Abstract
The remarkable resilience of cognitive functions to focal brain damage suggests that multiple degenerate neuronal systems can sustain the same function either via similar mechanisms or by implementing different cognitive strategies. In degenerate functional neuroanatomy, multiple degenerate neuronal systems might be present in a single brain where they are either co-activated or remain latent during task performance. In degeneracy over subjects, a particular function may be sustained by only one neuronal system within a subject, but by different systems over subjects. Degeneracy over subjects might have arisen from (ab)normal variation in neurodevelopmental trajectories or long-term plastic changes following structural lesions. We discuss how degenerate neuronal systems can be revealed using (1) intersubject variability, (2) multiple lesion studies and (3) an iterative approach integrating information from lesion and functional imaging studies.
Keywords: degeneracy, functional imaging, functional recovery, pluripotentiality, structure–function relationship
Introduction
The human brain shows an amazing ability to maintain and recover cognitive functions after focal cortical damage. This resilience suggests that there might not be a one-to-one mapping between neuronal structures and cognitive functions, but that multiple neuronal systems might be capable of producing the same behavioural response. In this paper, we explore structure–function relationships in terms of neuronal mechanisms that can mediate behavioural compensation after focal cortical damage. In the first section, we introduce degeneracy and pluripotentiality as two complementary concepts that characterize structure–function relationships. In the second section, we emphasize that structure–function relationships can be characterized at multiple levels of description. In the third section, we describe methodological approaches that enable us to investigate and define structure–function relationships empirically, using functional imaging and neuropsychological lesion studies. In the fourth section, we discuss, in detail, examples in which the same cognitive function can be sustained by multiple systems and further categorize them on the basis of whether the degeneracy is within one brain (i.e. degenerate functional neuroanatomy) or in different brains of the same population (degeneracy over subjects). Finally, in the fifth section, we revisit the neuronal mechanisms sustaining functional recovery and discuss how behavioural compensation may arise from degeneracy within or between subjects.
(1) Structure–function relationships: degeneracy and pluripotentiality (Fig. 1)
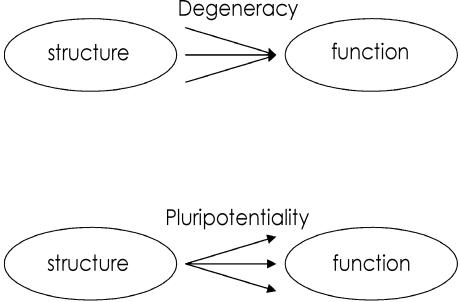
Fig. 1.
Structure–function relationships: degeneracy and pluripotentiality.
Degeneracy refers to many-to-one structure–function relationships. Edelman and colleagues have defined it as ‘the ability of elements that are structurally different to perform the same function or yield the same output’ (Edelman & Gally, 2001). The order of degeneracy is defined as the number of disjoint elements or structural configurations that are sufficient to produce the same output (Price & Friston, 2002).
Degeneracy is a ubiquitous characteristic of biological systems at multiple organizational levels, ranging from molecular to functional brain architectures. Prominent examples include the genetic system with different nucleotide triplets coding for the same protein and immunology with different antibodies binding the same antigen. At the level of cognitive anatomy, degeneracy means that, for instance, multiple sets of brain regions can sustain the same cognitive task as measured by behavioural parameters.
Anecdotally, in neurology and neuropsychology, the ability of two neuronal systems to perform the same task has also been referred to as redundancy (Lee & van Donkelaar, 1995; Cirstea & Levin, 2000). However, the concept of redundancy should be distinguished from degeneracy. Redundancy has been formally defined in information theoretic terms as the statistical dependency among the states of a system (Shannon & Weaver, 1949; Barlow, 2001). For example, if two brain structures co-activate to perform the same function, this would be redundant or inefficient functioning, because activation in one structure would suffice and could predict the state of the other system. Thus, whereas degeneracy characterizes a structure–function relationship, redundancy refers to how the systems are functioning. Obviously, the redundant use of multiple structural configurations requires that multiple structural elements can sustain the same function. Thus redundant functioning of a system necessitates degeneracy (Friston & Price, 2003).
Degeneracy plays an important role in both the ontogenesis of an individual as well as the phylogenesis of species. First, it affords a biological system robustness to damage or failure because if one structure is lesioned, the remaining ones can still support a particular function. Second, it facilitates selection, thus enabling evolutionary progress and neurodevelopment. This implies that degenerate structural sets in biological systems are not simply duplicates but structurally different, i.e. non-isomorphic elements that provide the necessary variability on which selection processes can operate. Unlike duplicates that can only support redundant functioning, degenerate structural sets can produce the same output in one context but different outputs in another context. Therefore, degeneracy is almost invariably associated with its complementary counterpart: pluripotentiality. Pluripotentiality refers to a one-to-many structure–function relationship, in which the same structural configuration can perform multiple functions. In terms of cognitive functional neuroanatomy, it means that the same brain region can take part in multiple cognitive functions. It is intuitively obvious that degeneracy, in the context of pluripotentiality, can only arise in biological systems that are endowed with a certain degree of complexity. Thus, neuronal systems combining the organizational principles of functional segregation and integration allow for multiple structural configurations that can produce the same and different behavioural responses depending on the cognitive context (Tononi et al. 1994, 1999; Edelman & Gally, 2001).
(2) Structure–function relationships: levels of description
Structure–function relationships depend implicitly on the descriptive level at which the structural and functional elements are specified. In terms of cognitive functional neuroanatomy, structural elements can constitute sets of multiple brain regions, single brain regions, neuronal populations/assemblies or single neurons. Similarly, functional elements can be specified at multiple levels by decomposing cognitive processes into subprocesses depending on the depth of task analysis or the underlying cognitive model. For instance, sentence comprehension encompasses many cognitive processes: word recognition, working memory, phrase structure building and syntactic parsing, thematic/pragmatic analysis, retrieval of semantic knowledge, etc. (Hagoort et al. 2003). It is well recognized in cognitive neuroscience that complex cognitive processes can often be accomplished in multiple ways, i.e. by engaging different subprocesses or using different strategies. Thus, the sentence ‘the postman was bitten by the dog’ can easily be understood by simply combining the semantic content of the single words rather than by formally assigning a syntactic structure to the lexical items. Many cognitive models even postulate multiple routes that can be used to yield the same task performance. For instance, based on neuropsychological data, it has been suggested that the appropriate action for an object can be retrieved either directly from visual structural features or indirectly through accessing semantic (contextual, associative) knowledge (Rumiati & Humphreys, 1998; Phillips et al. 2002). Similarly, reading of familiar, regularly spelled words might be accomplished via either spelling–sound relationships or lexical semantic processes. Although the limitations of these box-and-arrow models that decompose complex cognitive functions into dissociable subprocesses are well recognized, they demonstrate that cognitive functions – like structural elements – can in principle be characterized at multiple levels of description.
This is important, because the order of degeneracy (i.e. the number of disjoint elements that can perform the same operation) can change with either the structural or the functional level of description. For example, there might be only one set of cortical regions but multiple degenerate neuronal populations within the same regions that can sustain word reading. Conversely, there might be two degenerate sets of brain regions that can sustain word reading. However, there might be only one set of regions that can sustain reading if reading (e.g. by presenting pseudowords) is enforced via spelling–sound relationships (i.e. the subprocess of interest is the orthographic phonological translation rather than reading per se; Seidenberg & McClelland, 1989). Thus, the order of degeneracy is determined by both the structural and the functional levels as specified by their neurophysiological and psychological/behavioural measures.
Multiple levels of structure–function mappings preclude a straightforward relationship between neurophysiological (e.g. functional imaging) and psychological/cognitive measures. For example, the dual route model of reading cannot be disproved by functional imaging studies that fail to demonstrate two dissociable systems for reading, because the two strategies might be implemented at the lower level of neuronal populations. Conversely, functional imaging evidence for two anatomically dissociable systems (e.g. engaged by different subjects or trials) cannot refute a single route model, because the same reading strategy might be implemented in the two degenerate neuronal systems with stochastic variability in activation across trials or subjects. Evidence for a dual route model of reading can, however, be provided when the two neuronal systems show a double dissociative activation pattern for cognitive tasks that stress the two hypothetical reading strategies. For example, a double dissociation in activation for (1) reading pseudowords that rely on spelling–sound relationships and (2) reading familiar but irregularly spelled words that rely on lexico-semantic processing could provide evidence for a dual route model for reading (Marshall & Newcombe, 1973; Fiez & Petersen, 1998; Fiez et al. 1999; Hagoort et al. 1999; Mechelli et al. 2003; Shallice, 2003).
(3) Methodological approaches to characterizing structure–function relationships: functional imaging and lesion studies
Three fundamental methodological approaches can be distinguished to investigate structure–function relationships. The first manipulates the cognitive function while measuring its effect on neuronal structures indirectly using functional imaging. The second approach perturbs the neuronal structure by inducing permanent or transient lesions and measures the effect on psychological functions or behavioural performance. The third approach combines functional imaging and lesion methods to investigate which neuronal structures can sustain a particular function/task after the ‘normal’ neuronal system has been lesioned. In the following, each of these three approaches will be discussed in terms of their ability to reveal structure–function relationships that are characterized by either pluripotentiality (one-to-many structure–function mappings) or degeneracy (many-to-one structure–function mappings).
Functional imaging of normal subjects
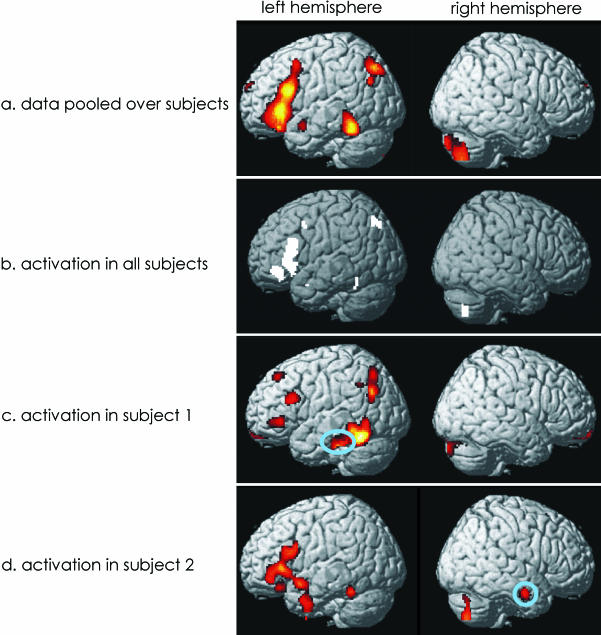
Functional imaging can identify a set of regions that are engaged for one task relative to another. Ignoring sensitivity issues, it enables us to define a system of regions that are sufficient for a particular function in normal subjects. However, functional imaging cannot distinguish areas that are necessary for task performance from those that simply reflect processing that is not necessary for a particular task. For instance, if two functionally distinct cognitive tasks activate (relative to their specific baselines) two sets of cortical regions that are in part overlapping, the regions of the intersection might either have two functions (i.e. be pluripotential) or reflect common processes such as increased attentional demands or general executive processes. With respect to degeneracy, functional imaging can neither determine whether multiple degenerate neuronal systems are activated redundantly nor disclose degenerate neuronal systems that are either inhibited by the prepotent system or not activated. Nevertheless, hypotheses about degenerate neuronal systems can be generated based on the inherent intertrial or intersubject variability in functional activations. These ‘idiosyncratic’ subject or trial-specific activations are usually discarded as uninteresting random noise in functional imaging experiments. However, they might reflect subjects engaging different degenerate neuronal systems and might thus enable us to define candidate regions (see Fig. 2).
Fig. 2.
Normal activation patterns during a semantic paradigm. (a) Regions that were consistently activated across all 12 subjects (P < 0.05 corrected). (b,c) Single subject data to illustrate intersubject variability. Circles highlight activations that are specific to only one subject.
Permanent and transient lesion studies
Lesion studies measure the effect on task performance (defined as response accuracy) that is caused by perturbing, i.e. lesioning, a particular part of the neuronal structure. Lesions can either be permanently induced by ischaemia, hypoxia, etc., or transiently by transcranial magnetic stimulation (TMS), electrical or pharmacological methods. Lesion studies are based on the fundamental rationale that a lesion-induced decline in a particular cognitive function demonstrates that the lesioned part was necessary or made an important contribution to this cognitive function. They also allow us to infer that a region is pluripotent (i.e. participates in multiple functions), if lesioning of this particular part of the system induces impaired performance on several cognitive tasks that do not share common cognitive components. This presupposes a thorough task analysis that decomposes complex cognitive processes into multiple dissociable components (e.g. based on additive factor logic using reaction time measurements). Single lesions cannot reveal multiple degenerate neuronal systems, because if only one system is lesioned, the cognitive function can be supported by the remaining degenerate systems and response accuracy will be maintained. However, lesioning all other degenerate elements will impair response accuracy and thus reveal the functional contribution of a particular neuronal element. Consequently, the measured functional contribution of a degenerate neuronal element depends on the state (i.e. lesioned or intact) of another neural element. In this sense, the contribution is second-order or higher. Only multiple lesion experiments can therefore fully assess the functional contributions of neural elements in a complex neuronal system endowed with degeneracy.
In order to quantify the contributions of elements and their interactions in complex neuronal systems, several methodological approaches have been proposed that formally analyse the performance of a particular task over multiple lesion configurations. This functional contribution analysis (FCA; Aharonov et al. 2003) aims to find the functional contributions of the elements such that they best predict the performance of the entire system, even in untested lesion configurations. The functional contributions (together with their adjoint performance prediction function) are iteratively adjusted to minimize this prediction error. In FCA, functional contributions are therefore only operationally defined and the solution might not be unique. To overcome these shortcomings, Keinan et al. (2004) have recently introduced the Multi-Lesion Shapley value Analysis (MSA), which incorporates fundamental game-theoretic concepts. The MSA determines the functional contribution of a particular element within a system by first quantifying the marginal contribution of a particular element to a subset of elements (i.e. the performance difference that is caused by removing this element from the subset) and then summing over the marginal contributions of this element to all possible subsets within the system. As FCA and MSA are both based on analysing performance scores over multiple lesion configurations, they are able to reveal degeneracy in neuronal systems. For instance, using the MSA, second-order degeneracy could be inferred if the average marginal contribution of two elements together is smaller than the sum of the marginal contributions of each of the two elements when the other one is lesioned.
Although lesion-based approaches theoretically permit reasonable inferences, they face two practical limitations with respect to human studies. First, unguided by any a priori hypotheses, the identification of degenerate neuronal systems requires a variety of lesions in many experiments. Second, lesions to some parts of the human brain are rare either because they are resistant to ischaemic damage or they are too deep to be reached by TMS (Price & Friston, 2002).
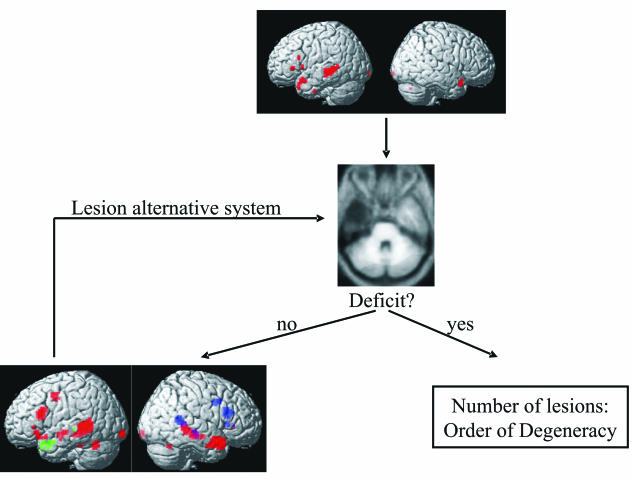
Combining functional imaging and lesion studies (Fig. 3)
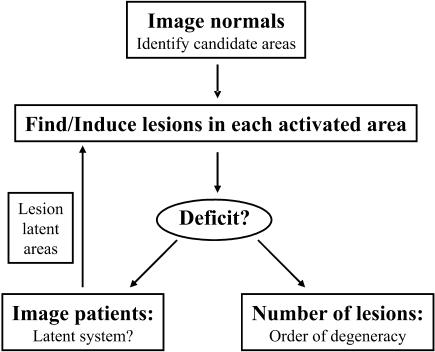
Fig. 3.
Identification of degenerate neuronal systems by combining functional imaging and neuropsychological lesion data in an iterative procedure.
Combining functional imaging and lesion studies offers a practically more feasible approach. Functional imaging can identify the candidate regions that are involved in a particular task. Its inherent intersubject/trial variability might even be used to generate hypotheses about potential degenerate neuronal systems. Guided by functional imaging data, lesion studies can test whether a behavioural deficit arises from damage to (1) a single region in this system or (2) a combination of regions in this system. In case (1), the single region plays a necessary role in this particular task. In case (2), we can conclude that there are degenerate neuronal systems each sufficient for task performance. The order of degeneracy (i.e. the number of disjoint elements that produce the same output, see Section 1) is operationally defined as the minimum number of regions that need to be damaged before a task performance deficit is observed. However, lesioning all the regions within the normal system might not result in a behavioural deficit, if there is a latent degenerate neuronal system that is not activated or even inhibited in normal subjects but activates only after lesioning the prepotent system. To identify these latent degenerate neuronal systems, we therefore have to perform functional imaging of subjects with lesions but intact performance. In conclusion, an iterative approach integrating information from functional imaging and lesion studies may allow us to identify degenerate neuronal systems in a practically feasible way (Price & Friston, 1999; Price & Friston, 2002).
(4) Degeneracy within and between subjects
Degeneracy has been introduced as a many-to-one mapping between structure and function. Two types of degeneracy can be distinguished depending on whether the degeneracy is constituted within or between subjects (i.e. expressed at the level of the individual or the population). In the first case of degenerate functional neuroanatomy, a particular function can be sustained by multiple neuronal configurations that simultaneously coexist within the same subject. In the second case of degeneracy over subjects, there is no degenerate organization within a single brain but a particular function can be sustained by distinct brain structures in different subjects or in the same subject at different times, for instance due to functional reorganization. The second case can be described as degeneracy in a more general sense because the brains of different subjects exhibit anatomical homologies. Below we present several examples of (1) degenerate functional neuroanatomy and (2) degeneracy over subjects and discuss how they can be detected using the methodological approaches outlined in the previous section.
Degenerate functional neuroanatomy
Degenerate neuronal systems within a single brain can function in three ways:
The multiple neuronal systems may activate in parallel and produce the same output in a redundant way. Therefore, applying TMS to one part of the normal system should neither cause a behavioural deficit in terms of response accuracy nor reveal novel regions that were not activated in normal subjects.
Only one of several degenerate neuronal systems might be engaged, possibly reflecting subjects using different strategies. For example, subjects might perform a semantic decision task via imagery or retrieval of episodic knowledge. Analysis of intertrial or intersubject variability in functional activations could provide evidence for multiple neuronal systems that are each sufficient for task performance. These candidate regions then need to be further investigated using lesion methods.
Only one prepotent degenerate neuronal system is consistently involved in task performance, while the other neuronal systems remain latent. In this case, applying TMS to a part of the normal system should have minimal effect on response accuracy but functional imaging of subjects with lesions to the prepotent system would reveal compensatory activations in other parts of the system or even in novel regions. For example, lesioning of primary motor cortex is associated with contralesional hyperexcitabilty and decreased intracortical inhibition as measured by evoked motor potentials (Liepert et al. 2000; Shimizu et al. 2002). Furthermore, functional imaging has revealed increased contralesional activations in pre/motor areas (Johansen-Berg et al. 2002; Johansen-Berg, 2003; Lee et al. 2003). Although the relationship between these different neurophysiological measures and their relevance for functional recovery remains disputed, they might, in part, be explained by disinhibition mechanisms.
Degeneracy over subjects
Degeneracy over subjects means that a task is sustained by only one system in a particular subject, but that the same task is supported by distinct neuronal systems in different subjects or in the same subject at different times, for example following functional reorganization. It thus describes a specific case of intersubject variability in structure–function mapping that can be found within the normal population and would be increased within populations with pathological abnormalities. Degeneracy over subjects can be detected by investigating intersubject variability in functional activations of normal subjects or by comparing functional activations of different cohorts (e.g. normal subjects and patients). Functional imaging will ideally reveal a double dissociation in activation pattern, with some brain regions being more activated in one cohort and/or other areas more activated in the other cohort. As there is only one neuronal system sufficient for task performance within each subject, lesioning one necessary part of this system will initially induce a behavioural deficit, but functional recovery might subsequently result from long-term plastic changes.
There are several mechanisms that can account for degeneracy over subjects:
Degeneracy over subjects can arise from neurodevelopmental trajectories that differ due to (epi)genetic variability, experience-dependent factors or simply stochastic processes. Genetic abnormalities and profound perturbation to sensory experience (e.g. peripheral deafness, blindness, paralysis) can also modify developmental trajectories beyond normal variation and lead to abnormal functional neuroanatomy. For instance, Fig. 4 illustrates that both sighted and early blind subjects activate a fronto-temporal system for semantic retrieval. In addition, blind subjects activate extrastriate areas that are coupling with frontal and semantic regions. Thus, visually deprived subjects might engage a more extensive neuronal system in higher cognitive tasks possibly due to reduced experience-dependent pruning processes that normally lead to sparser connectivity (Noppeney et al. 2003).
Structural brain lesions and functional abnormalities (e.g. epilepsy) in the developing or mature brain can induce functional reorganization of the neuronal systems sustaining a particular task. For instance, in neurologically normal subjects, sentence comprehension activates a left-lateralized fronto-temporal system extending into the left temporal pole (see Fig. 5). Nevertheless, patients who had undergone left anterior temporal lobe resection or refractory epilepsy do not exhibit any sentence comprehension deficits. Those matched to normal subjects for reading ability show decreased activation in undamaged areas of the normal left hemispheric system and increased activation in several right frontal and temporal regions. Critically, activation in several right-hemispheric regions is predicted significantly by the duration and the onset age of epilepsy, highlighting the influence of the epileptic process on the language system especially during early neurodevelopment. Although the functional contributions need to be further assessed using lesion methods, these functional imaging results suggest that in addition to the normal left hemispheric system, sentence comprehension in patients can be sustained by a right hemispheric system that has emerged primarily due to functional reorganization during early neurodevelopment (Noppeney et al., unpubl. observations).
Fig. 4.
General semantic activations (i) common to blind and sighted subjects (red); and (ii) increased for blind subjects (green) are rendered on an averaged normalized brain (top) or presented on sagittal and axial slices of an EPI image (P < 0.05 corrected).
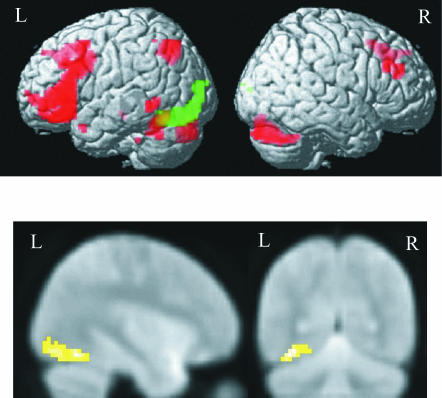
Fig. 5.
Combining functional magnetic resonance imaging and patients study to identify the neuronal systems sustaining sentence comprehension. Top: sentence activation for all normal subjects is rendered on an averaged normalized brain (P < 0.1 corrected). Middle: axial slice of an averaged patient's structural image demonstrating location and size of the left temporal pole lesion. Bottom: sentence activation (i) common to control subjects and patients (red), (ii) decreased for patients relative to control subjects (green) and (iii) increased for patients relative to control subjects (blue) are rendered on an averaged normalized brain (P < 0.1 corrected).
(5) Degeneracy as a neuronal mechanism for recovery or maintenance of function after focal cortical damage
Despite its initial success, the localizationist paradigm faced growing criticism at the turn of the century. In particular, it was pointed out that cortical localization was not able to explain the fact that similar lesions are associated with different behavioural impairments in different subjects and that subjects can often – at least in part – recover a function after insult. To explain these inconsistencies, various holistic theories of brain functioning were developed that incorporated gestalt theoretic concepts (Goldstein, 1934) or invoked – at least to some degree – cortical equipotentiality (Lashley, 1929).
Below we illustrate how maintenance and recovery of function in patients can be explained by different neuronal mechanisms within the framework of degeneracy. First, maintenance and recovery of a particular cognitive function can be explained by degenerate functional neuroanatomy, i.e. degeneracy within subjects, because after lesions to one of multiple degenerate systems, the function can still be sustained by the remaining systems. In this case, plastic changes, induced by the lesion, are immediate and rely on unmasking of a pre-existing but functionally latent system (e.g. disinhibition in the context of degeneracy). For instance, after lesions to the prepotent system, subjects might engage a different strategy (see Section 2). We would therefore expect increased activation in an alternative neuronal system immediately after applying TMS to a component of the predominant system within normal subjects. Second, recovery of function can be explained by degeneracy over subjects. In this case, only one system within a normal brain is capable of performing this particular task. However, an alternative system can emerge due to functional reorganization induced by the underlying pathological process. This type of functional reorganization might explain why impairments due to small strokes generally resolve in the first months (see recent studies investigating the association between functional deficits and lesions at stroke onset using diffusion- and perfusion-weighted imaging; Hillis et al. 2001, 2004). Plastic changes are then mediated by reorganization mechanisms with longer time-courses (possibly long-term potentiation, axonal regeneration and sprouting; Chen et al. 2002), which might also interact with neurodevelopment. Therefore, activation in a patient might in part be predicted by characteristic features of the underlying pathological process (e.g. stroke, closed head injury, tumour, penetrating missile injury), lesion location as well as the onset or duration of the illness. For instance, although it has been suggested that language recovery in patients with acute aphasia depends on restored activation in unaffected left-hemispheric regions (Karbe et al. 1998; Heiss et al. 1999), chronic post-stroke aphasia (Weiller et al. 1995; Thulborn et al. 1999; Gold & Kertesz, 2000; Blasi et al. 2002) or slowly evolving brain lesions (Thiel et al. 2001) have been shown to benefit from interhemispheric reorganization.
In summary, both degenerate functional neuroanatomy and degeneracy over subjects can, in principle, account for behavioural compensation after focal cortical damage and are easily dissociated based on the different time-courses of the plastic changes.
Conclusion
We have invoked here the concept of degeneracy to explain behavioural compensation after focal cortical damage. Neither functional imaging nor single lesion studies alone can characterize ‘many-to-one’ structure–function relationships: functional imaging can only determine the number of brain areas engaged in task performance but not whether these areas are part of one or more co-activated neuronal systems that are each sufficient for task performance. Conversely, single lesion studies cannot detect multiple systems, because lesioning only one of several sufficient systems will not result in a behavioural deficit. However, integrating information from functional imaging and lesion methods can reveal degenerate structure–function relationships. In degenerate functional neuroanatomy, a particular function can be sustained by multiple systems within one subject. In degeneracy over subjects, a particular function can be sustained by only one neuronal system within a subject, but by different systems in different subjects. Degeneracy over subjects might have arisen from (ab)normal variation in neurodevelopmental trajectories or long-term plastic changes following structural lesions. Although both forms of degeneracy can mediate functional recovery, they can easily be distinguished based on the different time-courses of their neuronal mechanisms.
Acknowledgments
This article is based on a talk given at a symposium of the Anatmoical Society of Great Britain and Ireland in January 2004, entitled ‘Functional anatomy of the human brain’, organized by John Marshall.
References
- Aharonov R, Segev L, Meilijson I, Ruppin E. Localization of function via lesion analysis. Neural. Comput. 2003;15:885–913. doi: 10.1162/08997660360581949. [DOI] [PubMed] [Google Scholar]
- Barlow H. Redundancy reduction revisited. Network. 2001;12:241–253. [PubMed] [Google Scholar]
- Blasi V, Young AC, Tansy AP, Petersen SE, Snyder AZ, Corbetta M. Word retrieval learning modulates right frontal cortex in patients with left frontal damage. Neuron. 2002;36:159–170. doi: 10.1016/s0896-6273(02)00936-4. [DOI] [PubMed] [Google Scholar]
- Chen R, Cohen LG, Hallett M. Nervous system reorganization following injury. Neuroscience. 2002;111:761–773. doi: 10.1016/s0306-4522(02)00025-8. [DOI] [PubMed] [Google Scholar]
- Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000;123:940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gally JA. Degeneracy and complexity in biological systems. Proc. Natl. Acad. Sci. USA. 2001;98:13763–13768. doi: 10.1073/pnas.231499798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Petersen SE. Neuroimaging studies of word reading. Proc. Natl Acad. Sci. USA. 1998;95:914–921. doi: 10.1073/pnas.95.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA, Balota DA, Raichle ME, Petersen SE. Effects of lexicality, frequency, and spelling-to-sound consistency on the functional anatomy of reading. Neuron. 1999;24:205–218. doi: 10.1016/s0896-6273(00)80833-8. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Price CJ. Degeneracy and redundancy in cognitive anatomy. Trends Cogn. Sci. 2003;7:151–152. doi: 10.1016/s1364-6613(03)00054-8. [DOI] [PubMed] [Google Scholar]
- Gold BT, Kertesz A. Right hemisphere semantic processing of visual words in an aphasic patient: an fMRI study. Brain Language. 2000;73:456–465. doi: 10.1006/brln.2000.2317. [DOI] [PubMed] [Google Scholar]
- Goldstein K. Der Aufbau des OrganismusEinführung in die Biologie unter besonderer Berücksichtigung der Erfahrungen am kranken Menschen. The Hague: Nijhoff; 1934. [Google Scholar]
- Hagoort P, Indefrey P, Brown C, Herzog H, Steinmetz H, Seitz RJ. The neural circuitry involved in the reading of German words and pseudowords: a PET study. J. Cogn. Neuro sci. 1999;11:383–398. doi: 10.1162/089892999563490. [DOI] [PubMed] [Google Scholar]
- Hagoort P, Wassenaar M, Brown C. Real-time semantic compensation in patients with agrammatic comprehension: electrophysiological evidence for multiple-route plasticity. Proc. Natl Acad. Sci. USA. 2003;100:4340–4345. doi: 10.1073/pnas.0230613100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. [see comments.] Ann. Neurol. 1999;45:430–438. doi: 10.1002/1531-8249(199904)45:4<430::aid-ana3>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Kane A, Tuffiash E, et al. Reperfusion of specific brain regions by raising blood pressure restores selective language functions in subacute stroke. Brain Lang. 2001;79:495–510. doi: 10.1006/brln.2001.2563. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Work M, Barker PB, Jacobs MA, Breese EL, Maurer K. Re-examining the brain regions crucial for orchestrating speech articulation. Brain. 2004;127:1479–1487. doi: 10.1093/brain/awh172. [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Rushworth MF, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl Acad. Sci. USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen-Berg H. Motor physiology: a brain of two halves. Curr. Biol. 2003;13:R802–R804. doi: 10.1016/j.cub.2003.09.049. [DOI] [PubMed] [Google Scholar]
- Karbe H, Thiel A, Weber-Luxenburger G, Herholz K, Kessler J, Heiss WD. Brain plasticity in poststroke aphasia: what is the contribution of the right hemisphere? Brain Lang. 1998;64:215–230. doi: 10.1006/brln.1998.1961. [DOI] [PubMed] [Google Scholar]
- Keinan A, Sandbank B, Hilgetag CC, Meilijson I, Ruppin E. Fair attribution of functional contribution in artificial and biological networks. Neural Computation. 2004;16:1887–1915. doi: 10.1162/0899766041336387. [DOI] [PubMed] [Google Scholar]
- Lashley KS. Brain Mechanisms and Intelligence. Chicago: University of Chicago Press; 1929. [Google Scholar]
- Lee L, Siebner HR, Rowe JB, et al. Acute remapping within the motor system induced by low-frequency repetitive transcranial magnetic stimulation. J. Neurosci. 2003;23:5308–5318. doi: 10.1523/JNEUROSCI.23-12-05308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RG, van Donkelaar P. Mechanisms underlying functional recovery following stroke. Can. J. Neurol. Sci. 1995;22:257–263. doi: 10.1017/s0317167100039445. [DOI] [PubMed] [Google Scholar]
- Liepert J, Hamzei F, Weiller C. Motor cortex disinhibition of the unaffected hemisphere after acute stroke. Muscle Nerve. 2000;23:1761–1763. doi: 10.1002/1097-4598(200011)23:11<1761::aid-mus14>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Marshall J, Newcombe F. Patterns of paralexia: a psycholinguistic approach. J. Psycholinguistic Res. 1973;2:175–198. doi: 10.1007/BF01067101. [DOI] [PubMed] [Google Scholar]
- Mechelli A, Gorno-Tempini ML, Price CJ. Neuroimaging studies of word and pseudoword reading: consistencies, inconsistencies, and limitations. J. Cogn. Neurosci. 2003;15:260–271. doi: 10.1162/089892903321208196. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Friston K, Price C. Effects of visual deprivation on the organisation of the semantic system. Brain. 2003;126:1620–1627. doi: 10.1093/brain/awg152. [DOI] [PubMed] [Google Scholar]
- Phillips JA, Humphreys GW, Noppeney U, Price CJ. The neural substrates of action retrieval: an examination of semantic and visual routes to action. Vision Cogn. 2002;9:662–684. [Google Scholar]
- Price CJ, Friston KJ. Scanning patients with tasks they can perform. Hum. Brain Mapp. 1999;8:102–108. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<102::AID-HBM6>3.0.CO;2-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ, Friston KJ. Degeneracy and cognitive anatomy. Trends Cogn. Sci. 2002;6:416–421. doi: 10.1016/s1364-6613(02)01976-9. [DOI] [PubMed] [Google Scholar]
- Rumiati RI, Humphreys GW. Recognition by action: dissociating visual and semantic routes to action in normal observers. J. Exp. Psychol. Hum. Percept. Perform. 1998;24:631–647. doi: 10.1037//0096-1523.24.2.631. [DOI] [PubMed] [Google Scholar]
- Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol. Rev. 1989;96:523–568. doi: 10.1037/0033-295x.96.4.523. [DOI] [PubMed] [Google Scholar]
- Shallice T. Functional imaging and neuropsychology findings: how can they be linked? Neuroimage. 2003;20(Suppl. 1):S146–S154. doi: 10.1016/j.neuroimage.2003.09.023. [DOI] [PubMed] [Google Scholar]
- Shannon CE, Weaver W. The Mathematical Theory of Communication. Chicago: University of Illinois Press; 1949. [Google Scholar]
- Shimizu T, Hosaki A, Hino T, et al. Motor cortical disinhibition in the unaffected hemisphere after unilateral cortical stroke. Brain. 2002;125:1896–1907. doi: 10.1093/brain/awf183. [DOI] [PubMed] [Google Scholar]
- Thiel A, Herholz K, Koyuncu A, et al. Plasticity of language networks in patients with brain tumors: a positron emission tomography activation study. Ann. Neurol. 2001;50:620–629. doi: 10.1002/ana.1253. [DOI] [PubMed] [Google Scholar]
- Thulborn KR, Carpenter PA, Just MA. Plasticity of language-related brain function during recovery from stroke. Stroke. 1999;30:749–754. doi: 10.1161/01.str.30.4.749. [DOI] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl Acad. Sci. USA. 1994;91:5033–5037. doi: 10.1073/pnas.91.11.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM. Measures of degeneracy and redundancy in biological networks. Proc. Natl Acad. Sci. USA. 1999;96:3257–3262. doi: 10.1073/pnas.96.6.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Isensee C, Rijntjes M, et al. Recovery from Wernicke's aphasia: a positron emission tomographic study. Ann. Neurol. 1995;37:723–732. doi: 10.1002/ana.410370605. [DOI] [PubMed] [Google Scholar]