Abstract
Muscles have two major roles in locomotion: to generate force and to absorb/generate power (do work). Economical force generation is achieved by short-fibred pennate muscle while the maximum power output of a muscle is architecture independent. In this study we tested the hypothesis that there is an anatomical and structural separation between the force-generating anti-gravity muscles and the propulsive (limb/trunk moving) muscles of the equine forelimb. Muscle mass and fascicle length measurements were made on the thoracic limb extrinsic muscles of six fresh horse cadavers. Physiological cross-sectional area and maximum isometric force were then estimated. Maximum power was estimated from muscle volume and published contraction velocity data. The majority of extrinsic forelimb muscles were large with long fascicles arranged in parallel to the long axis of the muscle. Muscles arranged in this way are optimised for doing work. The architecture of serratus ventralis thoracis (SVT) was unique. It had short (48 ± 17 mm) fascicles, arranged at about 45° to the long axis of the muscle, which would suggest a force-generating, anti-gravity role. The muscle belly of SVT was sandwiched between two broad, thick sheets of aponeurosis. Hence, SVT could make a significant contribution to the overall elastic properties of the thoracic limb.
Keywords: architecture, force, forelimb, horse, power
Introduction
The equine thoracic limb functions as a passive spring system. Distal thoracic limb muscles generally have short-fibres and long tendons (Hermanson & Cobb, 1992; Hermanson, 1997; Brown et al. 2003a), which are stretched at the beginning of stance, storing elastic strain energy and then releasing it later in stance (Alexander & Bennet-Clark, 1977). Much of the length change required for the work of locomotion occurs not in the muscles fibres themselves but by elastic recoil of the associated tendon and muscle aponeurosis. The force–length properties are thus predominantly passive (and hence fixed, Monti et al. 2003) and the muscle–tendon unit acts as the spring in a spring–mass system (Farley et al. 1993; Lindstedt et al. 2002). Tendon springs are particularly important in cursorial locomotion as they facilitate the exchange of kinetic, potential and elastic strain energy and reduce the amount of work that muscles must perform in order to move an animal' limbs and centre of mass (COM) (Alexander, 2002).
In contrast to steady-state locomotion, activities such as acceleration, running uphill and jumping require mechanical work in order to achieve increases in the kinetic and/or potential energy of the whole animal (Mero & Komi, 1986; Roberts et al. 1998). The maximum power output of a muscle is approximately one-tenth of the product of Fmax (maximum isometric force generation capacity) and Vmax (maximum contraction velocity) and occurs at approximately 35% of Vmax (Woledge et al. 1985). Fmax is proportional to the physiological cross-sectional area of the muscle (PCSA) and maximum isometric stress is similar for all vertebrate skeletal muscle (0.2–0.3 MPa, see Wells, 1965). Maximum contraction velocity, however, can vary widely between species (see Woledge et al. 1985 for Vmax in a range of different species) and in different fibre types (Curtin & Woledge, 1988; Lou et al. 2002). Vmax for intact horse muscle has not yet been published but ‘skinned’ fibre experiments reveal that Vmax ranges from 0.33 Lo s−1 (Lo = muscle fibre resting length) in type I fibres, to 1.33 Lo s−1 in type IIA fibres, and to 3.2 Lo s−1 in type IIB fibres (equine soleus muscle at 15 °C, Rome et al. 1990). This would indicate a higher rate of shortening at physiological temperatures, as a 10 °C rise in temperature can increase Vmax by 2 about times (Gasser & Hill, 1924). Horse muscle contains a mixture of fibre types but they are predominantly type IIA (Snow, 1983). Thus, Vmax for a mixed-fibre muscle would be close to 1.33 Lo s−1 (at 15 °C), which would scale to approximately 5 Lo s−1 at 37 °C. Because the maximum power output attainable by a muscle is a direct function of the number of cross bridges active within the muscle, it is directly proportional to muscle volume or mass (Biewener & Roberts, 2000) and hence larger muscles (with a high proportion of type IIB fibres) will have an increased capacity for powerful contraction.
Muscle role changes under different locomotor conditions. For example, in turkeys running on level ground, gastrocnemius has been found to contract almost isometrically during the stance phase (generating high forces), but perform work (shortened) when running on inclines (Roberts et al. 1997). There is greater scope for specialization of limb muscles in quadrupeds because the front and hind legs can serve different roles in locomotion. In horses, and most other mammalian quadrupeds, 57% of the vertical impulse is applied through the thoracic limbs, and only 43% through the hindlimbs (Merkens et al. 1993; Witte et al. 2004) yet the majority of the locomotor muscle mass resides in the hindlimb (Buchner et al. 1997; Kearns et al. 2002). It would therefore appear that the thoracic limbs are optimized for support and the hindlimbs for propulsion, a ‘rear-wheel drive’ system (Merkens & Schamhardt, 1988; Merkens et al. 1993).
In small, non-cursorial mammals, the limb and trunk communicate via the sterno-clavicular, acromio- clavicular and coraco-clavicular articulations. Although the clavicle constrains motion of the limb, the scapula undergoes a large amount of rotation (retraction) during gait, accounting for more than 50% of step length (see Fischer et al. 2002). Horses do not have clavicles; their thoracic limb is attached to the trunk via a synsarcosis, i.e. there is only muscle attachment with no bony articulation. This arrangement is of particular value as it frees the motion of the limb on the trunk (the scapula slides along the rib cage), increasing effective limb length. Long legs are beneficial as they permit animals to use slower steps and thus use slower, more economical muscle fibres to achieve the same speed as an animal with shorter legs. There are substantial muscle groups whose role it is to attach the thoracic limb to the trunk; the extrinsic muscles (Fig. 1). The total ground reaction force (GRF) is transferred via the extrinsic muscles to the trunk with no scaling due to moment arms at joints. The extrinsic muscles must therefore not only be able to withstand the GRF but also move the limb and perform/absorb work during locomotion. The role of individual extrinsic muscles has been estimated from their anatomical position (see, for example, Dyce et al. 1996) but there are no published data on their internal architecture or volume. These data are required in order to determine their capacity to fulfil specific locomotor roles. From existing data on the specialization of proximal and distal limb muscle architecture in other mammals (Alexander, 1974; Gillis & Biewener, 2001; Daley & Biewener, 2003; Biewener et al. 2004), we hypothesize that the extrinsic muscles will be large and fusiform with a high muscle-to-tendon ratio.
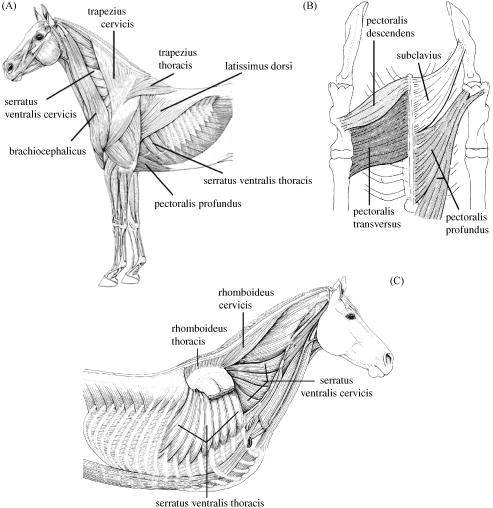
Fig. 1.
(A) Lateral view of extrinsic thoracic limb muscle anatomy. (B) Ventral view of extrinsic thoracic limb muscle anatomy. Deep view of extrinsic thoracic limb muscle anatomy (Figures adapted from König & Liebich, 2004).
Internal (muscle–tendon) forces can be investigated in vivo using surgically implanted strain gauges or force transducers (Riemersma et al. 1988; van Weeren et al. 1992). However, many muscles, particularly those of the proximal limb, do not have sufficient tendon in which to implant a strain gauge. There are also obvious ethical issues associated with experimenting on live animals and hence the method has been used in relatively few studies (e.g. Biewener et al. 2004). Alternatively, internal forces can be investigated non-invasively using either inverse or forwards dynamics to determine individual muscle forces or joint/segment motion, respectively. This is often done in conjunction with using computer-based models.
Existing models of the equine thoracic limb have focused on the distal section (Bartel et al. 1978; van den Bogert et al. 1989; Meershoek et al. 2001; Wilson et al. 2001, 2003; Brown et al. 2003b). This is partly because the distal limb is where most athletic injuries are known to occur but also because the computational methods become increasingly more complex as you move up the limb; as the number of muscles and joints increases it is more difficult to determine individual muscle forces. Extrinsic muscles are, however, important in deploying and modulating the mechanics of the predominantly passive thoracic limb and hence are also likely to be important in the prevention of injury. To our knowledge, none of the existing equine thoracic limb models has included data on muscles proximal to the elbow joint (with the exception of biceps brachii, Wilson et al. 2003).
In this study we set out to determine the volume and architecture of the extrinisic muscles of the equine thoracic limb. These data can then be used to make estimates of capacity for force development and maximum power output. Our ultimate aim is to generate new data to enhance existing models of the equine thoracic limb and to construct new, whole-animal models of equine locomotion.
Materials and methods
A thoracic limb extrinsic muscle dissection was performed on six fresh horse cadavers euthanased for reasons unrelated to musculo-skeletal pathology and obtained from the Royal Veterinary College, UK. Body mass, height (from dorsal thoracic vertebral spines to ground) and breed of each cadaver was obtained from hospital records (Table 1). Extrinsic muscles were dissected with the cadaver in a recumbent position. In order to gain access to the extrinsic thoracic limb muscles, the following intrinsic muscles were removed and discarded: triceps brachii, deltoideus, brachialis, biceps brachii and extensor carpi radialis. The extrinsic muscles (inferred primary biomechanical function in parentheses) were identified as shown in Table 2.
Table 1.
Subject data
| Horse | Age (years) | Mass (kg) | Height (cm) | Breed |
|---|---|---|---|---|
| A | 33.0 | 400 | 152 | WC |
| B | 24.0 | 450 | 154 | TBX |
| C | 1.0 | 470 | – | TBX |
| D | 18.0 | 500 | – | TB |
| E | 1.0 | 270 | – | TB |
| F | 6.0 | 600 | 162 | SF |
| G | 6.0 | 600 | 160 | TB |
WC, Welsh Cob; TBX, Thoroughbred Cross; TB, Thoroughbred; SF, Selle Francais. Height was not available for subjects C, D and E.
Table 2.
Equine thoracic limb extrinsic muscles and their primary biomechanical functions as reported in Sisson & Grossman (1975), Nickel et al. (1986) and Dyce et al. (1996)
| Muscle | Origin | Insertion | Function |
|---|---|---|---|
| Trapezius cervicis | Ligamentum nuchae from C2 to T10/11 | Spine of scapula | Scapular elevation and limb protraction |
| Trapezius thoracis | Ligamentum nuchae from C2 to T10/11 | Spine of scapula | Scapular elevation and limb retraction |
| Rhomboideus cervicis | From C2 to T7/8 | Medial aspect of scapula cartilage | Scapular elevation and limb protraction |
| Rhomboideus thoracis | From C2 to T7/8 | Medial aspect of scapula cartilage | Scapular elevation and limb retraction |
| Latissimus dorsi | From supraspinous ligament via a broad aponeurosis stretching from T3/4 to L5 and from the thoraco-lumbar fascia | Medial aspect of teres tubercle with tensor fascia antibrachii and teres major | Limb retraction, when limb fixed advances trunk |
| Omotransversarius | Fascia of lateral shoulder region | By separate digitations onto transverse processes of C2–4 | Limb protraction and lateral flexion of neck |
| Brachiocephalicus cranial and caudal | Mastoid process and transverse processes of varying proximal cervical vertebrae | Deltoid tuberosity between biceps brachii and brachialis | Limb protraction and lateral flexion of neck towards active side |
| Subclavius | Lateral aspect of sternum from costal cartilages 1–4 | Lateral tuberosity of humerus | Adduction and retraction of limb |
| Pectoralis profundus | Lateral sternum, cartilages and distal extremities of ribs 4/5–9 | Medial tuberosity of humerous & insertion of biceps brachii. Some fibres fuse with the tendon sheet of coracobrachialis and supraspinatus then pass onto lateral tuberosity of humerus | Adduction and retraction of limb |
| Pectoralis transversus | Ventral aspect of sternum between 1st and 6th costal cartilages | Antibrachial fascia | Adduction and protraction of the limb |
| Pectoralis descendens | Lateral aspect of manubrium sternum | Brachial fascia and crest of humerus with brachiocephalicus | Adduction and protraction of the limb |
| Serratus ventralis cervicis | Transverse processes of C3/4–C7 | Deep surface of scapula and cranial aspect of scapula cartilage | Suspends limb from trunk, raises thorax, retraction of limb via scapular rotation |
| Serratus ventralis thoracis | Middle section of ribs 1–8/9 | Deep surface of scapula and caudal aspect of scapula cartilage | Suspends limb from trunk, raises thorax, limb protraction via scapular rotation |
Each muscle was exposed and cleared of fascia. An incision was made along the muscle belly at 90° to the internal tendon or (if no internal tendon) from tendon of origin to tendon of insertion. Muscle fascicles were thus revealed and their lengths recorded (from random areas and depths across the belly) to the nearest millimetre using a flexible tape measure. The minimum number of measurement sites across a muscle was 10, and the maximum 20 (for larger muscles). Finally, the tendon of origin and insertion were removed so that muscle belly mass could be recorded (to the nearest gram) using a set of electronic scales (EKS®). Heavier muscles (> 2000 g) were weighed in several segments. Muscle mass and fascicle length data were then scaled assuming geometric similarity. In geometrically similar animals muscle mass scales as a fraction of body mass and fascicle length scales as a fraction of (body mass)1/3.
Muscle volume was determined by dividing muscle mass by muscle density (1.06 g cm−3, Mendez & Keys, 1960). The contribution of the non-contractile tissue to muscle volume was not taken into account (see Ker et al. 1988). PCSA was estimated by dividing muscle volume by muscle fascicle length. Serratus ventralis thoracis (SVT) was the only extrinsic muscle with pennate fascicles. Pennation angle was not taken into account as this is highly variable across the muscle and is also dependent on the length of the muscle (i.e. how the cadaver was placed for storage).
Maximum isometric force generation capacity of each muscle was estimated by multiplying muscle PCSA by the maximum isometric stress of skeletal muscle (taken as 0.3 MPa, Woledge et al. 1985). The maximum power output of each muscle was calculated as one-tenth of the product of Fmax (as estimated above) and Vmax (5 Lo s−1, see Introduction).
Results
Mean muscle data are provided in Table 3. The data from subject E were not used to calculate means as its body mass was approximately half that of the other subjects. It was not possible to separate brachiocephalicus and omotransversarius at their origin and thus the muscles were considered as one (BO). In all subjects brachiocephalicus had several smaller cranial heads originating from the spines of the cervical vertebrae that it passed over. The fascicle lengths of each of the heads were noted and included in the calculation of mean fascicle length for the whole muscle. Owing to damage inflicted during the post-mortem of subject B, no measurements were taken for SVT, serratus ventralis cervicis (SVC) or rhomboideus; however, all other muscles were intact and were similar in mass and fascicle length to the same muscles in the other subjects.
Table 3.
Muscle data
| Function | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abduction/adduction | Protraction | Retraction | Anti- gravity | Mass (g) | Volume (cm3) | FL (mm) | Range (mm) | PCSA (cm2) | Force (N) | Power (W) | |
| Pectoralis transversus | +++ | + | – | – | 1541 | 1434 | 200 | 115–280 | 77 | 2310 | 231 |
| Pectoralis descendens | |||||||||||
| Pectoralis profundus | +++ | – | + | – | 2837 | 2649 | 461 | 240–630 | 60 | 1800 | 415 |
| Serratus ventralis cervicis | – | – | + | + | 2101 | 1954 | 292 | 170–470 | 72 | 2160 | 315 |
| Serratus ventralis thoracis | – | + | – | +++ | 2991 | 2781 | 49 | 35–65 | 577 | 17310 | 424 |
| Brachiocephalicus & Omotransversarius | – | +++ | – | – | 2426 | 2349 | 693 | 170–995 | 62 | 1860 | 644 |
| Subclavius | + | – | + | – | 1303 | 1217 | 519 | 350–640 | 23 | 690 | 179 |
| Trapezius | + | + | + | – | 678 | 631 | 191 | 45–425 | 42 | 1260 | 120 |
| Latissimus Dorsi | – | – | +++ | – | 1828 | 1705 | 378 | 95–600 | 53 | 1590 | 301 |
| Rhomboid cervicis | + | + | – | – | 555 | 503 | 311 | 100–490 | 15 | 450 | 70 |
| Rhomboid thoracis | + | – | + | – | 433 | 409 | 139 | 50–160 | 24 | 720 | 50 |
| Protraction total | 2426 | 2349 | 62 | 1860 | 644 | ||||||
| Retraction total | 1828 | 1705 | 53 | 1590 | 301 | ||||||
Data presented are the mean of those obtained from six cadavers (not including subject E as its body mass was approximately half that of the other subjects). Muscle function was determined during dissections from observation of site of origin and insertion and orientation of muscle fascicles. The symbols +++ indicate primary function; +, secondary function; –, no contribution to the particular function. Abbreviations: FL, fascicle length; PCSA, physiological cross-sectional area. Between 10 and 20 separate measurements of fascicle length were recorded for each muscle. Fascicle length range is the range of all fascicle length measurements in all subjects. Muscle force was calculated assuming 0.3 MPa as the maximum isometric stress of skeletal muscle (Woledge et al. 1985). Vmax for equine muscle was estimated as 5 Lo s−1 (see Introduction for details).
The majority of the extrinsic muscles (BO, SVC, pectoralis profundus, latissimus dorsi and subclavius) were large (mean muscle volume ranged from 1220 to 2650 cm3) with long fascicles (mean fascicle length ranged from 292 to 693 mm) orientated in parallel with the long axis of the muscle. Peak isometric force generation capacity was estimated for these muscles and ranged from 690 to 2160 N. SVT was the largest of the extrinsic muscles studied (mean volume 2780 cm3) with the shortest fascicles (mean length 49 ± 16 mm), which were orientated at approximately 45° to the long axis of the muscle tendon unit. Peak isometric force generation capacity of SVT was estimated at 17 300 N. Pectoralis transversus and pectoralis descendens were smaller muscles (total volume 1430 cm3) with medium-length fascicles (range 115–280 mm) orientated in the transverse plane (i.e. the plane of limb abduction/adduction). Trapezius, rhomboideus cervicis and rhomboideus thoracis were the smallest extrinsic muscles (mean volume ranged from 409 to 631 cm3), with medium-length fascicles (mean fascicle length ranged from 139 to 311 mm). In both muscle groups fascicles inserted onto the dorsal aspect of the scapula and thus fascicle orientation ranged from vertical (middle fascicles) to almost cranio-caudal (the more cranial or caudal fascicles). None of the extrinsic muscles had a long tendon of insertion compared with muscle belly length. However, both SVT and latissimus dorsi had substantial sheet-like aponeuroses.
Information on anatomical position and muscle fascicle orientation (with respect to the primary plane of limb motion) was used to determine the primary muscles of limb protraction (BO) and retraction (latissimus dorsi). Maximum isometric force-generating capacity of the primary limb protractor was estimated to be 1860 N and maximum power 644 W. Maximum isometric force-generating capacity of the primary limb retractor was estimated to be 1590 N and maximum power 301 W.
Muscle mass and fascicle length data were scaled assuming geometric similarity (Tables 4 and 5). When scaled, muscle masses and muscle fascicle lengths were similar in all subjects. Six of the 10 extrinsic muscles were heaviest in subject A. There was no consistent rank of muscle mass or fascicle length between the other animals. The SVT showed the greatest amount of intersubject variation in mass and BO was variable in both mass and fascicle length.
Table 4.
Muscle mass as a fraction of body mass
| Subject | Subject mass (kg) | Pectoralis transversus and descendens | Pectoralis profundus | Serratus ventralis cervicis | Serratus ventralis thoracis | Brachio- cephalicus & Omotrans- versarius | Subclavius | Trapezius | Latissimus dorsi | Rhomboid cervicis | Rhomboid thoracis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 400 | 3.90 | 7.50 | 4.50 | 7.75 | 6.63 | 3.40 | 1.51 | 4.43 | 1.46 | 1.15 |
| B | 450 | 3.64 | 6.67 | – | – | 8.56 | 2.43 | 1.49 | 3.82 | – | – |
| C | 470 | 3.53 | 5.73 | 5.08 | 7.25 | 8.78 | 2.55 | 1.90 | 4.15 | 1.38 | 1.27 |
| D | 500 | 3.38 | 5.63 | 4.83 | 6.84 | 7.87 | 2.56 | 1.63 | 3.95 | 1.27 | 1.21 |
| E | 270 | 3.50 | 5.57 | 4.98 | 7.08 | 8.60 | 2.46 | 1.78 | 4.09 | 1.31 | 1.19 |
| F | 600 | 2.63 | 5.17 | 4.00 | 5.17 | 4.05 | 2.57 | 1.07 | 3.66 | 1.10 | 0.91 |
| G | 600 | 2.76 | 5.17 | 3.50 | 4.63 | 5.00 | 2.75 | 1.09 | 3.08 | 1.02 | 0.37 |
| Mean | 2.92 | 5.92 | 4.48 | 6.45 | 7.07 | 2.67 | 1.75 | 3.88 | 1.26 | 1.04 | |
| SD | 0.47 | 0.86 | 0.62 | 1.25 | 1.90 | 0.34 | 0.32 | 0.43 | 0.17 | 0.34 |
The data were scaled assuming geometric similarity. Owing to damage inflicted during post-mortem, data for several muscles were not available for subject B.
Table 5.
Muscle mass as a fraction of (body mass)1/3
| Subject | Subject mass (kg) | Pectoralis transversus and descendens | Pectoralis profundus | Serratus ventralis cervicis | Serratus ventralis thoracis | Brachio- cephalicus & Omotrans- versarius | Subclavius | Trapezius | Latissimus dorsi | Rhomboid cervicis | Rhomboid thoracis |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 400 | 2.52 | 6.94 | 4.68 | 0.63 | 6.56 | 6.81 | 2.32 | 4.54 | 6.18 | 1.13 |
| B | 450 | 3.07 | 6.69 | – | – | 8.99 | 6.42 | 2.82 | 5.06 | – | – |
| C | 470 | 2.99 | 5.92 | 4.08 | 0.75 | 11.33 | 6.42 | 3.58 | 5.79 | 4.25 | 2.55 |
| D | 500 | 3.00 | 6.26 | 4.39 | 0.78 | 11.95 | 6.33 | 3.86 | 5.92 | 4.26 | 2.48 |
| E | 270 | 3.15 | 6.03 | 4.56 | 0.70 | 9.53 | 6.50 | 3.61 | 5.72 | 4.08 | 2.45 |
| F | 600 | 1.70 | 6.07 | 3.25 | 0.64 | 9.37 | 7.19 | 1.86 | 3.51 | 2.89 | 1.73 |
| G | 600 | 2.54 | 4.82 | 2.91 | 0.67 | 9.87 | 7.35 | 1.57 | 4.39 | 3.32 | 1.41 |
| Mean | 2.71 | 6.10 | 3.98 | 0.69 | 9.66 | 6.72 | 2.80 | 4.99 | 4.15 | 2.03 | |
| STD | 0.51 | 0.68 | 0.73 | 0.06 | 1.74 | 0.41 | 0.91 | 0.89 | 1.13 | 0.62 |
The data were scaled assuming geometric similarity. Owing to damage inflicted during post-mortem, data for several muscles were not available for subject B.
Extrinsic muscle volume and fascicle length data were plotted with existing thoracic limb intrinsic muscle data (see Fig. 2A,B; biceps brachii: Wilson et al. 2003; carpal and digital flexors and extensors: Brown et al. 2003a; triceps brachii and supraspinatus, J. Watson, unpublished data). There was a distinct proximal-to-distal reduction in both muscle volume and muscle fascicle length. The exceptions were SVT, which had the largest volume but shortest fascicles of the extrinsic muscles, and triceps brachii, which had the largest volume of all thoracic limb muscles and the longest fascicles of the intrinsic muscles.
Fig. 2.
(A) Mean muscle volume and (B) fascicle length for equine thoracic limb muscles. Muscle abbreviations: BO, brachiocephalicus and omotransversarius; SC, subclavius; PP, pectoralis profundus; LD, latissimus dorsi; RC, rhomboid cervicis; SVC, serratus ventralis cervicis; PTD, pectoralis transversus and descendens; TP, trapezius; RT, rhomboid thoracis; SS, supraspinatus; SVT, serratus ventralis thoracis; LOT, long head of triceps; LAT, lateral head of triceps; MT, medial head of triceps; BBM, medial head of biceps; BBL, lateral head of biceps; FCR, flexor carpi radialis; DDFH, humeral head of deep digital flexor; DDFU, ulnar head of deep digital flexor; FCU, flexor carpi ulnaris; DDFR, radial head of deep digital flexor; SDF, superficial digital flexor; CDE, common digital extensor; ECR, extensor carpi radialis; LDE, lateral digital extensor; APL, abductor pollicis longus; UL, ulnaris lateralis). Muscle groups: extrinsic muscles (diagonal stripes); triceps brachii (white), biceps brachii (grey), carpal and digital flexors (black), carpal and digital extensors (horizontal stripes). Data for triceps and supraspinatus were kindly provided by J. Watson (unpublished data), data for biceps are from Wilson et al. (2003) and carpal and digital flexors and extensor data are from Brown et al. (2003a). All muscle data are derived from horses of similar mass (400–600 kg).
Discussion
We undertook this study to determine the structural and functional anatomy of the thoracic limb extrinsic muscles in horses. In general, the extrinsic muscles were large with long fascicles orientated parallel to the long axis of the muscle belly and thus we were able to accept our original hypothesis. In addition, bony attachment was most often via extremely short tendons or aponeurotic sheets, which were particularly substantial in SVT and latissimus dorsi (thoracolumbar fascia). This pattern of muscle volume and architecture is optimal for high-speed contraction over a wide range of motion, but at the expense of force development. The largest extrinsic muscles (BO, SVT, latissimus dorsi, pectoralis profundus and SVC) however should have a greater capacity for power production during contraction because maximum power is directly proportional to muscle volume.
It is often necessary to compare data from different sized animals both within and between species. To determine whether there were any systematic differences between horses of different breed and size, we assumed geometric similarity and scaled muscle mass and fascicle length accordingly (Tables 4 and 5). When scaled, there was very little intersubject variation in muscle mass or fascicle length. The greatest intersubject variation was seen in muscles SVT and BO. The insertion of SVT interdigitates with the origin of external oblique abdominus (EOA, see Fig. 1C). If parts of EOA were included in our measurements of SVT, this could have contributed to the observed variability. Variations in BO may also be due to difficulty in defining individual muscles. Brachiocephalicus and omotransversarius merge at the junction of the neck and shoulder so they could not be weighed separately. The brachiocephalic portion had longer fascicles than omotransversarius and this could have influenced mean fascicle length, depending on where the majority of the fascicle length measurements were taken from.
In order to visualize proximo-distal specialization of muscle architecture in the equine thoracic limb, we plotted extrinsic muscle volume and fascicle length data with equivalent intrinsic muscle data (Fig. 2A,B). There was a clear proximal-to-distal reduction in both muscle volume and muscle fascicle length. In comparison with extrinsic muscles, thoracic limb intrinsic muscles are small, with highly pennate fascicles and a low muscle-to-tendon length ratio (Hermanson & Hurley, 1990; Hermanson, 1997; Biewener, 1998; Brown et al. 2003a). Pennate muscles usually have shorter fibres and hence larger PCSAs than equally sized parallel-fibred muscles and thus are usually better for developing the high forces that are required for support of the COM. If pennate muscles are able to remain isometric with length change occurring in the series elastic elements, then length change is also highly economical. In this arrangement, elastic energy can be stored within the long tendons and aponeuroses during limb loading and released towards the end of stance. This reduces the work required from the muscles on account of kinetic and potential energy fluctuations of the COM, and thus reduces the metabolic cost of locomotion (Alexander, 2002).
Proximo-distal functional specialisation of pelvic and thoracic limb muscle architecture has been observed in other animals (camel: Alexander et al. 1982; antelope: Alexander & Maloiy, 1989; human: Thorpe et al. 1999; dog: Shahar & Milgram, 2001; Pasi & Carrier, 2003) and may be the optimum arrangement for achieving low-cost, high-speed locomotion. In contrast, birds (Roberts et al. 1997) and some primates (Alexander, 1991; Thorpe et al. 1999) have comparatively large distal limb muscles with long fibres. We compared fascicle length in selected thoracic limb muscles of horses and chimpanzees (Thorpe et al. 1999). In spite of large differences in both size (500 vs. 37 kg) and locomotor behaviour, their muscle fascicle lengths were remarkably similar. In fact, all intrinsic flexor muscles (biceps brachii, deep digital flexor, superficial digital flexor and flexor carpi ulnaris) had longer fascicles in the chimpanzee. This difference was most noticeable in biceps brachii, in which fascicles were over 20 times longer in the chimpanzee than in the horse (mean fascicle length for lateral head of biceps was 125 mm and 6 mm, respectively). In contrast to horses, chimpanzees are able to adapt their locomotion to the changing requirements of a complex three-dimensional environment. Thus, in the chimpanzee thoracic limb, selection has been for the adaptability of having muscles optimized for developing force over a wide range of motion.
Steady state locomotion requires little net work to be done by muscles because much of the metabolic cost of locomotion is due to supporting the body against gravity (Kram & Taylor, 1990). In contrast, moving on an incline, jumping and accelerating from stationary require increased work production from muscles (hence powerful contractions) as work must be done to elevate and/or accelerate the COM. Each of the above activities has a major role in equine athletics; however, the muscular source of this extra work is as yet unknown. We defined the primary (+++) and secondary (+) functions of the extrinsic muscles by virtue of their anatomical position (Table 3). The only extrinsic muscles capable of doing the work of protraction were brachiocephalicus, omotransversarius and SVT. The long fascicles of BO were consistent with a limb moving function. However, SVT had extremely short fascicles (approximately 50 mm), which could limit its role in limb protraction. This is because, although work output is independent of fascicle length for muscles of similar mass, the fascicles of SVT are so short compared with aponeurosis length that they would have a limited ability to compensate for stretch of the aponeurosis and hence to control the position of the limb. Biceps brachii is also capable of affecting limb protraction. However, Wilson et al. (2003) have shown that thoracic limb protraction is a largely passive process in the horse. They liken thoracic limb protraction to a catapult mechanism, in which energy is stored relatively slowly in elastic tissues (internal tendon of biceps brachii and lacertus fibrosus) during limb loading but is released quickly at toe-off, protracting the limb.
SVT is a large flat muscle sandwiched between two broad sheets of aponeurosis. The primary role of SVT is suspending the trunk from the limb (as there is no bony articulation between the thoracic limb and axial skeleton). When standing, this support is probably achieved with very little muscle activity, as muscles also produce force passively (without being stimulated) when their fibres (and, more importantly, parallel elastic elements) are elongated. Supporting the relatively massive trunk against gravitational and inertial loads during high-speed locomotion requires the muscle to resist much higher forces. We estimated maximum isometric force-generating capacity for SVT to be in excess of 17 kN. Peak vertical force at gallop is at least 8 kN per limb for a 500-kg horse (McGuigan & Wilson, 2003), and thus SVT alone might easily resist this. In addition, the double aponeurosis of SVT was at least 14 times longer (approximately 500 mm) than fascicle length. An 8% stretch of the aponeurosis would equate to an absolute length change of 42 mm, which is almost half the length change that occurs in the rest of the limb at gallop (McGuigan & Wilson, 2003). Thus, the SVT muscle–tendon unit is not only capable of developing high forces (particularly during isometric contraction or active stretch) but could also make a significant contribution to the overall elastic properties of the limb. The muscle–tendon unit would function as a spring in series with the leg and should be taken into account when studying the mechanics of locomotion.
The primary muscle of limb retraction (latissimus dorsi) was capable of developing similar levels of force but less than half the power of its antagonist (BO). Triceps brachii is, however, also a retractor and extensor and is by far the largest of all thoracic limb muscles (Fig. 2A). The combination of large muscle volume and medium-length fascicles (Fig. 2B) would suggest that triceps brachii has the capacity to be an extremely powerful muscle. If, however, as force records suggest (Back et al. 1995a,b ), most of the work of propulsion is done by the hindlimb, then these muscles are likely to function in roles other than active limb retraction. For instance, triceps may have a role in extending the limb during jumping.
Inverse dynamics approaches in trotting horses have shown that there is an extensor moment at the shoulder throughout most of the stance phase (Clayton et al. 2000). Thus, the net joint moment and angular velocity of the limb are in opposite directions and energy is being absorbed. EMG recordings reveal that triceps brachii and latissimus dorsi are active at the end of swing and at hoof contact (Preedy, 1998). During this time the muscles are active while lengthening and could well function to slow forward motion of the limb prior to hoof contact and control movement of the trunk in the stance period immediately following that. The substantial aponeurosis and thoraco-lumbar fascia associated with latissimus dorsi might also contribute to the above functions. Latissimus dorsi is, however, also active at the end of stance. At this stage a flexor moment is being generated while the shoulder is flexing (Clayton et al. 2000), which suggests that the limb is being actively retracted. Latissimus dorsi and triceps brachii might play a greater role in actively retracting the limb at higher speeds or when accelerating. However, we are unable to prove or disprove this hypothesis at the present time as published EMG data and inverse dynamics studies are limited to walking and trotting gaits. Pectoralis profundus could also play some role in limb retraction as it is active during early swing and through most of the stance phase (Preedy, 1998). However, the anatomical position of pectoralis profundus suggests that it is primarily an adductor of the limb (along with the pectoralis transversus and pectoralis descendens) and thus most likely functions to stabilize the proximal limb just prior to and during foot contact. Triceps brachii is a biarticular muscle; it crosses the caudal aspect of the elbow joint and thus exerts an extensor moment at the elbow. This extensor moment is opposed by biceps brachii (Clayton et al. 2000), ensuring that the elbow joint remains supported throughout stance.
Cursorial animals increase stride length by allowing the scapula to move freely on the rib cage. The lack of bony articulation and hence a clear center of rotation between the scapula and ribcage means that we cannot easily estimate muscle moments across that joint. SVT, SVC, trapezius and rhomboideus all insert onto the scapula and thus will have varying roles in scapula rotation (protracting and retracting the limb) and translation (increasing stride length). A more important role for these muscles might be in stabilizing the scapula during the varied loads imposed during dynamic movement.
We estimated maximum capacity for power in the extrinsic muscles using a value of 5 Lo s−1 for Vmax in horse muscle (see Introduction). We did not determine fibre type for the muscles studied but measurements of fibre type on other horse muscle show a non-homogeneous distribution of type I fibres in individual muscles from thoroughbred horses, with greater concentrations in the deeper and more cranial sections of the muscle belly [61 : 39 (Type I: Type II) at 8 cm depth in equine gluteus medius, Snow, 1983]. Thus, depending on the actual proportion of fast- to slow-twitch muscle fibres in each of the extrinsic muscles, Vmax and thus power may have been either under- or overestimated in this study. These differences would not, however, affect our broader conclusions on the role of the individual extrinsic muscles. The estimates are also of the maximum feasible power output for the muscle, which is unlikely to be attained during life. Pennation angle also affects the maximum capacity for force of a muscle. We did not measure pennation angle here as the majority of extrinsic muscles have parallel fibres. Furthermore, pennation angle measurements made on cadaveric material are one-dimensional and only apply at the resting length of the muscles. Emerging techniques such as three-dimensional ultrasound are likely to help uncover the relationship between pennation angle and muscle force in contracting muscles.
The thoracic limb extrinsic muscles have limited scope for power production as the muscles are small (mean total extrinsic muscle mass approximately 17 kg per limb, mean retractor mass 1.8 kg) compared with the total locomotor muscle mass [approximately 42% of total body mass (200 kg) Gunn, 1987]. They may, however, contribute to power absorption because muscles working eccentrically (i.e. those being actively stretched) can absorb up to 15 times more energy than during concentric contractions (McFadyen & Winter, 1988). Power absorption in locomotion is required for deceleration, moving downhill, jumping and for dissipating and damping of high-impact forces, which can be damaging to soft tissue structures. For example, in horses, vestigial muscles such as the superficial digital flexor may be important in attenuating the high-frequency forces experienced by the hoof at impact (Wilson et al. 2001). EMG and joint moment/power data for the equine shoulder joint suggest that the thoracic limb retractors are being actively stretched and thus absorbing energy at the end of swing phase and at the beginning of stance. Furthermore, muscles inserting onto the freely moveable scapula are required to stabilize the trunk–limb interaction during weight-bearing. The wide variation in extrinsic muscle internal architecture between muscles optimized for length change (most extrinsic muscles) and those optimized for economical force generation (SVT) supports the need to consider these muscles in musculoskeletal models of animal locomotion.
In light of the above, the bulk of propulsive muscle mass required for accelerating, jumping and running uphill must reside elsewhere in the body, most likely in the proximal hindlimb and back (Alexander, 1985). The equine rump is large compared with the equivalent shoulder region, which suggests that the muscles attaching the hindlimb to the trunk are larger than their thoracic limb counterparts. Specific data on muscle internal architecture and geometry (i.e. muscle paths) of equine proximal hindlimb muscles are not available and thus we are as yet unable to speculate on their functional capacity.
In conclusion, equine thoracic limb extrinsic muscles are large with long, parallel fascicles and a low muscle-to-tendon length ratio. This would equate with a capacity for doing work. The exception was serratus ventralis thoracis which, although large, had very short fascicles compared with aponeurosis length. This muscle may contribute to the overall elastic properties of the limb.
Acknowledgments
This work was funded by the BBSRC and HBLB. Thanks to Renate Weller, Justine Robilliard and Jo Watson for assistance in data collection and Roger Woledge for comments on the manuscript.
References
- Alexander RM. The mechanics of jumping by a dog (Canis familiaris) J. Zool. Lond. 1974;173:549–573. [Google Scholar]
- Alexander RM, Bennet-Clark HC. Storage of elastic strain energy in muscle and other tissues. Nature. 1977;265:114–117. doi: 10.1038/265114a0. [DOI] [PubMed] [Google Scholar]
- Alexander RM. Elastic structures in the back and their role in galloping in some animals. J. Zool. Lond. A. 1985;207:467–482. [Google Scholar]
- Alexander RM, Maloiy GMO. Locomotion of African mammals. Symp. Zool. Soc. Lond. 1989;61:163–180. [Google Scholar]
- Alexander RM. Elastic mechanisms in primate locomotion. Z. Morph. Anthropol. 1991;78:315–320. [PubMed] [Google Scholar]
- Alexander RM, Maloiy GMO, Ker RF, Jayes AS, Warui CN. The role of tendon elasticity in the locomotion of the camel. J. Zool. Lond. 1982;177:265–303. [Google Scholar]
- Alexander RM. Tendon elasticity and muscle function. Comp. Biochem. Physiol. A. Mol. Integr. Physiol. 2002;133:1001–1011. doi: 10.1016/s1095-6433(02)00143-5. [DOI] [PubMed] [Google Scholar]
- Back W, Schamhardt HC, Savelberg HHCM. How the horse moves: significance of graphical representations of equine thoracic limb kinematics. Equine Vet. J. 1995a;27:31–38. doi: 10.1111/j.2042-3306.1995.tb03029.x. [DOI] [PubMed] [Google Scholar]
- Back W, Schamhardt HC, Savelberg HHCM. How the horse moves: significance of graphical representations of equine hindlimb kinematics. Equine Vet. J. 1995b;27:39–45. doi: 10.1111/j.2042-3306.1995.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Bartel DL, Schryver HF, Lowe JE, Parker RA. Locomotion in the horse: a procedure for computing the internal forces in the digit. Am. J. Vet. Res. 1978;11:1721–1727. [PubMed] [Google Scholar]
- Biewener AA. Muscle-tendon stresses and elastic energy storage during locomotion in the horse Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 1998;120:73–87. doi: 10.1016/s0305-0491(98)00024-8. [DOI] [PubMed] [Google Scholar]
- Biewener AA, Roberts TJ. Muscle and tendon contributions to force, work and elastic energy savings: a comparative perspective. Ex. Sports Sci. Rev. 2000;2803:99–107. [PubMed] [Google Scholar]
- Biewener AA, McGowan C, Card GM, Baudinette RV. Dynamics of muscle function in tammar wallabies (M. eugenii) during level versus incline hopping. J. Exp. Biol. 2004;207:211–223. doi: 10.1242/jeb.00764. [DOI] [PubMed] [Google Scholar]
- van den Bogert AJ, Schamhardt HC, Crowe A. Simulation of quadrupedal locomotion using a rigid body model. J. Biomech. 1989;22:33–41. doi: 10.1016/0021-9290(89)90182-6. [DOI] [PubMed] [Google Scholar]
- Brown NA, Kawcak CE, McIlwraith CW, Pandy MG. Architectural properties of distal thoracic limb muscles in horses, Equus caballus. J. Morph. 2003a;258:106–114. doi: 10.1002/jmor.10113. [DOI] [PubMed] [Google Scholar]
- Brown NA, Pandy MG, Kawcak CE, McIlwraith CW. Force- and moment-generating capacities of muscles in the distal thoracic limb of the horse. J. Anat. 2003b;203:101–113. doi: 10.1046/j.1469-7580.2003.00206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner HH, Savelberg HH, Schamhardt HC, Barneveld A. Inertial properties of Dutch Warmblood horses. J. Biomech. 1997;30:653–658. doi: 10.1016/s0021-9290(97)00005-5. [DOI] [PubMed] [Google Scholar]
- Clayton HM, Hodson E, Lanovaz JL. The thoracic limb in walking horses: net joint moments and powers. Equine Vet. J. 2000;34:295–299. doi: 10.2746/042516400777032174. [DOI] [PubMed] [Google Scholar]
- Curtin NA, Woledge RC. Power output and force/velocity relationship of live fibres from white myotomal muscle of the dogfish Scyliorhinus canicula. J. Exp. Biol. 1988;140:187–197. [Google Scholar]
- Daley MA, Biewener AA. Muscle force–length dynamics during level versus incline locomotion: a comparison of in vivo performance of two guinea fowl ankle extensors. J. Exp. Biol. 2003;206:2941–2958. doi: 10.1242/jeb.00503. [DOI] [PubMed] [Google Scholar]
- Dyce KM, Sack WO, Wensing CGG. Textbook of Veterinary Anatomy. London: Saunders; 1996. [Google Scholar]
- Farley CT, Glasheen J, McMahon TA. Running springs: speed and animal size. J. Exp. Biol. 1993;185:71–86. doi: 10.1242/jeb.185.1.71. [DOI] [PubMed] [Google Scholar]
- Fischer MS, Schilling N, Schmidt M, Haarhaus D, Witte H. Basic limb kinematics of small therian mammals. J. Exp. Biol. 2002;205:1315–1338. doi: 10.1242/jeb.205.9.1315. [DOI] [PubMed] [Google Scholar]
- Gasser HS, Hill AV. The dynamics of muscular contraction. Proc. Roy. Soc. B. 1924;96:398–437. [Google Scholar]
- Gillis GB, Biewener AA. Hindlimb muscle function in relation to speed and gait: in vivo patterns of strain and activation in a hip and knee extensor of the rat (Rattus norvegicus) J. Exp. Biol. 2001;204:2717–2731. doi: 10.1242/jeb.204.15.2717. [DOI] [PubMed] [Google Scholar]
- Gunn HM. Muscle, bone and fat proportions and muscle distribution of thoroughbreds and other horses. In: Gillespie JR, Robinson NE, editors. Equine Exercise Physiology 2. Proceedings of the 2nd International Conference on Equine Exercise Physiology; Cambridge : ICEEP Publications. 1987. pp. 253–264. [Google Scholar]
- Hermanson JW, Hurley KJ. Architectural and histochemical analysis of the biceps brachii muscle of the horse. Acta Anat. (Basel). 1990;137:146–156. doi: 10.1159/000146875. [DOI] [PubMed] [Google Scholar]
- Hermanson JW, Cobb MA. Four forearm flexor muscles of the horse, Equus caballus: anatomy and histochemistry. J. Morph. 1992;212:269–280. doi: 10.1002/jmor.1052120306. [DOI] [PubMed] [Google Scholar]
- Hermanson JW. Architecture and the division of labor in the extensor carpi radialis muscle of horses. Acta Anat.(Basel) 1997;159:127–135. doi: 10.1159/000147975. [DOI] [PubMed] [Google Scholar]
- Kearns CF, McKeever KH, Abe T. Overview of horse body composition and muscle architecture: implications for performance. Vet. J. 2002;164:224–234. doi: 10.1053/tvjl.2001.0702. [DOI] [PubMed] [Google Scholar]
- Ker RF, Alexander RMcN, Bennett MB. Why are mammalian tendons so thick? J. Zool. Lond. 1988;216:309–324. [Google Scholar]
- König HE, Liebich HG. Veterinary Anatomy of Domestic Mammals. Stuttgart, New York: Schattauer; 2004. [Google Scholar]
- Kram R, Taylor CR. Energetics of running: a new perspective. Nature. 1990;6281:265–267. doi: 10.1038/346265a0. [DOI] [PubMed] [Google Scholar]
- Lindstedt SL, Reich TE, Keim P, LaStayo PC. Do muscles function as adaptable locomotor springs? J. Exp. Biol. 2002;205:2211–2216. doi: 10.1242/jeb.205.15.2211. [DOI] [PubMed] [Google Scholar]
- Lou F, Curtin NA, Woledge RC. Isometric and isovelocity contractile performance of red muscle fibres from the dogfish Scyliorhinus canicula. J. Exp. Biol. 2002;205:1585–1595. doi: 10.1242/jeb.205.11.1585. [DOI] [PubMed] [Google Scholar]
- McFadyen BJ, Winter DA. An integrated biomechanical analysis of normal stair ascent and descent. J. Biomech. 1988;21:733–744. doi: 10.1016/0021-9290(88)90282-5. [DOI] [PubMed] [Google Scholar]
- McGuigan MP, Wilson AM. The effect of gait and digital flexor muscle activation on limb compliance in the thoracic limb of the horse Equus cabllus. J. Exp. Biol. 2003;206:1325–1336. doi: 10.1242/jeb.00254. [DOI] [PubMed] [Google Scholar]
- Meershoek LS, van den Bogert AJ, Schamhardt HC. Model formulation and determination of in vitro parameters of a noninvasive method to calculate flexor tendon forces in the equine thoracic limb. Am. J. Vet. Res. 2001;62:1585–1593. doi: 10.2460/ajvr.2001.62.1585. [DOI] [PubMed] [Google Scholar]
- Mendez J, Keys A. Density and composition of mammalian muscle. Metabolism. 1960;9:184–188. [Google Scholar]
- Merkens HW, Schamhardt HC. Evaluation of equine locomotion during differing degrees of experimentally induced lameness (II). Distribution of ground reaction force patterns of the concurrently loaded limbs. Equine Vet. J. 1988;6(Suppl.):107–112. doi: 10.1111/j.2042-3306.1988.tb04656.x. [DOI] [PubMed] [Google Scholar]
- Merkens HW, Schamhardt HC, van Osch GJ, van den Bogert AJ. Ground reaction force patterns of Dutch Warmblood horses at normal trot. Equine Vet. J. 1993;25:134–137. doi: 10.1111/j.2042-3306.1993.tb02923.x. [DOI] [PubMed] [Google Scholar]
- Mero A, Komi PV. Force-, EMG-, and elasticity-velocity relationships at submaximal, maximal and supramaximal running speeds in sprinters. Eur. J. Appl. Physiol. Occup. Physiol. 1986;55:553–561. doi: 10.1007/BF00421652. [DOI] [PubMed] [Google Scholar]
- Monti RJ, Roy RR, Zhong H, Edgerton VR. Mechanical properties of rat soleus aponeurosis and tendon during variable recruitment in situ. J. Exp. Biol. 2003;206:3437–3445. doi: 10.1242/jeb.00550. [DOI] [PubMed] [Google Scholar]
- Nickel R, Schummer A, Seiferle E, Frewein J, Wilkens H, Wille H. The Locomotor System of the Domestic Animals. Berlin: Verlag Paul Parey; 1986. [Google Scholar]
- Pasi BM, Carrier DR. Functional trade-offs in the limb muscles of dogs for running vs. fighting. J. Evol. Biol. 2003;16:324–332. doi: 10.1046/j.1420-9101.2003.00512.x. [DOI] [PubMed] [Google Scholar]
- Preedy D. Honours Dissertation. Bristol University; 1998. Analysis of horse thoracic limb muscle activity (EMG) at walk and trot. [Google Scholar]
- Riemersma DJ, Schamhardt HC, Hartman W, Lammertink JL. Kinetics and kinematics of the equine hind limb: in vivo tendon loads and force plate measurements in ponies. Am. J. Vet. Res. 1988;49:1344–1352. [PubMed] [Google Scholar]
- Roberts TJ, Marsh RL, Weyand PG, Taylor CR. Muscular force in running turkeys: the economy of minimising work. Science. 1997;275:1113–1115. doi: 10.1126/science.275.5303.1113. [DOI] [PubMed] [Google Scholar]
- Roberts TJ, Chen MS, Taylor CR. Energetics of bipedal running II: Limb design and running mechanics. J. Exp. Biol. 1998;201:2753–2762. doi: 10.1242/jeb.201.19.2753. [DOI] [PubMed] [Google Scholar]
- Rome LC, Sosnicki AA, Goble DO. Maximum velocity of shortening of three fibre types from horse soleus muscle: implications for scaling with body size. J. Physiol. 1990;431:173–185. doi: 10.1113/jphysiol.1990.sp018325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahar R, Milgram J. Morphometric and anatomic study of the hind limb of a dog. Am. J. Vet. Record. 2001;62:928–933. doi: 10.2460/ajvr.2001.62.928. [DOI] [PubMed] [Google Scholar]
- Sisson S, Grossman JD. The Anatomy of the Domestic Animals. Vol. 1. Philadelphia: W.B. Saunders; 1975. [Google Scholar]
- Snow DH. Skeletal muscle adaptations: a review. In: Snow DH, Persson SGB, Rose RJ, editors. Equine Physiology. Cambridge: Granta Editions; 1983. p. 160. [Google Scholar]
- Thorpe SKS, Crompton RH, Günther MM, Ker RF, Alexander RMcN. Dimensions and moment arms of the hind- and thoracic limb muscles of common chimpanzees (Pan troglodytes) Am. J. Phys. Anthropol. 1999;110:179–199. doi: 10.1002/(SICI)1096-8644(199910)110:2<179::AID-AJPA5>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- van Weeren PR, Jansen MO, van den Bogert AJ, Barneveld A. A kinematic and strain gauge study of the reciprocal apparatus in the equine hind limb. J. Biomech. 1992;25:1291–1301. doi: 10.1016/0021-9290(92)90284-8. [DOI] [PubMed] [Google Scholar]
- Wells JB. Comparison of mechanical properties between slow and fast mammalian muscles. J. Physiol. 1965;178:252–269. doi: 10.1113/jphysiol.1965.sp007626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson AM, McGuigan MP, Su A, Van den Bogert AJ. Horses damp the spring in their step. Nature. 2001;414:895–898. doi: 10.1038/414895a. [DOI] [PubMed] [Google Scholar]
- Wilson AM, Watson JC, Lichtwark GA. A catapult mechanism for rapid limb protraction. Nature. 2003;421:35–36. doi: 10.1038/421035a. [DOI] [PubMed] [Google Scholar]
- Witte TH, Knill K, Wilson AM. Determination of peak vertical ground reaction force from duty factor during field locomotion in the horse (Equus caballus) J. Exp. Biol. 2004;207:3639–3648. doi: 10.1242/jeb.01182. [DOI] [PubMed] [Google Scholar]
- Woledge RC, Curtin NA, Homsher E. Energetic aspects of muscle contraction. Monogr. Physiol. Soc. 1985;41:1–357. [PubMed] [Google Scholar]