Abstract
The regeneration and repair of cartilage damaged by injury or disease, a major goal of orthopaedic science, depends on understanding the structure and function of both the extracellular matrix and the chondrocytes. In this study, we explored the in situ organization and potential interactions between chondrocytes in the superficial zone of adult rabbit articular cartilage. Some chondrocytes in this zone were observed close together and appeared to be paired whereas others were solitary. The shared surfaces of a chondrocyte pair were separated by a narrow plate of extracellular matrix, into which extended small cytoplasmic projections from both cells. Furthermore, the spatial distribution of major cellular landmarks, such as the nucleus and centrosome as well as some intracellular proteins such as connexin-43, tended to be mirrored about this matrix plate. Fluorescence recovery after photobleaching revealed the fluorescent dye calcein–AM dye can pass between paired cells, and that the passage of this dye can be inhibited by the gap junction blocker octanol. These results illustrate that rapid cellular communication is possible between cells in the superficial layer of adult articular cartilage, which challenges the current thinking that these chondrocytes function in isolation.
Keywords: cell communication, chondrocyte, chondron, gap junction, hemichannel
Introduction
Articular cartilage is a specialized connective tissue that covers the ends of the bones in synovial joints, functions to transmit loads across joints and is essential for normal joint function. Adult cartilage is aneural and avascular and consists of a small number of cells (chondrocytes) that are responsible for the synthesis and maintenance of a large volume of extracellular matrix (Palfrey & Davies, 1966; Meachim & Stockwell, 1979; Ghadially, 1983; Buckwalter & Mankin, 1998). Morphologically, adult articular cartilage can be divided into four distinct zones: a superficial (or tangential) zone, a transitional (or intermediate) zone, a deep (or radial) zone and a calcified cartilage zone (Meachim & Stockwell, 1979; Ghadially, 1983). Each zone contains a distinct subpopulation of chondrocytes that differs in morphology, distribution within the matrix as well as metabolic activity (Palfrey & Davies, 1966; Meachim & Stockwell, 1979; Kuettner et al. 1982; Bayliss et al. 1983; Ghadially, 1983; Aydelotte & Kuettner 1988; Aydelotte et al. 1988). Chondrocytes typically range in shape from discoidal to spheroidal and are surrounded by an extracellular matrix rich in collagen proteins and in proteoglycans.
A chondrocyte, along with its surrounding pericellular matrix, is the basic functional unit of articular cartilage and is referred to as a chondron (Benninghoff, 1925; Poole, 1997). According to Poole (1997), a chondron consists of a chondrocyte linked at its surface to a transparent pericellular glycocalyx and enclosed by a fibrillar pericellular capsule. In canine articular cartilage, chondrons may contain one, two or multiple chondrocytes (often in columns) and typically exhibit some degree of polarity within the surrounding (interterritorial) extracellular matrix (Poole et al. 1988; Poole, 1997). It has been speculated that the chondron functions as a fluid-filled ‘bladder’ to absorb mechanical loads and provide hydrodynamic protection for the chondrocyte (Benninghoff, 1925; Szirmai, 1969). This presumption is supported by recent reports in which the mechanical properties of chondrons were found to be intermediate between the interterritorial matrix and the chondrocyte (Guilak et al. 1999; Knight et al. 2001).
Historically, the study of connective tissues has focused primarily on the extracellular matrix and only in the last decade has the chondrocyte received close scrutiny. In many connective tissues, including tendon, ligament, meniscus and bone, the cells are interconnected via cellular processes containing gap junctions to form a so-called ‘cytomatrix’ that may function to communicate and co-ordinate cellular responses to regional differences in mechanical or humoral stimuli (Oliani et al. 1995; Tanji et al. 1995; McNeilly et al. 1996; Lo et al. 2002a,b). In contrast, chondrocytes seem to have no physical linkages in vertebrates. Curiously, chondrocytes are known to express the gap junction protein connexin-43 in situ (Jones et al. 1993; Schwab et al. 1998), and have the capacity to form functional gap junctions in dense monolayer and micromass cultures (Donahue et al. 1995; D’Andrea & Vittur, 1996; Zhang et al. 2002). The movement of molecules between chondrocytes via gap junctions has been documented using microinjection (Donahue et al. 1995) and between other connective tissue cells by the technique of fluorescence recovery after photobleaching (FRAP) (Wade et al. 1986; Hunter et al. 2003).
The superficial zone of articular cartilage characteristically has one to several layers of flattened, disc-shaped cells, relatively low proteoglycan content and densely packed bundles of collagen fibres orientated parallel to the articulating surface. Studies of developing cartilage suggest that the spatial distribution of cells within the superficial zone is unequal throughout the matrix (Meachim & Stockwell, 1979; Ghadially, 1983; Schwab et al. 1998; Schumacher et al. 2002). That is, some cells are further apart and some are closer to one another, which gives the impression that some cells occur in pairs. Hence, the current study was undertaken to determine if chondrocytes are arranged in pairs in adult articular cartilage and if neighboring cells in this zone have the capacity to communicate in vivo. Here we demonstrate that paired cells are present in the superficial zone of adult rabbit articular cartilage and that these paired cells are functionally linked. Functionally linked cellular units may be important in maintaining the superficial zone of cartilage and possibly in co-ordinating the activities of cells in injured cartilage tissue.
Materials and methods
Thin shavings of articular cartilage (∼30–50 µm thick) were cut with a no. 15 scalpel blade from the surface of the femoral condyle of skeletally mature New Zealand white rabbits (n = 16). Tissue samples were collected and processed for immunohistochemistry, confocal microscopy and transmission electron microscopy as outlined below. Tissue shavings were prepared so that sections were orientated either perpendicular or parallel to the articular surface. Samples for immunohistochemistry were fixed immediately in cold methanol at −20 °C for 15 min; samples for electron microscopy were fixed in Karnovsky's fixative for a minimum of 24 h at 4 °C. Fresh shavings for FRAP analysis were maintained in calcium-free phosphate-buffered saline (PBS, Sigma, St. Louis, MO, USA) supplemented with 2 µm EGTA at room temperature.
Immunofluorescence histochemistry
A panel of antibodies was chosen to highlight a variety of subcellular landmarks and to explore their distribution in solitary and paired cells. Briefly, fixed tissue samples were rinsed in PBS (pH 7.1, 20 °C) and incubated en bloc (37 °C, 1 h) in a primary antibody directed against: (a) actin microfilaments (1 : 100, Boehringer Mannheim), (b) vimentin intermediate filaments (1 : 100, Boehringer Mannheim), (c) the microtubule subunit β-tubulin (1 : 100, Clone TUB2.1, Sigma), (d) detyrosinated tubulin (1 : 100, ID5, a kind gift of Dr Denys Wheatley, Aberdeen, UK), (e) gamma tubulin (1 : 100, Sigma), (f) centrosome (1 : 100, human auto-antibody M4491) (Mack et al. 1998) or (g) connexin-43 (1 : 100, Transduction Laboratories, Lexington, KY, USA). Following incubation in the primary antibody, all samples were rinsed three times in PBS and incubated in Cy™-3-conjugated secondary antibody (1 : 100, donkey anti-mouse IgG; Jackson ImmunoResearch, West Grove, PA, USA) for 30 min at 37 °C. Samples were mounted in 90% glycerol containing the anti-fade reagent N,N-paraphenylenediamine. Sections were studied by wide-field fluorescence microscopy (Zeiss Axiophot) and laser-scanning confocal microscopy (Zeiss LSM 510). Single confocal images and stacks of serial optical sections (∼30 µm thick) were made using a ×25 0.8 NA multi-immersion objective (Plan-Neofluor, Zeiss). Higher magnification images were obtained using a ×63, 1.2 NA water-immersion objective (C-Apochromat, Zeiss). The fluorescence intensity of cells was measured using the calibrated measuring tool in Image J software (v1.30, http://rsb.info.nih.gov/ij/). Digital deconvolution of wide-field and confocal image stacks was done using AutoDeblur software (v9.2, Autoquant Imaging Inc., Watervliet, NY, USA).
Electron microscopy
Tissues fixed in Karnovsky's were washed twice in 0.1 m cacodylate buffer containing 5 mm calcium and post-fixed for 1 h with 2% osmium tetraoxide in 0.1 m cacodylate buffer, dehydrated in ethanol and embedded in Spurr's resin (J.B. EM Services Ltd). Thin sections (approximately 90 nm) were cut with a diamond knife on a Reichert ultramicrotome. Sections were stained with uranyl acetate and lead citrate and examined on a Hitachi H-7000 TEM microscope.
Fluorescence recovery after photobleaching
Fresh cartilage shavings were incubated in Ca2+-free PBS containing the dye calcein-AM (5 µm, Molecular Probes, Eugene, OR, USA), which passes freely into the cells in its non-fluorescent form and upon esterase cleavage becomes captured inside the cells and is intensely fluorescent. Calcein-AM-stained shavings were washed three times in Ca2+-free PBS, placed on a coverslip, covered with a drop of 2% low-melting-point agarose (NuSieve®-GTG® agarose, FMC Bioproducts, Rockland, ME, USA) in Ca2+-free PBS (to immobilize the sample), and examined by confocal microscopy at room temperature. Although the configuration of our confocal microscope did not control temperature, previous studies have demonstrated functional gap junction activity at temperatures both lower and higher than 37 °C (Tadvalkar & Pinto da Silva, 1983; Spray & Bennett, 1985; Chen & DeHaan, 1993; Rozental et al. 1995; Stelling & Jacob, 1997; Srinivas et al. 2001). Some cartilage shavings were also prepared in Ca2+-free PBS medium containing the gap junction blocker 1-octanol at a concentration of 1.0 mm (Contreras et al. 2002; Hunter et al. 2003).
Chondrons within the cartilage shavings were located using transmitted light. The chondrons selected for FRAP analysis had a characteristic ‘walnut-in-the-shell’ morphology containing a pair of closely neighboring chondrocytes (Fig. 1). Prior to photobleaching, an initial image was obtained to define the baseline fluorescence of the cells. One chondrocyte within a chondron or solitary chondrocytes were chosen randomly and outlined exclusively as the region of interest. These cells were repeatedly photobleached with 40–60 pulses of an argon laser (operated at 488 nm and 6.25 mW) until the calcein fluorescence was clearly darkened but still visible [approximately 35% of the original fluorescence; further photobleaching seemed to affect viability adversely (data not shown)]. Three subsequent images of the same cell were acquired 5, 10 and 15 min after photobleaching. The fluorescence intensity of individual chondrocytes was evaluated as the mean pixel intensity in the manually defined region of interest directly over the cell using Image J software.
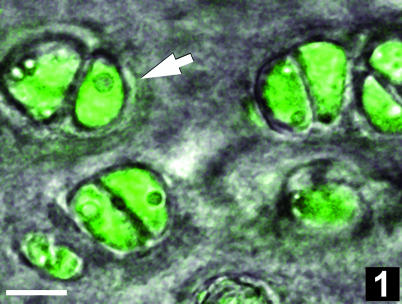
Fig. 1.
Fluorescence photomicrograph of a tangential section through the superficial zone of rabbit femoral condyle articular cartilage. Note the walnut-in-the-shell appearance of chondrocyte pairs: chondrocytes exhibit green fluorescence with the vital dye calcein-AM; the pericellular matrix delimiting the chondron (arrow) is visible in the transmitted light channel of the confocal microscope (composite fluorescence–differential interference contrast image). Scale bar, 10 µm.
As a small amount of photobleaching normally occurs when exposing a confocal photomicrograph, the fluorescence in the cells that were repeatedly photobleached was normalized to that of a ‘non-neighboring’ cell in the same image field (to account for the photographic exposure). The normalized fluorescence intensity values were then used to calculate the percentage recovery of fluorescence 15 min after photobleaching using the following formula:
where T0 is the initial fluorescence, Tb is the fluorescence after photobleaching and T15 is the fluorescence 15 min after recovery.
To verify that the FRAP technique achieved the desired results in situ, we reproduced previously published experiments in which isolated chondrocytes in monolayer culture exhibited intercellular communication and the inhibitory effects of octanol (Donahue et al. 1995). For these experiments, cartilage slices were obtained aseptically, minced and sequentially digested with 0.05% (w/v) hyaluronidase, 1% (w/v) Pronase® and 0.4% collagenase (Type II, Sigma) in HBSS at 37 °C (Kuettner et al. 1982). Isolated chondrocytes were seeded on coverslips and cultured in 24-well plates with DMEM/F-12 supplemented with 10% fetal bovine serum and 1% antibiotics/antimycotics at 37 °C with 5% CO2. Chondrocytes were stained with calcein-AM and examined by confocal microscopy either in Ca2+-free PBS or the same medium containing 1.0 mm octanol.
Measurement of intercellular distances
To define the mean distance separating chondrocytes in situ, the ‘edge-to-edge’ distances between paired or solitary cells and neighboring cells were measured. A ‘near neighbour’ was designated as any cell within a 50-µm radius of the selected cell to which a straight line could be drawn without passing through another cell. Intercellular distances were measured using the calibrated measuring tool in Image J software.
Results
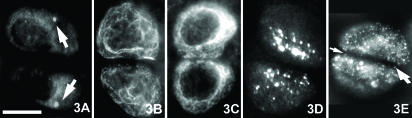
The apparent pairing of chondrocytes in the superficial zone of rabbit femoral condyle articular cartilage was more evident in sections cut tangential to the surface than in sections cut in the usual surface-to-deep orientation. Solitary or paired chondrocytes were observed within chondrons by fluorescence and electron microscopy (Figs 1 and 2). In all cartilage samples, neither the chondrons nor the chondrocytes were evenly distributed within the interterritorial matrix and the spatial distribution of chondrons and cells in the superficial zone was inhomogeneous from the middle to the edge of the joint surface. At the light microscopy level, the minimal spacing among solitary chondrocytes and their nearest neighbours averaged 14.1 ± 6.3 µm (n = 100); the minimal spacing between paired cells within a chondron averaged 2.4 ± 1.0 µm (n = 100). Optical confocal image stacks confirmed that some solitary cells had no obvious partners in any dimension, whereas others had near neighbours in a different optical plane and were not truly solitary. In many (though not all) chondrocyte pairs, the thin plate of extracellular matrix between the cells formed a plane about which was mirrored the nucleus, centrosome, primary cilum (as detected by detyrosinated tubulin staining) and the constellation of connexin-43-staining foci (Figs 2 and 3). The cytoskeletal elements beta-tubulin (Fig. 3C), actin and vimentin were distributed throughout the cytoplasm without any clear symmetry about the matrix plate.
Fig. 2.
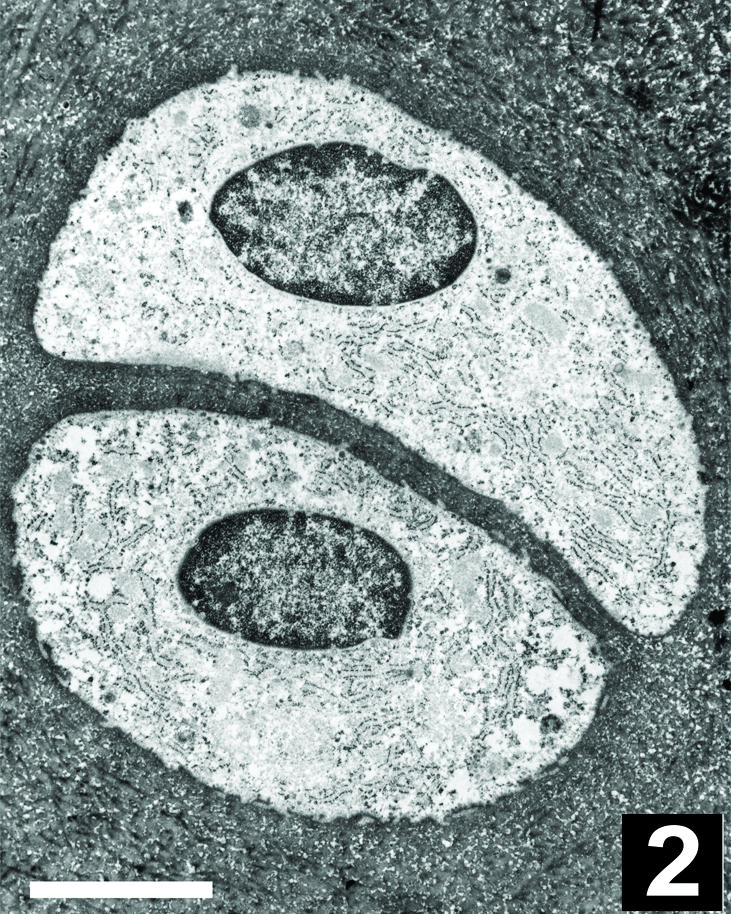
Electron micrograph of a chondrocyte pair similar to that shown in Fig. 1. Note the two chondrocytes within a chondron are surrounded by finely woven fibres of extracellular matrix with a thin plate of matrix separating their apposing surfaces. Scale bar, 5 µm.
Fig. 3.
Fluorescent photomicrographs of chondrocyte pairs immunostained for: (A) centrosome, (B) primarily cilia (detyrosinated tubulin), (C) beta tubulin and (D) the gap junction protein connexin-43. Note the mirrored arrangement of centrosome and connexin-43 foci in paired chondrocytes. (E) Digitally deconvolved, maximum intensity projection image of connexin-43 staining foci. Note the presence of small foci of connexin-43 staining in the septal plate (arrows) between paired chondrocytes. Scale bar, 12 µm.
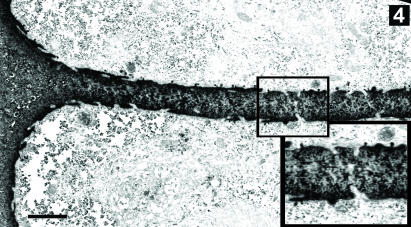
At the electron microscopy level, the structure of the chondron was clearly visible: a finely woven pericellular matrix surrounded and separated adjacent cells (Figs 2 and 4). Centrosomes and primary cilia were observed in electron micrographs of both solitary and paired chondrocytes. The matrix plate between the apposing surfaces of paired cells measured a few micrometres (∼1–5 µm) in thickness. At high magnification, however, numerous small cellular projections extended from the apposing surfaces of adjacent cells and formed an interwoven meshwork (Fig. 4 inset). Owing to their convoluted paths, it was difficult to follow individual cell projections for long distances. In some cases, the cell projections of apposing cells came within 20 nm of one another, yet no direct contact or surface specializations such as gap junctions were observed between paired chondrocytes.
Fig. 4.
Electron micrograph of the apposing surfaces of a chondrocyte pair. Note the small cytoplasmic projections extending from all surfaces of the chondrocytes (arrows), and the close proximity of the processes in the thin plate of matrix separating the apposing surfaces (inset). Scale bar, 3 µm. Inset magnification doubled.
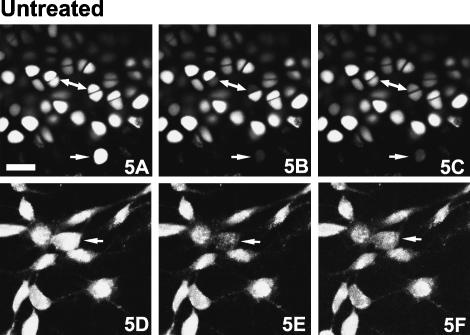
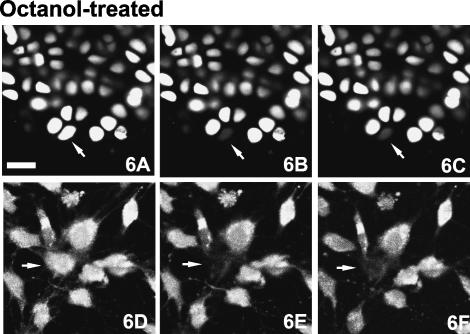
As the convoluted arrangement of the cell projections might obscure structures involved in intercellular communication, we studied the distribution of the gap junction protein connexin-43 by immunofluorescence. Figure 3(D) illustrates that this protein is synthesized by both cells of a chondrocyte pair, and that this protein is preferentially distributed towards the shared surface of these cells. By contrast, solitary chondrocytes expressed connexin-43 evenly throughout the cell (data not shown). Digital deconvolution facilitated three-dimensional inspection of the distribution of connexin-43, a few foci of which were visible within the septal plate (Fig. 3E). The close apposition of the paired cells and the presence of the gap junction protein connexin-43 suggested that these chondrocytes might communicate through the exchange of material, possibly through the presence of gap junctions or hemichannels. To test this hypothesis, we stained cartilage samples with calcein-AM and performed FRAP using the confocal microscope. Two-cell chondrons were identified in situ, the calcein-AM fluorescence of one cell was photobleached and the fluorescence recovery was monitored for over a 15-min interval (Fig. 5A–C). A similar photobleaching experiment was performed with solitary chondrocytes in situ and with chondrocytes grown in monolayer culture (Fig. 5D–F) where gap junctions are known to exist. Fluorescence recovery was observed consistently in chondrocytes in monolayer culture (∼30% recovery) and in paired cells in situ (∼25% recovery), but not in solitary cells in situ (Table 1). When chondrocytes in situ or in vitro were exposed to octanol, an agent that disrupts gap junction activity, fluorescence recovery was minimal both in situ and in monolayer culture (Fig. 6, Table 1). These results confirm that intercellular communication is not restricted to cultured chondrocytes and can occur between paired chondrocytes, but not between solitary chondrocytes and other cells, in situ.
Fig. 5.
Fluorescence recovery after photobleaching (FRAP) experiments. Confocal photomicrographs of chondrocytes in situ (A–C) and chondrocytes in monolayer culture (D–F). Time-series showing fluorescence just before (A and D – baseline), just after (B and E), and 15 min after intense photobleaching (C and E). Note the bleaching and fluorescence recovery of two chondrocytes in a two-cell chondron (double-ended arrow) and the lack of recovery of a solitary chondrocyte (single-headed arrow). Calcein-AM fluorescence. Scale bar, 25 µm.
Table 1.
Percentage recovery of fluorescence after photobleaching
| In situ pairs | In situ pairs octanol | In situ solitary | Monolayer cells | Monolayer cells octanol | |
|---|---|---|---|---|---|
| Recovery (%)* | 26.4 ± 1.4 | 1.5 ± 0.8 | 7.7 ± 0.5 | 29.2 ± 1.1 | 1.3 ± 0.7 |
Fluorescence intensity values normalized before calculation (see Methods).
Mean ± SE (n = 9, 7, 6, 9 and 9).
Fig. 6.
Flourescence recovery after photobleaching (FRAP) experiments in the presence of the gap-junction-disrupting agent octanol. Time-series experiments (as in Fig. 5) of chondrocytes in situ (A–C), and in monolayer culture (D–F). Confocal photomicrographs just before (A and D – baseline), just after (B and E), and 15 min after intense photobleaching (C and E). Scale bar, 25 µm.
Discussion
Historically, the cells within connective tissues, perhaps due to their relatively low numbers, have merited less attention than the abundant extracellular matrix. In many connective tissues, the cells were once thought to exist as isolated islands within the sea of matrix. Recently it has become clear that this is not the case in tissues such as ligaments, tendons, menisci and certain regions of the intervertebral disc. In these tissues, the cells are interconnected via gap junctions to form a ‘cytomatrix’ (Bruehlmann et al. 2002; Lo et al. 2002a,b). It has been suggested that this cytomatrix serves to link all the cells of the tissue together and thereby co-ordinate their activities, including their response to environmental cues such as mechanical loads. The major finding of the present study is that at least some chondrocytes in adult articular cartilage of the rabbit are also linked together. Although this linkage may only be confined to paired cells in the superficial zone, communication between these chondrocytes may help co-ordinate their activity and their responses to mechanical or humoral cues.
The arrangement of chondrocytes in pairs in articular cartilage has been documented previously, though primarily in immature and developing cartilage (Ghadially, 1983; Clark & Rudd, 1991). In adult human articular cartilage, chondrocyte pairing and clustering have been reported to varying degrees in the knee and ankle (Schumacher et al. 2002). A possible explanation for the presence of chondrocyte pairs in adult cartilage is that pairs form during development, possibly after a complete division of a cell and the incomplete division of the chondron microenvironment, and simply persist into adulthood. Alternatively, the clustering of chondrocytes in injured cartilage and in cartilage diseases such as osteoarthritis (Ghadially, 1983) suggest that adult chondrocytes might be able to organize themselves into pairs or groups after development, possibly by recapitulating the events of development (Morrison et al. 2000). Regardless of how the pairing develops, it is clear that the microenvironment moderates the metabolism of the chondrocytes (e.g. Hing et al. 2002), and hence increasing the number of cells in a chondron undoubtedly increases the biological complexity of these units.
Several features of the paired cells observed in the present study are worthy of discussion. First, the cytoarchitecture of paired cells in a chondron is often mirrored. Such an organization may be required for the establishment and maintenance of the shared surface, which may involve shuttling cellular constituents to and from the shared surface. Second, the FRAP studies clearly indicate that pairs of cells are functionally interconnected in situ. Whereas the exact nature of this in situ interconnection remains unclear, the concentration of connexin-43 at the paired surface and the octanol blockade provides strong evidence in support of the presence of functional gap junctions or hemichannels (Donahue et al. 1995; Li et al. 1996; Contreras et al. 2002; Hunter et al. 2003). The lack of direct ultrastructural evidence for gap junctions may be due to their relatively low abundance, their transient and dynamic nature (Laird, 1996), the complex and convoluted arrangement of the cell projections, or a fixation artefact. As we have been able to demonstrate gap junctions in a variety of dense connective tissues using Karnovsky's fixative and electron microscopy (data not shown), it seems unlikely that an artefact of fixation explains our inability to verify the presence of full gap junctions. Alternatively, gap junctions may not need to form completely to be functional: hemichannels (Li et al. 1996; Goodenough & Paul, 2003) on the apposing surfaces may open transiently and allow molecules to flow between cell pairs. Although this study has focused on chondrocyte pairs separated by only nanometres at the electron microscopic level, the average edge-to-edge distances measured by confocal microscopy were greater. This is undoubtedly due to the greater resolving power of the electron microscope, which can readily visualize small structures such as the cell processes. As the chances are poor of cutting a thin section through two ellipsoidal bodies where they come into intimate contact, our inability to identify a gap junction by electron microscopy may be due to insufficient sampling. Alternatively, the larger near-neighbour distances between paired cells measured by light microscopy might reflect real biological variations in cell spacing (possibly even movement) that depend on the functional or metabolic state of the cells, chondrons or tissue.
The identification of paired chondrocytes capable of intercellular communication raises several intriguing questions. For example, (1) do paired cells within a chondron differ in their biosynthetic activities, (2) do paired cells function synergistically in sensing and responding to the environment, and (3) do paired and solitary cells have similar or dissimilar functions in the tissue? While the answers to these questions warrant further investigation, the present study establishes that, like other connective tissues, at least some cells within adult articular cartilage have the capacity to communicate directly and thus function together. These results imply that not all chondrocytes function in isolation, which challenges the prevailing thinking regarding adult articular chondrocytes.
Acknowledgments
J.R.M. is an Investigator of the Arthritis Society and the Canadian Arthritis Network; J.R.M. and J.B.R. receive support from the National Science and Engineering Research Council of Canada (NSERC) and the Canadian Institute of Health Research (CIHR). We gratefully acknowledge Dr Donald Welsh for his valuable input regarding gap junctions, and Dr Sabina Breuhlmann for critically reading the manuscript.
References
- Aydelotte MB, Greenhill RR, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. II. Proteoglycan metabolism. Connect. Tissue Res. 1988;18:223–234. doi: 10.3109/03008208809016809. [DOI] [PubMed] [Google Scholar]
- Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect. Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- Bayliss MT, Venn M, Maroudas A, Ali SY. Structure of proteoglycans from different layers of human articular cartilage. Biochem. J. 1983;209:387–400. doi: 10.1042/bj2090387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninghoff A. Form und Bau der Gelenkknorpel in Ihren Bezeihungen zur Funktion Zweiter Teil. Der Aufbau des Gelenkknorpels in seinen Bezeihungen zur Funktion. Z. Zellforchung. 1925;2:783–862. [Google Scholar]
- Bruehlmann SB, Rattner JB, Matyas JR, Duncan NA. Regional variations in the cellular matrix of the annulus fibrosus of the intervertebral disc. J. Anat. 2002;201:159–171. doi: 10.1046/j.1469-7580.2002.00080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter JA, Mankin HJ. Articular Cartilage: Tissue Design and Chondrocyte–Matrix Interactions. 1998. pp. 477–486. Instructional Course Lecture 47, AAOS Iowa US. [PubMed] [Google Scholar]
- Chen YH, DeHaan RL. Temperature dependence of embryonic cardiac gap-junction conductance and channel kinetics. J. Membr. Biol. 1993;136:125–134. doi: 10.1007/BF02505757. [DOI] [PubMed] [Google Scholar]
- Clark JM, Rudd E. Cell patterns in the surface of rabbit articular cartilage revealed by the backscatter mode of scanning electron microscopy. J. Orthop. Res. 1991;9:275–283. doi: 10.1002/jor.1100090216. [DOI] [PubMed] [Google Scholar]
- Contreras JE, Sanchez HA, Eugenin EA, et al. Metabolic inhibition induces opening of unapposed connexin-43 gap-junction hemichannels and reduces gap-junctional communication in cortical astrocytes in culture. Proc. Natl Acad. Sci. USA. 2002;99:495–500. doi: 10.1073/pnas.012589799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Andrea P, Vittur F. Gap junctions mediate intercellular calcium signalling in cultured articular chondrocytes. Cell Calcium. 1996;20:389–397. doi: 10.1016/s0143-4160(96)90001-9. [DOI] [PubMed] [Google Scholar]
- Donahue HJ, Guilak F, Vander Molen MA, et al. Chondrocytes isolated from mature articular cartilage retain the capacity to form functional gap-junctions. J. Bone Miner. Res. 1995;10:1359–1364. doi: 10.1002/jbmr.5650100913. [DOI] [PubMed] [Google Scholar]
- Ghadially FN. Fine Structure of Synovial Joints: a Text and Atlas of the Ultrastructure of Normal and Pathological Articular Tissues. London: Butterworths; 1983. [Google Scholar]
- Goodenough DA, Paul DL. Beyond the gap: functions of unpaired connexon channels. Nat. Rev. Mol. Cell Biol. 2003;4:285–294. doi: 10.1038/nrm1072. [DOI] [PubMed] [Google Scholar]
- Guilak F, Jones WR, Ting-Beall HP, Lee GM. The deformation behavior and mechanical properties of chondrocytes in articular cartilage. Osteoarthritis Cartilage. 1999;7:59–70. doi: 10.1053/joca.1998.0162. [DOI] [PubMed] [Google Scholar]
- Hing WA, Sherwin AF, Poole CA. The influence of the pericellular microenvironment on the chondrocyte response to osmotic challenge. Osteoarthritis Cartilage. 2002;10:297–307. doi: 10.1053/joca.2002.0517. [DOI] [PubMed] [Google Scholar]
- Hunter CJ, Matyas JR, Duncan NA. The three-dimensional architecture of the notochordal nucleus pulposus: novel observations on cell structures in the canine intervertebral disc. J. Anat. 2003;202:279–291. doi: 10.1046/j.1469-7580.2003.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SJ, Gray C, Sakamaki H, et al. The incidence and size of gap junctions between the bone cells in rat calvaria. Anat. Embryol. 1993;187:343–352. doi: 10.1007/BF00185892. [DOI] [PubMed] [Google Scholar]
- Knight MM, Ross JM, Sherwin AF, Lee DA, Bader DL, Poole CA. Chondrocyte deformation within mechanically and enzymatically extracted chondrons compressed in agarose. Biochim. Biophys. Acta. 2001;1526:141–146. doi: 10.1016/s0304-4165(01)00118-0. [DOI] [PubMed] [Google Scholar]
- Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J. Cell Biol. 1982;93:743–750. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird DW. The life cycle of a connexin: gap-junction formation, removal, and degradation. J. Bioenerg. Biomembr. 1996;28:311–318. doi: 10.1007/BF02110107. [DOI] [PubMed] [Google Scholar]
- Li H, Liu TF, Lazrak A, et al. Properties and regulation of gap-junctional hemichannels in the plasma membranes of cultured cells. J. Cell Biol. 1996;134:1019–1030. doi: 10.1083/jcb.134.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo IK, Chi S, Ivie T, Frank CB, Rattner JB. The cellular matrix: a feature of tensile bearing dense soft connective tissues. Histol. Histopathol. 2002a;17:523–537. doi: 10.14670/HH-17.523. [DOI] [PubMed] [Google Scholar]
- Lo IK, Ou Y, Rattner JP, et al. The cellular networks of normal ovine medial collateral and anterior cruciate ligaments are not accurately recapitulated in scar tissue. J. Anat. 2002b;200:283–296. doi: 10.1046/j.1469-7580.2002.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack GJ, Rees J, Sandblom O, Balczon R, Fritzler MJ, Rattner JB. Autoantibodies to a group of centrosomal proteins in human autoimmune sera reactive with the centrosome. Arthritis Rheum. 1998;41:551–558. doi: 10.1002/1529-0131(199803)41:3<551::AID-ART22>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- McNeilly CM, Banes AJ, Benjamin M, Ralphs JR. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J. Anat. 1996;189:593–600. [PMC free article] [PubMed] [Google Scholar]
- Meachim G, Stockwell RA. The matrix. In: Freeman MAR, editor. Adult Articular Cartilage. 2. London: Pitman Medical; 1979. pp. 1–67. [Google Scholar]
- Morrison SL, Campbell CK, Wright GM. Chondrogenesis of the branchial skeleton in embryonic sea lamprey, Petromyzon marinus. Anat. Rec. 2000;260:252–267. doi: 10.1002/1097-0185(20001101)260:3<252::AID-AR50>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Oliani SM, Girol AP, Smith RL. Gap junctions between mast cells and fibroblasts in the developing avian eye. Acta Anat. (Basel) 1995;154:267–271. doi: 10.1159/000147778. [DOI] [PubMed] [Google Scholar]
- Palfrey AJ, Davies DV. The fine structure of chondrocytes. J. Anat. 1966;100:213–226. [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW. Chondrons extracted from canine tibial cartilage: preliminary report on their isolation and structure. J. Orthop. Res. 1988;6:408–419. doi: 10.1002/jor.1100060312. [DOI] [PubMed] [Google Scholar]
- Poole CA. Articular cartilage chondrons: form, function and failure. J. Anat. 1997;191:1–13. doi: 10.1046/j.1469-7580.1997.19110001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozental R, Mehler MF, Morales M, Andrade-Rozental AF, Kessler JA, Spray DC. Differentiation of hippocampal progenitor cells in vitro: temporal expression of intercellular coupling and voltage- and ligand-gated responses. Dev. Biol. 1995;167:350–362. doi: 10.1006/dbio.1995.1029. [DOI] [PubMed] [Google Scholar]
- Schumacher BL, Su JL, Lindley KM, Kuettner KE, Cole AA. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat. Rec. 2002;266:241–248. doi: 10.1002/ar.10063. [DOI] [PubMed] [Google Scholar]
- Schwab W, Hofer A, Kasper M. Immunohistochemical distribution of connexin-43 in the cartilage of rats and mice. Histochem. J. 1998;30:413–419. doi: 10.1023/a:1003220225670. [DOI] [PubMed] [Google Scholar]
- Spray DC, Bennett MV. Physiology and pharmacology of gap-junctions. Annu. Rev. Physiol. 1985;47:281–303. doi: 10.1146/annurev.ph.47.030185.001433. [DOI] [PubMed] [Google Scholar]
- Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap-junction channel subtypes. Proc. Natl Acad. Sci. USA. 2001;98:10942–10947. doi: 10.1073/pnas.191206198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelling JW, Jacob TJ. Functional coupling in bovine ciliary epithelial cells is modulated by carbachol. Am. J. Physiol. 1997;273:C1876–C1881. doi: 10.1152/ajpcell.1997.273.6.C1876. [DOI] [PubMed] [Google Scholar]
- Szirmai J. Structure of cartilage. In: Engel ATL, editor. Ageing of Connective and Skeletal Tissue. Stockholm: Nordiska Bokhendelns; 1969. pp. 163–184. [Google Scholar]
- Tadvalkar G, Pinto da Silva P. In vitro, rapid assembly of gap-junctions is induced by cytoskeleton disruptors. J. Cell Biol. 1983;96:1279–1287. doi: 10.1083/jcb.96.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji K, Shimizu T, Satou T, Hashimoto S, Bonilla E. Gap junctions between fibroblasts in rat myotendon. Arch. Histol. Cytol. 1995;58:97–102. doi: 10.1679/aohc.58.97. [DOI] [PubMed] [Google Scholar]
- Wade MH, Trosko JE, Schindler M. A fluorescence photobleaching assay of gap-junction-mediated communication between human cells. Science. 1986;232:525–528. doi: 10.1126/science.3961495. [DOI] [PubMed] [Google Scholar]
- Zhang W, Green C, Stott NS. Bone morphogenetic protein-2 modulation of chondrogenic differentiation in vitro involves gap junction-mediated intercellular communication. J. Cell Physiol. 2002;193:233–243. doi: 10.1002/jcp.10168. [DOI] [PubMed] [Google Scholar]