and the total number of branch points of all blood vessels in the superficial retinal vascular plexus. The mean volume and endothelial surface area of these vessels were significantly greater in the hypoxic than in the control group. The mean number of blood vessel branch points was also significantly greater in the hypoxic group. Our findings demonstrate that chronic systemic hypoxia, in the absence of other pathological processes, causes angiogenesis in the adult rat retina and provide an in vivo model for investigating this important process in the adult retina, in particular pathways specific to this tissue.
Keywords: morphometry, retina, stereology, vasculature, volume
Introduction
Retinal hypoxia secondary to local vascular abnormalities occurs in many ocular diseases and is an important stimulus to neovascularization; collectively these diseases are known as the proliferative retinopathies. The new vessels formed in these conditions are abnormal in their structure, location and function and frequently lead to loss of vision. However, the retinal oxygen supply is also threatened in any circumstance where there is sustained systemic hypoxaemia (Wangsa-Wirawan & Linsenmeier, 2003), including chronic lung diseases, late onset of left to right shunting in congenital heart disease and residence at high altitude. In these conditions retinal hypoxia can occur in the absence of any other disease directly affecting the blood vessels of the eye (Wangsa-Wirawan & Linsenmeier, 2003). Little is known about the adaptive responses of the retinal vasculature in such circumstances.
Hypoxia of a tissue or organ does not inevitably lead to angiogenesis; in some adult tissues it does not lead to new vessel formation, for example in skeletal muscle (Banchero, 1987; Olfert et al. 2001). There are also important differences in the mechanisms of angiogenesis between organs (Pettersson et al. 2000; Carmeliet et al. 2001; LeCouter et al. 2001; Yancopoulos et al. 2000). Thus, the mechanisms controlling any hypoxia-induced new vessel formation in the retina might well be different from those operating in other tissues. To date, there are no reports of angiogenesis in the adult retinal circulation in response to global hypoxia, as distinct from the response seen to local vascular disease and ischaemia.
In the proliferative retinopathies, hypoxia is an important stimulus for new vessel formation (Ashton, 1957; Shimizu et al. 1981; Aiello et al. 1994; Jampol et al. 1994; Campochiaro, 2000; Funatsu et al. 2003). However, in these disease conditions other pathological processes such as inflammation, glycosylation of proteins and alteration of shear stress on blood vessels also operate and each of these stimuli can lead to increased vascular endothelial growth factor (VEGF) and induce angiogenesis independently of tissue hypoxia (Adamis, 2002; Lu et al. 1998; Isner, 1999; Griffioen & Molema, 2000; Treins et al. 2001; Rivilis et al. 2002; Urbich et al. 2003). It is possible that the neovascularization observed in retinal diseases might not be the result of hypoxia alone but may also be dependent, in part or in whole, on these other stimuli.
Investigation of the vascular response of the adult retina to hypoxia is hampered by the absence of a reliable in vivo animal model of hypoxia acting in the absence of other disease processes. One of the most widely used approaches to investigating retinal hypoxia is the retinopathy of prematurity model. However, this produces angiogenesis in the developing retina and the extent to which the mechanisms that regulate angiogenesis in the developing retinal circulation are replicated in the mature retinal circulation is uncertain (Campochiaro, 2000). In other organs there is clear evidence that differences exist between the two states. For example, mice deficient in the endothelial cell nitric oxide synthase gene have normal vascular development but in adulthood have impaired angiogenesis of limb vessels in response to ischaemia or VEGF administration (Murohara et al. 1998). Also, placental growth factor (PlGF)-deficient mice demonstrate normal vascular development in utero and early postnatal life, but impaired angiogenesis and collateral growth during ischaemia, inflammation, wound healing and cancer (Carmeliet et al. 2001). Thus, although new vessel formation in the developing and adult organs shares some common regulatory mechanisms, it is clear that important differences exist between them. Existing adult animal models of retinal neovascularization, such as laser photocoagulation of retinal vessels, result in tissue destruction and inflammation in addition to hypoxia (Saito et al. 1997). Thus, these experimental approaches do not mimic the conditions found in global or systemic hypoxia of the mature adult retina.
We hypothesized that chronic systemic hypoxia in adult rats, induced by exposure to a hypoxic environment, would lead to intra-retinal angiogenesis in the absence of any pre-existing retinal disease. To explore this hypothesis, we used stereological morphometry to quantify the total volume of blood vessels, total endothelial surface area and total number of blood vessel branch points in the superficial vascular plexus of the retina in adult rats exposed to sustained hypoxia.
Methods
Experimental protocol and tissue preparation
All experiments were approved by the Institutional Ethics Committee and conducted under licence. Adult male (290–390 g, 12–14 weeks old) specific pathogen-free Sprague–Dawley rats (Harlan, Bicester, UK) were randomly assigned to hypoxic conditions (n = 9) or room air (n = 10) for 14 days. Hypoxia (FiO2 = 0.10) was induced using a normobaric opaque perspex environmental chamber, as previously described (Howell et al. 2003). In brief, the FiO2 and FiCO2 were monitored continuously and maintained at approximately 0.10 (range 0.098–0.102) and at less than 0.005, respectively. The gases in the chamber were continuously recirculated via a pump through soda lime (Sigma Chemicals, Dublin, Ireland), a cooled condensing unit (Glo-Therm, Dublin, Ireland) and an activated carbon filter (Carbon Cap 150, Whatman Ltd, Maidstone, UK) in order to remove excess CO2, humidity and ammonia, respectively. The animals were fed ad libitum. The chamber was opened daily for 30 min to replenish food and water and to change the cage bedding. Otherwise the environmental conditions were maintained constant. Ten control animals were exposed to room air for the same duration. They were kept in the same location, fed ad libitum and had their bedding changed on the same schedule as the hypoxic group.
Animals were weighed prior to entry into the experimental protocol. Following the 2-week exposure period they were again weighed, anaesthetized via an intraperitoneal injection of 60 mg kg−1 sodium pentobarbitone (Sagatal, Rhône-Merieux), anticoagulated (1000 iu kg−1 heparin) and killed by exsanguination. Venous blood was obtained for haematocrit determination and a canula was inserted into the ascending aorta. The circulation was flushed by perfusion with 0.15 m calcium-free normal saline containing 2 iu mL−1 of heparin via the aortic canula at a pressure of 85 mmHg. The circulation was then perfusion fixed with 4% paraformaldehyde in 0.1 m PBS at the same perfusion pressure for 1 h. The eyes were then enucleated, the extraocular tissue was removed and the volume of each eye was measured by displacement. In order to ensure adequate retinal fixation the cornea was removed and the eye was placed in 4% paraformaldehyde at 4 °C for 12 h. The vitreous was then removed and one of the eyes from each rat was embedded in resin and the other was placed in Optimal Cutting Temperature compound (Sigma Chemicals, Dublin, Ireland) and frozen at −70 °C.
Following enucleation each eye was assigned a new random identifier number in order to blind the observer during collection and analysis of images. The randomization key was stored separately until analysis was completed.
Owing to technical difficulties two frozen eyes from the hypoxic rats were lost as analysis was not possible. As a result, two additional hypoxic rats were used to achieve n = 7 for frozen hypoxic eyes. Similarly, three frozen eyes were lost from the control group as analysis was not possible for technical reasons. As a result, three additional control rats were used to achieve n = 7 for frozen control eyes.
Stereological analysis of resin-embedded eyes
A brief description of the stereological methods is given below; more extensive details are available as supplement material on the journal website.
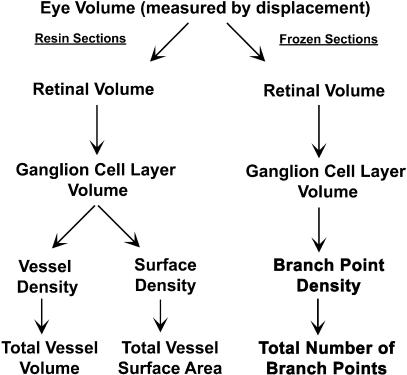
Resin-embedded eyes were used to calculate the volume and endothelial surface area of blood vessels in the ganglion cell layer of the retina. In calculating the volume and endothelial surface area of blood vessels in this layer of the retina, all blood vessels including arterioles, venules and capillaries between the internal limiting membrane and the inner plexiform layer were included.
Each resin-embedded eye was serially sectioned using a vertical uniform random (VUR) strategy as required for stereological analysis (Gundersen, 1986; Gundersen et al. 1988; Bolender et al. 1993; Weibel & Cruz-Orive, 1997). One-micrometre-thick sections were collected for analysis and stained with 10% toluidine blue. To determine the volume and endothelial surface area of the vascular compartment of interest, a cascade approach was used (Bolender et al. 1993; Gundersen, 1986; Gundersen et al. 1988; Weibel & Cruz-Orive, 1997) (Fig. 1). Volume fractions of specific tissues and retinal layers in the eye were estimated by point counting (Bolender et al. 1993; Gundersen, 1986; Gundersen et al. 1988; Weibel & Cruz-Orive, 1997) and the absolute volume estimated using the eye volumes measured by displacement.
Fig. 1.
Diagram illustrating the experimental design and cascade approach to calculating the volume, surface area and the total number of branch points of blood vessels in the superficial retinal vascular plexus.
Stereological analysis of frozen eyes
The total number of blood vessel branch points in the ganglion cell layer of the retina was estimated in those eyes that were frozen at −70 °C. Frozen sections (20 µm thick) were collected from each eye at intervals of 400 µm, beginning at a random start point. These sections were stained with biotinylated griffonia simplicifolia isolectin B4 (Vector Laboratories, Burlingame, CA, USA) and counterstained with 1 µL mL−1 propidium iodide (Molecular Probes, Eugene, OR, USA) in HEPES-buffered saline.
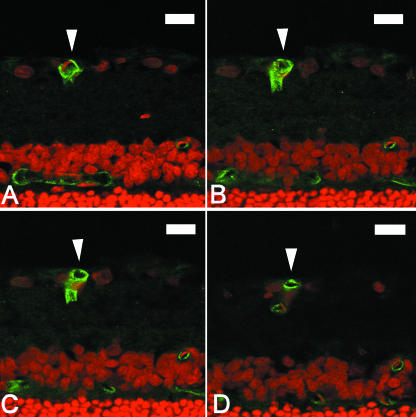
The blood vessel branch point density in the ganglion cell layer was estimated in each eye using the double dissector technique as previously described for the lung (Hopkins et al. 2001; Howell et al. 2003). The total number of blood vessel branch points was estimated using the ganglion cell layer volume for each eye. This was estimated using a cascade approach as described above and in more detail in the supplement material on the journal website. A branch point was defined as where two, immediately adjacent vessels shared a common wall. An example of a typical branch point is illustrated in Fig. 2. No distinction was made between arterial or venous branch points.
Fig. 2.
(A–D) Four serial optical sections obtained by confocal microscopy from the same 20-µm-thick frozen section of retina. Each optical section is 2 µm thick and each image is separated by a distance of 2 µm. Cell nuclei are stained with propidium iodide (red). Blood vessels are stained with biotinylated griffonia simplicifolia isolectin B4 and labelled with FITC (green). A typical blood vessel branch point is demonstrated. In (A) a single blood vessel is visible in the retinal ganglion cell layer (indicated by arrow). In (B) and (C) the vessel is seen to branch to form two individual vessels sharing a common wall. In (D) the vessel has completely separated to form two distinct vessels. Scale bar = 20 µm.
Statistical analysis
All values shown are means ± standard error of the mean (SEM); n represents the number of animals studied in each group. To test for statistically significant differences between means of the two groups unpaired t-tests were used. A data value of P = 0.05 was accepted as statistically significant.
Results
The mean haematocrit in the hypoxic group was 57.8 ± 0.7%, significantly greater (P < 0.05) than that (43.9 ± 0.8%) of the control group. The change in weight was also significantly different (P < 0.05) with the control group gaining weight (+27.7 ± 3.9 g) and the hypoxic group losing weight (−43.6 ± 4.7 g). There was no significant difference in the mean whole eye volume between the control (0.134 ± 0.003 cm3) and hypoxic (0.131 ± 0.002 cm3) groups.
The retinal volume and ganglion cell layer volume in resin-embedded control eyes were 1.23 (± 0.07) × 10−2 cm3 and 1.80 (± 0.11) × 10−3 cm3, respectively. The corresponding values for resin-embedded hypoxic eyes were 1.14 (± 0.06) × 10−2 cm3 and 1.81 (± 0.14) × 10−3 cm3, respectively. The retinal volume and ganglion cell layer volume in frozen control eyes were 1.75 (± 0.05) × 10−2 cm3 and 2.24 (± 0.12) × 10−3 cm3, respectively. The corresponding values for frozen hypoxic eyes were 1.70 (± 0.06) × 10−2 cm3 and 2.35 (± 0.15) × 10−3 cm3, respectively. There was no statistically significant difference between control and experimental groups embedded in the same material (wax or resin). However, the differences between retinal and ganglion cell layer volumes when resin-embedded control eyes were compared with wax-embedded control eyes were statistically significant. Similar statistically significant differences were noted when comparing experimental eyes prepared in these two ways. These differences can be accounted for by a tissue processing artefact, because resin embedding required tissue dehydration whereas tissue processing for frozen sectioning did not. The differences observed do not, however, affect the interpretation of the results as resin-embedded hypoxic eyes were only compared with resin-embedded control eyes and likewise for frozen hypoxic and control eyes.
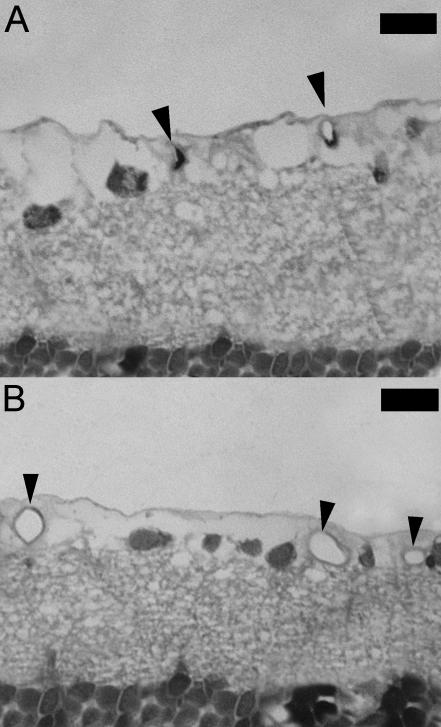
Figure 3 shows images of resin sections obtained from control and hypoxic eyes demonstrating typical vessels in the ganglion cell layer of the retina. At no point were preretinal neovascularization, intra-retinal haemorrhage or vitreous haemorrhage observed. Vessels in the two groups differed only in size and frequency. Their histological structures appeared identical and normal and no obvious difference in tortuousity was observed between groups.
Fig. 3.
(A) Photomicrograph of a 1-µm-thick resin section of the retina from a control rat. Typical blood vessels (indicated by arrows) of the superficial retinal vascular plexus are seen in the ganglion cell layer. (B) Photomicrograph of the inner retina from a hypoxic rat showing an increase in size and number of vessels (indicated by arrows) in the superficial retinal vascular plexus. Scale bar = 20 µm.
Table 1 gives the mean values for volume fraction (volume of blood vessels per unit volume of ganglion cell layer), total volume (of blood vessels), endothelial surface density (surface area of endothelium per unit volume of ganglion cell layer), total endothelial surface area (vessel surface area), branch point density (number of branch points per unit volume of ganglion cell layer) and total number of blood vessel branch points in the ganglion cell layer of the retina of the control and hypoxic groups. All these parameters were significantly increased in the hypoxic group, demonstrating that angiogenesis had occurred.
Table 1.
Mean values for volume fraction, total volume, endothelial surface density, total endothelial surface area of blood vessels, branch point density and total number of blood vessel branch points in the ganglion cell layer of the retina of the control and hypoxic groups. n = number of eyes examined in each experimental group
| Control (n = 7) | Hypoxic (n = 7) | |
|---|---|---|
| Volume fraction (%) | 10.25 (1.0) | 18.0 (2.0)* |
| Total volume (×10−4 cm3) | 1.84 (0.19) | 3.36 (0.55)* |
| Endothelial surface density (cm2 cm−3) | 281.0 (21.9) | 400.7 (27.4)* |
| Total endothelial surface area (cm−2) | 0.50 (0.04) | 0.74 (0.09)* |
| Branch point density (×106 cm−3) | 14.6 (0.8) | 18.2 (0.8)* |
| Total number of branch points (×103) | 32.9 (2.8) | 42.4 (2.4)* |
Values are mean (± SEM).
A statistically significant difference between the two groups (P = 0.05, unpaired t-test).
Discussion
The major stimuli to neovascularization in disease conditions are hypoxia, chronic inflammation, metabolic disturbance and sustained alteration of shear stress, each of which acting alone is sufficient to cause angiogenesis (Gille et al. 1997; Lu et al. 1998; Carmeliet & Jain, 2000; Cho et al. 2001; Rivilis et al. 2002; Urbich et al. 2003). These stimuli are not uniformly effective in all organs and, in particular, chronic hypoxia fails to cause angiogenesis in some adult tissues (Banchero, 1987; Olfert et al. 2001). Under conditions of reduced PaO2 the retina can become profoundly hypoxic (Wangsa-Wirawan & Linsenmeier, 2003). Thus, in conditions where there is systemic hypoxaemia, including chronic lung diseases, late onset of left to right shunting in congenital heart disease and residence at high altitude, retinal hypoxia can occur in the absence of any other disease directly affecting the eye and, in particular, in the absence of retinal vascular disease. The present series of experiments was undertaken to determine whether such hypoxia could cause angiogenesis in the adult retina.
We used a well-established animal model of chronic environmental hypoxia and confirmed that systemic hypoxia had been produced by demonstrating increased haematocrit and typical loss of weight in all rats (LaManna et al. 1992; Harik et al. 1995; Ooi et al. 2000; Howell et al. 2003). We have shown in previously published papers that this protocol resulted in the development of classic hypoxic pulmonary hypertension, including right ventricular hypertrophy and hypoxic vascular remodelling in rats, and polycythaemia, confirming the presence of sustained hypoxaemia (Ooi et al. 2000; Howell et al. 2003). It must be noted that while the retina was exposed to chronic hypoxia it was also exposed to the systemic consequences of chronic hypoxia, such as elevated erythropoietin concentrations and polycythaemia. The extent to which these other factors might account for the changes that we observed remains to be determined. Nonetheless, this retinal hypoxia resulting from systemic hypoxia is clearly distinct from that which results from local vascular occlusion of retinal vessels, such as occurs in many occular diseases.
Although the hypoxic conditions did not change the volume of the whole eye or the retina, there was a significant structural change in the retinal circulation, as indicated by the increased total endothelial cell surface area and volume of the vessels in the ganglion cell layer. This increased vessel volume and endothelial surface area could have resulted either from increased diameter of pre-existing vessels or from new vessel formation. However, our demonstration of an increase in the number of blood vessel branch points in the hypoxic group demonstrates unequivocally that angiogenesis had taken place and that vascular volume and surface area increased, at least in part, as a result of new vessel formation.
Thus, our results demonstrate unequivocally for the first time that chronic systemic hypoxia can cause angiogenesis in the adult retina, in the absence of any disease process. Under conditions of physiological hypoxia, such as on ascent to high altitude, angiogenesis is clearly a beneficial adaptation as it increases the total surface area for gas and solute exchange, reduces the diffusion distance from blood vessels to cells and increases the total volume of blood contained in the retinal circulation. In addition, the formation of new vascular pathways can reduce the velocity of blood flow while increasing total volume of blood flow per unit time. Acting together, these changes will serve to return oxygen delivery to the retina towards normal values and, as in other organs, establish a new stable vasculature in which the vessels have normal morphology and function (LaManna et al. 1992; Harik et al. 1995; Howell et al. 2003).
Our demonstration of hypoxia-induced angiogenesis within the retina provides a model that will allow investigation of the underlying mechanisms. Investigation of the mechanisms that operate in the retina is required given the recent demonstrations that tissue-specific pathways exist. LeCouter et al. (2001) have reported an angiogenic mitogen specific for endocrine organs. This growth factor, which is structurally dissimilar to VEGF, is only expressed in steroidogenic endocrine organs, exerts angiogenic effects solely within these organs and is induced by hypoxia (LeCouter et al. 2001). It seems likely that there is a large class of tissue-specific angiogenic factors, and it has been suggested that targeting of these putative molecules may allow tissue-specific anti-angiogenic therapy in disease conditions (Carmeliet et al. 2001).
One aspect of our methods that merits specific comment is the use of stereological techniques to detect angiogenesis in the retinal circulation. These have been widely used as a method of obtaining quantitative information in three dimensions about structural changes in the vascular bed of other organs from two-dimensional histological images, particularly in the lung and nervous system (Bolender et al. 1991; LaManna et al. 1992; Harik et al. 1995; Hopkins et al. 2001; Howell et al. 2003). Stereology has been used previously to evaluate changes in the retinal circulation of the rat during diabetes (Anderson et al. 1995, 1996). We employed this technique because it allowed us to quantify multiple variables relating to the structure of the retinal circulation, namely volume, endothelial surface area and branching. These variables cannot be measured using other commonly applied microscopy techniques. Quantification of these parameters allowed unequivocal demonstration of new vessel formation.
In clinical conditions such as diabetic retinopathy or central retinal vein occlusion, neovascular complications result from structurally weak new vessels that are located on the surface of the retina beyond the inner limiting membrane. Such vessels were not observed in the present study. One possible explanation is that vascular remodelling may have provided an adequate compensatory physiological response, restored adequate retinal oxygen delivery, relieved tissue hypoxia and thus removed the stimulus to continued new vessel formation. This might have prevented the proliferation of vessels on the surface of the retina. This would be in keeping with the effects of chronic hypoxia in other organs where angiogenesis produces well-organized, structurally and functionally normal new vessels and does not lead to abnormal vessel growth (LaManna et al. 1992; Harik et al. 1995; Howell et al. 2003). This may also be the reason that the changes of high altitude retinopathy were not observed in these animals, because increased permeability of vessels is a feature of the early stages of new vessel formation, which resolves once the new vessels have matured. In contrast, angiogenesis may proceed to preretinal neovascularization in pathological conditions because of a number of additional disease-related factors that are not present in systemic hypoxia.
In conclusion, we have demonstrated for the first time that angiogenesis occurs in the superficial vascular bed of the mature adult rat retina in response to chronic systemic hypoxia in the absence of local ocular disease. Our findings may prove to be of use in elucidating the pathways and mediators involved in the response of the adult rat circulation to hypoxia and in determining the similarities and differences between the pathways operating in the developing and mature retinal circulations. In addition, this approach will allow an investigation of potential tissue-specific pathways regulating angiogenesis in the retina. Because, in other tissues, the mechanisms producing hypoxia-induced angiogenesis are major contributors to angiogenesis in disease, the insights gained with this model are likely to be of relevance to new vessel formation in the proliferative retinopathies.
Acknowledgments
This work was supported in part by the Health Research Board of Ireland
Supplementary material
Supplementary details of the methods employed are available on the journal website. These include details of the hypoxic chamber, stereological techniques used and a supplementary figure.
References
- Adamis A. Is diabetic retinopathy an inflammatory disease? Br. J. Ophthalmol. 2002;86:363–365. doi: 10.1136/bjo.86.4.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiello LP, Avery RL, Arrigg PG, et al. Vascular endothelial growth factor in ocular fluid of patients with diabetic retinopathy and other retinal disorders. N. Engl. J. Med. 1994;331:1480–1487. doi: 10.1056/NEJM199412013312203. [DOI] [PubMed] [Google Scholar]
- Anderson HR, Stitt AW, Gardiner TA, Archer DB. Diabetic retinopathy: morphometric analysis of basement membrane thickening of capillaries in different retinal layers within arterial and venous environments. Br. J. Ophthalmol. 1995;79:1120–1123. doi: 10.1136/bjo.79.12.1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson HR, Gardiner T, Archer D. Stereological analysis of changes in the 3-dimensional structure of the retinal vasculature during diabetes. Acta Stereol. 1996;15:103–111. [Google Scholar]
- Ashton N. Retinal vascularization in health and disease. Am. J. Ophthalmol. 1957;44:7–17. doi: 10.1016/0002-9394(57)90426-9. [DOI] [PubMed] [Google Scholar]
- Banchero N. Cardiovascular responses to chronic hypoxia. Annu. Rev. Physiol. 1987;49:465–476. doi: 10.1146/annurev.ph.49.030187.002341. [DOI] [PubMed] [Google Scholar]
- Bolender RP, Charleston J, Mottet K, McCabe JT. Quantitative morphology of the nervous system: expanding horizons. Anat. Rec. 1991;231:407–415. doi: 10.1002/ar.1092310403. [DOI] [PubMed] [Google Scholar]
- Bolender RP, Hyde DM, Dehoff RT. Lung morphometry: a new generation of tools and experiments for organ, tissue, cell and molecular biology. Am. J. Physiol. 1993;265:L521–L548. doi: 10.1152/ajplung.1993.265.6.L521. [DOI] [PubMed] [Google Scholar]
- Campochiaro PA. Retinal and choroidal neovascularization. J. Cell Pathol. 2000;184:301–310. doi: 10.1002/1097-4652(200009)184:3<301::AID-JCP3>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. doi: 10.1038/35025220. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Moons L, Luttun A, et al. Synergism between vascular endothelial growth factor and placental growth factor contributes to angiogenesis and plasma extravasation in pathological conditions. Nat. Med. 2001;7:575–583. doi: 10.1038/87904. [DOI] [PubMed] [Google Scholar]
- Cho M, Hunt TK, Hussain MZ. Hydrogen peroxide stimulates macrophage vascular endothelial growth factor release. Am. J. Physiol. Heart Circ. Physiol. 2001;280:H2357–H2363. doi: 10.1152/ajpheart.2001.280.5.H2357. [DOI] [PubMed] [Google Scholar]
- Funatsu H, Yamashita H, Ikeda T, Mimura T, Eguchi S, Hori S. Vitreous levels of interleukin-6 and vascular endothelial growth factor are related to diabetic macular edema. Ophthalmology. 2003;110:1690–1696. doi: 10.1016/S0161-6420(03)00568-2. [DOI] [PubMed] [Google Scholar]
- Gille J, Swerlick RA, Caughman SW. Transforming growth factor-alpha-induced transcriptional activation of the vascular permeability factor (VPF/VEGF) gene requires AP-2-dependent DNA binding and transactivation. EMBO J. 1997;16:750–759. doi: 10.1093/emboj/16.4.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffioen AW, Molema G. Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol. Rev. 2000;52:237–268. [PubMed] [Google Scholar]
- Gundersen HJ. Stereology of arbitrary particles. A review of unbiased number and size estimators and the presentation of some new ones, in memory of William R Thompson. J. Microsc. 1986;143:3–45. [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, et al. The new stereological tools: Disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Harik SI, Hritz MA, Lamanna JC. Hypoxia induced brain angiogenesis in the adult rat. J. Physiol. 1995;485:525–530. doi: 10.1113/jphysiol.1995.sp020748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins N, Cadogan E, Giles S, McLoughlin P. Chronic airway infection leads to angiogenesis in the pulmonary circulation. J. Appl. Physiol. 2001;91:919–928. doi: 10.1152/jappl.2001.91.2.919. [DOI] [PubMed] [Google Scholar]
- Howell K, Preston RJ, McLoughlin P. Chronic hypoxia causes angiogenesis in addition to remodelling in the adult rat pulmonary circulation. J. Physiol. 2003;547:133–145. doi: 10.1113/jphysiol.2002.030676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isner JM. Cancer and atherosclerosis: the broad mandate of angiogenesis. Circulation. 1999;99:1653–1655. doi: 10.1161/01.cir.99.13.1653. [DOI] [PubMed] [Google Scholar]
- Jampol LM, Ebroon DA, Goldbaum MH. Peripheral proliferative retinopathies: an update on angiogenesis, etiologies and management. Surv. Ophthalmol. 1994;38:519–540. doi: 10.1016/0039-6257(94)90146-5. [DOI] [PubMed] [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J. Appl. Physiol. 1992;72:2238–2243. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- LeCouter J, Kowalski J, Foster J, et al. Identification of an angiogenic mitogen selective for endocrine gland endothelium. Nature. 2001;412:877–884. doi: 10.1038/35091000. [DOI] [PubMed] [Google Scholar]
- Lu M, Kuroki M, Amano S, et al. Advanced glycation end products increase retinal vascular endothelial growth factor expression. J. Clin. Invest. 1998;101:1219–1224. doi: 10.1172/JCI1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murohara T, Asahara T, Silver M. Nitric oxide synthase modulates angiogenesis in response to tissue ischaemia. J. Clin. Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olfert IM, Breen EC, Mathieu-Costello O, Wagner PD. Chronic hypoxia attenuates resting and exercise-induced VEGF, flt-1, and flk-1 mRNA levels in skeletal muscle. J. Appl. Physiol. 2001;90:1532–1538. doi: 10.1152/jappl.2001.90.4.1532. [DOI] [PubMed] [Google Scholar]
- Ooi H, Cadogan E, Sweeney M, Howell K, O'Regan RG, McLoughlin P. Chronic hypercapnia inhibits hypoxic pulmonary remodelling. Am. J. Physiol. Heart Circ. Physiol. 2000;278:H331–H338. doi: 10.1152/ajpheart.2000.278.2.H331. [DOI] [PubMed] [Google Scholar]
- Pettersson A, Nagy JA, Brown LF, et al. Heterogeneity of the angiogenic response induced in different normal adult tissues by vascular permeability factor/vascular endothelial growth factor. Lab. Invest. 2000;80:99–115. doi: 10.1038/labinvest.3780013. [DOI] [PubMed] [Google Scholar]
- Rivilis I, Milkiewicz M, Boyd P, et al. Differential involvement of MMP-2 and VEGF during muscle stretch- versus shear stress-induced angiogenesis. Am. J. Physiol. Heart Circ. Physiol. 2002;283:H1430–H1438. doi: 10.1152/ajpheart.00082.2002. [DOI] [PubMed] [Google Scholar]
- Saito Y, Park L, Skolik SA, et al. Experimental preretinal neovascularization by laser-induced venous thrombosis in rats. Curr. Eye Res. 1997;16:26–33. doi: 10.1076/ceyr.16.1.26.5116. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kobayashi Y, Muraoka K. Midperipheral fundus involvement in diabetic retinopathy. Ophthalmology. 1981;88:601–612. doi: 10.1016/s0161-6420(81)34983-5. [DOI] [PubMed] [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, et al. Regulation of vascular endothelial growth factor expression by advanced glycation end products. Retinal hypoxia in long-term diabetic cats. J. Biol. Chem. 2001;276:43836–43841. doi: 10.1074/jbc.M106534200. [DOI] [PubMed] [Google Scholar]
- Urbich C, Stein M, Reisinger K, Kaufmann R, Dimmeler S, Gille J. Fluid shear stress-induced transcriptional activation of the vascular endothelial growth factor receptor-2 gene requires Sp1-dependent DNA binding. FEBS Lett. 2003;535:87–93. doi: 10.1016/s0014-5793(02)03879-6. [DOI] [PubMed] [Google Scholar]
- Wangsa-Wirawan ND, Linsenmeier RA. Retinal oxygen: fundamental and clinical aspects. Arch. Ophthalmol. 2003;121:547–557. doi: 10.1001/archopht.121.4.547. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Cruz-Orive LM. Morphometric methods. In: Crystal RG, West JB, Weibel ER, Barnes PJ, editors. The Lung: Scientific Foundations. 2. Philadelphia, PA: Lippincott-Raven; 1997. pp. 333–344. [Google Scholar]
- Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]